Introduction
Bitter acids (BA), which can be found in the female
inflorescences of the hop plant Humulus lupulus L. in high
amounts, are traditionally used for beer brewing as natural
preserving agent and to add its typical bitterness and flavor
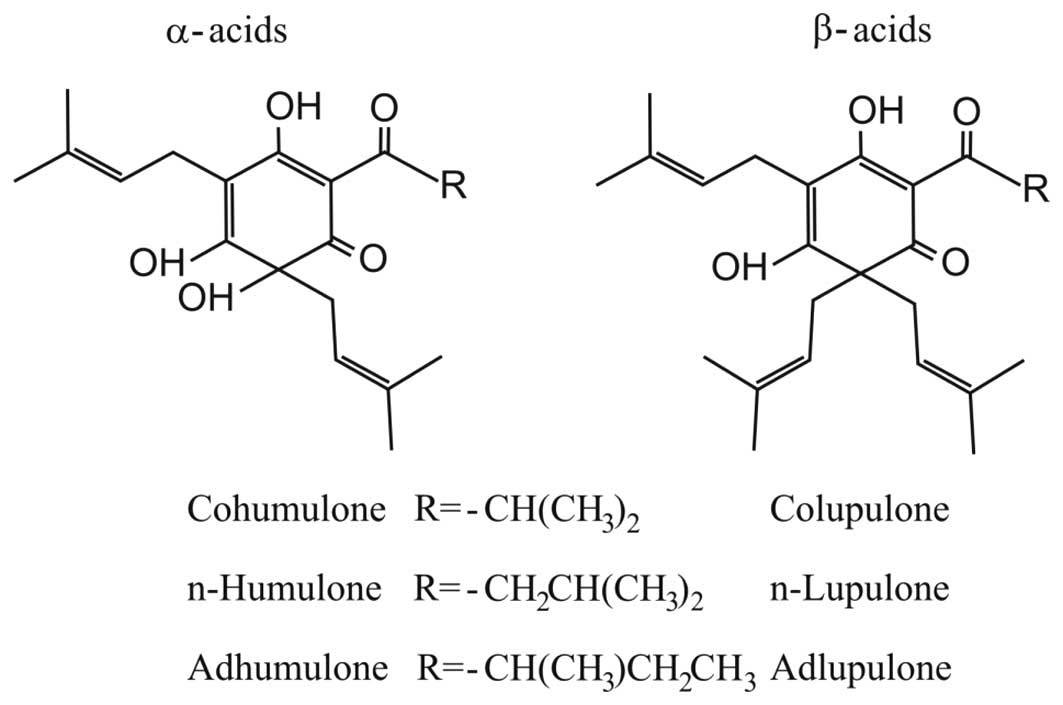
(1). BA consist of two structurally
related series, α-acids (or humulones) and β-acids (or lupulones),
which are both characterized as prenylated acylphloroglucinols.
Both groups are mainly composed of three analogues, and the main
structural difference is that α-BA are substituted with one
isoprenyl side chain at C-6 whereas β-BA contain two isoprenyl side
chains at this position (Fig. 1).
Recent studies have shown that both α- and β-BA exhibit various
potent biological properties with promising effects for cancer
therapy and prevention. BA induce apoptosis in fast-growing tumor
cells in vitro and inhibit chemical induced tumor promotion
in vivo (2–6). However, so far no studies are
available, which assessed the effects of BA in hepatocellular
carcinoma (HCC).
HCC is the fifth most frequent cancer worldwide
(7). In most cases, patients have a
background of chronic liver disease leading to liver cirrhosis,
which is the main risk factor for the development of HCC (8,9). HCC
represents the cancer with the third highest mortality (10), and up until now, surgical resection
or transplantation are the most promising treatment options of HCC.
However, there is a high rate of disease recurrence since most
patients are diagnosed at an advanced stage (8). Thus, small molecules which are able to
target angiogenesis, apoptosis and specific signal transduction
pathways have gained attention in cancer therapy (11).
The aim of this study was to assess the effect of BA
on HCC cells in vitro. We used hop extracts enriched with α-
or β-BA, respectively, to obtain first insight into whether
biological effects vary between these two groups of BA.
Materials and methods
Cell isolation and cell culture
HCC cell lines HepG2 (ATCC HB-8065), PLC (ATCC
CRL-8024), and Hep3B (ATCC HB-8064) were maintained in high glucose
DMEM supplemented with penicillin (400 U/ml), streptomycin (50
μg/ml), L-glutamine (300 μg/ml) and 10% fetal calf serum (FCS;
Sigma, Deisenhofen, Germany) and passaged at a 1:5 ratio every 3
days. Generation of conditioned medium (CM) from activated hepatic
stellate cells was done as described (12).
