Introduction
Squamous cell carcinoma of the head and neck (SCCHN)
is the 6th most common cancer type worldwide (1). The majority of cancers of the upper
aerodigestive tract are of squamous cell origin (2). In Germany, >15,000 new cases of
oral, pharyngeal and laryngeal carcinoma are reported each year
(3). It is well-known that tobacco
and alcohol consumption, as well as human papillomavirus (HPV)
infection are associated with the development SCCHN.
The epidermal growth factor receptor (EGFR) is a
basic cellular regulator of essential functions that regulate the
survival, migration and proliferation of cells. EGFR signaling is
impaired in various cancers. In SCCHN, EGFR is found to be
overexpressed in 90% of cases (4).
Its overexpression is an early event in SCCHN tumorgenesis and
correlates with poor prognosis (4,5). Over
the past decade, the knowledge of EGF overexpression in these
tumors has led to the introduction of antibodies targeting EGFR for
the treatment of head and neck cancer. For example, drugs such as
the EGFR antibody, Cetuximab© or the tyrosine kinase
inhibitors, Gefitinib© and Erlotinib©, target
EGFR and are often used in SCCHN therapy (6). The EGF ligand stimulates the
proliferation of keratinocytes. Therefore, EGF promotes DNA
synthesis and the progression from the G1 to the S phase of the
cell cycle (7). Another mediator of
the cell cycle is serotonin (5-HT). 5-HT is a growth factor, which
regulates DNA synthesis. The mitogenic effect of 5-HT is thought to
act via 5-HT1A and 5-HT1B (8).
In SCCHN therapy, platinum agents (cisplatin and
carboplatin), taxanes (docetaxel) and antimetabolic agents
(5-fluorouracil) are commonly used as chemotherapeutic drugs. These
cytostatic drugs affect cells in the S or M phase. One challenge of
SCCHN chemotherapy is that a small percentage of tumor cells do not
proliferate (9). These cells arrest
in the G0 phase of the cell cycle and are not affected by
chemotherapy. This could be one reason for tumor recurrence at a
later date. The recruitment of these G0-arresting cells into the
active cell cycle and thus, proliferation, may increase the
efficacy of chemotherapeutic agents.
Former in vitro experiments performed by
Hambek et al(10), have
provided evidence that the treatment of tumor cells with EGF and
5-HT can decrease the amount of dormant G0/G1 cells, resulting in
more active, dividing cells that are consequently more sensitive to
chemotherapeutic treatment. The aim of this study was to
investigate whether tumor cell stimulation with EGF and 5-HT can
affect tumor growth in xenografts.
Materials and methods
Cell culture
Detroit 562 cells (CCL-138; American Type Culture
Collection) were cultured in Eagle’s minimum essential medium (10%
FCS, 0.5 mM sodium pyrovate, 25 mg gentamycin) at 37°C and 5%
CO2. For injection, cells were detached with Accutase
(PAA Laboratories) and the concentration of living cells was
determined using Cedex XS cell counter (Innovatis). The cells were
diluted in Lactated Ringer’s solution in a concentration of
5×106 cells/0.1 ml. The injection solution was
transferred on ice to where the animals were housed.
Mice, tumor xenografts and treatment
Mice were housed in a pathogen-free facility for a
12-h light-dark cycle and with free access to food and water.
Six-week-old female NMRI-Foxn1nu mice (Harlan) were anesthetized
with forane (Baxter) evaporated with Dräger Forena Vapor (19.3).
Five million cells (100 μl) were subcutaneously (s.c.) injected
into the flank of each mouse. One day after tumor cell injection,
treatment was performed with 15 μg EGF (murine EGF; mEGF) (315-09;
PeproTech), human EGF (hEGF) (100-009; RELIATech GmbH), or 200 μg
serotonin (B21263; Alfa Aesar). The control mice were treated with
Lactated Ringer’s solution. Each treatment group consisted of 5
mice. Mice were treated as described above, daily for a period of
10 days. The tumor size was measured on days 4, 8 and 12 after
tumor cell injection using a digital caliper. The tumor volume was
calculated with the following formula: V = π/6 × length ×
width2. All mice were sacrificed by the 12th day after
tumor cell transplantation or before the tumors ulcerated.
Staining
After sacrifice, tumors were etched. One tumor was
directly frozen in liquid nitrogen and the second was fixed in
Notoxhisto (Quartett) and embedded in paraffin. Ki67 and EGFR
staining was performed on the frozen sections. Immunohistological
staining for CD31 was carried out on the paraffin-embedded
sections. CD31 is a marker for lymphatic and blood vessels. Ki67
(rabbit, dilution 1/200) (Ki681C01; DCS), EGFR (rat, dilution
1/200) (ab231; Abcam) and CD31 (rat, dilution 1/20) (DIA-310;
Dianova) primary antibodies were used for the staining procedure.
