Introduction
Over the last few decades, systemic chemotherapy for
colon cancer has markedly changed worldwide. Combinations of
5-fluorouracil (5-FU) with other cytotoxic agents, such as
oxaliplatin and irinotecan, have improved the prognosis of patients
with advanced colon cancer. Furthermore, the addition of
molecular-targeted agents, such as anti-VEGF antibody (bevacizumab)
and anti-EGFR antibody (cetuximab/panitumumab), have resulted in
dramatic improvements in the survival of patients with advanced
colon cancer and are currently regarded as first-line chemotherapy
regimens (1–4). Although 5-FU is still a key drug in
these regimens, the inherent or acquired resistance to 5-FU in
colon cancer is a critical problem. A number of studies have
reported that 5-FU metabolism-related factors, such as thymidylate
synthase (TS), folate co-factors, dihydropyrimidine dehydrogenase
(DPD) and orotate phosphoribosyltransferase (OPRT), are associated
with the response to or toxicity of 5-FU (5–8).
However, there are still no reliable biomarkers for the sensitivity
or resistance to 5-FU chemotherapy.
Mammalian heat shock proteins (HSPs) have been
classified into four main families based on their molecular
weights: HSP90, HSP70, HSP60, and small HSPs (15–30 kDa), including
HSP27. These proteins are well known as molecular chaperons in
protein-protein interactions, as anti-apoptotic proteins and
contributors to cell survival (9,10).
Many studies have reported that HSP27 expression contributes to the
malignant properties of cancer cells, including tumorigenicity,
treatment resistance, and apoptosis inhibition (11–15).
In colon cancer, certain studies have reported that HSP27
expression participates in the resistance to doxorubicin or
irinotecan in vitro (16,17),
and HSP27 has recently been considered as a prognostic marker in
clinicopathological studies (18–20).
Our previous study also indicated that HSP27 expression
participates in the degree of resistance to 5-FU, a key drug for
the treatment of colorectal cancer, in experiments performed in
vitro (21). In the present
study, we sought to clarify whether HSP27 can be used as a clinical
biomarker for 5-FU chemotherapy or as a treatment target for 5-FU
resistance using a xenograft model.
Materials and methods
Drug, cell lines and cell culture
conditions
The anticancer drug, 5-FU, was purchased from Kyowa
Hakko Kogyo Co., Ltd. (Tokyo, Japan). The human colon cancer cell
line, HCT116, was obtained from the American Type Culture
Collection (Rockville, MD). The cells were grown in Roswell Park
Memorial Institute (RPMI)-1640 medium (Gibco, MA). Each culture was
supplemented with 10% fetal bovine serum (CSL Ltd., Melbourne,
Australia) and 1% penicillin/streptomycin. The cells were cultured
at 37°C with 5% CO2.
Stable transfection with short hairpin
RNA (shRNA)
An oligonucleotide for a short hairpin RNA targeting
the human HSP27 site (5′-UAGCCAUGCUCGUCCUGCCUU-3′) was designed
(21), so as to contain
5′-BamHI and 3′-EcoRI overhangs. The annealed
oligonucleotide was then ligated into a linearized U6
promoter-driven vector (pSIREN-DNR-DsRed-Express Vector; Clontech,
Mountain View, CA). As the negative control, an oligonucleotide for
a scrambled shHSP27 (5′-ACGUAAGGCGCGUAACGGGTT-3′) containing
5′-BamHI and 3′-EcoRI overhangs was also constructed
and ligated to the pSIREN vector.
For transfection, HCT116 cells expressing high
levels of HSP27 under normal culture conditions were plated in
6-well plates at a density of 1×106 cells per well and
allowed to grow overnight. The cells were transfected with a
mixture of 5 μg of siRNA-encoding plasmids (the HSP27 target
sequence plasmid and the negative control plasmid) and 20 μl of
Lipofectamine 2000 (Invitrogen Life Technologies, Inc., Carlsbad,
CA) in 2 ml of serum-free medium, according to the manufacturer’s
instructions. After incubation at 37°C for 6 h, the medium was
replaced with fresh growing medium. The transfected cells,
containing a red fluorescent protein, were verified by
visualization in living cells using fluorescence microscopy. Some
of the transfected clones with different levels of HSP27 expression
were monoclonally obtained and named HCT116-shRNA/HSP27-1 to -4.
The control cells, HCT116-scramble/HSP27 and HCT116-mock cells,
were established in a similar manner.
Quantitative real-time PCR (qRT-PCR)
analysis of 5-FU metabolic enzymes
Total RNA was extracted from the cells in each
group, and qRT-PCR analysis was performed using a
fluorescence-based, real-time detection method [ABI PRISM 7900
Sequence Detection System (TaqMan); Applied Biosystems, Foster
City, CA]. The mRNA expression of DPD, OPRT and TS was analyzed in
the transfected cells that were either treated or not with 5-FU
(1.28 μg/ml) in culture medium for 24 h. The enzyme expression
levels of the transfected cells were assessed relative to the
levels of the gene transcript in the mock transfected cells
(interest/β-actin). The sequences of the primers and probes were as
follows: DPD: 51F (19 bp), AGGACGCAAGG AGGGTTTG; 134R (20 bp),
GTCCGCCGAGTCCTTACTGA; probe-71T (29 bp), CAGTGCCTACAGTCTCGAGTCTGCC
AGTG. OPRT: 496F (25 bp), TAGTGTTTTGGAAACTG TTGAGGTT; 586R (20 bp),
CTTGCCTCCCTGCTCTCTGT; probe-528T (27 bp), TGGCATCAGTGACCTTCAAGCCC
TCCT. TS: 764F (18 bp), GCCTCGGTGTGCCTTTCA; 830R (17 bp),
CCCGTGATGTGCGCAAT; probe-785T (21 bp), TCGCCAGCTAC GCCCTGCTCA.
β-actin: 592F (18 bp), TGAGCGCGGCTACAGCTT; 651R (22 bp), TCCTTAA
TGTCACGCACGATTT; probe-611T (18 bp), ACCACCA CGGCCGAGCGG. The 25-μl
PCR reaction mixture contained 600 nmol/l of each primer, 200
nmol/l each of dATP, dCTP and dGTP, 400 μmol/l of dUTP, 5.5 μmol/l
of MgCl2 and 1X TaqMan buffer A containing a reference
dye (all reagents were supplied by Applied Biosystems). The PCR
conditions were 50°C for 10 sec and 95°C for 10 min, followed by 42
cycles at 95°C for 15 sec and 60°C for 1 min.