Chemicals
Supercritical carbon dioxide hop extracts were
provided by Nateco2 (Wolnzach, Germany). α-rich extract
(α-extract) contained 57.2% α-acids (humulone, cohumulone and
adhumulone) and 18.3% β-acids (lupulone, colupulone and
adlupulone), whereas β-rich extract (β-extract) contained 13.0%
α-acids (humulone, cohumulone and adhumulone) and 51.9% β-acids
(lupulone, colupulone and adlupulone) as revealed by HPLC analysis
(data not shown). Stock solutions of the BA containing extracts
(100 mg/ml) were prepared by dissolving the extracts in DMSO. After
a centrifugation step, unsolved compounds were removed, and the
obtained stock solutions were aliquoted and stored at −20°C prior
to use. Samples indicated as controls were treated with an
according concentration of DMSO. Final concentration of DMSO did
not exceed 0.1% in all experiments. To ensure that the bitter acids
and not concomitant substances are responsible for the observed
activity, the BA from the β-extract were further purified.
Therefore, the extract was solved in hexane and the obtained
solution was transferred to a separating funnel and liquid-liquid
extraction was done with sodium hydroxide (pH 12.0). The sodium
hydroxide solution (containing deprotonated BA) was acidified with
hydrochloric acid (37%) followed by liquid-liquid extraction with
petroleum ether as second phase. Subsequently, purified BA as well
as the fraction containing remnant lipophilic compounds (not
soluble at pH 12.0) were dried using a rotary evaporator.
Separation into purified BA and remnant lipophilic
compounds was checked by thin layer chromatography using silica gel
plates (Merck, Darmstadt, Germany) and a solvent mixture of
cyclohexane, ethyl acetate and glacial acetic acid (ratio 70:29:1).
For visualization of purification success anisaldehyde spray
reagent was used as described (13)
(data not shown). Both fractions (purified BA and remnant
lipophilic compounds) were pharmacologically tested in a
proliferation assay.
Proliferation assay
Quantification of cell proliferation was measured
applying colorimetric XTT assay (Roche Diagnostics, Mannheim,
Germany) as described (12).
Migration assay
Migratory capacity of HCC cells was quantified using
Cultrex 96 Well Cell Migration assay (Trevigen, Gaithersburg, USA)
as described (14).
Quantification of ERK1/2 and NFκB
activity
Phospho-p44/42 and phospho-p65 were quantified
applying PathScan phospho- sandwich ELISA (Cell Signalling
Technology, Beverly, USA) as described (14).
AP-1 reporter gene assay
For transfections, HCC cells (2×105 cells
per well) were seeded in 6-well plates and transfected with 0.5 μg
pAP-1 luc plasmid (Stratagene, La Jolla, USA) using Lipofectamine
Plus reagent (Invitrogen, Karlsruhe, Germany) according to the
manufacturer’s instructions. For normalization of transfection
efficiency, cells were contransfected with 0.2 μg of pRL-TK plasmid
resulting in a renilla luciferase acitivity (Promega, Mannheim,
Germany). After 24 h, transfection-medium was removed and cells
were stimulated with the two hop extracts for 20 h. Subsequently,
cells were lysed and the luciferase activities were measured using
a luminometric assay (Promega).
Statistical analysis
Values are presented as mean ± SEM. Comparison
between groups was made using the Student’s unpaired t-test. A
p<0.05 was considered statistically significant. All
calculations were performed using the statistical computer package
GraphPad Prism version 4.00 for Windows (GraphPad Software, San
Diego, CA, USA).
