Introduction
Head and neck squamous cell carcinoma (HNSCC)
represents the sixth most common cancer in developed countries and
is responsible for approximately 350,000 cancer deaths per year
(1). In addition, the incidence of
oral tumors is increasing around the world (2). Despite recent advances in early
detection and diagnosis, current treatments of oral cancer are
unsatisfactory mainly due to the development of distant metastasis
and the emergence of treatment-resistant recurrences, resulting in
an unchanged 5-year survival rate over the last two decades
(3,4). It is now established that tumor
persistence and recurrence could be due to the presence of cancer
stem cells (CSCs) in some cancers. The cancer stem cell theory of
carcinogenesis postulates that normal stem or progenitor cells can
eventually become malignant because of the occurrence of genetic
and epigenetic alterations (5).
Based on their similarity to normal stem cells, CSCs exhibit
extensive capability of self-renewal and are likely to be more
resistant to therapy (5). Moreover,
they have the ability to generate a tumor after injection of a very
small number of cells and to reconstitute the cellular
heterogeneity typical of the original tumor (6,7). The
presence of CSCs has now been evidenced in solid tumors from
various origins including head and neck (5,8,9). This
rare HNSCC CSC population can be isolated by using cell surface
antigens such as CD44 (8) or CD133
(10). The propensity of CSCs to
efflux the vital DNA binding Hoechst 33342 dye, due to an increased
expression of multiple drug resistance transporter proteins, has
also been used as a means to isolate them as a side population (SP)
(6). Alternatively, HNSCC CSCs can
be isolated on the basis of the expression of the aldehyde
dehydrogenase 1 (ALDH1) enzymatic activity (11,12),
which is now recognized as a CSC marker in tumors from various
origins (13). This last method was
described as more specific as the isolated cells appear to be more
tumorigenic than the CD44+ CSCs (12).
Although primary cancer cells obtained from patients
represent the most physiologically relevant material to isolate
CSCs, this source presents two main disadvantages. First, it
constitutes a very limited resource due to the relatively small
size of head and neck tumors and the small percentage of CSCs
within the tumor. Second, the human cells need to be administered
in immunocompromised animals and, depending on the degree of host
immunocompetence, these xenotransplantation assays have been shown
to underestimate or overestimate the frequency of cells with
tumorigenic potential (14).
In the present study, the objective was to isolate
ALDH1high cells in a murine established HNSCC cell line
and to determine if these cells would exhibit cancer stem cell-like
properties. In addition, we examined the effect of a hypoxic
environment on the CSC population in vitro.
Materials and methods
Cell culture
The SCC-VII cell line is derived from murine oral
squamous cell carcinoma and is able to induce progressive tumors in
syngeneic hosts. SCC-VII cells were maintained at 37°C under 5%
CO2, in Dulbecco’s modified Eagle’s medium (Invitrogen,
Cergy Pontoise, France) supplemented with 10% fetal calf serum
(FCS) (Dutscher, Brumath, France), penicillin (100 U/ml) and
streptomycin (100 μg/ml).
The oralspheres (SCC-VII CSCs) were cultured on
ultra-low attachment flasks (Corning, Sigma-Aldrich) in serum-free
DMEM/F12 medium (Invitrogen) with penicillin (100 U/ml) and
streptomycin (100 μg/ml) supplemented with 10 ng/ml bFGF
(Peprotech), 20 ng/ml EGF (Peprotech), 4 μg/ml, heparin
(Sigma-Aldrich), 4 mg/ml bovine serum albumin (Sigma-Aldrich), 20
μg/ml insulin (Sigma-Aldrich) and N2 supplement (Invitrogen).
Oralspsheres were dissociated mechanically and enzymatically, using
Accumax reagent (Sigma-Aldrich). This reagent that combines
protease, collagenolytic and DNase activities was used to generate
single cell suspension for ALDH1 labeling and flow cytometry
analysis.
Hypoxia/normoxia
In normoxia, the oralspheres were grown in a
humidified 21% O2 and 5% CO2 environment. For
hypoxia experiments, the oralspheres were grown in a humidified 1%
O2 and 5% CO2 environment using a MiniGalaxy
A incubator (RS Biotech, Ltd). The medium was replaced twice per
week after being prewarmed and equilibrated for 4 h in 1%
O2.