Incubation was carried out for 1 h at room temperature. Afterwards,
we proceeded with the DCS Detection Line system (AD050POL-K,
PD000POL-R), according to the supplier’s instruction. Staining was
performed with DAB reagent (DC137C100). The Fuchsin
Substrate-Chromogen system (K0625; Dako) and HistoGreen (E109;
Linaris). Images were taken under a Zeiss Axioplan 2 with an
AxioCam ICc1 camera. Statistical analysis was performed with BIAS
for windows version 9.12 using one-way ANOVA. The animal
experiments were approved by Regierungspräsidium Darmstadt, Hessen
F66/08.
Results
Increased volume in EGF-treated
tumors
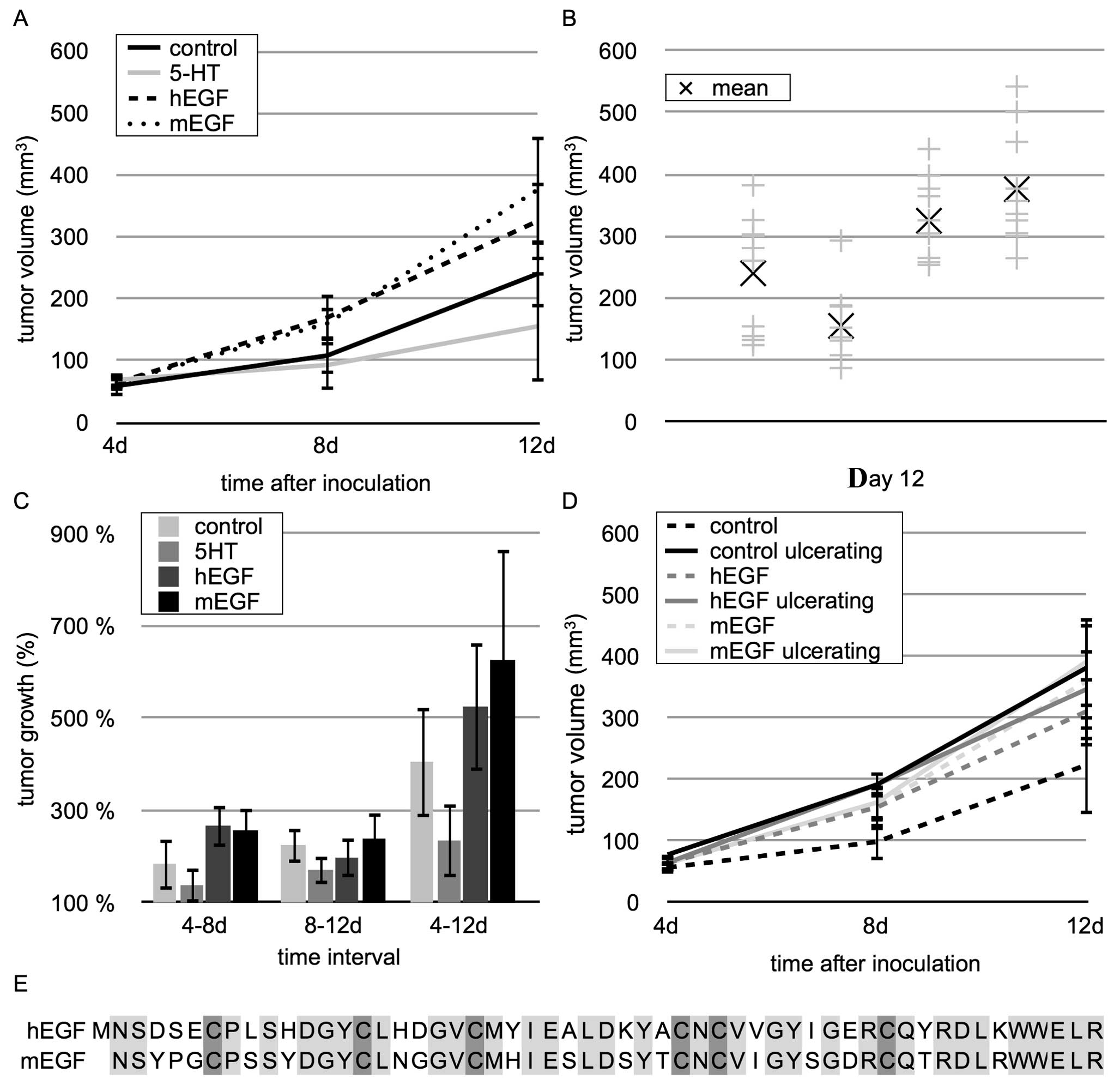
The daily injection regime of EGF led to an enhanced
tumor volume in both groups of mEGF- and hEGF-injected mice
(Fig. 1A). After 12 days, the mean
tumor volume in the hEGF-treated mice reached 325±63
mm3, whereas in the control mice, the mean tumor volume
was only 240±89 mm3. The mean tumor volume in the
mEGF-treated mice was 376±88 mm3, which was comparable
to that of the hEGF-treated mice (Fig.
