Introduction
The ubiquitin-proteasome pathway is involved in
tumorigenesis (1). E2-EPF is a
24-kDa protein that is a member of the E2 family of
ubiquitin-conjugating enzymes (2),
which, together with an E1 ubiquitin-activating enzyme and an E3
ubiquitin ligase, catalyze the addition of ubiquitin to substrate
proteins (3). Multiple rounds of
ubiquitinylation result in substrate polyubiquitinylation that can
target proteins for proteasome-dependent destruction. E2-EPF was
discovered in 1992 and was highly expressed in human cancer tissues
compared with normal tissues (4,5).
Large-scale meta-analysis of cancer microarray data identifies that
E2-EPF is one of commonly activated genes in multiple cancer
(6). Though discovered in 1992,
candidate substrates and cognate E3 ligases for E2-EPF were unclear
until Jung et al demonstrated that the stability of a von
Hippel-Lindau (VHL) tumorsuppressor gene product is dependent on
E2-EPF levels in 2006 (7). As we
know, human solid tumors contain hypoxic regions that have
considerably lower oxygen tension than normal tissues. Hypoxia
offers resistance to radiotherapy and anticancer chemotherapy, as
well as predispose to increased tumor metastases. Furthermore,
hypoxia induces hypoxia inducible factor (HIF)-1, which in turn
increases tumor angiogenesis (8).
The von Hippel-Lindau tumor suppressor, pVHL, forms part of an E3
ubiquitin ligase complex that targets specific substrates for
degradation, including HIF-1α (9).
Their study suggested a role for E2-EPF in the stabilization of
HIF-1α by specifically targeting pVHL for degradation under
normoxic conditions. E2-EPF expression level correlates inversely
with pVHL level in most tumor cell lines. In vitro and in
vivo, forced expression of E2-EPF boosts tumor-cell
proliferation, invasion and metastasis through effects on the
pVHL-HIF pathway (7).
Thereafter, increased expression of E2-EPF
expression in multiple cancers including breast cancer (10) and esophageal squamous cell carcinoma
(11) was reported and their roles
in tumor cell growth and metastasis were explored. Cellular factors
that regulate the expression of E2-EPF gene were also revealed.
Growth factors and serum induce expression of Egr-1 and SRF,
respectively. Egr-1 and SRF can bind the promotor region of E2-EPF,
therefore increase the HIF-1α protein level under non-hypoxic
conditions through the Egr-1/SRF- E2-EPF-VHL pathway (12).
Here, we report the expression profile for E2-EPF in
cervical tumor and normal tissue specimens and address that it has
an essential role in cancer cell proliferation, invasion,
tumorigenicity and chemosensitivity to topoisomerase
inhibitors.
Materials and methods
Experimental samples
Samples from cervical cancer and normal cervical
tissues were obtained with informed consent form patients
undergoing surgery or biopsy in Tokyo Medical University Hospital.
The protocols used here have been approved by the ethics committees
of the respective institutions where their study was carried out
and conform to the provisions of the Declaration of Helsinki in
1995 (as revised in Edinburgh 2000). Totally, 13 normal cervical
tissues, and 75 cervical carcinoma tissues were analysed. The
clinical stage of tumor tissues was classified by the FIGO 2009
standard. Tumor histological grades, as well as the status of
chemotherapy were recorded. Sample tissues were minced into small
pieces with scissors; washed in 0.9% sterile saline to avoid
contamination of red blood cells, snap-frozen and stored at −80°C
until used.
Total RNA isolation and real-time PCR
analysis
Total RNA was isolated using Isogen reagent (Nippon
Gene, Tokyo, Japan) and was reverse transcribed into cDNA with High
Capacity cDNA Reverse Transcription Kit (Applied Biosystems, USA).
Real-time PCR was carried out for quantitative estimation with
TaqMan Gene Expression Master Mix (Applied Biosystems). E2-EPF was
amplified with the following primer pair: forward:
5′-CGACCTCCAGGTCACCAT-3′; reverse: 5′-GGAACAGACCTCCAGCATATGG-3′ and
with a probe 5′-CCCCTCAGGGCCCTC-3′. The reaction was cycled in the
StepOne Plus real-time PCR system (Applied Biosystems) with the
following parameters: denaturation for 1 cycle at 95°C for 10 min,
40 cycles of 95°C for 15 sec, 50°C for 10 sec and 60°C for 1 min.
The mRNA level of each PCR product was estimated by the StepOne
software v2.0 and normalized to the GAPDH mRNA level.
Cell culture, transfection and single
clone collection
Human cervical squamous cell carcinoma cell line
SiHa which was obtained from the American Type Culture Collection
was incubated in MEM-n supplemented with 10% FBS in presence of 5%
CO2. E2-EPF shRNA and control shRNA plasmid vector were
purchased from Santa Cruz. Transfection was done when cells were
~70–80% confluency. All the procedures are followed by the
manufacturer’s protocol. Forty-eight hours after transfection,
cells were selected by puromycin (Santa Cruz, USA) with
concentration of 0.5 μg/ml for 2 weeks. The positive cells were
harvested and implanted into 96-well plates with single one in each
well and changed medium every week. The positive clone were
selected and subjected for real-time PCR detection of mRNA and
western blot examination of protein expression.
