Introduction
Pediatric brainstem glioma (BSG) is now the main
cause of death in pediatric patient with solid tumors, which
constitute 15–20% of all central nervous system (CNS) neoplasms
that developed in childhood (1). In
particular, typical pediatric BSG is characterized by malignant
feature, about 80% of these tumors are ‘diffuse intrinsic’ or
‘diffusely infiltrative’ category, leading to diffuse increase of
the brainstem and making complete resection nearly impossible
(2). Despite recent attempts to
optimize or combine radiotherapy and chemotherapy, the poor
prognosis of these tumors has not changed in the past two decades
(3). These circumstances leave the
median survival after diagnosis at less than 1 year (1).
Although adult intracranial gliomas account for more
than 40% of all intracranial tumors (4,5), it is
noteworthy that adult patients with BSG are extremely rare and
accounts for less than 2% of adult gliomas (6–8).
Moreover, compared to pediatric BSG, adult BSG is typically much
less invasive, and there is a clear and intact boundary between the
BSG and surrounding tissue (9).
Thus, the therapeutic effect of surgery is better. Very different
from pediatric BSG, adult subtype has a median survival period up
to 7.3 years after diagnosis (6).
These and other data suggest that BSG is a unique glioma subtype
with biological and molecular pathology mechanisms different from
common gliomas (6). Discerning the
key differences between pediatric and adult BSG may prove
indispensable to the fight against pediatric BSG (10), a fight that has not yielded many
positive victories in recent years (1).
Molecular genetic analysis will provide insight into
gliomagenesis, but the precise pattern of genetic abnormalities
within the group of pediatric BSG is still poorly documented.
Existing researches on this issue were fragmented and limited in
scope (11). Nevertheless, to our
knowledge, no study has examined the molecular mechanisms of BSG
malignant progression from the aspect of the striking difference
between pediatric and adult types of BSG. Also, we speculate that
the central role of miRNA in modulating gene expression will
present a comprehensive perspective for understanding the molecular
bases of pediatric BSG.
microRNAs (miRNAs) are a set of small non-coding RNA
molecules (12). Partial
complementary pairing between the 3′-untranslated region (3′UTR) of
the messenger RNA (mRNA) and the 5′ seed region of a miRNA is
essential for the post-transcriptional modulation of the target
gene expression (13). Given the
diversity and abundance of target genes, miRNAs appear to
functionally interact with various components of cellular signal
transduction pathways known as tumor suppressor genes or oncogenes
to generate a complex combinatorial network (14,15),
suggesting that miRNAs play central role in carcinogenesis
(16).
We previously reproduced the major characteristics
of two kinds of human BSG in rat orthotopic C6 cell BSG models,
respectively (10). In the current
study, we modified the former models, replaced by athymic rats
using suspensions of human malignant glioma cell line. With the
modified model, we examined, for the first time, the expression
profile of aberrant miRNAs of pediatric BSG compared to adult type.
Human BSG tissues were utilized to verify the microarray results by
qRT-PCR and in situ hybridization.
Materials and methods
Cell culture, experimental animals and
model making
The LN229 cell line was obtained from the China
Academia Sinica Cell Repository. All cells were maintained in
Dulbecco’s modified Eagle’s medium (DMEM, Gibco) supplemented with
10% fetal bovine serum (Gibco), at 37°C with 5% CO2 in a
humidified incubator.
Pediatric male athymic rats (3-week old, body weight
45±5 g) and adult male athymic rats (10-week old, body weight
275±50 g) were purchased from the Cancer Institute of The Chinese
Academy of Medical Science. All animal procedures were approved by
the Capital Medical University Animal Care and Use Committee.
The BSG model was modified from our previous
description (10). In detail,
animals were anesthetized with 10% chloral hydrate administered
intraperitoneally, and then positioned in a stereotaxic frame. A
midline incision was made and the lambda suture was identified. A
bur hole was drilled 0.5 mm to the right of and 1 mm posterior to
the lambda, taking care to keep the integrity of the dura mater.
The LN229 cells were trypsinized, washed twice, resuspended, and
diluted in DMEM (without FBS) to a concentration of
2.0×105 cells/15 μl. Using a Hamilton syringe with a
27-gauge needle, LN229 cells were then implanted into the pons (15
μl, 10-mm depth from the dura mater in adult rats and 5 μl, 7 mm in
young rats). All rats were assessed by MRI (Philips Achieva 1.5T
Nova).
Tissue preparation and RNA
extraction
After anesthesia and transcardial perfusion with 4%
paraformaldehyde (to eliminate some of the red blood cells), the
animals were killed by decapitation. Immediately the brain tissue
was isolated, the edema and normal brain tissue around the tumor
focus tissue was strictly selected, the cerebral pia mater was
cleared and blood vessels were removed under a microscope, quickly
stored into liquid nitrogen or disruption. Each frozen tissue piece
was homogenized in 1 ml TRIzol reagent (Invitrogen) for RNA
extraction, as previously described (17).
miRNA microarray analysis
The RNA was purified from total RNA using a mirVana
miRNA isolation kit (AM1561, Ambion). The miRNA expression profiles
were performed at Beijing CapitalBio Corp. using miRNA Microarray
system (Version 2.2) with Agilent miRNA Complete Labeling and Hyb
kit (p/n 5190-0456), containing 723 human and 76 human viral miRNAs
catalogued in the Sanger miRNA database v10.1 (Agilent
Technologies). Purified RNA quality was verified using the 2100
Bioanalyzer (Agilent Technologies). With a sample input of 100 ng,
purified RNA was treated with calf intestinal alkaline phosphatase
(CIP) for 5′ end dephosphorylation, and then labeled with
Cyanine3-pCp (Cy3) using T4 RNA Ligase to the 3′ end of RNA. The
generated fluorescent miRNA was hybridized to the microarray probes
at 55°C for 20 h. The microarray slide was subsequently washed and
scanned using the Agilent scanner to obtain microarray images.