1B).
In all tumors, the rate of intratumoral connective
tissue was constant at 33–67% of the tumor tissue (±4%, data not
shown) indicating that growth increase was caused by tumor
proliferation and not by edema or an increase in connective tissue.
The maximal growth increase was measured between days 4 and 8
(Fig. 1C). During this time,
hEGF-stimulated tumors grew by 267±46%, the tumors of the
mEGF-treated mice grew by 257±47% and the control tumors only
increased by 185±38%; however, this did not reach statistical
significance (p>0.05). The tumors of the 5-HT-treated mice
showed a lower growth increase compared to the control tumors and
reached a final volume of 155±54 mm3 (Fig. 1A).
EGF treatment increases the risk of tumor
ulceration
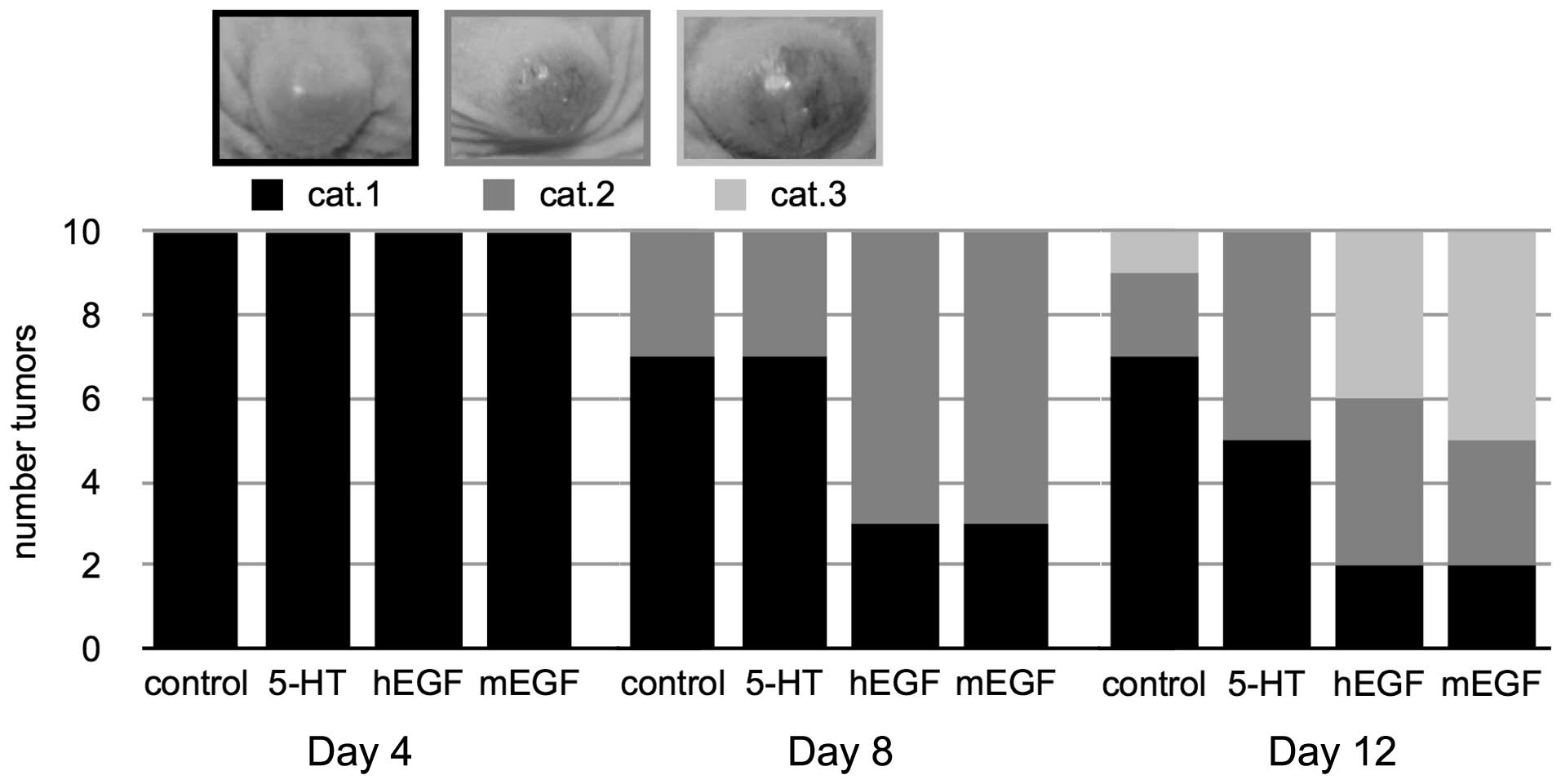
The experiment was terminated by the 12th day or
when tumor ulceration occured. After this treatment period, 1
control tumor, 4 tumors from the hEGF-treated mice and 5
mEGF-treated tumors had ulcerated (Fig.