Western blot analysis
Total cell lysates were extracted with lysis buffer
with standard methods. The quantity of cell lysates was determined
using a Bio-Rad DC protein assay kit (Bio-Rad Laboratories,
Hercules, CA), and 20 mg of lysates in total were resolved in Ready
Gel (Bio-Rad Laboratories) and transferred to an Immuno-Blot™
polyvinylidene fluoride membrane (Bio-Rad Laboratories). The
membrane was blocked in phosphate-buffered saline (PBS) containing
5% non-fat milk powder for 2 h at room temperature, then incubated
at 4°C overnight with 1:2000-diluted anti-human HSP27 mouse
monoclonal antibody (G3.1; Lab Vision Corp., Fremont, CA) or
1:5000-diluted anti-human β-actin mouse monoclonal antibody (AC74;
Sigma, St. Louis, MO). The membranes were incubated for 30 min with
a 1:5000-diluted horseradish peroxidase-conjugated anti-mouse
immunoglobulin G (IgG) (Promega Corp., Madison, WI). Bound
complexes were detected using the ECL-Plus reagent (Amersham
Biosciences Corp., Cardiff, UK) according to the manufacturer’s
instructions. The density of the band was measured using NIH
imaging (Scion) software and normalized to β-actin. Each experiment
was performed in triplicate.
Xenograft model
Female nude mice with a BALB/cA genetic background
were purchased from CLEA Japan Co. Ltd., Tokyo, Japan. The mice
were maintained under specific pathogen-free conditions using an
Isorack in our experimental animal center and fed sterile food and
water. Six- to eight-week-old mice weighing 20–22 g were used for
the experiments. To analyze the effect of HSP27 knockdown against
5-FU treatment, HCT116-mock, HCT116-scramble/HSP27, and
HCT116-shRNA/HSP27 cells (3×106 cells per injection),
which were resuspended in 100 μl of PBS, were injected into the
subcutaneous tissues of the bilateral dorsum of ether-anesthetized
mice using a 1-ml syringe and a 27-gauge tuberculin needle (Terumo
Co., Tokyo, Japan). The tumors were measured (length and width)
twice a week using a sliding caliper by the same observer. When the
inoculated tumors reached 5 mm in length, 5-FU (200 μl saline
solution) was administered intraperitoneally at a dose of 60 mg/kg
on days 1, 5 and 9 (n=5 mice per group). As the control, saline
(200 μl) was also administered intraperitoneally (n=5 mice). The
estimated tumor volume (EV) was calculated as EV = length ×
width2 × 1/2. EV was plotted against the day since 5-FU
treatment initiation to derive a xenograft growth curve. All the
mice were sacrificed at three weeks after the initial treatment.
Tumors were collected and fixed in 4% paraformaldehyde (Sigma) at
room temperature for 24 h before processing for sectioning and
immunohistochemical staining or the preparation of tumor lysates
for western blot analysis. All animal studies were conducted in
accordance with the institutional guidelines approved by the Animal
Care and Use Committee of our university.
Immunohistochemical staining
Anti-human HSP27 rabbit polyclonal antibody
(Stressgen Bioreagents Corp., Victoria, BC, Canada) was used for
the immunohistochemical staining of the tumors from the mice.
Immunohistochemical staining was performed according to the
standard streptavidin-biotin peroxidase complex method using a
Histofine™ SAB-PO[M] kit (Nichirei, Tokyo, Japan). Briefly,
deparaffinized sections were placed in methanol containing 1%
hydrogen peroxide for 15 min to block endogenous peroxidase
activity. After washing with PBS, the sections were incubated with
anti-HSP27 antibody (1:100 diluted in PBS) overnight at 4°C in a
moist chamber, and then incubated with biotinylated goat
anti-rabbit IgG and peroxidase-conjugated streptavidin (Stressgen
Bioreagents Corp.) for 30 min each at room temperature. After a
final washing with PBS, the sections were immersed in a PBS
solution containing 0.02% 3,3′-diaminobenzidine and 0.1%
hematoxylin and mounted in balsam.
Detection of caspase-3 activities for
apoptosis analysis
The transfected cells were treated with or without
5-FU (1.28 μg/ml) in the culture medium for 24 h. The caspase-3
activities were then measured using an ApoAlert Caspase-3
Colorimetric assay kit (Clontech), according to the manufacturer’s
instructions. The cells (2×106) were centrifuged at 400
× g for 5 min. After the extraction of the cell lysates using cell
lysis buffer, the supernatants were centrifuged for 10 min at 4°C
to precipitate the cellular debris and were then incubated with
reaction buffer/dithiothreitol (DTT) mix for 30 min on ice and
caspase-3 substrate for 1 h at 37°C. The samples were read at 405
nm on a microplate reader.
Statistical analysis
Each value was expressed as the mean ± standard
deviation. Statistical analysis was performed using the Student’s
t-test or the Mann-Whitney U test. The regression analysis was
performed using the Statistical Package for Social Sciences (SPSS)
13.0J for Windows (SPSS, Chicago, IL). P<0.05 was considered to
indicate a statistically significant difference.