ALDH1high cell selection
The ALDEFLUOR kit (StemCell Technologies SARL,
Grenoble, France) was used to isolate the cell population with a
high aldehyde dehydrogenase 1 (ALDH1) enzymatic activity. Single
cell suspensions obtained from freshly trypsinized SCC-VII cells or
dissociated oralspheres were suspended in ALDEFLUOR assay buffer.
Then, 5 μl of ALDH substrate (BAAA) were added to the cell
suspension and the samples were incubated during 40 min at 37°C. As
a negative control for each sample of cells, an aliquot was treated
with 5 μl of diethylaminobenzaldehyde (DEAB), a specific ALDH
inhibitor. Fluorescence-activated cell sorting (FACS) was used to
analyze the ALDH1high cell population. The sorting gates
were established using the ALDEFLUOR-stained cells treated with
DEAB.
Animals and tumor model
Six- to eight-week-old female C3H/HeOuJ mice
(Charles River, L’arbresle, France) were used to assess the in
vivo stem cell properties of the ALDH1high
population, compared to the ALDH1low population. All the
surgical procedures and the care given to the animals were
performed in accordance with institutional guidelines. After
anesthetization of the mice, 1,000 or 250 ALDH1high or
ALDH1low SCC-VII cells (50 μl) mixed with 50 μl of
Matrigel (BD Biosciences, NJ, USA) were injected subcutaneously
using a 25-gauge needle. Tumor growth was monitored and after
visual detection, the mice were sacrificed and tumor formation was
assessed. Tumor volumes were estimated using the formula: length ×
width2 × π/6 (15).
Tumor dissociation