2). This destruction of the surface epithelium is a common
event in SCCHN patients (11) and
also in xenograft models. We graded the ulceration process into 3
categories. At the beginning, the tissue beyond the tumor was
unremarkable or showed a rose shading and was assigned to category
1 (cat. 1). Category 2 (cat. 2) was characterized by red marbling
and violet spots under the skin. Finally, tumor ulceration occured
[category 3 (cat. 3)]. In our experiment, the tumors of the
EGF-treated mice ulcerated more frequently than those of the
control group.
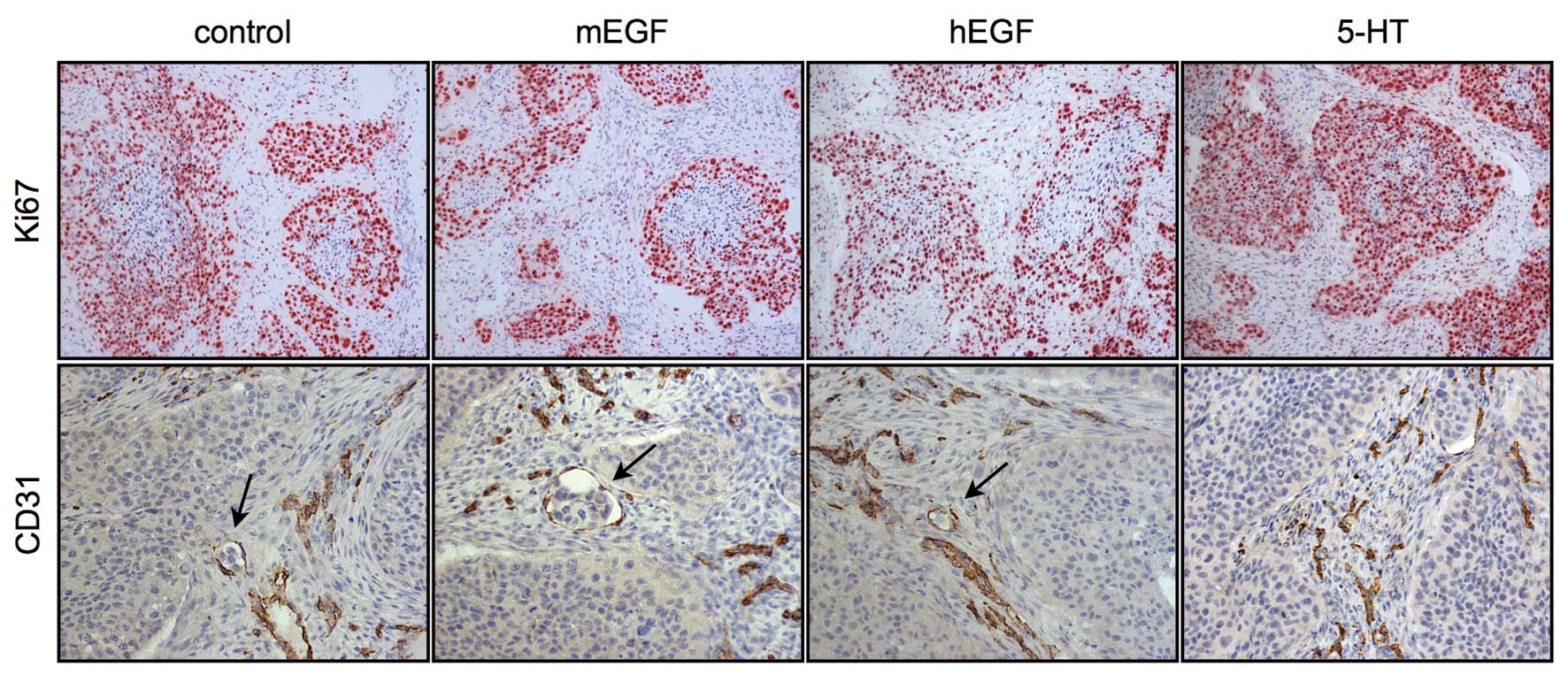
The amount of vessels in the intratumoral connective
tissue remained equal in the control and hEGF-treated tumors (6±4%,
data not shown) (sample images shown in Fig. 3). In the 5-HT-treated mice, the
tumor ulceration rate was comparable to that of the control mice.
We did not observe any vessel invasion in this group (Fig. 1D).