Results
5-FU sensitivity of
shRNA/HSP27-transfected cells in vitro
The 5-FU-resistant colon cancer cells, HCT116, were
transfected with HSP27 shRNA ligated to the
pSIREN-DNR-DsRed-Express vector (shRNA/HSP27-transfected cells),
scrambled shRNA ligated to the pSIREN vector as the control
(scramble/HSP27-transfected cells), or an empty vector
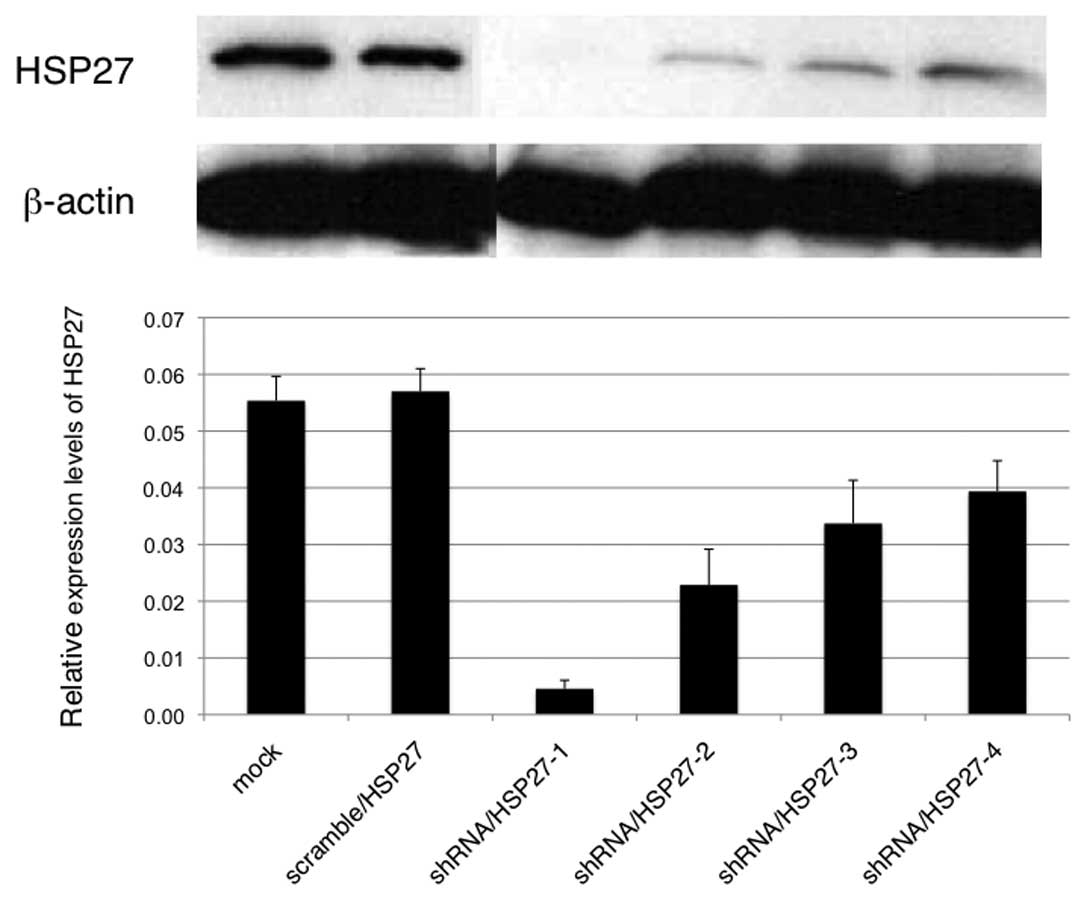
(mock-transfected cells). Transfected cells with various HSP27
expression levels were obtained by cloning (Fig. 1; shRNA/HSP27-1 to -4). To evaluate
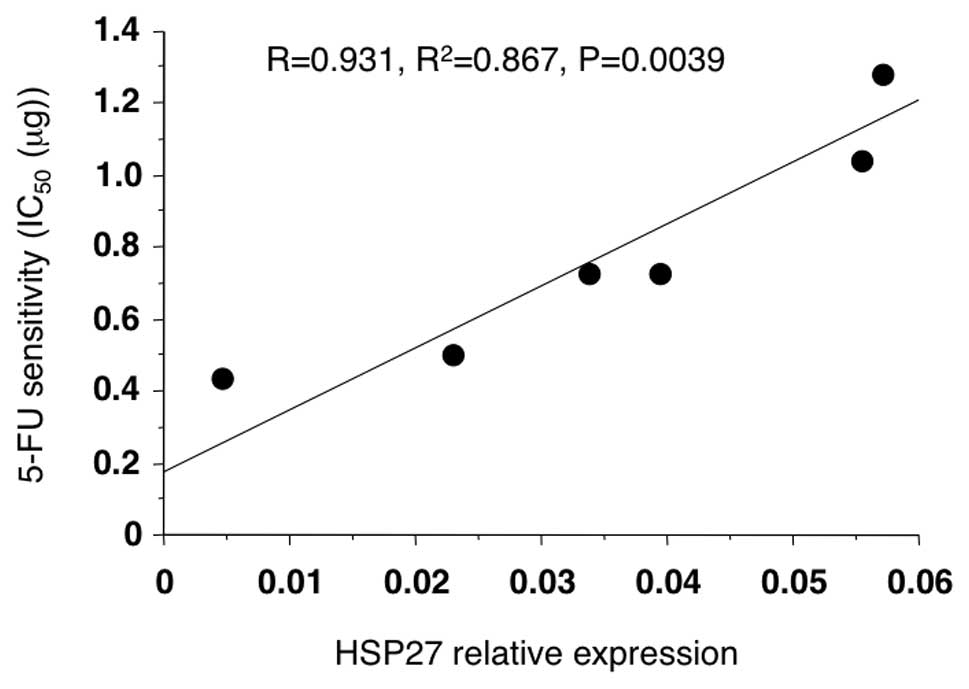
the 5-FU sensitivity, the transfected cells were first treated with
5-FU in vitro, and then the IC50 of each
transfectant was measured using an MTT assay. A significant
correlation between the HSP27 protein levels and the
IC50 of 5-FU was observed (Fig. 2; R=0.931, P=0.0039).