Microarray data analyses
The microarray image information was converted into
spot intensity values using Scanner Control Software Rev 7.0
(Agilent Technologies). The signal after background subtraction was
exported directly into the GeneSpring GX10 software (Agilent
Technologies) for quantile normalization. The mean normalized
signal from biological replicates was used for comparative
expression analysis. Unpaired t-test with Benjamini-Hochberg
correction was performed to determine differentially expressed
miRNAs between pediatric and adult BSG subtypes. The fold-changes
(log2 transformed) of expression signals between pediatric and
adult BSG subtypes were calculated from the normalized values.
Hierarchical clustering was performed with Pearson correlation
using Cluster 3.0 software by Average linkage clustering.
Dendrograms and expression pattern maps were generated by TreeView
software from the Stanford University.
Patients and specimens
The study was performed according to a protocol
approved by the Capital Medical University Ethics Committee. Human
BSG tissue samples were obtained from Sanbo Brain Hospital
affiliated to Capital Medical University after informed consent
from patients diagnosed with BSG. One WHO-I and 1 WHO-II adult BSG,
1 WHO-III and 2 WHO-IV pediatric BSG tissues were resected during
surgery and immediately frozen in liquid nitrogen for subsequent
total RNA extraction.
Quantitative RT-PCR (qRT-PCR)
The expression levels of specific miRNAs were
validated by quantitative RT-PCR using the Hairpin-it™ miRNAs qPCR
Quantitation kit (GenePharma Co., Ltd., Shanghai, China). Briefly,
the 20 μl reverse transcriptase reactions included purified total
RNA (2–10 ng per reaction) were incubated in a PTC-200 thermal
cycler (Bio-Rad Laboratories, Hercules, CA, USA) in a 96-well plate
for 30 min at 16°C, followed by 30 min at 42°C, 10 min at 85°C, and
then held at 4°C. All reverse transcriptase reactions, including
no-template controls and RT minus controls, were run in duplicate.
Real-time PCR was performed using a DNA Engine Opticon 2 system
(Bio-Rad Laboratories). The following real-time PCR protocol was
used: denaturation program (95°C for 3 min), amplification and
quantification program (40 cycles of 95°C for 12 sec and 62°C for
40 sec), and melting curve program (from 62°C to 95°C, read every
0.2°C, hold 2 sec). U6 RNA (GenePharma) was used for normalization.
Data are shown as fold-change (2−ΔΔCT).
Fluorescence in situ hybridization
(FISH)
To study the spatial and temporal expression of
miRNAs with high sensitivity and resolution, FISH was performed
with in situ hybridization kit (Boster, Wuhan, China).
LNA/DNA oligos had the following sequences: LNA-miR-106b 5′-ATC TGC
ACT GTC AGC ACT TTA-3′, LNA-miR-20a 5′-CTA CCT GCA CTA TAA GCA CTT
TA-3′, scramble 5′-GTG TAA CAC GTC TAT ACG CCC A-3′, U6 5′-CAC GAA
TTT GCG TGT CAT CCT T-3′. DEPC water (0.1%) was used for all
solutions and appliances necessary for FISH. After deparaffinized
and deproteinated, the tissue sections were pre-hybridized for 4 h
at 42°C, followed by incubation with LNA-miRNA hybridization
solution (containing probe) overnight at 42°C. Then the sections
were washed with gradient SSC thoroughly (2X SSC, 0.5X SSC and 0.2X
SSC) to remove the background signals, followed by treatment with
biotinylated digoxin antibody at 37°C for 1 h, and Cy3-avidin (used
to label miRNA) at 37°C for 30 min. Nuclei were counterstained with
DAPI, then sections were detected under FV1000 fluorescence
microscope (Olympus, Tokyo, Japan) and analyzed using IPP5.1
(Olympus).