After excision, the tumors were washed in PBS and
DMEM-F12 containing the penicillin/streptomycin (500 U/ml) and
amphotericine B (1.25 μg/ml). Then, each tumor was cut into small
fragments using sterile scissors and incubated at 37°C with DMEM
F-12 containing 1.5 mg/ml collagenase I, 20 μg/ml hyaluronidase and
0.006% of DNase I. After 1 h of digestion, cells were filtered
first through a 100-μm nylon sieve, followed by centrifugation at
1,000 rpm for 5 min and then filtered through a 40-μm nylon sieve
and centrifuged again. The pellet was suspended in supplemented
serum-free DMEM/F12 medium, as described above for culture of
oralspheres.
Statistical analysis
The results are expressed either as median [95%
confidence intervals (CI)] or as mean ± standard error. Comparison
of tumor volumes between different mice groups was performed using
the Mann-Whitney test, which is a non-parametric, two-tailed
probability test. P-values were considered to be statistically
significant when <0.05.
Results
Isolation of ALDH1high cells
from the murine SCC-VII squamous cell carcinoma cell line
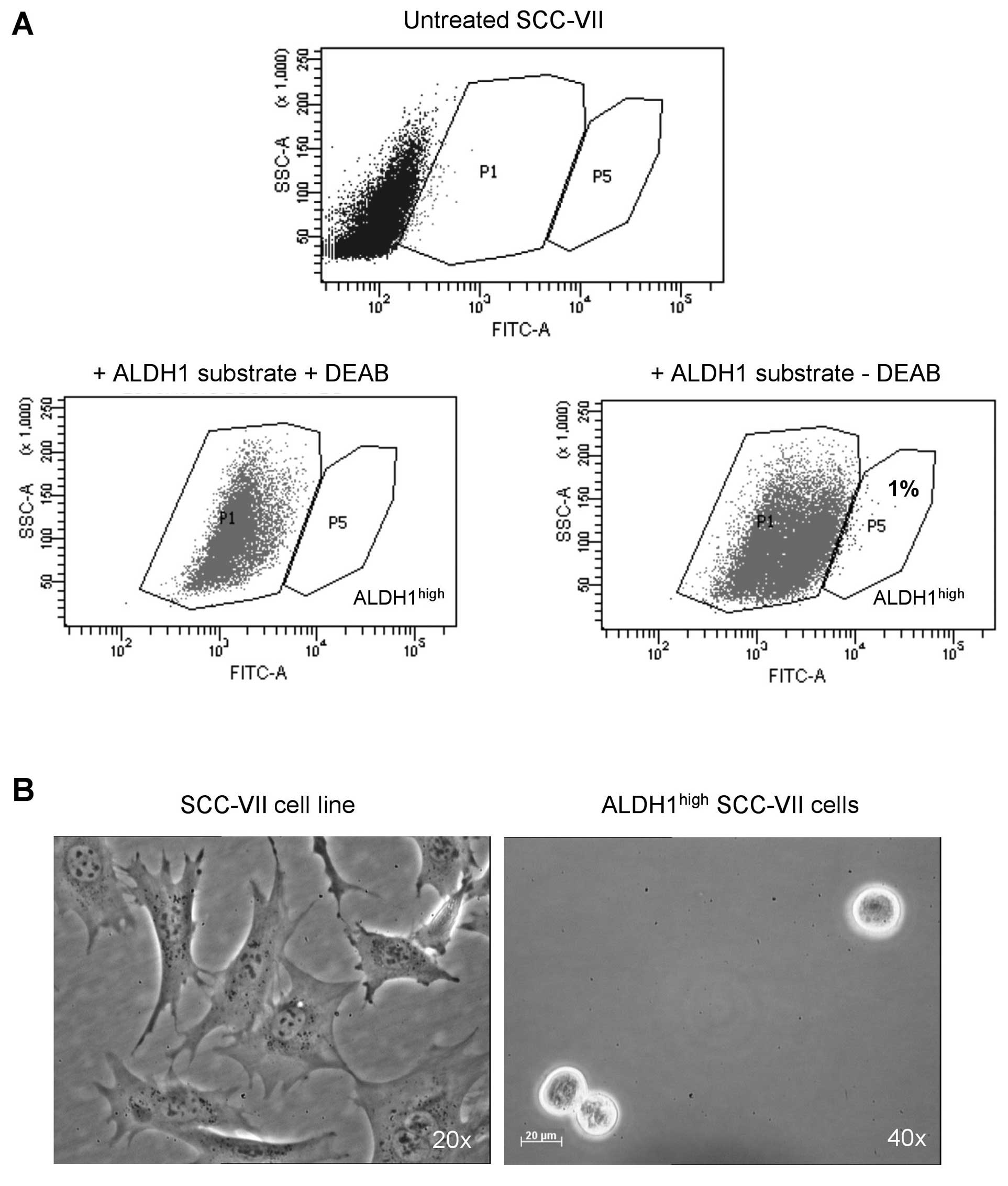
Using the ALDEFLUOR assay and FACS analysis,
ALDH1high and ALDH1low cells were isolated
from the SCC-VII cell line. Untreated cells exhibited some
autofluorescence and the entry of the fluorescent ALDEFLUOR
substrate within the cells shifted them to the P1 gate (Fig. 1A). In the presence of the ALDH1
enzyme, substrate cleavage increased its fluorescence, shifting the
cells to the P5 gate, which was not observed in the presence of