5-FU sensitivity of
shRNA/HSP27-transfected cells in xenograft model
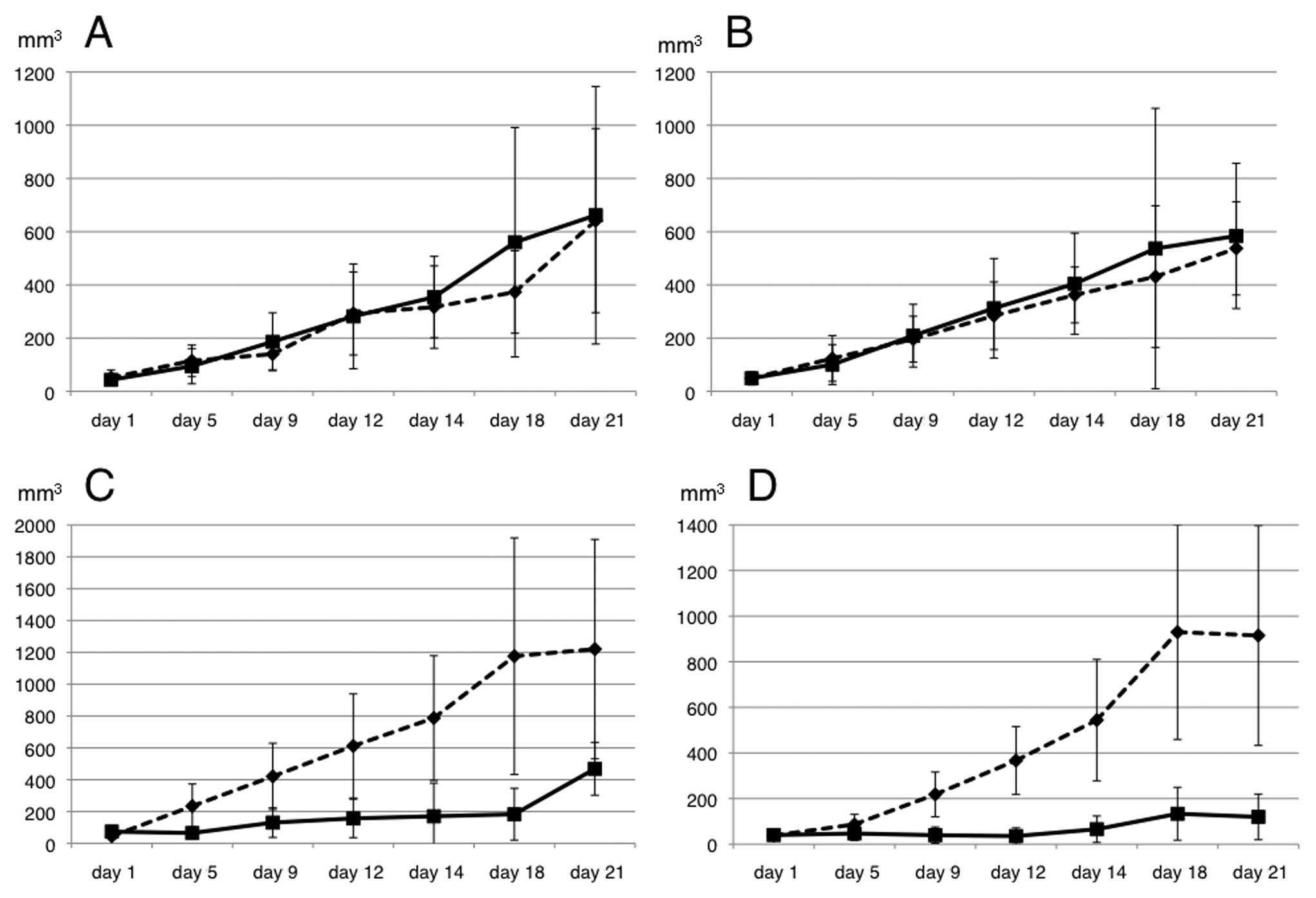
To evaluate the 5-FU sensitivity in the xenograft
model, the transfected cells were injected into the subcutaneous
tissues of the bilateral dorsum of ether-anesthetized mice. When
the inoculated tumors reached 5 mm in length, 5-FU was administered
intraperitoneally at a dose of 60 mg/kg on days 1, 5 and 9 (n=5
mice per group). Saline (200 μl) was also administered
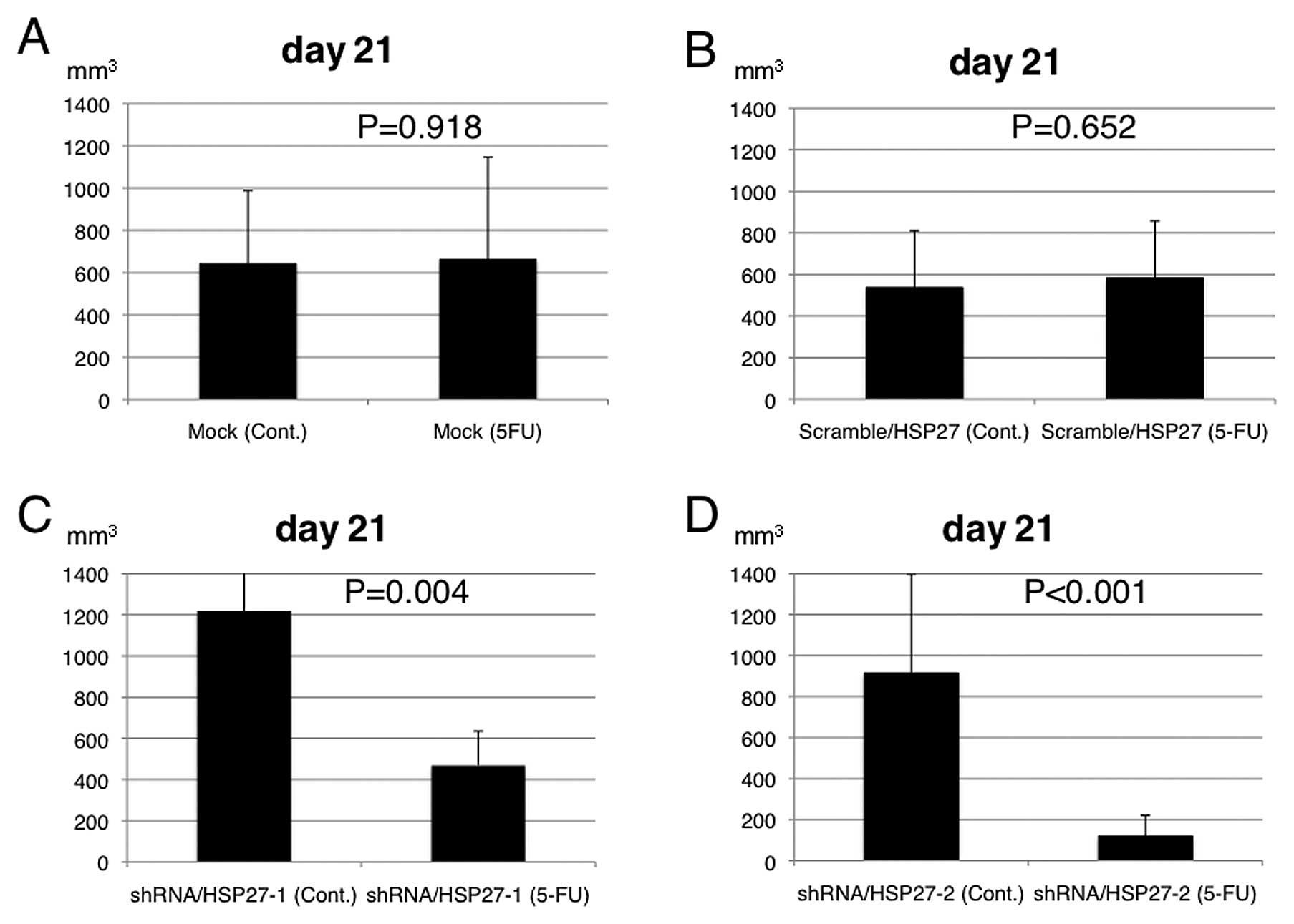
intraperitoneally in the control group (n=5 mice). The EV following
5-FU treatment was significantly suppressed in the mice injected
with the shRNA/HSP27-transfected cells, compared to those injected
with the scramble/HSP27- and mock-transfected cells (Figs. 3 and 4).