Results
Assessment of pediatric BSG and adult BSG
orthotopic models
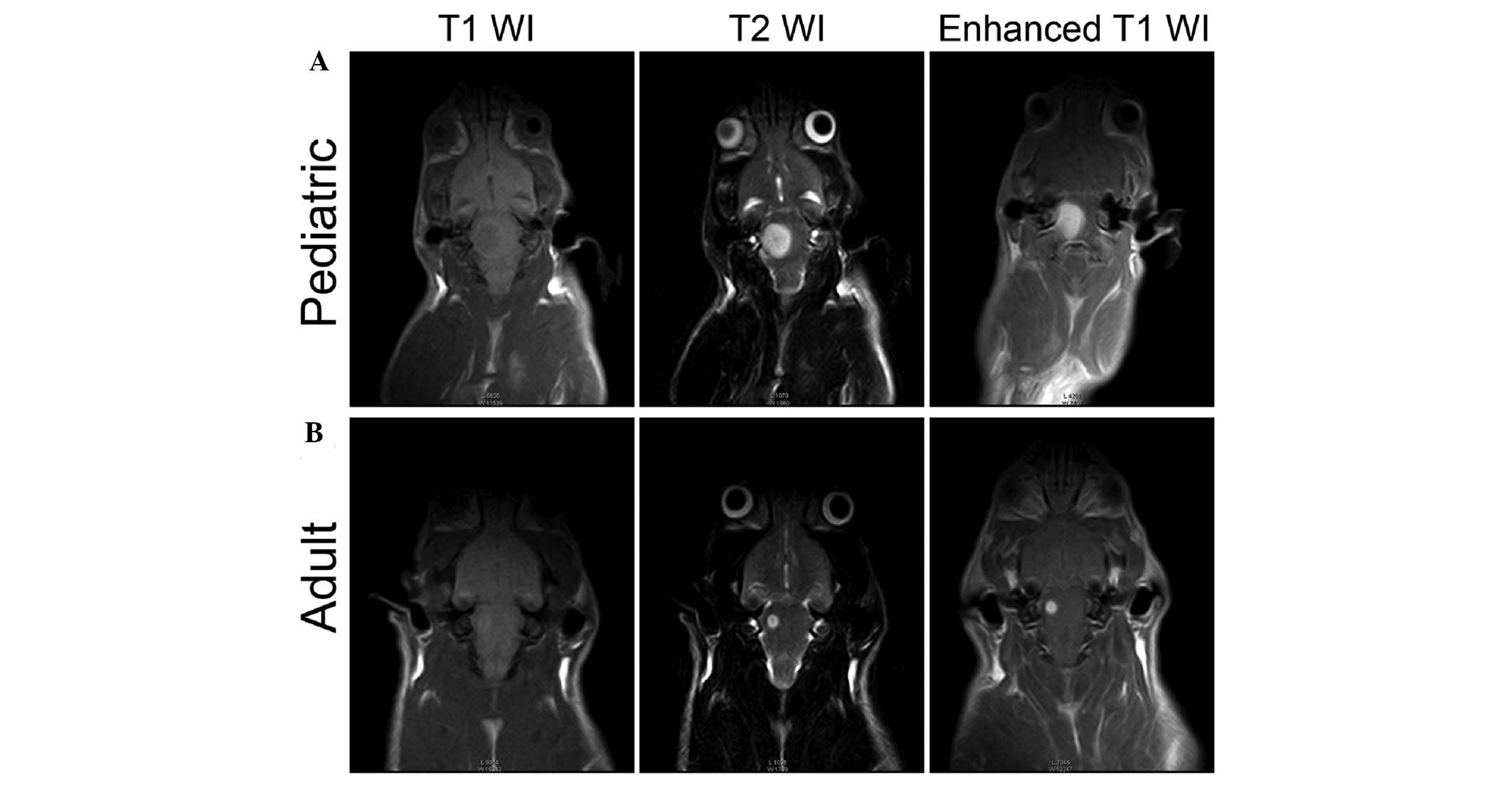
The orthotopic models simulating the BSG
heterogeneity were modified from our previous description (10). The orthotopically injected LN229
cells into the pons of animals was imaged after 2 weeks of tumor
growth. The MRI scan revealed that human glioma cells in the
brainstems of pediatric group progress more extensively than in the
adult group. They grew rapidly and enlarged to occupy most parts of
the pons of pediatric group (Fig.
1A). In adult group, the lesions grew relatively focally
(Fig. 1B). These results suggest
that the quality of the models we developed meet the requirements
for subsequent study.
The microarray analysis for the
expression profile of aberrant miRNAs of pediatric BSG compared to
adult type in orthotopic models
To identify miRNAs specifically involved in the
acquisition of malignant progression of pediatric BSG, we employed
a microarray to analyze the miRNA expression profiles in the two
groups of orthotopic models which could simulate the BSG
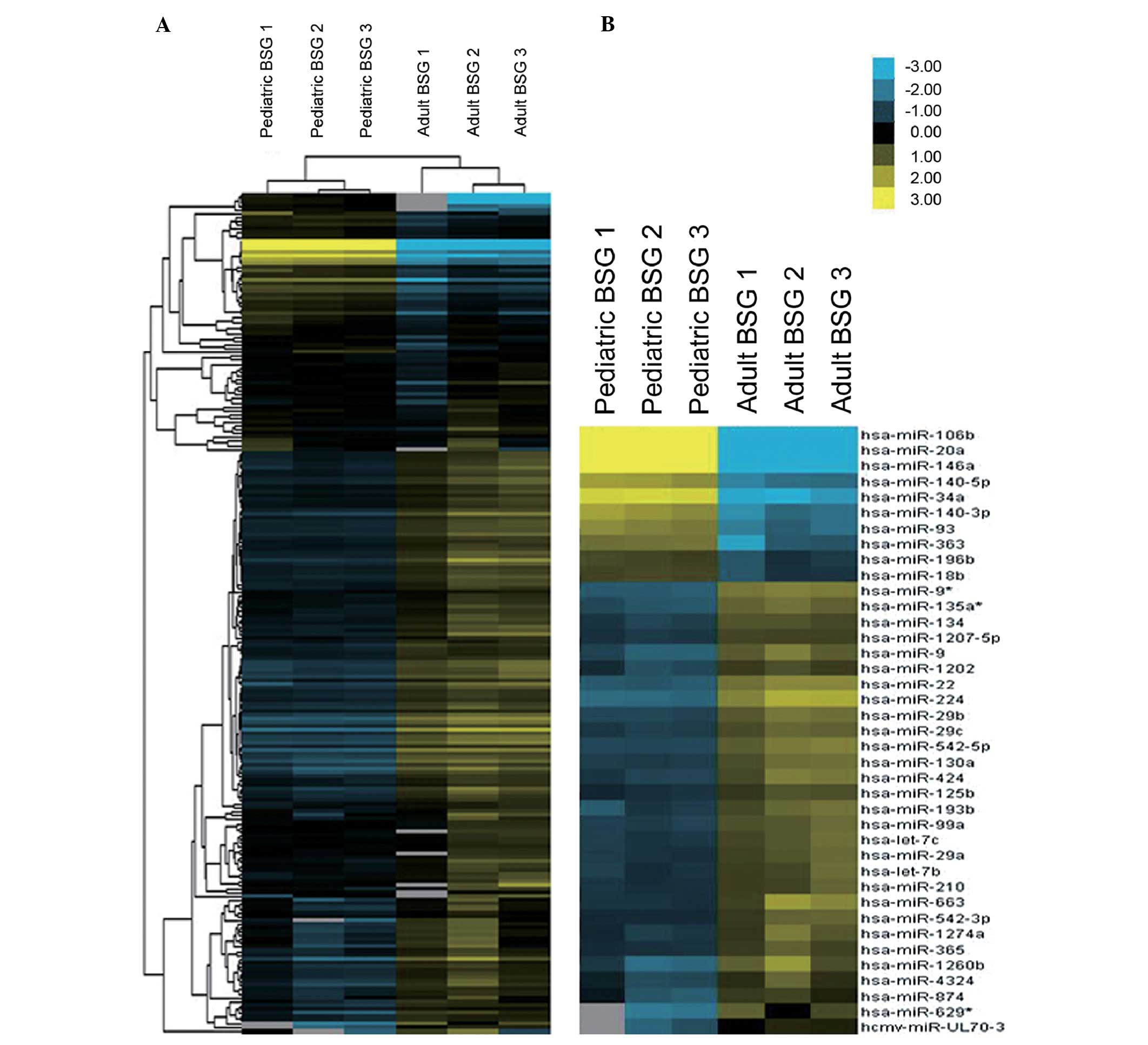
heterogeneity. Two hundred and sixteen miRNAs were detected in both
the pediatric BSG group and the adult BSG group (Fig. 2A).