Results
Bitter acids induce cell death of HCC
cells
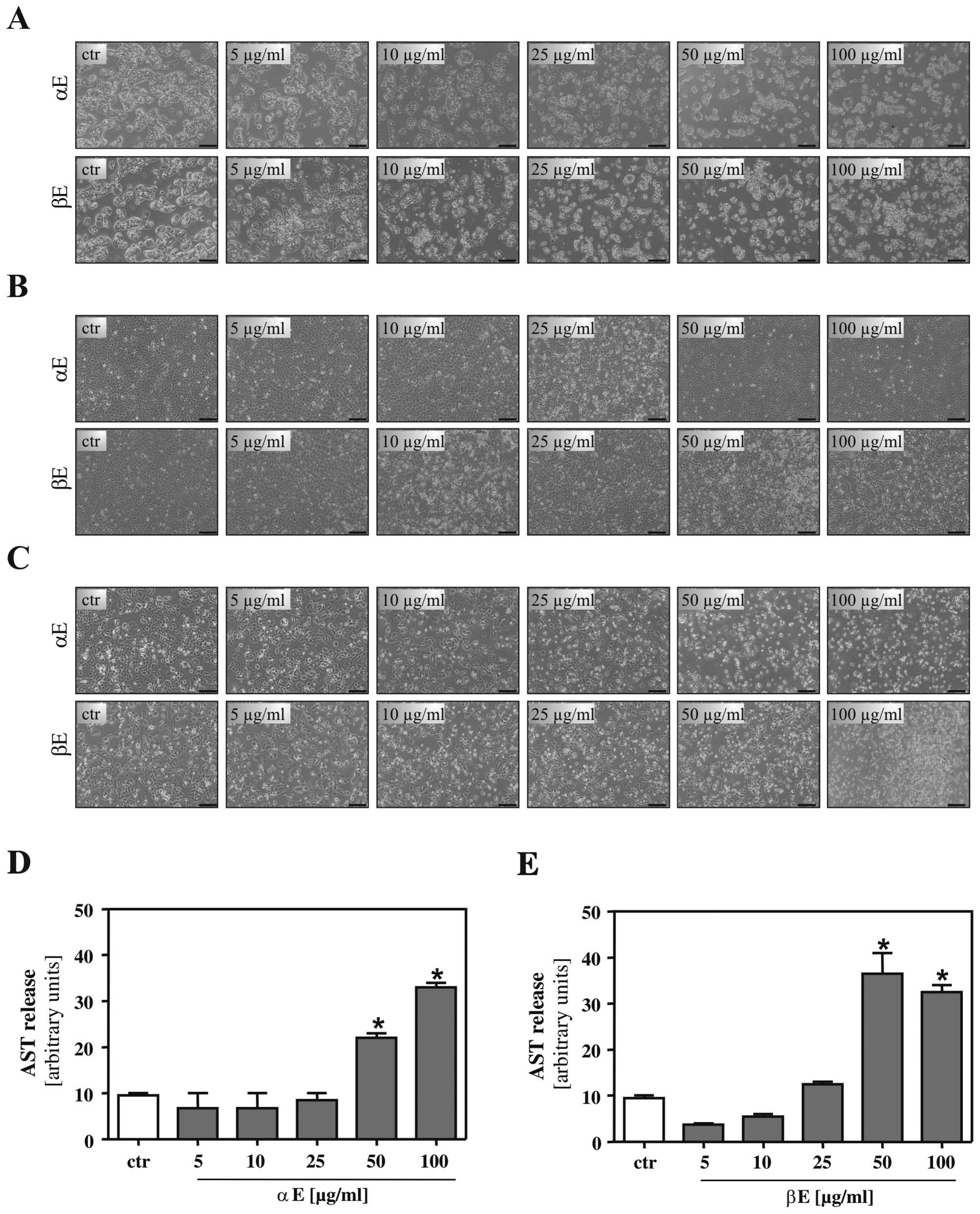
Our first aim was to define the effective dose range
of BA on different human HCC cell lines. Cells were incubated with
indicated concentrations of an α-rich extract (α-extract) and a
β-rich extract (β-extract) for 24 h. Here and in subsequent
experiments, control cells were treated with an equivalent amount
of the solvent DMSO. Microscopic analysis revealed no alterations
in BA treated HepG2 (Fig. 2A),
Hep3B (Fig. 2B) and PLC cells
(Fig. 2C) up to a concentration of
10 μg/ml. Higher concentrations led to first morphological changes,
and a concentration of 50 μg/ml caused cell bubbling in all three
HCC cell lines. In line with these data, aspartate transaminase
(AST) concentration in the supernatant of HepG2 cells treated with
the two hop extracts did only slightly differ from control cells up
to a concentration of 25 μg/ml, while AST levels were markedly
increased upon incubation with concentrations of 50 μg/ml
indicating cell injury (Fig. 2D and
E). Interestingly, β-extract showed more pronounced effects
than α-extract regarding morphological changes and induction of AST
release. Similar results were obtained for Hep3B and PLC cells
(data not shown).