The majority of xenograft tumor cells
express EGFR and Ki67
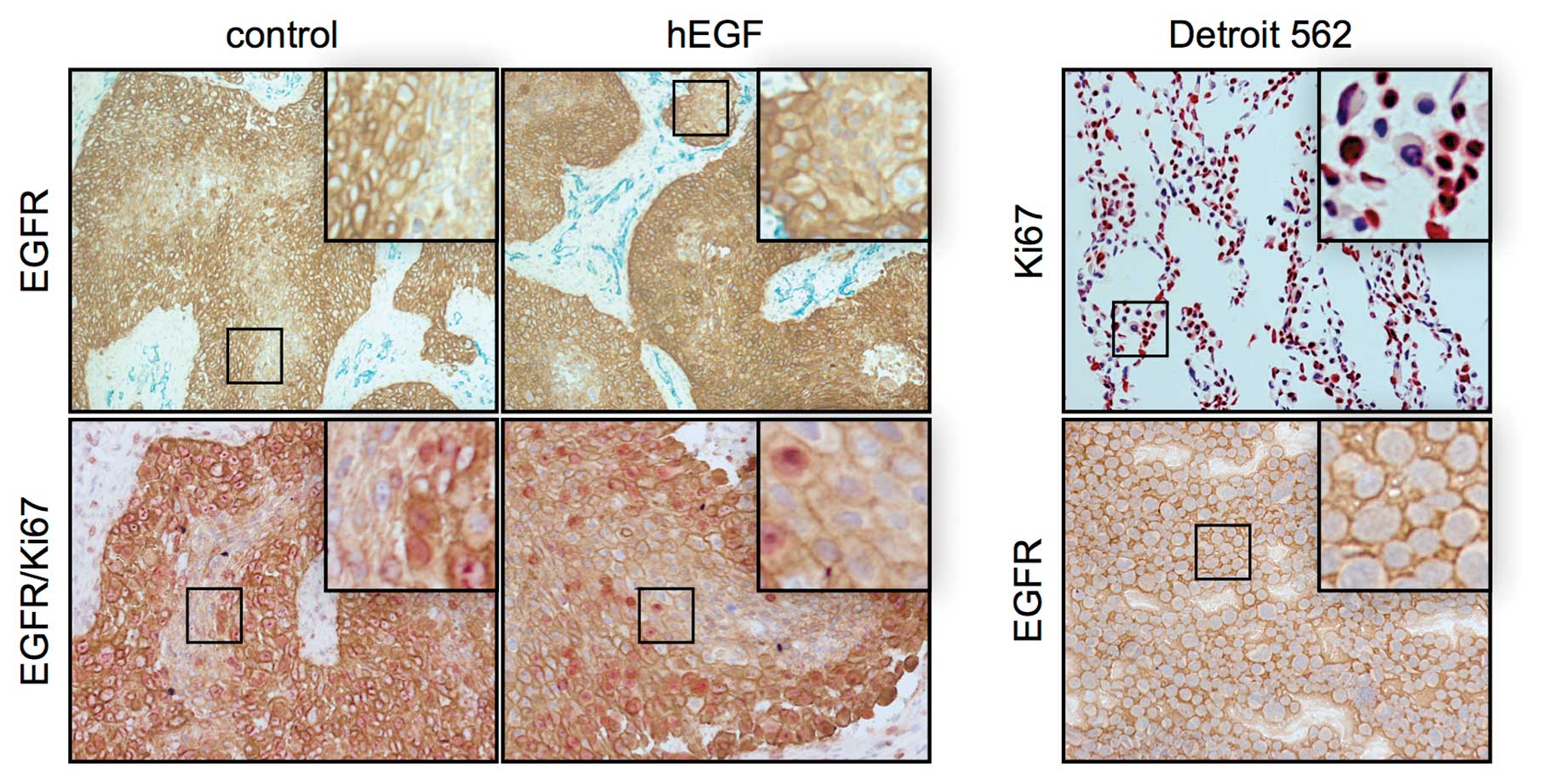
Detroit 562 cells overexpress EGFR (12). Immunohistological staining of EGFR
indicated that high levels of EGFR were present in the cell
cultures and tumor xenografts (Fig.
4). Almost all tumor cells expressed EGFR. In
vitro-cultured Detroit 562 cells had an equal amount of EGFR in
each cell. In the mouse xenografts, the level of EGFR expression
varied in the cells. The EGFR expression seemed to depend on the
localization of the cell. Tumor cells that were located next to the
necrotic core of the tumor cell nests had lower quantities of EGFR.
By contrast, most cells at the borders had a high EGFR expression.
The reduction in EGFR was potentially caused by necrosis or tumor
cell differentiation. In the skin, for instance, the amount of EGFR
is reduced during the differentiation process (7).
Seventy-four percent of the in vitro-cultured
Detroit 562 cells were Ki67-positive (Fig. 4). In the tumor xenografts, the
majority of tumor cells was also Ki67-positive. The results showed
that these cells were in the active cell cycle. However, a number
of Ki67-negative cells was localized in the middle of the tumor
cell nests. These cells were supposed to be quiescent in the G0
phase. The size of cell nests correlated with the number of inner
Ki-67-negative cells. In the EGF-stimulated mice, the number of
Ki-67-positive cells was much higher.