DEAB, the ALDH1 inhibitor. As expected, the majority of SCC-VII
cells exhibited low ALDH1 activity, with the percentage of
ALDH1high cells being 1±0.6%. Following sorting,
ALDH1high cells were cultured in suspension in
serum-free medium with bFGF and EGF. As shown in Fig. 1B, the ALDH1high cells
were able to grow as spheres in this medium.
Tumorigenicity of ALDH1high
and ALDH1low cells in syngeneic animals
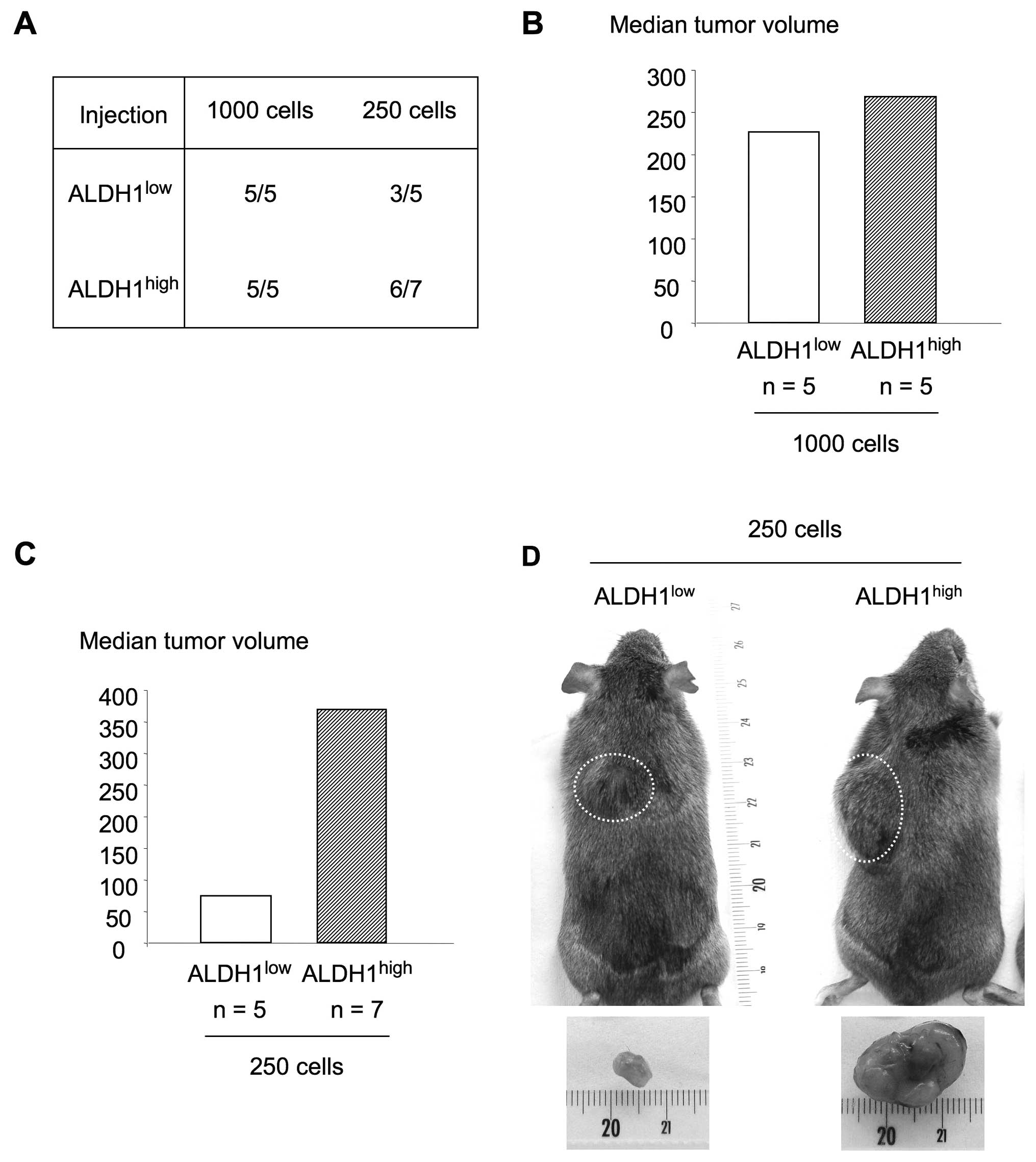
To evaluate the tumorigenicity of
ALDH1high and ALDH1low cells, the two cell
populations were injected subcutaneously in two different doses
(1,000 or 250 cells) in syngeneic C3H mice immediately after
sorting. Fig. 2A shows tumor
occurrence according to ALDH1 expression and number of injected
cells. At the highest cell number, both ALDH1high and
ALDH1low cells were able to induce tumors in 100% of
cases. The median tumor volumes were not statistically different
with 226.1 and 267.9 mm3 when generated by
ALDH1low and ALDH1high cells, respectively
(Fig. 2B). At the lowest number of
injected cells, tumors were developed in 3 out of 5 mice after
administration of ALDH1low cells and in 6 out of 7 mice
in the case of ALDH1high cells. Although the differences
in tumor size were not statistically significant, a 5-fold increase
in the median volume of tumors generated by ALDH1high
cells (368.4 mm3; 95% CI, 118–784) compared to the ones
generated by ALDH1low cells (74.2 mm3; 95%
CI, 0–333.4) was observed (Fig. 2C and
D). Taken together, these results suggest that
ALDH1high cell population is enriched in CSCs.