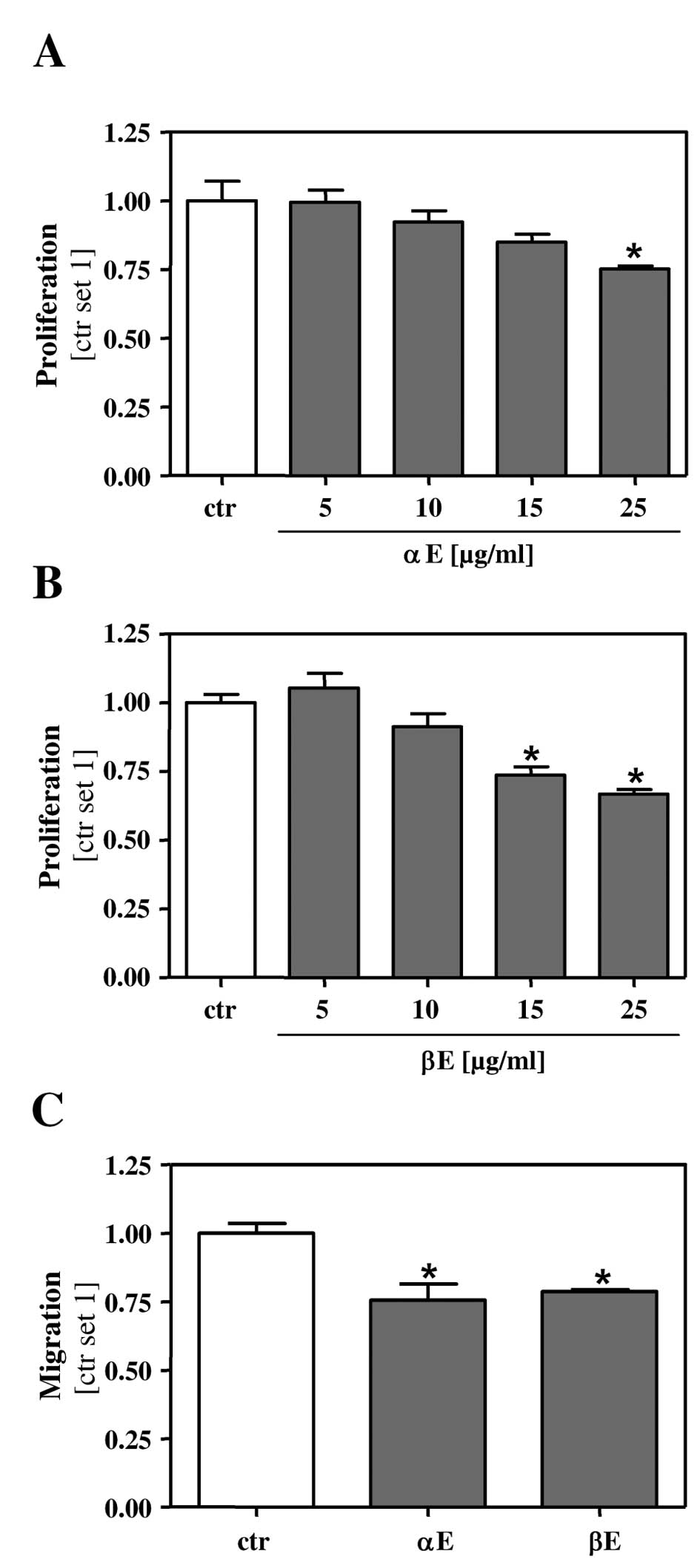
Bitter acids reduce proliferation and
block the migratory potential of HCC cells
Acquisition of a highly proliferative and invasive
phenotype is a main characteristic of tumor cells. We therefore
investigated the effect of the two hop extracts on cell cycle
synchronized HCC cells. Incubation for 24 h with 15 μg/ml β-extract
led to a significant inhibition of the proliferation rate (Fig. 3B) whereas a concentration of 25
μg/ml α-extract was required to achieve an anti-proliferative
effect on HCC cells (Fig. 3A).
Next, we analyzed the effect of the hop extracts on the migratory
capacity of HCC cells. Here, we applied a low concentration of
BA-extracts (5 μg/ml) to exclude interfering effects on cell
viability and proliferation, respectively. At this concentration
both α- and β-extract exhibited a similar inhibitory effect on HCC
cell migration (Fig. 3C).