The data from the pediatric BSG group are compared
with the data of adult BSG group to discover the differential
expressed miRNAs. Those miRNAs with FCAbsolute >3.5 and
corrected P-value <0.02 were identified as exhibiting
considerable upregulated expression, or, they may be considered as
exhibiting considerable downregulated expression likewise.
With these criteria, our research revealed 39 miRNAs
to be differentially expressed in the pediatric BSG group vs. adult
group, including 10 upregulated expression miRNAs and 29
downregulated expression miRNAs (Table
I, Fig. 2A). hsa-miR-20a,
hsa-miR-106b, hsa-miR-146a and hsa-miR-34a, were found to be the
most significantly upregulated miRNAs, while hsa-miR-224,
hsa-miR-22, hsa-miR-1260b and hsa-miR-9*, to be the most
significantly downregulated ones.
 | Table ISignificantly changed miRNAs with
corrected P-value and fold-change (absolute) by comparison between
pediatric BSG and adult BSG in orthotopic models. |
Table I
Significantly changed miRNAs with
corrected P-value and fold-change (absolute) by comparison between
pediatric BSG and adult BSG in orthotopic models.
| miRNA ID | FCAbsolute | Corrected
P-value |
|---|
| Positively
correlated miRNAs |
| hsa-miR-20a | 65.632 | 4.93E-05 |
| hsa-miR-106b | 44.682 | 4.93E-05 |
| hsa-miR-146a | 25.36153 | 4.93E-05 |
| hsa-miR-34a | 15.55422 | 1.26E-04 |
|
hsa-miR-140-3p | 13.98039 | 0.0011711 |
|
hsa-miR-140-5p | 13.59603 | 4.65E-04 |
| hsa-miR-93 | 10.21174 | 7.77E-04 |
| hsa-miR-363 | 9.115072 | 0.0076257 |
| hsa-miR-196b | 3.546545 | 0.0030593 |
| hsa-miR-18b | 3.512272 | 0.0030655 |
| Negatively
correlated miRNAs |
| hsa-miR-224 | 12.30044 | 5.50E-04 |
| hsa-miR-22 | 7.664119 | 2.87E-04 |
| hsa-miR-1260b | 6.506731 | 0.0076637 |
| hsa-miR-9* | 7.519141 | 4.93E-05 |
| hsa-miR-629* | 6.106636 | 0.01767 |
| hsa-miR-9 | 6.086732 | 0.0019944 |
| hsa-miR-135a* | 5.71923 | 3.92E-04 |
|
hsa-miR-542-5p | 5.628573 | 7.38E-04 |
| hsa-miR-663 | 4.729385 | 0.0084151 |
| hsa-miR-29b | 5.151732 | 5.50E-04 |
| hsa-miR-424 | 4.837116 | 0.0027836 |
| hsa-miR-193b | 4.67872 | 0.0036245 |
| hsa-miR-130a | 4.44713 | 0.0010245 |
| hsa-miR-99a | 4.273679 | 5.50E-04 |
| hsa-miR-29c | 4.105427 | 8.15E-04 |
| hsa-let-7c | 3.908946 | 0.0015876 |
| hsa-miR-134 | 3.791241 | 3.92E-04 |
| hsa-miR-1274a | 3.634825 | 0.0070172 |
| hsa-miR-29a | 3.613525 | 0.0015876 |
| hsa-let-7b | 3.535912 | 0.0035764 |
Quantitative RT-PCR validation of miR-20a
and miR-106b
Amongst the aberrantly expressed miRNAs according to
microarray analysis, the most considerable fold-changes are seen in
miR-20a and miR-106b (Table I,
Fig. 2B). Specifically, miR-106b
belongs to miR-106b-25 cluster, whereas its paralog miR-17–92
cluster contains miR-20a. These findings indicate that miR-20a and
miR-106b might be putative causative miRNAs, hence, chosen as
candidate for validation in human BSG to confirm the result
obtained from orthotopic models. Thus, we employed qRT-PCR on human
specimens of 3 pediatric BSG and 2 adult BSG. qRT-PCR analysis
verified the data screened using microarray: miR-20a and miR-106b
expression patterns behaved similarly in human specimens, both
levels were markedly higher in pediatric BSG in comparison with
adult ones, though the extent was slightly lower than the data
revealed by orthotopic models (Tables
II and III). There were no
significant differences between miR-20a or miR-106b levels in grade
III and grade IV pediatric BSG (Tables
IV and V), or grade I and grade
II adult BSG (Tables VI and
VII).