Western blot analysis
Cells were harvested and lysed on ice for 30 min in
M-PER tissue protein extraction reagent (Thermo Scientific, USA)
containing complete protease inhibitor mixture (Roche Diagnostics,
USA). The lysates were subjected to centrifugation at 14800 g for
15 min and the soluble fraction was collected. Protein
concentrations were measured using BCA protein assay kit (Thermo
Scientific). Equal amounts of protein (30 μg) were loaded and
probed with anti-E2-EPF antibody C-term (Abgent, USA). The
intensity of each band was normalized to the intensity of the
β-actin band. The lysate of HL-60 cells were used as an
E2-EPF-positive control.
Luciferase assays
Cells (2×104) of E2-EPF knockdown single
clone, E2-EPF(−), were seeded at 96-well plate and incubated
overnight. For each transfection, 50 ng HIF-1 luciferase reporter
vector (Panomics, USA) was mixed in 0.2 ml Opti-MEM (Invitrogen,
USA), and a precipitate was formed using Lipofectamine 2000
(Invitrogen) according to the protocol recommendations. After
transfection for 24 h, cells were harvested and extracts prepared
with Glo Lysis buffer (Promega, USA). Luciferase activity was
measured in extracts from triplicate samples using the Bright-Glo
Luciferase Assay system (Promega).
Proliferation and invasion assays
Cells (2×103) E2-EPF(−), control and
normal cells were implanted in triplicate into 96-well plate and
allow attaching overnight and the proliferation assay was performed
according to the instructions of CellTiter 96 Non-Radioactive Cell
Proliferation assay (Promega). Record the absorbance at 570 nm with
96-well plate reader.
The invasion assay was done following the
manufacturer’s protocol (BD Biosciences, UK). In brief, following
synchronization by serum starvation for 24 h, cell suspensions in
1% FBS MEM-n containing 2.5×104 cells was added into the
24-well BioCoat Matrigel invasion chambers and MEM-n containing 20%
FBS was added into the low chamber. After 24-h incubation, the
cells on the lower surface of the membrane are stained with
Diff-Quik™ stain kit. The number of cells was scored visually in 8
random, non-overlapping fields at magnification 10×10 using a light
microscope.
Chemotherapeutic drug treatments
Cells (4×104) of each well were implanted
into 96-well plate in triplicate. Drugs were diluted from distilled
water or dimethyl sulfoxide stock solutions into culture medium
working solutions and added at the following concentrations
(Table I). After 24 h of drug
treatment, survival cells were evaluated by the Cell Proliferation
assay (Promega). Drug resistance was represented as calculated
using the following formula: (absorbance of treated
cells)/(absorbance of untreated cells) × 100%.
 | Table IConcentration of different drugs used
for chemotherapeutic sensitivity test. |
Table I
Concentration of different drugs used
for chemotherapeutic sensitivity test.
| Cisplatin (μM) | Doxorubicin (nM) | Paclitaxel (nM) | Doxetaxel (nM) | Topotecan (nM) | Etoposide
(μg/ml) |
|---|
| 0 | 0 | 0 | 0 | 0 | 0 |
| 2.5 | 50 | 10 | 3 | 1 | 1 |
| 10 | 200 | 20 | 10 | 2 | 10 |
| 40 | 800 | 50 | 20 | 4 | 30 |
Cell cycle analysis
Approximately 2×106 E2-EPF(−), control
and normal cells were fixed overnight in 1.5 ml of 70% ethanol.
Thereafter, cells were centrifuged, the supernatant discarded, and
the cell pellet resuspended and washed twice with PBS. After
another centrifugation step, cells were resuspended in 300 μl PBS
staining solution containing 100 μg/ml propidium iodide
(Invitrogen/Molecular Probes) and 100 μg/ml freshly prepared RNase
A (Qiagen, USA) and were incubated at room temperature for 30 min.
FACS analysis was performed using a FACSCalibur (BD Biosciences) at
488 nm. The data were analyzed using CellQuest Pro and ModFit
software.
Tumor cell implantation experiments
In vivo experiments were done in accordance
with the guidelines for the Care and Use of Laboratory Animals of
China-Japan Friendship Hospital. E2-EPF(−), control and normal
cells were s.c. injected into the back of 4-week-old female nude
mice with cells at 2×106 per mouse. Tumor growth was
monitored every 3 days after 6 weeks of inoculation. Mice were
sacrificed at day 90 and tumors were excised and weighted.
Statistical analysis
Data are presented as the mean ± SD. p<0.05 was
considered statistically significant. E2-EPF mRNA expression in
cervical tumor and normal tissues and tumor weight in in
vivo tumorigenesis were compared using Student’s t-test.
Luciferase assay, MTT assay, invasion assay and chemotherapeutic
sensitivity assay was analysed using ANOVA test.