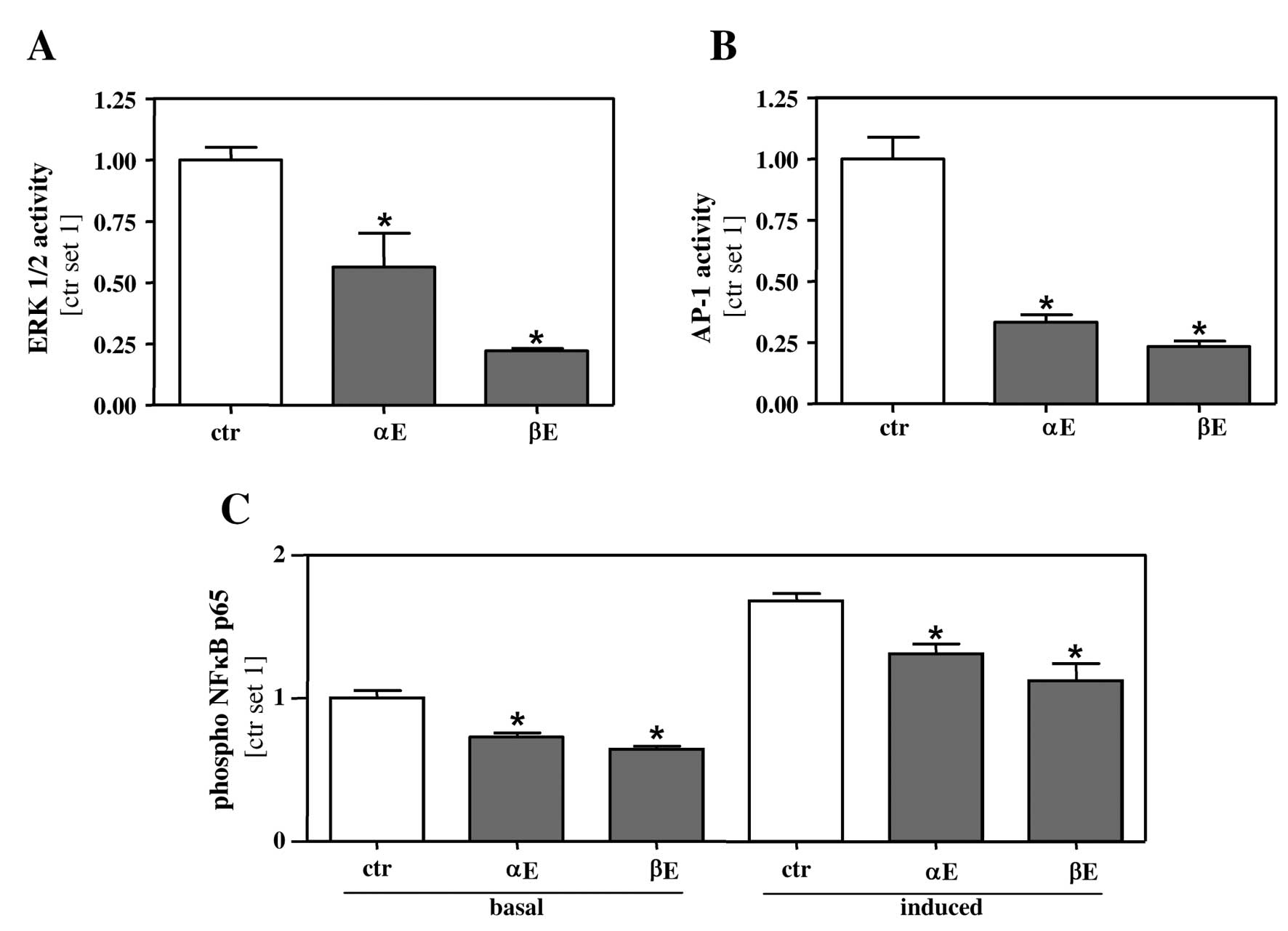
Bitter acids inhibit ERK1/2, AP-1 and
NFκB activity in HCC cells
To get insight into the molecular mechanism by which
BA affect proliferation and migration of HCC cells, we analyzed
different pathways, which are known to affect HCC progression.
Extracellular signal-regulated kinase (ERK1/2) is often deregulated
in HCC, and its induction correlates with poor prognosis of HCC
patients (15–17). Stimulation with 5 μg/ml of both hop
extracts significantly reduced the amount of phosphorylated ERK1/2
in HepG2 cells (Fig. 4A).
Interestingly, the inhibitory effect of β-extract was more potent
compared to α-extract. Once activated, ERK1/2 pathway induces
different transcription factors including the activator protein-1
(AP-1) (15). Therefore, we next
applied a reporter gene assay to assess AP-1 activity in HepG2
cells stimulated with the two hop extracts. Both of them markedly
inhibited AP-1 activity in HepG2 cells (Fig. 4B). Moreover, ERK-activation can also
directly affect the transcription factor NFκB (18,19),
and we and others have shown that NFκB plays an important role in
HCC progression (12,20,21).
We therefore investigated the influence of BA on NFκB activity.