 | Table IIThe expression changes of miR-20a
between human specimens of adult and pediatric BSG. |
Table II
The expression changes of miR-20a
between human specimens of adult and pediatric BSG.
| Group | Specimen |
2−ΔCT | RQ (fold) | P-value |
|---|
| Adult | 2 |
9.88±0.460521444 | 1 | |
| Pediatric | 3 |
6.59±0.18594354 |
9.46072±0.37015 | <0.01 |
 | Table IIIThe expression changes of miR-106b
between human specimens of adult and pediatric BSG. |
Table III
The expression changes of miR-106b
between human specimens of adult and pediatric BSG.
| Group | Specimen |
2−ΔCT | RQ (fold) | P-value |
|---|
| Adult | 2 |
8.51500±0.21380 | 1 | |
| Pediatric | 3 |
5.09111±0.42923 |
11.04985±0.92818 | <0.01 |
 | Table IVThe expression changes of miR-20a
between human specimens of adult BSGs with different WHO
classification. |
Table IV
The expression changes of miR-20a
between human specimens of adult BSGs with different WHO
classification.
| Group | Specimen |
2−ΔCT | RQ (fold) | P-value |
|---|
| Adult WHO-I | 1 |
9.79667±0.58731 | 1 | |
| Adult WHO-II | 1 |
9.96333±0.40550 |
0.90668±0.19905 | >0.01 |
 | Table VThe expression changes of miR-106b
between human specimens of adult BSGs with different WHO
classification. |
Table V
The expression changes of miR-106b
between human specimens of adult BSGs with different WHO
classification.
| Group | Specimen |
2−ΔCT | RQ (fold) | P-value |
|---|
| Adult WHO-I | 1 |
8.56333±0.18502 | 1 | |
| Adult WHO-II | 1 |
8.46667±0.27025 |
1.07978±0.18849 | >0.01 |
 | Table VIThe expression changes of miR-20a
between human specimens of pediatric BSGs with different WHO
classification. |
Table VI
The expression changes of miR-20a
between human specimens of pediatric BSGs with different WHO
classification.
| Group | Specimen |
2−ΔCT | RQ (fold) | P-value |
|---|
| Pediatric
WHO-III | 1 |
6.56667±0.19732 | 1 | |
| Pediatric
WHO-IV | 2 |
6.60167±0.19813 |
0.97748±0.05130 | >0.01 |
 | Table VIIThe expression changes of miR-106b
between human specimens of pediatric BSGs with different WHO
classification. |
Table VII
The expression changes of miR-106b
between human specimens of pediatric BSGs with different WHO
classification.
| Group | Specimen |
2−ΔCT | RQ (fold) | P-value |
|---|
| Pediatric
WHO-III | 1 |
5.15000±0.42930 | 1 | |
| Pediatric
WHO-IV | 2 |
5.06167±0.46684 |
1.07915±0.10707 | >0.01 |
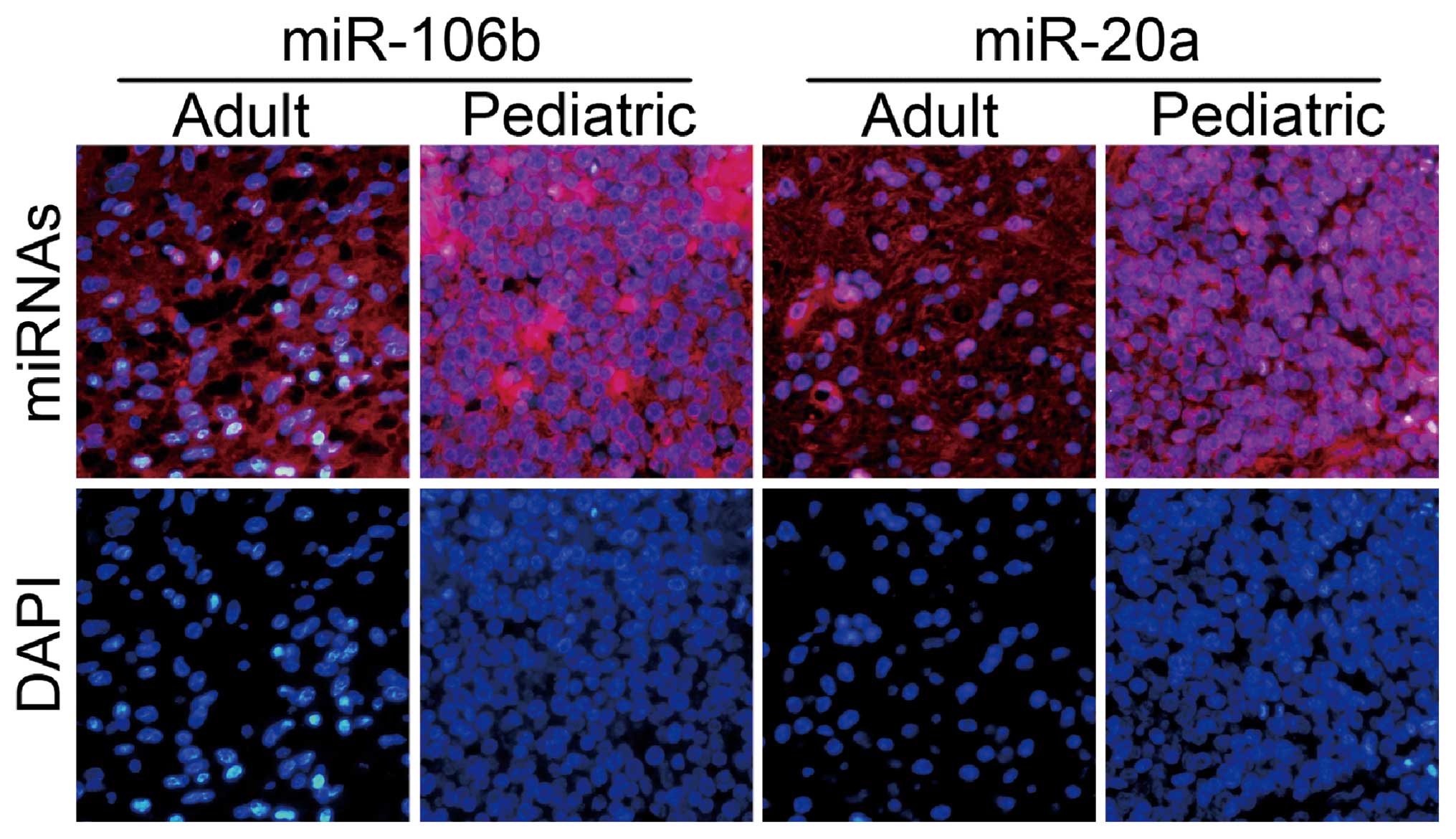
Fluorescence in situ hybridization
validation of miR-20a and miR-106b
To more firmly identify the changes of miRNA
expression obtained from orthotopic models, FISH was further
applied to localize the expression patterns of miR-20a and miR-106b
using LNA probes. As expected, the staining pattern of sections
from human pediatric BSG specimens were particularly striking,
whereas low in situ signals observed in adult ones,
concurred with our qRT-PCR data (Fig.
3).
Discussion
Brainstem glioma (BSG) is an entity which commonly
occurs in pediatric patients (1,18). The
benign property of adult BSG has provided a unique perspective to
comparatively understand pediatric BSG (10). Nevertheless, BSG in adults are
poorly understood because they are quite unusual (6). Besides the distinction in incidence,
biological behavior, invasion pattern, prognosis and treatment
outcome, many studies indicated the concept that these tumors form
a homogeneous group have changed, and now it should be recognized
that BSGs are a heterogenous group with differing genetic
characteristics (10).
Many clinical reports have shown that the majority
of pediatric BSG are high grade gliomas (WHO grades III and IV)
revealed by histopathological examination (2,19,20).