Results
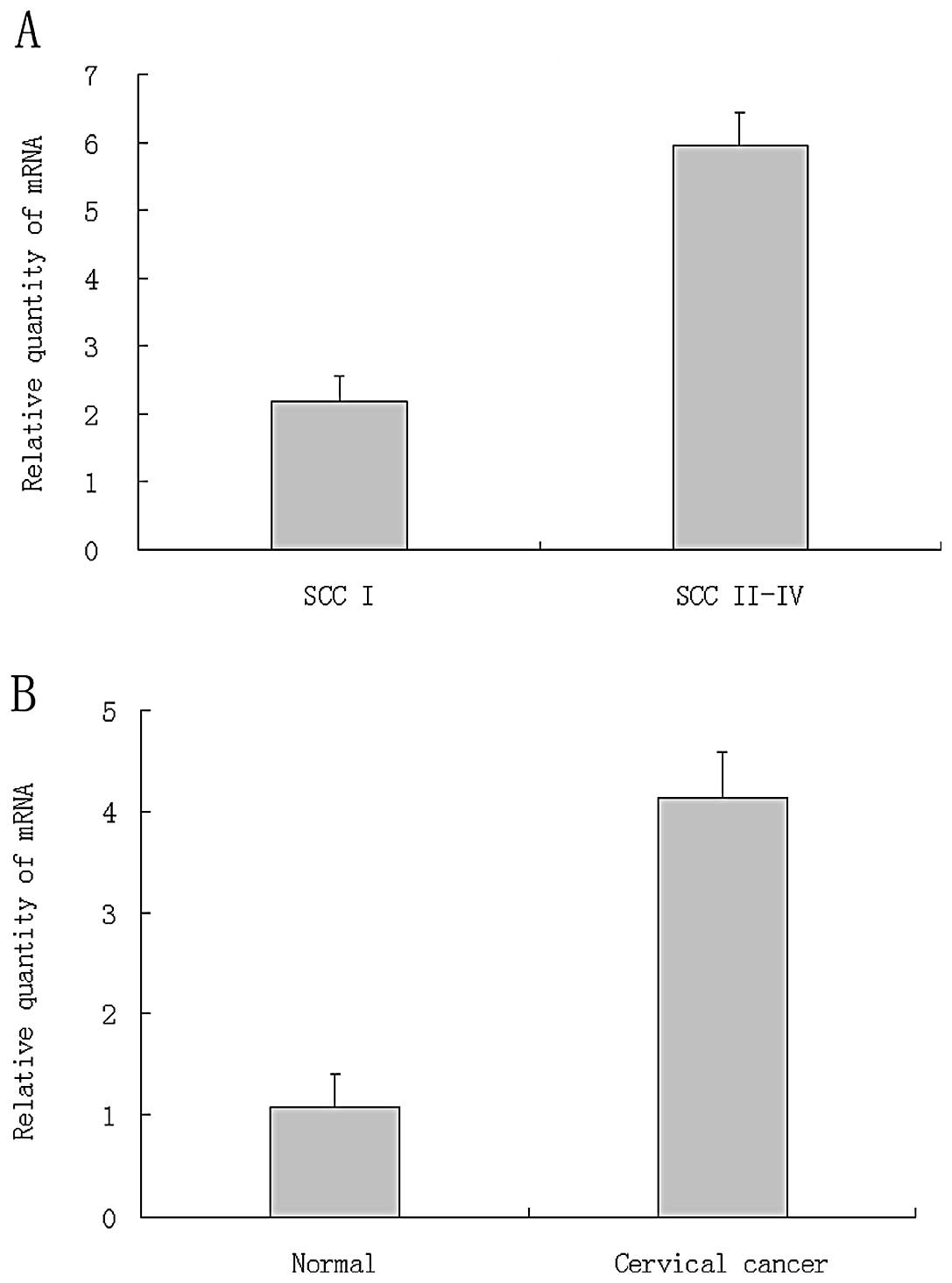
E2-EPF mRNA are overexpressed in cervical
tumor specimens
E2-EPF was significantly overexpressed in tumor
tissues relative to normal tissues (Fig. 1A). The average relative expression
level in tumor tissues is ~4-fold of the normal tissues.
Relationship between expression level of E2-EPF and clinical stage
of the tumor tissues was analyzed. The relative expression level of
E2-EPF in tumors at stage I was significantly lower than the tumors
at stage II–IV (Fig. 1B).