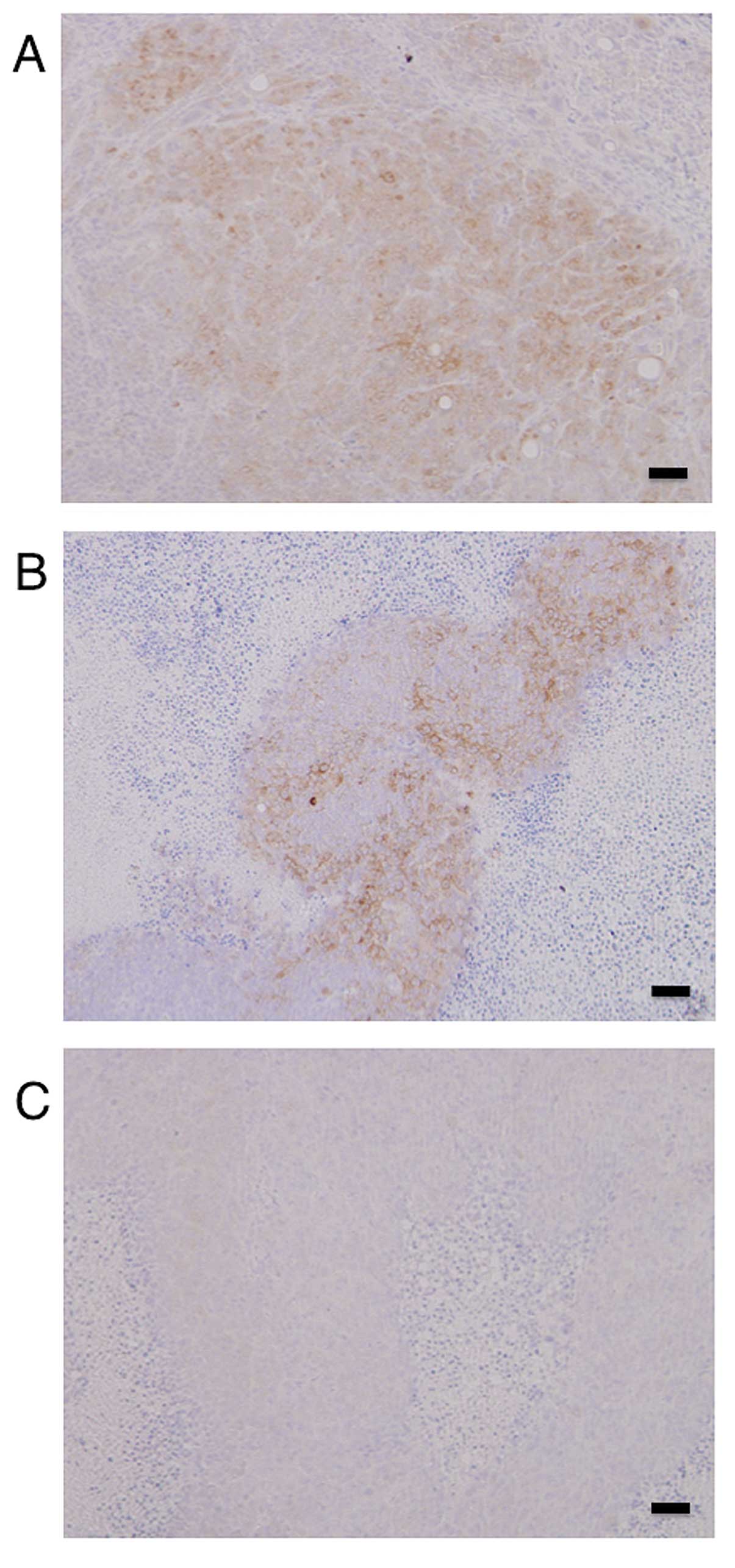
Immunohistochemistry of the tumors in the xenograft
model showed high expression levels of HSP27 in the mock- and
scramble/HSP27-transfected cells, but not in the
shRNA/HSP27-transfected cells (Fig.
5).