Both hop extracts significantly attenuated basal NFκB activity at a
concentration of 5 μg/ml. We further assessed the effect of both
hop extracts on induced NFκB activity. To induce NFκB activation,
HepG2 cells were stimulated with conditioned medium from activated
hepatic stellate cells (HSC). Activated HSC which are regarded as
the major profibrogenic cell type in the liver, secrete a variety
of proinflammatory cytokines, and previously, we identified their
influence on tumor aggressiveness (12). Importantly both hop extracts
significantly alleviated HSC induced NFκB activity at
concentrations as low as 5 μg/ml (Fig.
4C).
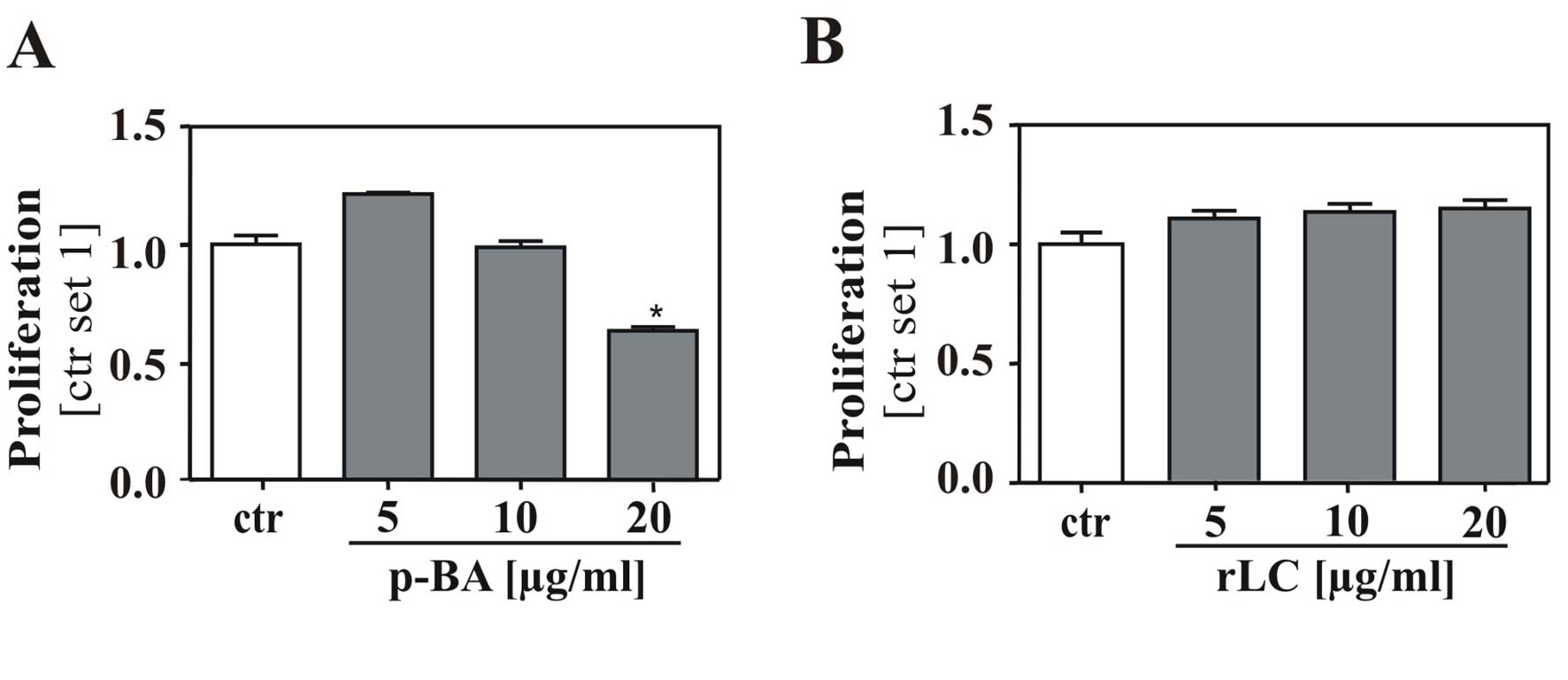
Bitter acids but not remnant lipophilic
compounds of hop extracts affect proliferation of HCC cells
Finally, we analyzed whether concominant compounds
present in the α- and β-extracts contribute to the observed
anti-tumorous effects on HCC cells. We separated BA from the
β-extract by liquid-liquid extraction, and HepG2 cells were
stimulated with the obtained purified bitter acids (pBA) and the
remnant lipophilic compounds (rLC) at equimolar concentrations.