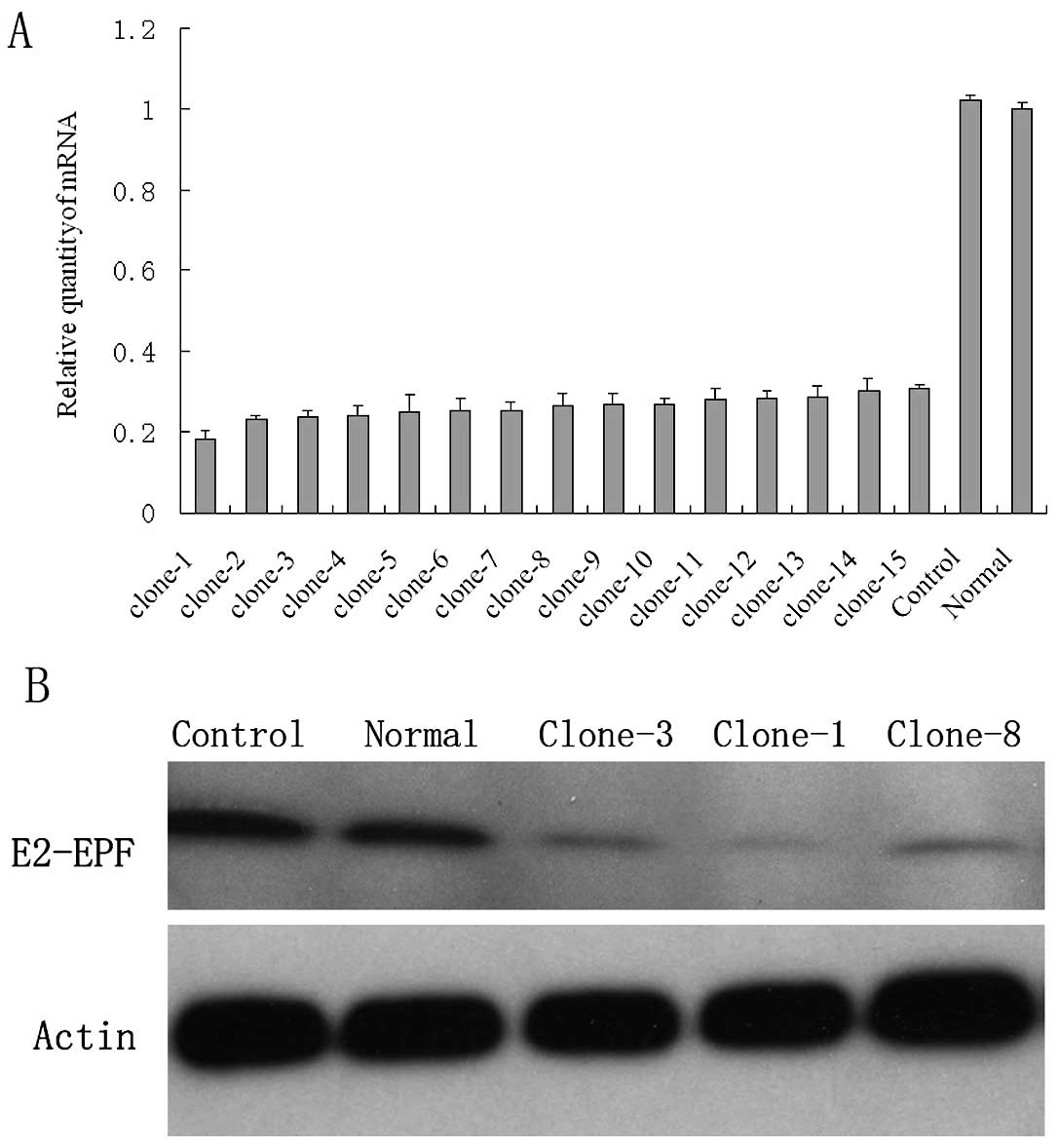
Establishment of E2-EPF low expression
clones
After antibiotics selection, the survival cells were
seeded into 96-well plates for E2-EPF(-) single clone selection.
All together, >15 low expression clones were selected and
subjected for real-time PCR examination of mRNA level and western
blot detection of protein levels. Fig.
2A shows the relative mRNA level of the representative clones.
shRNA decreased the E2-EPF mRNA by more than 80% in some clones.
Fig. 2B shows the relative protein
level. The E2-EPF(−) clones show hardly any protein expression.
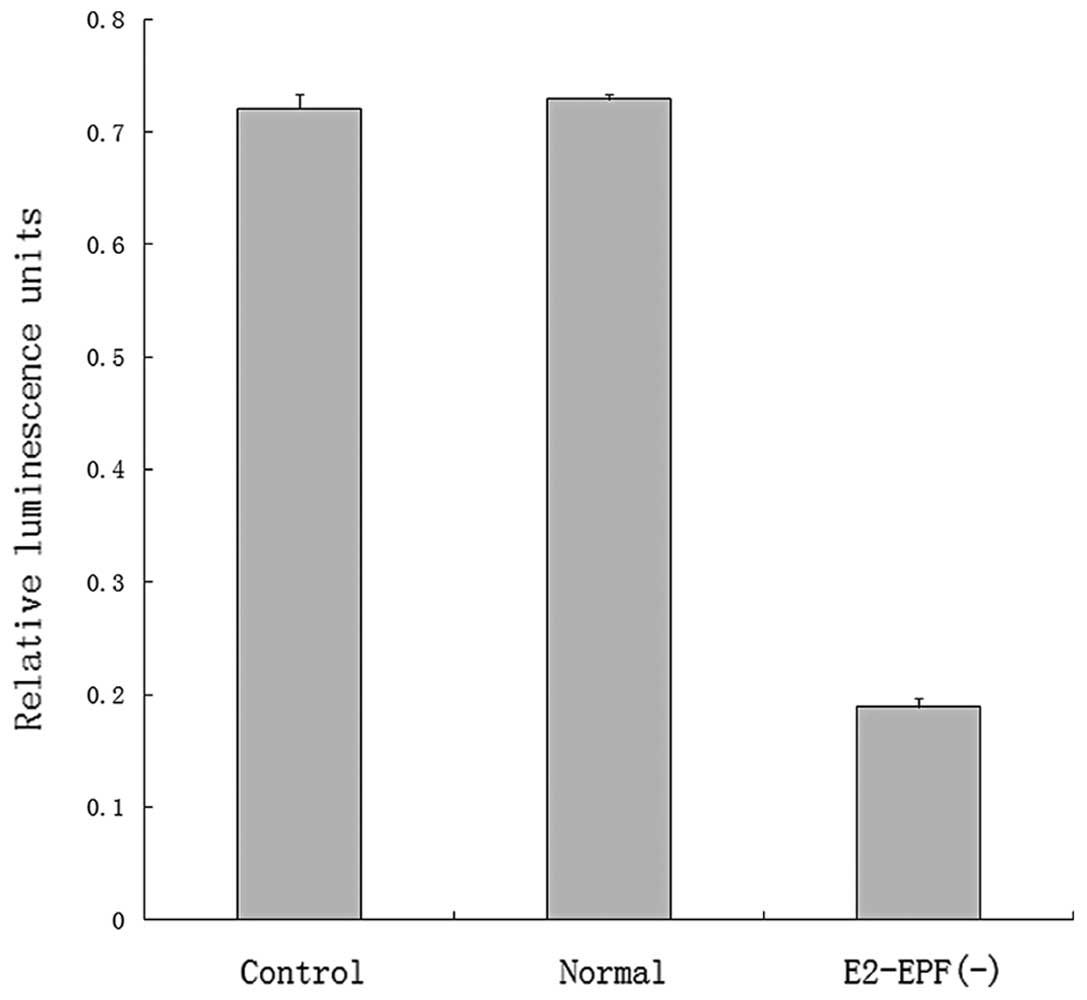
Decreased expression of E2-EPF is