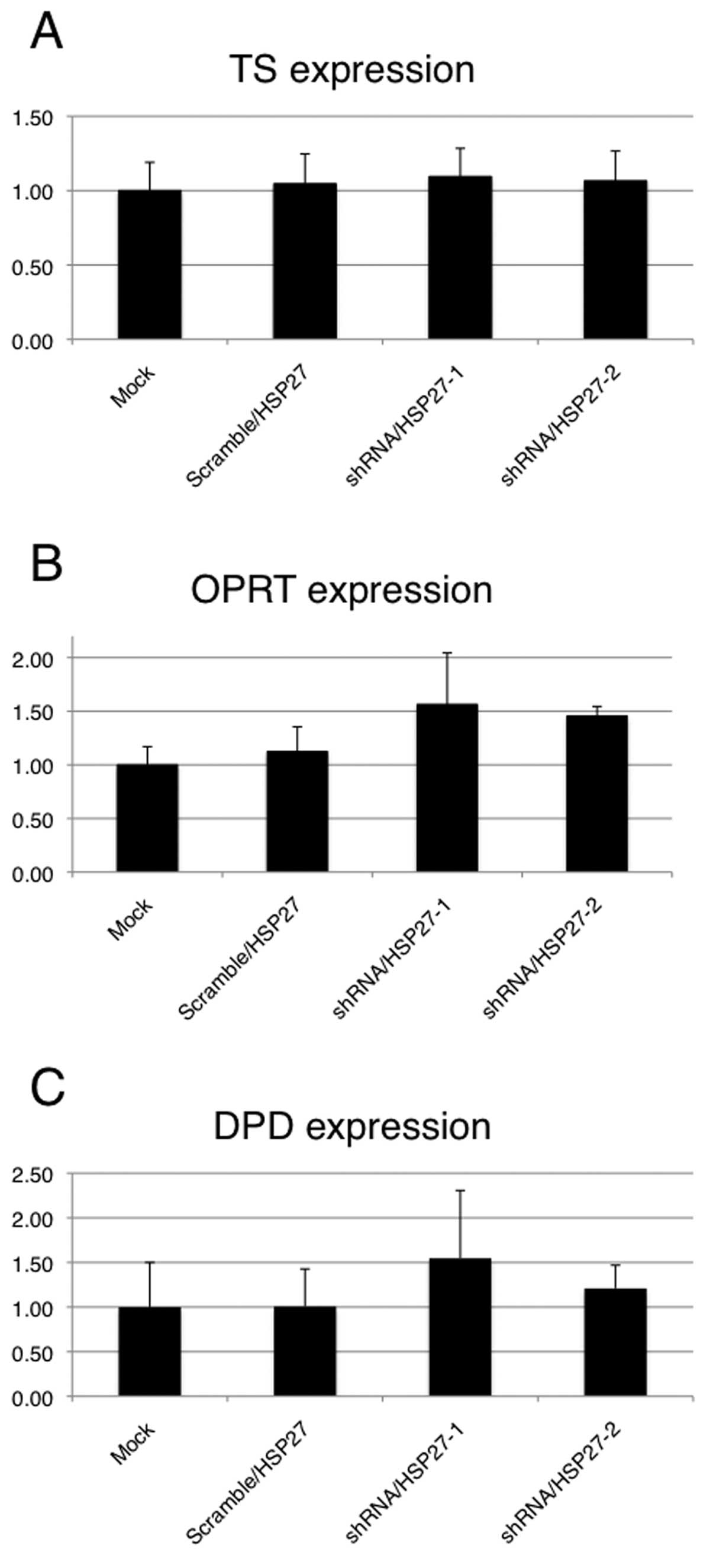
Expression of 5-FU metabolic enzymes in
shRNA/HSP27-transfected cells
To evaluate whether HSP27 expression levels affect
the principal 5-FU metabolic enzymes (TS, DPD and OPRT) in the
transfected cells treated with 5-FU, a qRT-PCR analysis of 5-FU
metabolic enzymes was performed. These enzyme expression levels
were assessed relative to the levels of the gene transcript in the
mock-transfected cells as the control. Although no significant
differences in the mRNA expressions of the three metabolic enzymes
were observed between the shRNA/HSP27-transfected cells and the
mock- or scramble/HSP27-transfected cells, the expression of DPD
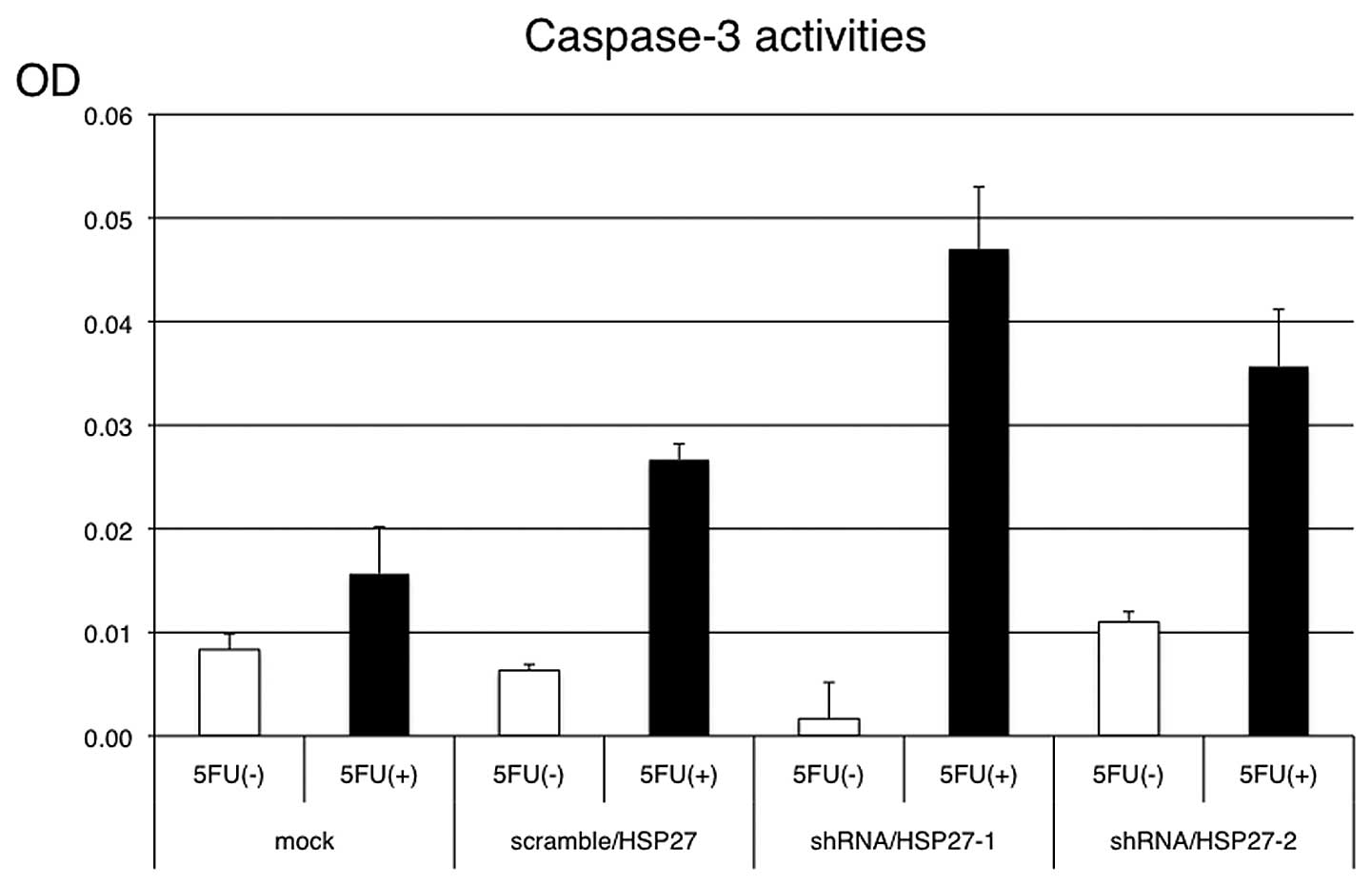
and OPRT was higher in the shRNA/HSP27-transfected cells (Fig. 6). Under the same conditions with
5-FU treatment, apoptosis induction was more frequently observed in
the shRNA/HSP27-transfected cells than in the mock- or
scramble/HSP27-transfected cells (Fig.