Correspondingly, Guillamo et al have shown that benign
histology (WHO grades I and II) was found in up to 82% in adult
group (6). Moreover, in the
previous study we also observed that despite the application the
suspension of C6 cells was identical, after the establishment of
orthotopic BSG model, malignant grade of pediatric BSG was
significantly higher than the adult group. A huge degree of
difference in cell proliferation and apoptosis has also been
reported (10). In addition, a
retrospective analysis was undertaken on 40 BSG patients (10
children and 30 adults) with the identical malignant grade (WHO
II), median survival was 35.2 months in the adult group, having far
better prognosis than the pediatric patients with the survival of
16.5 months (21). Therefore, in
the current study, we performed genetic analysis of pediatric BSG
compared to adult type by miRNA microarrays to see if there were
any genetic characteristics that could afford relevant information
in terms of management of patients and etiology of pediatric
BSG.
Indeed, existing research on the oncogenic role of
miRNAs in brain tumors has achieved some remarkable results
(22), such as pituitary adenoma
(23), and medulloblastoma
(24). Ciafrè et al
(25) profiled the expression of
245 miRNAs in 10 glioblastoma (GBM) cell lines and nine freshly
resected GBM samples and observed a consistent pattern of miRNAs
whose expression differs from surrounding normal brain tissue.
However, most of these data were based on the analyses of
supratentorial gliomas. BSG has not been genetically well studied,
not to mention from the perspective of distinction between BSG
heterogeneity. On the other hand, tumor location could be
associated with specific tumorigenic pathway even within one
histological entity (26).
In the present study, a set of miRNAs were
identified as differentially expressed in pediatric BSG compared to
adult type, suggesting that altered miRNA expression may be
involved in the acquisition of malignant progression of pediatric
BSG. If we set 3.5-fold as the threshold of a differential
expression level, the number of miRNAs which have at least 3.5-fold
changes is not large. After data analysis, we found that, among the
differentially expressed miRNAs, the most representative were
miR-20a and miR-106b, with strong enrichment in pediatric BSG.
The development of satisfactory experimental models
for simulating BSG heterogeneity is critical to our understanding
of this tumor and the future discovery of novel therapies, for it
could be adapted to facilitate experimental intervention and
pre-clinical testing of new therapies (10,27).
Our previous models using C6 rat glioma cell lines have been
reported (10). A potential
limitation of rodent tumor-derived systems is that the response of
rodent cell lines to treatment with anticancer agents can differ
from that of human glioma cells (28). In the present study, altering of
human glioma cell line offers a distinct advantage with regard to
this concern (27). Furthermore, to
verify our above observations in BSG models that reflected
molecular distinction between two types of BSGs, we analyzed
miR-20a and miR-106b expression in tissue sections from BSG
patients, and found that the results were accordant. We presume
that miR-20a and miR-106b could have putative causative involvement
of acquisition of malignant progression of pediatric BSG.
Noteworthy, miR-106b belongs to miR-106b-25 cluster,
whereas its paralog miR-17–92 cluster contains miR-20a. In these
two miRNA families, the homologue miRNAs relations are: miR-106b
matches miR-17-5p sequence, miR-93 matches miR-20a, and miR-25
matches miR-92a-1 (29).