associated with the lower promoter activity of HIF-1 reporter
Luciferase assay showed that the promoter activity
of HIF-1 reporter was significantly decreased in the E2-EPF(−)
clone cells. This results indicates that HIF-1α level may be
downregulated in these cells (Fig.
3).
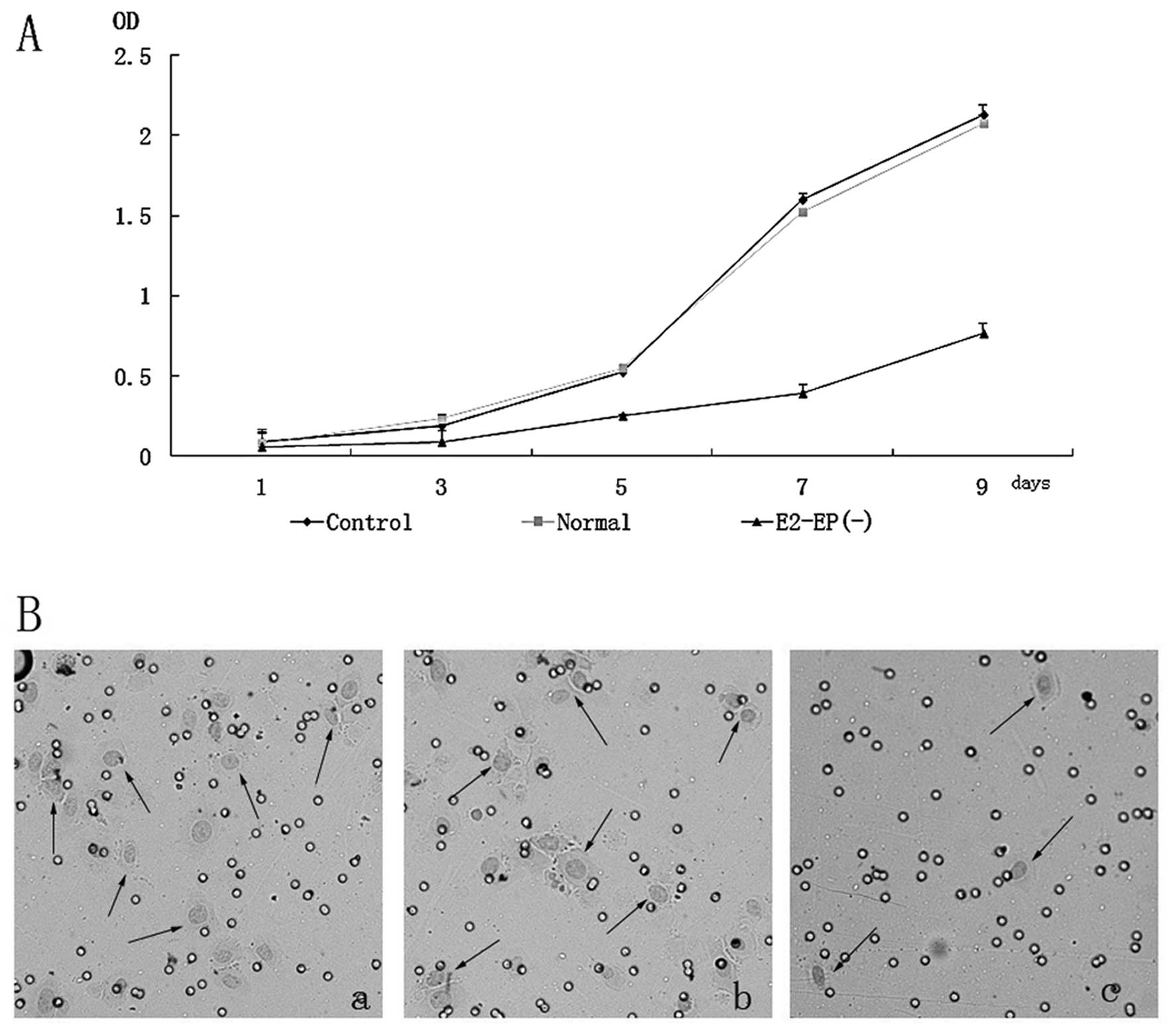
shRNA knockdown decreases growth rate and
aggressivity of cells
E2-EPF(−) clone cells displayed a lower
proliferative potential compared with normal and control cells
(Fig. 4A), indicating that E2-EPF
is involved in the growth control of SiHa cell. The aggressivity of
E2-EPF(−) clone cells was significantly decreased (Fig. 4B).