In vitro selection of oralspheres from
ALDH1high derived-tumors
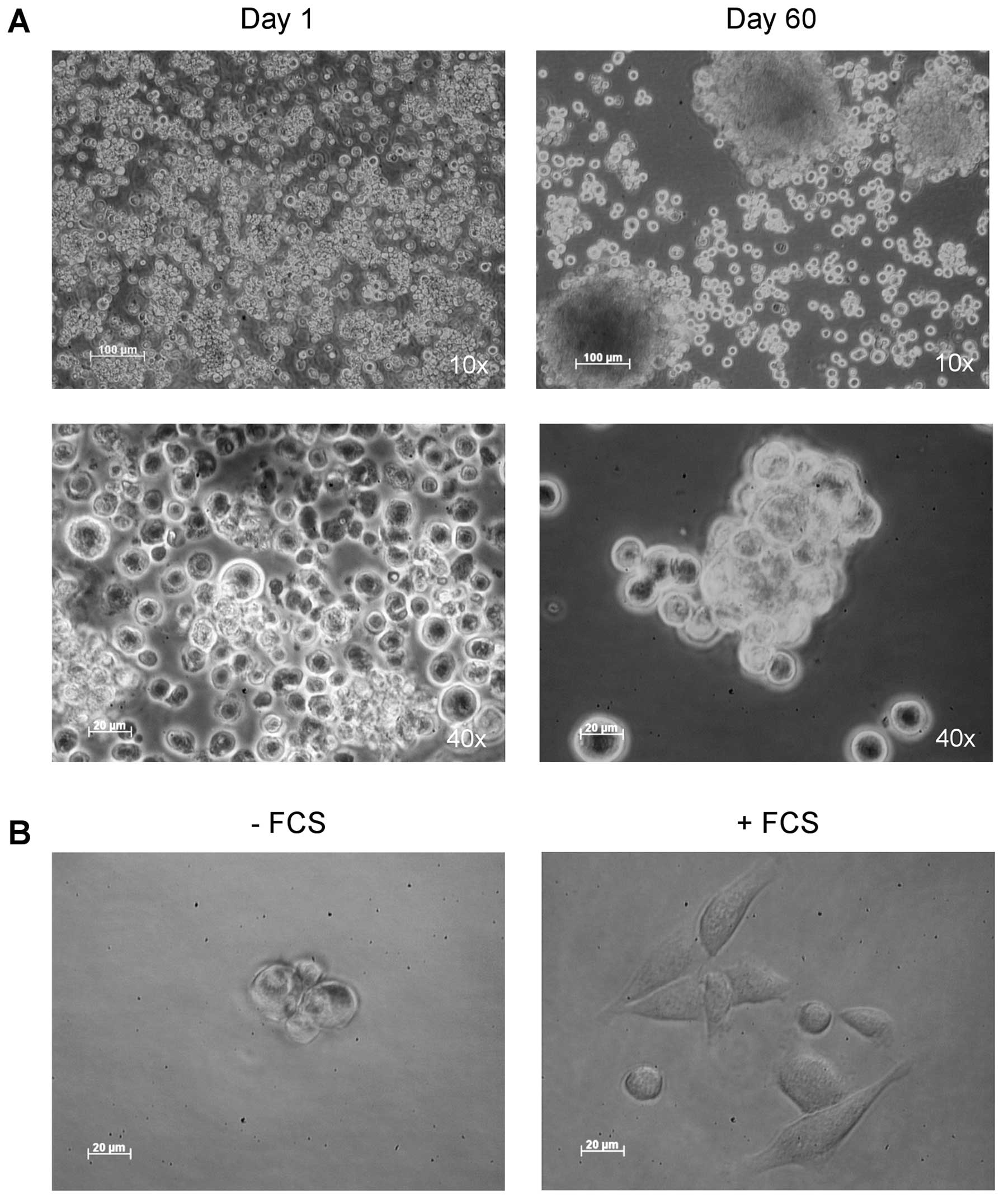
Following tumor dissociation, the cell suspension
was cultured in serum-free medium with bFGF and EGF. Spheres, which
we called oralspheres, formed by cells aggregates were able to grow
exponentially in these conditions (Fig.
3A). To determine the differentiation potential of these cells,
oralspheres were then cultured in standard medium in the presence
of 10% serum. After 24 h of culture, undifferentiated cells in
suspension attached to the plastic, gradually migrating from
oralspheres and differentiating into large and adherent cells
(Fig. 3B).
The percentage of ALDH1high cells was
determined in oralspheres, following an in vivo passage,
after 3 months of culture in serum-free medium. As shown in
Fig. 3C, a slight increase in this
percentage was observed with averages of 1% for the SCC-VII cell
line and 3% for oralspheres.