Accordingly, there may exist a coordination mechanism involving
miR-106b-25 and miR-17–92 clusters in BSG. The miR-106b-25 and
miR-17–92 synergistic control apoptosis is supported by the work of
Petrocca and colleagues, triggering a positive feedback loop that
would support MYC, E2F1, and miR-106b-25/miR-17–92 elevated
expression while inactivating the TGFα pathway (29). Also, both miR-106b-25/miR-17–92
could directly target p21/CDKN1A, so as to contribute to tumor cell
proliferation in part by regulating cell cycle progression and by
modulating checkpoint functions (30,31).
Thus, it is of interest to note that miR-106b-25 cluster is present
in self-renewing neural stem/progenitor cells (NSPCs) and does not
change its expression when cells are stimulated to undergo
differentiation (32). miR-20a was
identified to be involved in spontaneously progression of primary
WHO grade II gliomas to WHO grade IV secondary glioblastomas
(33), while our previous study
observed that miR-106b were overexpressed in five glioblastoma cell
lines (34).
The lack of knowledge on miRNA target genes hampers
the full understanding on the biological functions of miRNAs. The
target genes of miR-20a and miR-106b could be extracted using
databases such as TargetScan, and PicTar, which provide a composite
prediction of target genes from target prediction tools. Further
studies are required to clarify the role of these genes in cellular
biology. Besides, our previous finding indicated that the growth
pattern and invasiveness of pediatric BSG could depend not only on
the internal mechanism of the tumor cells, but also on the host
cellular environment created for the tumor to spread into the
adjacent brain tissue (10). The
acquisition of malignant progression of pediatric BSG compared to
adult patients could be attributed to the collaborative interaction
of aberrantly expressed miRNAs of tumor cells (such as miR-20a and
miR-106b) and the host cellular environment, which warrants further
investigation.
In conclusion, our results demonstrate that miR-20a
and miR-106b, at least in part, may play a role in acquired
malignant progression in pediatric BSG compared with the adult
group. The elucidation of the oncogenic role of these miRNA in
modulating gene expression will present a comprehensive perspective
for understanding the molecular bases and initiation of BSG
heterogeneity. Antagonizing the corresponding miRNAs, thus may
represent a possible antitumor approach for integrated cancer
therapy to alter the gloomy outlook in pediatric BSG.
Acknowledgements
This study was supported by the China National
Natural Scientific Found (81172399, 30872647), and the Beijing
Natural Science Found (7092041).
Abbreviations:
|
BSG
|
brain stemglioma
|
|
CNS
|
central nervous system
|
|
miRNA
|
microRNA
|
|
FCAbsolute
|
absolute value of the fold-change
|
|
3′ UTR
|
3′ untranslated region
|
|
WHO
|
World Health Organization
|
|
mRNA
|
messenger RNA
|
|
Cy3
|
Cyanine3-pCp
|
|
FISH
|
fluorescence in situ
hybridization
|
References
|
1
|
Hargrave D, Bartels U and Bouffet E:
Diffuse brainstem glioma in children: critical review of clinical
trials. Lancet Oncol. 7:241–248. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Laigle-Donadey F, Doz F and Delattre JY:
Brainstem gliomas in children and adults. Curr Opin Oncol.
20:662–667. 2008. View Article : Google Scholar
|
|
3
|
Guillamo JS, Doz F and Delattre JY: Brain
stem gliomas. Curr Opin Neurol. 14:711–715. 2001. View Article : Google Scholar
|
|
4
|
Mischel PS and Cloughesy TF: Targeted
molecular therapy of GBM. Brain Pathol. 13:52–61. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hwang JH, Smith CA, Salhia B and Rutka JT:
The role of fascin in the migration and invasiveness of malignant
glioma cells. Neoplasia. 10:149–159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guillamo JS, Monjour A, Taillandier L,
Devaux B, Varlet P, Haie-Meder C, Defer GL, Maison P, Mazeron JJ,
Cornu P and Delattre JY: Brainstem gliomas in adults: prognostic
factors and classification. Brain. 124:2528–2539. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mursch K, Halatsch ME, Markakis E and
Behnke-Mursch J: Intrinsic brainstem tumours in adults: results of
microneurosurgical treatment of 16 consecutive patients. Br J
Neurosurg. 19:128–136. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kesari S, Kim RS, Markos V, Drappatz J,
Wen PY and Pruitt AA: Prognostic factors in adult brainstem
gliomas: a multicenter, retrospective analysis of 101 cases. J
Neurooncol. 88:175–183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Epstein F and Wisoff JH: Surgical
management of brain stem tumors of childhood and adolescence.
Neurosurg Clin N Am. 1:111–121. 1990.PubMed/NCBI
|
|
10
|
Liu Q, Liu R, Kashyap MV, Agarwal R, Shi
X, Wang CC and Yang SH: Brainstem glioma progression in juvenile
and adult rats. J Neurosurg. 109:849–855. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gilbertson RJ, Hill DA, Hernan R, Kocak M,
Geyer R, Olson J, Gajjar A, Rush L, Hamilton RL, Finkelstein SD and
Pollack IF: ERBB1 is amplified and overexpressed in high-grade
diffusely infiltrative pediatric brain stem glioma. Clin Cancer
Res. 9:3620–3624. 2003.PubMed/NCBI
|
|
12
|
Lee RC, Feinbaum RL and Ambros V: The
C. elegans heterochronic gene lin-4 encodes small RNAs with
antisense complementarity to lin-14. Cell. 75:843–854. 1993.
|
|
13
|
Karginov FV, Conaco C, Xuan Z, Schmidt BH,
Parker JS, Mandel G and Hannon GJ: A biochemical approach to
identifying microRNA targets. Proc Natl Acad Sci USA.