E2-EPF knockdown increased the cells no.
in the G0/G1 phase
FACS analysis of propidium iodine-stained cells
revealed that E2-EPF knockdown increased the cells no. in the G0/G1
phase. The percentage of cells in G2/M, especially S phase are
significantly lower in E2-EPF low expression cells than the normal
and control cells. The representative results were shown in
Fig. 5.
E2-EPF knockdown increased SiHa cell
sensitivity to topoisomerase inhibitors
We used different kinds of chemotherapeutic drugs to
evaluate the effect of E2-EPF knockdown on cell chemosensitivity,
i.e., topotecan, etoposide, doxorubicin, cisplatin, paclitaxel and
doxetaxel. E2-EPF knockdown resulted in a significantly greater
antiproliferative effect in the topoisomerase I inhibitor
(topotecan, Fig. 6A) and II
(etoposide and doxorubicin, Fig. 6B and
C). In contrast, no significant sensitizing effects of E2-EPF
depletion were observed in cisplatin, paclitaxel or doxetaxel
(Fig. 6D-F).
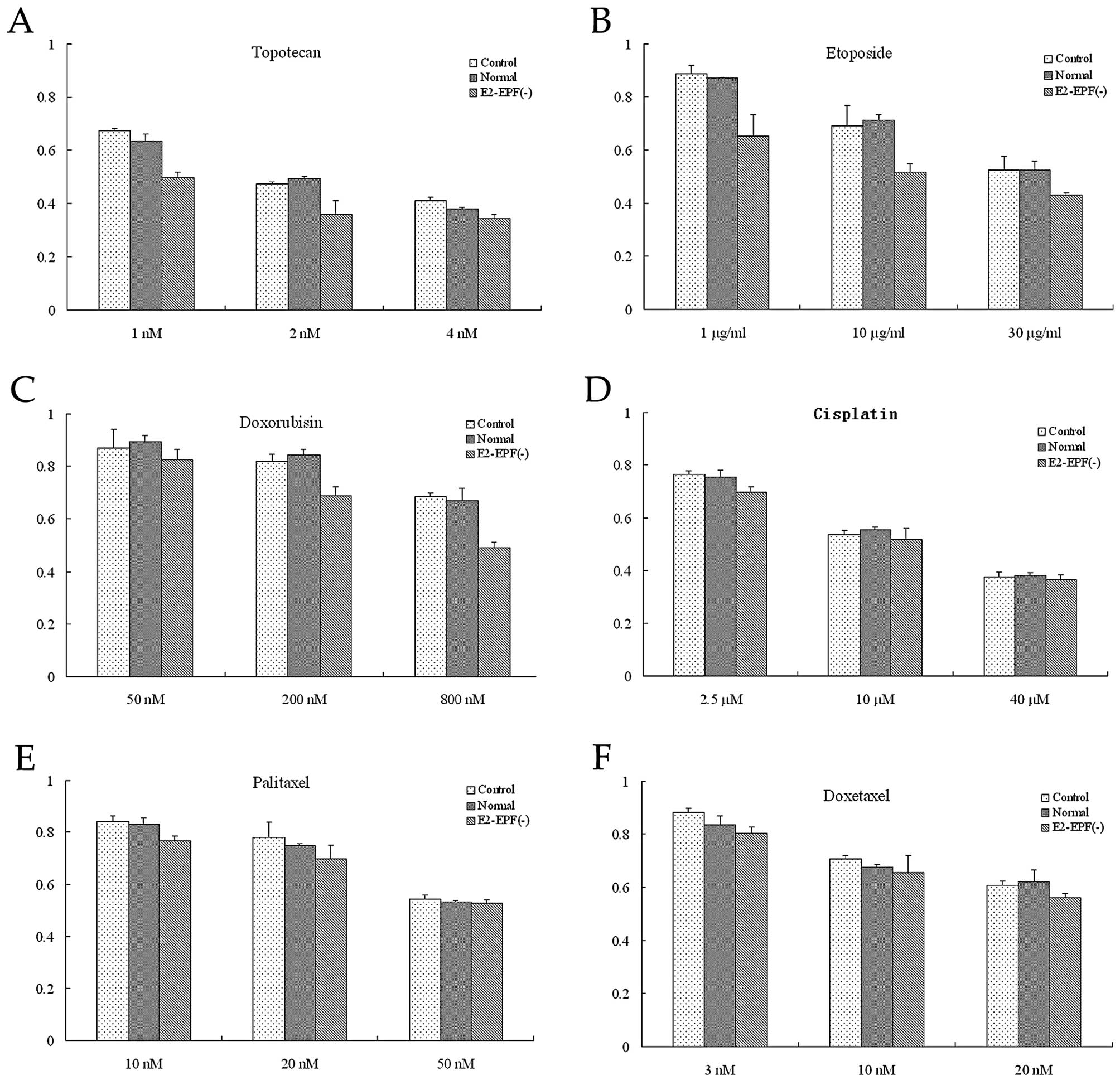 | Figure 6(A) Topotecan (1 or 2 nm) was added to
treat the control, normal and E2-EPF(−) cells. The relative
survival cell no. in E2-EPF(−) cell was significantly lower than
the control and normal cells, p<0.05. But no difference was
observed in the drug concentration of 4 nm. Among the three groups,
p>0.05. (B) Etoposide (1, 10 and 30 μg/ml) was added to treat
the three groups. The relative survival cell no. in E2-EPF(−) cell
was significantly lower than the control and normal cells,
p<0.05. (C) The relative survival cell no. in E2-EPF(−) cell was
significantly lower than the control and normal cells, in 200 and
800 nm Doxorubicin, p<0.05, but no difference was observed in
the drug concentration of 50 nm, p>0.05. (D) Cisplatin (2.5, 10
and 40 μM) was added to treat the three groups. There is no
significant difference of the relative survival cell no. among the
three groups, p>0.05. (E) Paclitaxel (10, 20 and 50 nm) was
added to treat the three groups. There is no significant difference
of the relative survival cell no. among the three groups,
p>0.05. (F) Doxetaxel (3, 10 and 20 nm) was added to treat the c
three groups. There is no significant difference of the relative
survival cell no. among the three groups, p>0.05. |
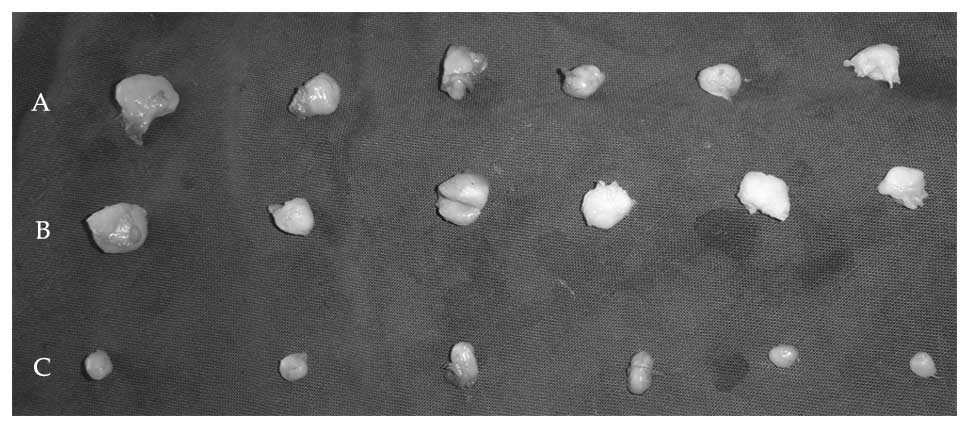
E2-EPF knockdown decreases the
tumorigenicity of SiHa cell line
To test whether E2-EPF knockdown affects the