7).
Discussion
The inherent or acquired resistance to 5-FU-based
chemotherapy remains a critical issue in colorectal cancer
treatment. Consequently, the identification of biomarkers for
chemo-sensitivity or resistance, such as the K-RAS and
B-RAF mutation status for anti-EGFR antibody, is important
for personalized chemotherapy and the avoidance of unsuitable
chemotherapy and adverse events. In addition, the identification of
a new treatment strategy for patients with resistance to
chemotherapy is required to improve the survival of colorectal
cancer patients.
HSP27 has been widely recognized as a
stress-activated, ATP-independent cytoprotective chaperone that is
associated with a number of functions, including chemoresistance in
several cancers. In colorectal cancer, HSP27 has been reported as a
clinical prognostic factor or as a factor of irinotecan resistance
in experiments performed in vitro (17–20).
Our previous study also reported that the overexpression of HSP27
reduced 5-FU sensitivity and the suppression of HSP27 expression
reduced 5-FU resistance in experiments performed in vitro
using colon cancer cells (21).
Furthermore, in prostate and bladder cancer cells, the inhibition
of HSP27 expression reportedly enhanced drug sensitivity in
xenograft models (22–24). Thus, HSP27 is considered to be a
predictor of cancer prognosis or a treatment target for several
cancers; however, the role of HSP27 in colorectal cancer, including
chemoresistance, remains uncertain due to the lack of evidence in
xenograft models. Recently, a phase I/II clinical trial of
monotherapy using the antisense oligonucleotide (ASO), OGX-427,
which inhibits HSP27 expression, in patients with prostate,
bladder, ovarian, breast, or non-small cell lung cancer, but not
colorectal cancer, was carried out in the United States and Canada,
and the therapy proved to be feasible and effective [J Clin Oncol
28 (Suppl): S15, abs. 3077, 2010]. Accordingly, the purpose of this
study was to verify that HSP27 can also serve as a target for the
treatment of colorectal cancer.
The present study demonstrates that the suppression
of HSP27 expression in HSP27 high-expressing colon cancer cells
reduces resistance to 5-FU chemotherapy in a xenograft model, and
that the induction of apoptosis caused by the suppression of HSP27
expression, which many studies have reported, may be connected to
5-FU sensitivity. Our findings are consistent with the findings of
several studies that link HSP27 and chemoresistance in different
cell types. Collectively, this evidence suggests a role of HSP27 in
the mediation of 5-FU resistance in colon cancer cells. HSP27 may
also serve as a reliable target for the clinical treatment of colon
cancer in patients with chemoresistance.
5-FU is well known to induce apoptosis in colon
cancer cells, predominantly through the mitochondrial pathway,
involving the release of cytochrome c and the subsequent
activation of the upstream initiator, caspase-9, and the downstream
effector, caspase-3 (25–27). We confirmed that the suppression of
HSP27 expression was associated with the induction of apoptosis in
colon cancer cells treated with 5-FU. Consequently, HSP27 may
function as a negative regulator of the cytochrome
c-dependent activation of procaspase-3, as previously
reported (28). However, whether
HSP27 is associated with 5-FU metabolic enzymes, remains unknown.
We then analyzed the correlation between HSP27 expression and the
principal 5-FU metabolic enzymes, such as TS, DPD and OPRT. The
mRNA expression of DPD and OPRT was higher after 5-FU treatment in
the shRNA/HSP27-transfected cells that had a high sensitivity to
5-FU comapred to the control cells, although the difference was not
significant. Changes in the TS mRNA expression levels were not
clearly observed in the shRNA/HSP27-transfected cells. DPD mediates
5-FU degradation by catabolizing 5-FU to fluoro-5,6-dihydrouracil.
The enzyme OPRT directly converts 5-FU to
5-fluorouridine-5′-monophosphate and inhibits normal RNA or DNA
synthesis in tumor cells. TS is inhibited by
5-fluoro-2′-deoxyuridine-5′-monophosphate (FdUMP) derived from
5-FU, leading to the inhibition of DNA synthesis. Consequently,
high TS levels in tumor tissues are considered to be associated
with the low efficacy of 5-FU treatment. In consideration of the
functions of these enzymes, shRNA/HSP27-transfected colon cancer
cells (with a suppressed HSP27 expression) may promote 5-FU
metabolism by increasing the expression of DPD and OPRT to remove
5-FU from the cytoplasm, in order to enable cell survival.
In conclusion, this study suggests that the
suppression of HSP27 expression in colon cancer cells promotes 5-FU
sensitivity by inducing apoptosis, while also accelerating 5-FU
metabolism to avoid cell death. Further investigation, such as an
HSP27 functional study examining HSP27 phosphorylation activity and
signal regulation, may lead to novel treatments for colorectal
cancer.
References
|
1
|
Van Cutsem E, Rivera F, Berry S, et al:
First BEAT investigators: Safety and efficacy of first-line
bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in
metastatic colorectal cancer: the BEAT study. Ann Oncol.