104:19291–19296. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cui Q, Yu Z, Purisima EO and Wang E:
Principles of microRNA regulation of a human cellular signaling
network. Mol Syst Biol. 2:462006.PubMed/NCBI
|
|
15
|
Wang X, Han L, Zhang A, Wang G, Jia Z,
Yang Y, Yue X, Pu P, Shen C and Kang C: Adenovirus-mediated shRNAs
for co-repression of miR-221 and miR-222 expression and function in
glioblastoma cells. Oncol Rep. 25:97–105. 2011.PubMed/NCBI
|
|
16
|
Bhatti I, Lee A, Lund J and Larvin M:
Small RNA: a large contributor to carcinogenesis? J Gastrointest
Surg. 13:1379–1388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song YJ, Tian XB, Zhang S, Zhang YX, Li X,
Li D, Cheng Y, Zhang JN, Kang CS and Zhao W: Temporal lobe epilepsy
induces differential expression of hippocampal miRNAs including
let-7e and miR-23a/b. Brain Res. 1387:134–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Selvapandian S, Rajshekhar V and Chandy
MJ: Brainstem glioma: comparative study of clinico-radiological
presentation, pathology and outcome in children and adults. Acta
Neurochir. 141:721–727. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jallo G: Brainstem gliomas. Childs Nerv
Syst. 22:1–2. 2006. View Article : Google Scholar
|
|
20
|
Salmaggi A, Fariselli L, Milanesi I,
Lamperti E, Silvani A, Bizzi A, Maccagnano E, Trevisan E, Laguzzi
E, Ruda R, et al: Natural history and management of brainstem
gliomas in adults. A retrospective Italian study. J Neurol.
255:171–177. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yin LX, Li DZ, Zhang JT, Wu Z and Zhang
LW: Comparison of clinical characteristics and surgical outcomes
between adults and children with astrocytomas of brain stem. Zhong
Guo Wei Qin Xi Shen Jing Wai Ke Za Zhi. 15:158–160. 2010.
|
|
22
|
Pang JC, Kwok WK, Chen Z and Ng HK:
Oncogenic role of microRNAs in brain tumors. Acta Neuropathol.
117:599–611. 2009. View Article : Google Scholar
|
|
23
|
Bottoni A, Zatelli MC, Ferracin M,
Tagliati F, Piccin D, Vignali C, Calin GA, Negrini M, Croce CM and
Degli Uberti EC: Identification of differentially expressed
microRNAs by microarray: a possible role for microRNA genes in
pituitary adenomas. J Cell Physiol. 210:370–377. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gokhale A, Kunder R, Goel A, Sarin R,
Moiyadi A, Shenoy A, Mamidipally C, Noronha S, Kannan S and Shirsat
NV: Distinctive microRNA signature of medulloblastomas associated
with the WNT signaling pathway. J Cancer Res Ther. 6:521–529. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ciafre SA, Galardi S, Mangiola A, Ferracin
M, Liu CG, Sabatino G, Negrini M, Maira G, Croce CM and Farace MG:
Extensive modulation of a set of microRNAs in primary glioblastoma.
Biochem Biophys Res Commun. 334:1351–1358. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miwa T, Hirose Y, Sasaki H, Ikeda E,
Yoshida K and Kawase T: Genetic characterization of adult
infratentorial gliomas. J Neurooncol. 91:251–255. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hashizume R, Ozawa T, Dinca EB, Banerjee
A, Prados MD, James CD and Gupta N: A human brainstem glioma
xenograft model enabled for bioluminescence imaging. J Neurooncol.
96:151–159. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yaz G, Kabadere S, Oztopcu P, Durmaz R and
Uyar R: Comparison of the antiproliferative properties of
antiestrogenic drugs (nafoxidine and clomiphene) on glioma cells in
vitro. Am J Clin Oncol. 27:384–388. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Petrocca F, Vecchione A and Croce CM:
Emerging role of miR-106b-25/miR-17–92 clusters in the control of
transforming growth factor beta signaling. Cancer Res.
68:8191–8194. 2008.PubMed/NCBI
|
|
30
|
Ivanovska I, Ball AS, Diaz RL, Magnus JF,
Kibukawa M, Schelter JM, Kobayashi SV, Lim L, Burchard J, Jackson
AL, et al: MicroRNAs in the miR-106b family regulate p21/CDKN1A and
promote cell cycle progression. Mol Cell Biol. 28:2167–2174. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Inomata M, Tagawa H, Guo YM, Kameoka Y,
Takahashi N and Sawada K: MicroRNA-17–92 down-regulates expression
of distinct targets in different B-cell lymphoma subtypes. Blood.
113:396–402. 2009.
|
|
32
|
Peck B, Schulze A, Oliveras-Ferraros C,
Vellon L, Joven J and Vazquez-Martin A: A role for the
cancer-associated miR-106b~25 cluster in neuronal stem cells. Aging
(Albany NY). 3:329–331. 2011.PubMed/NCBI
|
|
33
|
Malzkorn B, Wolter M, Liesenberg F,
Grzendowski M, Stuhler K, Meyer HE and Reifenberger G:
Identification and functional characterization of microRNAs
involved in the malignant progression of gliomas. Brain Pathol.
20:539–550. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou X, Ren Y, Moore L, Mei M, You Y, Xu
P, Wang B, Wang G, Jia Z, Pu P, Zhang W and Kang C: Downregulation
of miR-21 inhibits EGFR pathway and suppresses the growth of human
glioblastoma cells independent of PTEN status. Lab Invest.
90:144–155. 2010. View Article : Google Scholar : PubMed/NCBI
|