Effect of a hypoxic environment on
oralspheres in vitro
As CSCs are able to grow under extreme conditions,
we then analyzed the effect of hypoxia on the growth of this
population. To this aim, oralspheres were cultured under either
normoxia (21% O2) or hypoxia (1% O2) for 8
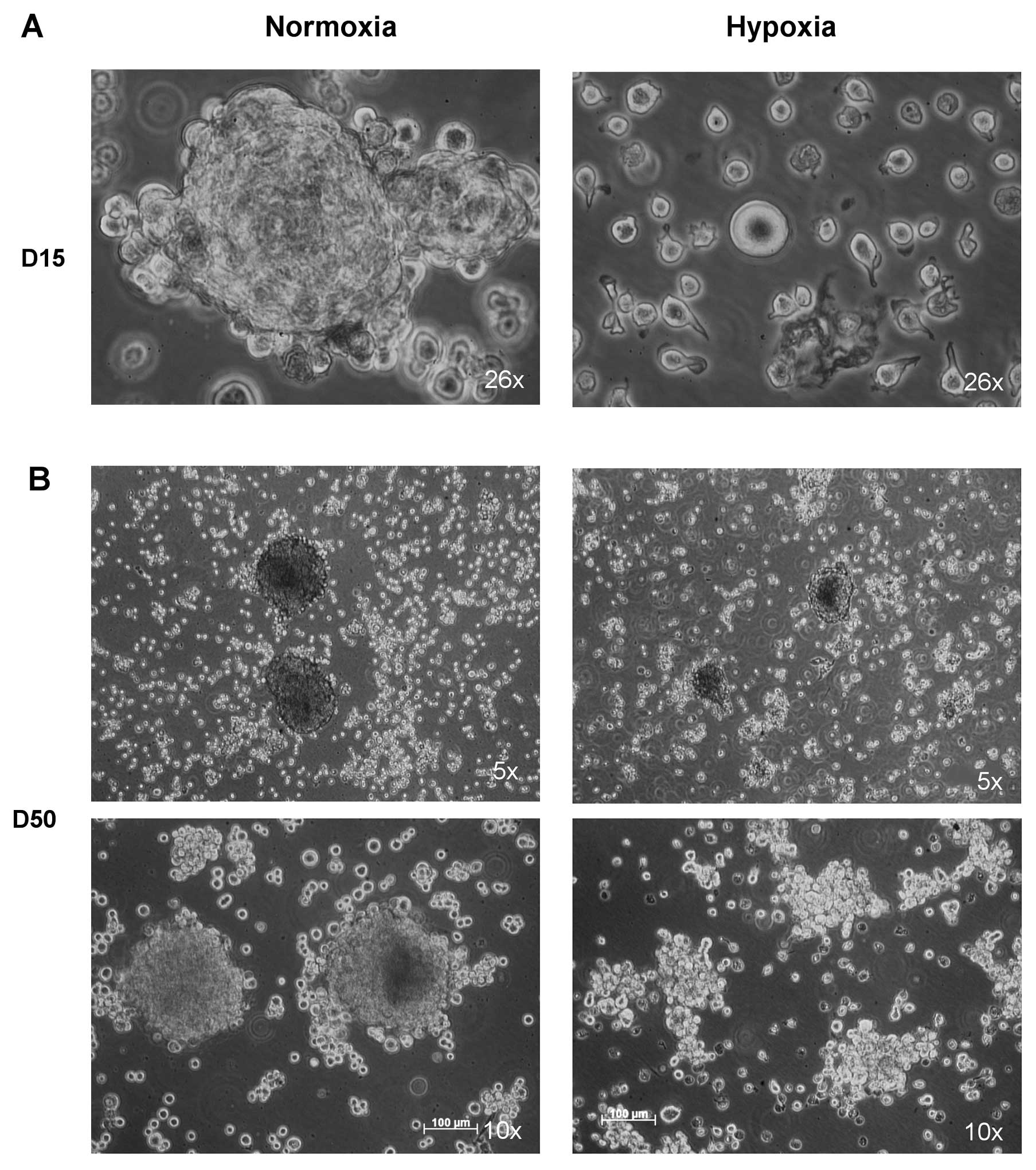
weeks. As observed in Fig. 4A,
after 15 days oralspheres did not exhibit large spheres under
hypoxia as compared to those in normoxia. In addition, under
hypoxic conditions, the number of cells decreased and some of them
appeared to degenerate and die. The cells finally recovered after 4
weeks under hypoxia, and some spheres could be observed (Fig. 4B). As shown in Fig. 4C and D, comparison of the percentage
of ALDH1high cells in oralspheres maintained in normoxia
and hypoxia revealed a significant increase of the percentage of
ALDH1high cells under the latter conditions with a mean
of 6.7% (±1.3) vs. 2.6% (±1.9), under the former conditions
(P<0.05).