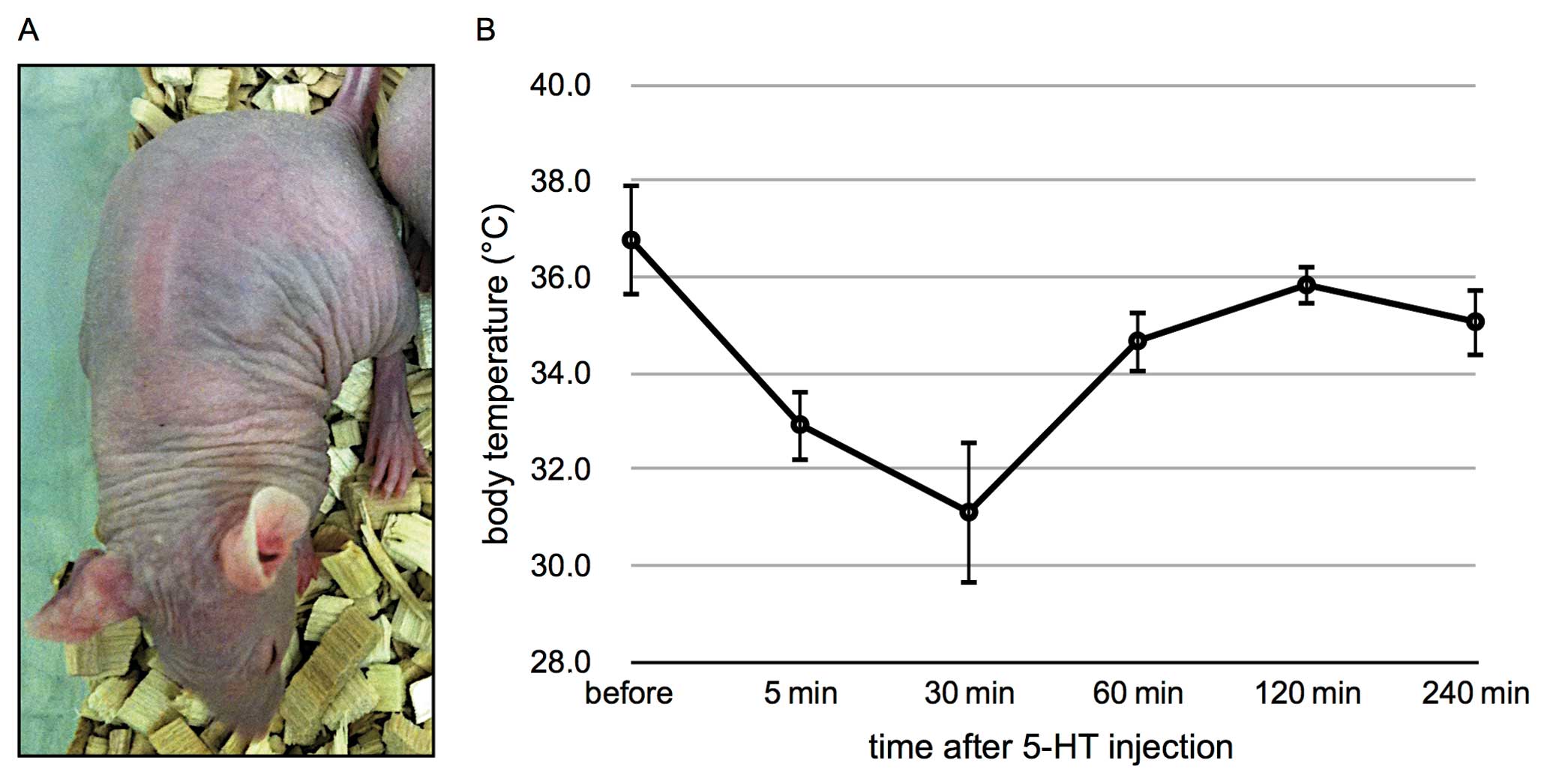
The 5-HT-treated mice presented with several severe
side-effects, such as depressed behavior, tremors, respiratory
depression and sporadic diarrhea. The skins of the mice had also
turned blue (Fig. 5A). Recovery
from these side-effects was observed 1 h following treatment.
Abdominal measurement of body temperature documented a decrease in
body temperature to 31°C within 30 min (Fig. 5B). Two hours after injection, normal
body temperature was measured. This was not observed in the
EGF-stimulated mice.
Discussion
Chemotherapeutic drugs affect cells in the S or M
phase. One challenge of SCCHN chemotherapy is that a small
percentage of tumor cells do not proliferate (9), and thus arrest in the G0 phase of the
cell cycle. Therefore, these cells are not affected by
chemotherapy. This could be one reason for tumor recurrence at a
later date. The recruitment of these G0 cells into the active cell
cycle and thus, proliferation, may increase the efficacy of
chemotherapeutic agents.