Importantly, treatment with pBA revealed the same
anti-proliferative effect as the original β-extract (Fig. 5A) whereas treatment with the remnant
lipophilic compounds alone had no influence on proliferation
(Fig. 5B). These findings confirmed
that BA, but not remnant hop compounds in the extracts account for
the observed effects on HCC cells.
Discussion
The aim of the present study was to analyze the
effects of bitter acids (BA) on tumorigenicity of HCC cells. To
obtain first insight into differential effects of α-BA and β-BA we
used hop extracts enriched with either one of these two related
series and found that both extracts exhibited a profound effect on
ERK1/2 as well as on AP-1 and NFκB activation. These signaling
pathways are known to be pathophysiologically relevant in HCC cells
(12,15,16,18,20,21),
and in line with this, both extracts also revealed inhibitory
effects on proliferation and migration of HCC cells in
vitro. Importantly, we could exclude that the observed effects
derived from remnant lipophilic compounds in hop extracts. However,
individual anti-tumorigenic effects were stronger upon stimulation
with β-extract compared to α-extract-mediated effects. One might
speculate that the additional isoprenyl side chain of β-BA, which
contributes to a higher lipophilicity and increased steric
hindrance, may positively influence their effects on HCC cells.
However, Cattor et al demonstrated a rapid and effective
uptake of α-BA into colon cancer cells in vitro (22). Furthermore, and notwithstanding the
slight differences between α- and β-BA regarding anti-tumorigenic
effects on HCC, it has to be noted that α-BA are the most abundant
type of BA in beer whereas β-BA are only present in trace amounts
(23). Still, different methods
(i.e., using liquid supercritical carbon dioxide) were developed to
isolate BA from hop cones in large quantities, and thus,
independent of beer intake extracts enriched for either α- or β-BA
may be potentially used as hepatoprotective dietary supplement.
Scarce information is available on the
bioavailability of BA in vivo, and comprehensive data
comparing α-BA and β-BA are missing. After a single oral uptake of
940 mg of isomerized α-BA (called META060) Desai and colleagues
detected serum concentrations of 4–15 μg/ml in men (24), which is within the dose range in
which BA exhibit anti-tumorigenic effects on HCC cells in the
present study.
With regards to the safety profile of BA, Farber
et al did not detect any toxic effects on liver, kidney,
bone marrow or myocardium in man after oral application of BA at a
dose of 5.0 g per day for 3 months (25). Still, it has to be considered that
HCC mainly develops in cirrhotic livers (7), which are characterized by an impaired
metabolic and detoxifying capacity. Therefore, special caution has
to be taken in patients with chronic liver disease, and further
studies in experimental fibrosis and HCC models are required.
Still, the present study indicates the potential of BA as
functional nutrient for both prevention and treatment of HCC.
Acknowledgements
We would like to thank Marina Fink and Ruth Schewior
(Department of Internal Medicine I, University Hospital Regensburg)
for excellent technical assistance. Further, this study was
supported in part by an unrestricted research grant from the Joh.
Barth & Sohn GmbH (Nuremberg, Germany). Financial relationships
of the authors with Joh. Barth & Sohn GmbH are as follows: C.H.
is a consultant, and M.S., C.D. and B.C. are working in the
laboratory of C.H. M.G. is an employee of Nateco2.
References
|
1
|
Van Cleemput, Cattoor K, De Bosscher K,
Haegeman G, De Keukeleire D and Heyerick A: Hop (Humulus
lupulus)-derived bitter acids as multipotent bioactive
compounds. J Nat Prod. 72:1220–1230. 2009.
|
|
2
|
Chen WJ and Lin JK: Mechanisms of cancer
chemoprevention by hop bitter acids (beer aroma) through induction
of apoptosis mediated by Fas and caspase cascades. J Agric Food
Chem. 52:55–64. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lamy V, Roussi S, Chaabi M, Gosse F,
Schall N, Lobstein A and Raul F: Chemopreventive effects of
lupulone, a hop {beta}-acid, on human colon cancer-derived
metastatic SW620 cells and in a rat model of colon carcinogenesis.
Carcinogenesis. 28:1575–1581. 2007.
|
|
4
|
Lamy V, Roussi S, Chaabi M, Gosse F,
Lobstein A and Raul F: Lupulone, a hop bitter acid, activates
different death pathways involving apoptotic TRAIL-receptors, in
human colon tumor cells and in their derived metastatic cells.