Discussion
It has been suggested that CSCs could be one of the
key determinants of treatment failure in HNSCC like in other cancer
types (16,17). So far, most studies regarding HNSCC
CSCs have been carried out in vitro with only a few studies
performed in an in vivo environment. Developing in
vivo models is needed to obtain a comprehensive understanding
of the biology of these cells (18). In vivo experiments have
generally been performed in human cancer cells and
immunocompromised animals, and these xenotransplantation assays
have been described to result in a great variability in the
frequency of cells with tumorigenic potential, depending on the
degree of host immunocompetence (14). For these reasons, we decided to
isolate CSCs in a syngeneic HNSCC murine model. Although different
markers are available for CSC isolation, their detection within the
total tumor bulk remains a challenge (18). ALDH1 was shown to select HNSCC cells
exhibiting high self-renewal capacity, chemo- and radioresistance
(16), and ALDH1high
cells appear to be more tumorigenic than the CD44+ CSCs
(12). Using this approach in the
SCC-VII cell line, we showed that ALDH1high cells
represent approximately 1±0.6% of the population, which is in
accordance with previously published results (12), while the majority of the cell
population presented a low ALDH1 activity. Growth in serum-free
conditions has been demonstrated as a suitable method by which
HNSCC CSCs can be efficiently cultured in vitro in an
undifferentiated state (6). This
was indeed the case for the SCC-VII ALDH1high cells, in
agreement with previously reported results (7,19).
Following selection of ALDH1high and ALDH1low
cells, the tumorigenic potential of both populations was evaluated
in a syngeneic C3H immunocompetent mouse model for oral cancer. For
the injection of 1,000 cells, tumor occurrences and volumes were
not significantly different between ALDH1high and
ALDH1low cells, suggesting that the highest tested cell
dose did not discriminate the tumorigenic potential of the two
populations. This observation also indicates that cells with a high
tumorigenic potential were present in the ALDH1low cell
population, strengthening the need for the identification of
additional markers of the CSCs. The 250 cell transplantation assay
allowed the discrimination between ALDH1high and
ALDH1low cells for both tumor volumes and occurrences,
suggesting that ALDH1high cells were enriched in CSCs.
As expected, these cells were able to grow as spheres in serum-free
medium and serum supplementation led to sphere adhesion and
differentiation (20).
Stem cell niches are often located in anatomical
regions characterized by hypoxic conditions, which are crucial for
the maintenance of an undifferentiated state for stem cells from
various origins (21). Das et
al have shown that CSCs localize in hypoxic areas of solid
tumors in vivo and that they migrate to areas of hypoxia in
nude mice (22). These data
reinforce the hypothesis that a CSC niche is characterized by a
hypoxic environment. According to these findings, we analyzed the
effect of hypoxia on the growth of the CSC population. Although
SCC-VII CSCs lost their capacity to grow as spheres and some of
them even degenerated during the first 15 days, they were then able
to recover after an adaptation period. Finally, we compared the
ALDH1high cell percentage after an 8-week culture in
normoxia or hypoxia. The percentage of ALDH1high cells
was significantly higher when the oralspheres were maintained in a
hypoxic instead of a normoxic environment, which demonstrated that
culturing these cells in hypoxia favoured the enrichment in
ALDH1high cells. These results suggest that, as for
colorectal cell line-derived CSCs (23), hypoxia is involved in the
maintenance of the stem-like phenotype.
Overall, this study reports for the first time the
isolation of HNSCC CSCs in a syngeneic mouse model and the use of
hypoxia as a method to further enrich the ALDH1high cell
population. These cells appear to be a suitable model to develop
new therapeutic strategies aiming at eradicating relapses in
HNSCC.
Acknowledgements
We would like to thank Dr Nathalie Mazure and Dr
Jacques Pouyssegur (CNRS UMR 6543, Nice) for the opportunity to use
the MiniGalaxy A incubator. We also thank Dr Pascal Staccini
(Département Informatique Médicale, Faculté de Médecine, Nice) for
statistical analysis of the results. This study was supported by
the CNRS and the PESSOA-EGIDE Program (2010–2011), the Portuguese
Foundation for Science and Technology and France Cancer. S.D. is
recipient of a fellowship from the Portuguese Foundation for
Science and Technology (SFRH/BD/39727/2007).