tumorigenic ability of SiHa cell line or not, certain number of
tumor cells were s.c. inoculated into nude mice to monitor the
tumor development. As shown in Fig.
7A, weight of tumors from E2-EPF low expression clone cells was
~1/4 of the normal and control cells. Typical tumors are shown in
Fig. 7B. These results indicate
that E2-EPF may play an important role in tumor development in
vivo.
Discussion
The importance of E2-EPF in human cancers has become
evident in recent years, including, breast cancer, colon cancer,
and renal cancer (13). To explore
the role of E2-EPF in cervical cancer, we examined the expression
level of E2-EPF in cervical tumor tissues and normal tissues. Our
study demonstrated that the E2-EPF protein was more highly
expressed in the cervical tumor tissues than in normal tissues.
Also, the expression level was positively correlated with the
clinical stage of tumor. These results indicate that E2-EPF high
expression may be associated with the tumor growth, cell invasion
and/or metastasis. It has been showed that E2-EPF was significantly
associated with poor prognosis of esophageal cancer (11). The role of E2-EPF in cervical cancer
prognosis is known and more cases of clinical specimens need to be
collected and analysed.
To further demonstrate the role of E2-EPF in
cervical cancer, we downregulated its expression with a silencing
shRNA plasmid vector. Cell growth and invasion were examined by MTT
assay and invasion assay, respectively. Interestingly,
downregulation of E2-EPF decreased the cell growth rate and
aggressivity which indicates that E2-EPF may play an important role
in the cell growth and invasion. This was further approved by cell
cycle analysis. In vivo study showed that the tumorigenicity
of the E2-EPF knockdown cell decreased dramatically. All these
results indicate that E2-EPF may be one of the key factors which is
involved in cell growth, tumor development. Targeting E2-EPF
pathway may be a potential therapeutic method of cervical cancer
treatment. Using bortezomib, a proteasome inhibitor, can decrease
the function HIF-1-VEGF pathway in SiHa tumor cell line (14). As previous study showed that E2-EPF
increased the expression of HIF-1α through degrading the tumor
suppressor pVHL (7). It is quite
promising to find new E2-EPF inhibitors which can be developed into
new anti-cancer drugs.