Apoptosis. 13:1232–1242. 2008. View Article : Google Scholar
|
|
5
|
Tobe H, Kubota M, Yamaguchi M, Kocha T and
Aoyagi T: Apoptosis to HL-60 by humulone. Biosci Biotechnol
Biochem. 61:1027–1029. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yasukawa K, Takeuchi M and Takido M:
Humulon, a bitter in the hop, inhibits tumor promotion by
12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in
mouse skin. Oncology. 52:156–158. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kirchner G, Kirovski G, Hebestreit A,
Scholmerich J, Schlitt HJ, Stoeltzing O and Hellerbrand C:
Epidemiology and survival of patients with hepatocellular carcinoma
in Southern Germany. Int J Clin Exp Med. 3:169–179. 2010.PubMed/NCBI
|
|
8
|
Bruix J, Boix L, Sala M and Llovet JM:
Focus on hepatocellular carcinoma. Cancer Cell. 5:215–219. 2004.
View Article : Google Scholar
|
|
9
|
Farazi PA and DePinho RA: Hepatocellular
carcinoma pathogenesis: from genes to environment. Nat Rev Cancer.
6:674–687. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Freise C, Ruehl M, Erben U, et al: A
hepatoprotective Lindera obtusiloba extract suppresses
growth and attenuates insulin like growth factor-1 receptor
signaling and NF-kappaB activity in human liver cancer cell lines.
BMC Complement Altern Med. 11:392011.PubMed/NCBI
|
|
12
|
Amann T, Bataille F, Spruss T, et al:
Activated hepatic stellate cells promote tumorigenicity of
hepatocellular carcinoma. Cancer Sci. 100:646–653. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saugspier M, Dorn C, Thasler WE, Gehrig M,
Heilmann J and Hellerbrand C: Hop bitter acids exhibit
anti-fibrogenic effects on hepatic stellate cells in vitro. Exp Mol
Pathol. 92:222–228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dorn C, Weiss TS, Heilmann J and
Hellerbrand C: Xanthohumol, a prenylated chalcone derived from
hops, inhibits proliferation, migration and interleukin-8
expression of hepatocellular carcinoma cells. Int J Oncol.
36:435–441. 2010.PubMed/NCBI
|
|
15
|
Ito Y, Sasaki Y, Horimoto M, et al:
Activation of mitogen-activated protein kinases/extracellular
signal-regulated kinases in human hepatocellular carcinoma.
Hepatology. 27:951–958. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmitz KJ, Wohlschlaeger J, Lang H, et
al: Activation of the ERK and AKT signalling pathway predicts poor
prognosis in hepatocellular carcinoma and ERK activation in cancer
tissue is associated with hepatitis C virus infection. J Hepatol.
48:83–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsuboi Y, Ichida T, Sugitani S, et al:
Overexpression of extracellular signal-regulated protein kinase and
its correlation with proliferation in human hepatocellular
carcinoma. Liver Int. 24:432–436. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakano H, Shindo M, Sakon S, Nishinaka S,
Mihara M, Yagita H and Okumura K: Differential regulation of
IkappaB kinase alpha and beta by two upstream kinases,
NF-kappaB-inducing kinase and mitogen-activated protein kinase/ERK
kinase kinase-1. Proc Natl Acad Sci USA. 95:3537–3542. 1998.
View Article : Google Scholar
|
|
19
|
Zhao Q and Lee FS: Mitogen-activated
protein kinase/ERK kinase kinases 2 and 3 activate nuclear
factor-kappaB through IkappaB kinase-alpha and IkappaB kinase-beta.
J Biol Chem. 274:8355–8358. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arsura M and Cavin LG: Nuclear
factor-kappaB and liver carcinogenesis. Cancer Lett. 229:157–169.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pikarsky E, Porat RM, Stein I, et al:
NF-kappaB functions as a tumour promoter in inflammation-associated
cancer. Nature. 431:461–466. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cattoor K, Bracke M, Deforce D, De KD and
Heyerick A: Transport of hop bitter acids across intestinal Caco-2
cell monolayers. J Agric Food Chem. 58:4132–4140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Foster BC, Kearns N, Arnason JT, Saleem A,
Ogrodowczyk C and Desjardins S: Comparative study of hop-containing
products on human cytochrome p450-mediated metabolism. J Agric Food
Chem. 57:5100–5105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Desai A, Konda VR, Darland G, et al:
META060 inhibits multiple kinases in the NF-kappaB pathway and
suppresses LPS-mediated inflammation in vitro and ex vivo. Inflamm
Res. 58:229–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Farber SM, Masten JM, Anderson HH, Gentry
RW and Chin YC: Tolerance and effects of lupulon in man. Dis Chest.
18:10–15. 1950. View Article : Google Scholar : PubMed/NCBI
|