In this study, we aimed to recruit non-cycling tumor
cells into the mitotic cycle in order to sensitize them to
chemotherapeutic agents. The majority of tumor cells express EGFR.
They all have the potential to process the EGF signal. Our results
showed that EGF stimulation enhanced tumor volume, indicating that
the application of extra EGF had a proliferative effect on tumor
cells. This effect did not differ in the hEGF- and mEGF-injected
mice, a fact which could be explained with the homologous amino
acid sequences. The increased tumor volume did not reach
significance, when compared to the control tumors, a fact which
could be caused by the small number of mice treated, the short
treatment interval and the way of application or dosaging. The
majority of resting cells was located in the center of the cell
nests. Further studies are required in order to examine these cells
in more detail. For tumor therapy, cells that can re-enter the cell
cycle are the main focus of interest, as these cells can survive
chemotherapy and lead to cancer recurrence. The arrest in the cell
cycle may be caused by the large distance from the blood vessels.
Consequently, chemotherapeutic agents cannot reach these cells. A
treatment procedure containing various cycles of EGF and cytostatic
drugs may potentially prove to be more effective.
EGFR is a multifunctional receptor involved in
proliferation, motility, angiogenesis and survival of tumor cells
(13). Three major pathways,
PI3K/Akt, Ras/Raf/MEK/MAPK and PLC/PKC, are involved in signal
transduction (7). Further analysis
will show which signaling cascade is activated. EGF-stimulated
xenografts have a higher risk of ulceration. It is hypothesized
that EGF application increases tumor cell invasiveness.
The amplitude of growth increase depends on the
number of EGF receptors on the cell surface (14). Compared with A431, Detroit 562
tumors have a 3.6-fold lower EGFR-binding activity (12,14),
which may result in lower growth induction compared to A431
xenografts.
Our results are consistent with the findings of
Ozawa et al(14) and
Ginsburg and Vonderhaar (15),
which showed that EGF treatment stimulated the growth of SCCHN
tumor xenografts, showing an increased tumor volume in the A431, NA
and Ca9-22 cell line mouse xenografts after treatment with murine
EGF. Factors such as gender-specific EGF host production (16), treatment interval, dosage and the
way of application (osmotic pump or injection) affect the results.
All EGFR ligands own the conserved EGF motive. It is characterized
by 6 cysteines that form disulfide bridges with each other
(17). The length of amino acid
sequences between the cysteines of hEGF and mEGF is identical
(CX7CX5CX10CXCX8C, X could be any amino acid). Furthermore, the
recombinant EGFs have 70% sequence homology (Fig. 1E). The tumors of the 5-HT-treated
mice showed a lower growth increase compared to the control tumors.
Pratesi et al(18) described
an increase in tumor growth after administration of 200 μg/day 5-HT
delivered by osmotic mini-pumps in small lung cancer cell
xenografts. 5-HT could have a dose-dependent effect, showing an
inhibition of tumor growth when administered at lower doses (20
μg/day). One possible reason for tumor growth decrease could
possibly be the reduction of blood flow in tumor vessels by 5-HT
which impairs oxygen supply (19).
5-HT-treatment possibly does inhibit tumor growth via impact on
tumor-feeding vessels. The decrease in tumor blood flow and,
consequently, a deficit in oxygen and nutrient supply reduce the
proliferation tempo (8). This
effect would overcome the prospective pro-mitotic effect of 5-HT on
tumor growth. 5-HT is not useful for our strategy to enhance
proliferation. Our observations suggest vertical invasion of
tumors. A possible active migration of tumor cells into the skin
could destroy its structure. The administration of EGF may lead to
an increased tumor cell motility. Seventy-four percent of the in
vitro-cultured Detroit 562 cells were Ki67-positive (Fig. 4). In tumor xenografts, the majority
of tumor cells was also Ki67-positive. The results indicate that
these cells are in the active cell cycle. A number of Ki67-negative
cells was localized in the middle of the tumor cell nests. These
cells were supposed to be quiescent. In the EGF-stimulated mice,
the number of Ki-67 positive cells was much higher. The size of
cell nests correlated with the number of inner Ki-67-negative
cells. It is possible that these resting cells are more
differentiated. Another explanation could be that they do not have
enough resources for mitosis. Oxygen diffuses into tissue and
reaches a distance of approximately 100 μm. Due to the large
distance to the feeding arterioles, these cells may stop cell
cycling to prolong their survival (20). Most Ki67-positive cells also express
high levels of EGFR. The Ki67-negative cells have reduced levels of
EGFR.
In conclusion, in the present study we show that it
is possible to stimulate tumor cells by EGF, and thus enhance cell
proliferation, resulting in a higher tumor growth compared to the
untreated control group. In our future investigations, we plan to
include a higher number of mice and an adjustment of the EGF
dosage, considering the heterogeneity of SCCHN tumors. Furthermore,
we plan to treat EGF-stimulated SCCHN mice with chemotherapeutic
drugs to investigate whether these mice show a better responce to
therapy compared to a non-stimulated control group. These data may
result in new clinical, stratified therapy regimes.