20:1842–1847. 2009.PubMed/NCBI
|
|
2
|
Van Cutsem E, Köhne CH, Hitre E, et al:
Cetuximab and chemotherapy as initial treatment for metastatic
colorectal cancer. N Engl J Med. 360:1408–1417. 2009.PubMed/NCBI
|
|
3
|
Tol J, Koopman M, Cats A, et al:
Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal
cancer. N Engl J Med. 360:563–572. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hurwitz H, Fehrenbacher L, Novotny W, et
al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for
metastatic colorectal cancer. N Engl J Med. 350:2335–2342. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kinoshita M, Kodera Y, Hibi K, et al: Gene
expression profile of 5-fluorouracil metabolic enzymes in primary
colorectal cancer: potential as predictive parameters for response
to fluorouracil-based chemotherapy. Anticancer Res. 27:851–856.
2007.
|
|
6
|
Okumura K, Shiomi H, Mekata E, et al:
Correlation between chemosensitivity and mRNA expression level of
5-fluorouracil-related metabolic enzymes during liver metastasis of
colorectal cancer. Oncol Rep. 15:875–882. 2006.
|
|
7
|
Aschele C, Debernardis D, Casazza S, et
al: Immunohistochemical quantitation of thymidylate synthase
expression in colorectal cancer metastases predicts for clinical
outcome to fluorouracil-based chemotherapy. J Clin Oncol.
17:1760–1770. 1999.
|
|
8
|
Soong R, Shah N, Salto-Tellez M, et al:
Prognostic significance of thymidylate synthase, dihydropyrimidine
dehydrogenase and thymidine phosphorylase protein expression in
colorectal cancer patients treated with or without
5-fluorouracil-based chemotherapy. Ann Oncol. 19:915–919. 2008.
View Article : Google Scholar
|
|
9
|
Bruey JM, Ducasse C, Bonniaud P, et al:
Hsp27 negatively regulates cell death by interacting with
cytochrome c. Nat Cell Biol. 2:645–652. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mehlen P, Schulze-Osthoff K and Arrigo AP:
Small stress proteins as novel regulators of apoptosis. Heat shock
protein 27 blocks Fas/APO-1- and staurosporine-induced cell death.
J Biol Chem. 271:16510–16514. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Andrieu C, Taieb D, Baylot V, et al: Heat
shock protein 27 confers resistance to androgen ablation and
chemotherapy in prostate cancer cells through eIF4E. Oncogene.
29:1883–1896. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sarto C, Valsecchi C, Magni F, et al:
Expression of heat shock protein 27 in human renal cell carcinoma.
Proteomics. 4:2252–2260. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song TF, Zhang ZF, Liu L, Yang T, Jiang J
and Li P: Small interfering RNA-mediated silencing of heat shock
protein 27 (HSP27) increases chemosensitivity to paclitaxel by
increasing production of reactive oxygen species in human ovarian
cancer cells (HO8910). J Int Med Res. 37:1375–1388. 2009.
View Article : Google Scholar
|
|
14
|
Ciocca DR, Fuqua SA, Lock-Lim S, Toft DO,
Welch WJ and McGuire WL: Response of human breast cancer cells to
heat shock and chemotherapeutic drugs. Cancer Res. 52:3648–3654.
1992.PubMed/NCBI
|
|
15
|
Vargas-Roig LM, Gago FE, Tello O, Aznar JC
and Ciocca DR: Heat shock protein expression and drug resistance in
breast cancer patients treated with induction chemotherapy. Int J
Cancer. 79:468–475. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garrido C, Mehlen P, Fromentin A, et al:
Inconstant association between 27-kDa heat-shock protein (Hsp27)
content and doxorubicin resistance in human colon cancer cells. The
doxorubicin-protecting effect of Hsp27. Eur J Biochem. 237:653–659.
1996. View Article : Google Scholar
|
|
17
|
Choi DH, Ha JS, Lee WH, et al: Heat shock
protein 27 is associated with irinotecan resistance in human
colorectal cancer cells. FEBS Lett. 581:1649–1656. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tweedle EM, Khattak I, Ang CW, et al: Low
molecular weight heat shock protein HSP27 is a prognostic indicator
in rectal cancer but not colon cancer. Gut. 59:1501–1510. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu Z, Zhi J, Peng X, Zhong X and Xu A:
Clinical significance of HSP27 expression in colorectal cancer. Mol
Med Rep. 3:953–958. 2010.PubMed/NCBI
|
|
20
|
Wang F, Zhang P, Shi C, Yang Y and Qin H:
Immunohistochemical detection of HSP27 and hnRNP K as prognostic
and predictive biomarkers for colorectal cancer. Med Oncol. Aug
23–2011.(Epub ahead of print).
|
|
21
|
Tsuruta M, Nishibori H, Hasegawa H, et al:
Heat shock protein 27, a novel regulator of 5-fluorouracil
resistance in colon cancer. Oncol Rep. 20:1165–1172.
2008.PubMed/NCBI
|
|
22
|
Kamada M, So A, Muramaki M, Rocchi P,
Beraldi E and Gleave M: Hsp27 knockdown using nucleotide-based
therapies inhibit tumor growth and enhance chemotherapy in human
bladder cancer cells. Mol Cancer Ther. 6:299–308. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rocchi P, So A, Kojima S, et al: Heat
shock protein 27 increases after androgen ablation and plays a
cytoprotective role in hormone-refractory prostate cancer. Cancer
Res. 64:6595–6602. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rocchi P, Beraldi E, Ettinger S, et al:
Increased Hsp27 after androgen ablation facilitates
androgen-independent progression in prostate cancer via signal
transducers and activators of transcription 3-mediated suppression
of apoptosis. Cancer Res. 65:11083–11093. 2005. View Article : Google Scholar
|
|
25
|
Sun Y, Tang XM, Half E, Kuo MT and
Sinicrope FA: Cyclooxygenase-2 overexpression reduces apoptotic
susceptibility by inhibiting the cytochrome c-dependent apoptotic
pathway in human colon cancer cells. Cancer Res. 62:6323–6328.
2002.PubMed/NCBI
|
|
26
|
Kluck RM, Bossy-Wetzel E, Green DR and
Newmeyer DD: The release of cytochrome c from mitochondria: a
primary site for Bcl-2 regulation of apoptosis. Science.
275:1132–1136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li P, Nijhawan D, Budihardjo I, et al:
Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9
complex initiates an apoptotic protease cascade. Cell. 91:479–489.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Concannon CG, Orrenius S and Samali A:
Hsp27 inhibits cytochrome c-mediated caspase activation by
sequestering both pro-caspase-3 and cytochrome c. Gene Expr.
9:195–201. 2001.PubMed/NCBI
|