Abbreviations:
|
ALDH
|
aldehyde dehydrogenase
|
|
CSCs
|
cancer stem cells
|
|
DEAB
|
diethylaminobenzaldehyde
|
|
DMEM
|
Dulbecco’s modified Eagle’s medium
|
|
FACS
|
fluorescence-activated cell
sorting
|
|
FCS
|
fetal calf serum
|
|
HNSCC
|
head and neck squamous cell
carcinoma
|
References
|
1
|
Argiris A, Karamouzis MV, Raben D and
Ferris RL: Head and neck cancer. Lancet. 371:1695–1709. 2008.
View Article : Google Scholar
|
|
2
|
Moore SR, Johnson NW, Pierce AM and Wilson
DF: The epidemiology of mouth cancer: a review of global incidence.
Oral Dis. 6:65–74. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Forastiere A, Koch W, Trotti A and
Sidransky D: Head and neck cancer. N Engl J Med. 345:1890–1900.
2001. View Article : Google Scholar
|
|
4
|
Forastiere AA, Trotti A, Pfister DG and
Grandis JR: Head and neck cancer: recent advances and new standards
of care. J Clin Oncol. 24:2603–2605. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tabor MH, Clay MR, Owen JH, et al: Head
and neck cancer stem cells: the side population. Laryngoscope.
121:527–533. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li HZ, Yi TB and Wu ZY: Suspension culture
combined with chemotherapeutic agents for sorting of breast cancer
stem cells. BMC Cancer. 8:1352008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prince ME, Sivanandan R, Kaczorowski A, et
al: Identification of a subpopulation of cells with cancer stem
cell properties in head and neck squamous cell carcinoma. Proc Natl
Acad Sci USA. 104:973–978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lim YC, Oh SY, Cha YY, Kim SH, Jin X and
Kim H: Cancer stem cell traits in squamospheres derived from
primary head and neck squamous cell carcinomas. Oral Oncol.
47:83–91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen H, Zhou L, Dou T, Wan G, Tang H and
Tian J: BMI1’s maintenance of the proliferative capacity of
laryngeal cancer stem cells. Head Neck. 33:1115–1125. 2011.
|
|
11
|
Chen YC, Chen YW, Hsu HS, et al: Aldehyde
dehydrogenase 1 is a putative marker for cancer stem cells in head
and neck squamous cancer. Biochem Biophys Res Commun. 385:307–313.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Clay MR, Tabor M, Owen JH, et al:
Single-marker identification of head and neck squamous cell
carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck.
32:1195–1201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ginestier C, Hur MH, Charafe-Jauffret E,
et al: ALDH1 is a marker of normal and malignant human mammary stem
cells and a predictor of poor clinical outcome. Cell Stem Cell.
1:555–567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Quintana E, Shackleton M, Sabel MS, Fullen
DR, Johnson TM and Morrison SJ: Efficient tumour formation by
single human melanoma cells. Nature. 456:593–598. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Auerbach R, Morrissey LW and Sidky YA:
Regional differences in the incidence and growth of mouse tumors
following intradermal or subcutaneous inoculation. Cancer Res.
38:1739–1744. 1978.PubMed/NCBI
|
|
16
|
Chen ZG: The cancer stem cell concept in
progression of head and neck cancer. J Oncol.
2009:8940642009.PubMed/NCBI
|
|
17
|
Frank NY, Schatton T and Frank MH: The
therapeutic promise of the cancer stem cell concept. J Clin Invest.
120:41–50. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sayed SI, Dwivedi RC, Katna R, et al:
Implications of understanding cancer stem cell (CSC) biology in
head and neck squamous cell cancer. Oral Oncol. 47:237–243. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dontu G, Abdallah WM, Foley JM, et al: In
vitro propagation and transcriptional profiling of human mammary
stem/progenitor cells. Genes Dev. 17:1253–1270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
et al: Identification and expansion of human
colon-cancer-initiating cells. Nature. 445:111–115. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cabarcas SM, Mathews LA and Farrar WL: The
cancer stem cell niche - there goes the neighborhood? Int J Cancer.
129:2315–2327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Das B, Tsuchida R, Malkin D, Koren G,
Baruchel S and Yeger H: Hypoxia enhances tumor stemness by
increasing the invasive and tumorigenic side population fraction.
Stem Cells. 26:1818–1830. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yeung TM, Gandhi SC and Bodmer WF: Hypoxia
and lineage specification of cell line-derived colorectal cancer
stem cells. Proc Natl Acad Sci USA. 108:4382–4387. 2011. View Article : Google Scholar : PubMed/NCBI
|