Acknowledgements
We thank Dr Alf Theisen and Erika Weith for their
excellent technical support. This project was supported by a grant
(Young Investigator Award) of the Medical School, Goethe
University.
References
|
1
|
Matzinger O, Zouhair A, Mirimanoff RO and
Ozsahin M: Radiochemotherapy in locally advanced squamous cell
carcinomas of the head and neck. Clin Oncol (R Coll Radiol).
21:525–531. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leemans CR, Braakhuis BJ and Brakenhoff
RH: The molecular biology of head and neck cancer. Nat Rev Cancer.
11:9–22. 2011. View
Article : Google Scholar
|
|
3
|
Robert Koch Institut. Krebs in Deutschland
2005/2006. Häufigkeiten und Trends. Westkreuz-Druckerei; Berlin:
pp. 24–44. 2010
|
|
4
|
Argiris A, Karamouzis MV, Raben D and
Ferris RL: Head and neck cancer. Lancet. 371:1695–1709. 2008.
View Article : Google Scholar
|
|
5
|
Cohen EE: Role of epidermal growth factor
receptor pathway-targeted therapy in patients with recurrent and/or
metastatic squamous cell carcinoma of the head and neck. J Clin
Oncol. 24:2659–2665. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sharafinski ME, Ferris RL, Ferrone S and
Grandis JR: Epidermal growth factor receptor targeted therapy of
squamous cell carcinoma of the head and neck. Head Neck.
32:1412–1421. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jost M, Kari C and Rodeck U: The EGF
receptor - an essential regulator of multiple epidermal functions.
Eur J Dermatol. 10:505–510. 2000.PubMed/NCBI
|
|
8
|
Vicaut E, Laemmel E and Stücker O: Impact
of serotonin on tumour growth. Ann Med. 32:187–194. 2000.
View Article : Google Scholar
|
|
9
|
Williams GH and Stoeber K: The cell cycle
and cancer. J Pathol. 226:352–364. 2012. View Article : Google Scholar
|
|
10
|
Hambek M, Werner C, Baghi M, Gstöttner W
and Knecht R: Enhancement of docetaxel efficacy in head and neck
cancer treatment by G0 cell stimulation. Eur J Cancer.
43:1502–1507. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kurago ZB, Lam-ubol A, Stone B and
Stetsenko A: Cancer-supporting factors consistently induced by
lipopolysaccharide - squamous cell carcinoma - monocyte
interactions. Arch Otolaryngol Head Neck Surg. 132:9062006.
View Article : Google Scholar
|
|
12
|
Bozec A, Lassalle S, Gugenheim J, Fischel
JL, Formento P, Hofman P and Milano G: Enhanced tumour
antiangiogenic effects when combining gefitinib with the
antivascular agent ZD6126. Br J Cancer. 95:722–728. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kalyankrishna S and Grandis JR: Epidermal
growth factor receptor biology in head and neck cancer. J Clin
Oncol. 24:2666–2672. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ozawa S, Ueda M, Ando N, Abe O, Hirai M
and Shimizu N: Stimulation by EGF of the growth of EGF
receptor-hyperproducing tumor cells in athymic mice. Int J Cancer.
40:706–710. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ginsburg E and Vonderhaar BK: Epidermal
growth factor stimulates the growth of A431 tumors in athymic mice.
Cancer Lett. 28:143–150. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stern LE, Falcone RA Jr, Kemp CJ, Braun
MC, Erwin CR and Warner BW: Salivary epidermal growth factor and
intestinal adaptation in male and female mice. Am J Physiol
Gastrointest Liver Physiol. 278:G871–G877. 2000.PubMed/NCBI
|
|
17
|
Schneider MR and Wolf E: The epidermal
growth factor receptor ligands at a glance. J Cell Physiol.
218:460–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pratesi G, Cervi S, Balsari A, Bondiolotti
G and Vicentini LM: Effect of serotonin and nicotine on the growth
of a human small cell lung cancer xenograft. Anticancer Res.
16:3615–3619. 1996.PubMed/NCBI
|
|
19
|
Shrivastav S, Joines WT and Jirtle RL:
Effect of 5-hydroxytryptamine on tissue blood flow and microwave
heating of rat tumors. Cancer Res. 45:3203–3208. 1985.PubMed/NCBI
|
|
20
|
Krishnamurthy S, Dong Z, Vodopyanov D,
Imai A, Helman JI, Prince ME, Wicha MS and Nör JE: Endothelial
cell-initiated signaling promotes the survival and self-renewal of
cancer stem cells. Cancer Res. 70:9969–9978. 2010. View Article : Google Scholar : PubMed/NCBI
|