FACS analysis revealed a marked increase in the
percentage of the G0/G1 phase cells and a obvious decrease of the S
phase after E2-EPF knockdown. It is reported that the
anaphase-promoting complex (APC) is an E3 ubiquitin ligase that
regulates mitosis and G1 by sequentially targeting cell cycle
regulators for ubiquitination and proteasomal degradation. E2-EPF
is a critical, unique component of the APC ubiquitination pathway
(15). E2-EPF acts as an APC/C
auxiliary factor that promotes mitotic exit (16). Our results showed that decreased
E2-EPF expression leading to the decreased cell number, mainly in
the S phase, which may be due to the function of E2-EPF in the APC
complex. Previous study using HeLa, the cervical adenocarcinoma
cell line, found that E2-EPF mRNA expression correlated with genes
involved in mitotic surveillance, but RNAi mediated knockdown of
the E2-EPF protein did not alter cell cycle distribution or affect
the proliferation (10). Our study
showed a quite different result using SiHa, the cervical squamous
cancer cell line. Therefore, we supposed that the role of E2-EPF in
cell growth and cell cycle is different depending on the cell type.
Evaluation of additional cell lines representative of other
cervical cancer cell lines for effects of E2-EPF depletion may
therefore be warranted.
Studies showed that E2-EPF ubiquitin carrier protein
associates with and targets pVHL for ubiquitin-mediated proteolysis
in cells, thereby stabilizing HIF-1α (7). The heterodimeric transcription factors
HIF-1 and HIF-2, composed of HIF-1α or HIF-2α and HIF-1β, increase
expression of a number of hypoxia-inducible genes including the
gene encoding vascular endothelial growth factor (VEGF), which
promotes tumor growth and vascularization (7,17).
Interestingly, recent study using the sporadic papillary renal cell
carcinoma found that multiple hypoxia-responsive elements within
the E2-EPF promoter, which demonstrated that E2-EPF is a hypoxia
inducible gene directly regulated via HIF-1 (13). Our study also demonstrated that
HIF-1α expression was decreased in the E2-EPF(−) clone cells, which
indicate that E2-EPF may also play an important role in cervical
cancer invasion and metastasis through effects of the pVHL-HIF
pathway.
Here we found that E2-EPF knockdown increased the
cell sensitivity to the topoisomerase I inhibitor (topotecan) and
II (etoposide and doxorubicin). The possible explanation for the
increased antiproliferative effect of Topo II inhibitors following
E2-EPF knockdown is that Topo II protein levels were increased.
Alternatively, E2-EPF may decrease drug sensitivity by involving in
the turnover of Topo II inhibitor-induced Topo II-DNA complexes,
thereby enabling repair of DNA damage (10). The mechanism of E2-EPF knockdown on
the increased sensitivity to the topoisomerase I inhibitor is still
unknown. These data suggest that combined administration of
topoisomerase directed drugs and E2-EPF inhibitors may enhance
their clinical effectiveness.
In conclusion, E2-EPF is overexpressed in cervical
squamous cancer specimens and its expression level positively
correlated with the clinical stage. Downregulation of E2-EPF by
RNAi results in a decreased expression level of HIF-1α. E2-EPF
knockdown decreases cell growth, cell invasion and tumorigenicity
of SiHa cell line and increases the chemosensitivity to
topoisomerase inhibitors. E2-EPF plays an important role in the
tumorigenesis and development. Targeting of E2-EPF pathway may be a
new therapeutic method of cervical tumor treatment.
Acknowledgements
We thank Chun-h. Xu for excellent technical
assistance. This study was partially supported by the Japan-China
Sasakawa Medical Fellowship.
References
|
1
|
Brahimi-Horn C and Pouyssegur J: When
hypoxia signalling meets the ubiquitin-proteasomal pathway, new
targets for cancer therapy. Crit Rev Oncol Hematol. 53:115–123.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu Z, Diaz LA, Haas AL and Giudice GJ:
cDNA cloning of a novel human ubiquitin carrier protein. An
antigenic domain specifically recognized by endemic pemphigus
foliaceus autoantibodies is encoded in a secondary reading frame of
this human epidermal transcript. J Biol Chem. 267:15829–15835.
1992.
|
|
3
|
Liu Z, Haas AL, Diaz LA, Conrad CA and
Giudice GJ: Characterization of a novel keratinocyte ubiquitin
carrier protein. J Biol Chem. 271:2817–2822. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Welsh JB, Zarrinkar PP, Sapinoso LM, et
al: Analysis of gene expression profiles in normal and neoplastic
ovarian tissue samples identifies candidate molecular markers of
epithelial ovarian cancer. Proc Natl Acad Sci USA. 98:1176–1181.
2001. View Article : Google Scholar
|
|
5
|
Wagner KW, Sapinoso LM, El-Rifai W, et al:
Overexpression, genomic amplification and therapeutic potential of
inhibiting the UbcH10 ubiquitin conjugase in human carcinomas of
diverse anatomic origin. Oncogene. 23:6621–6629. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rhodes DR, Yu J, Shanker K, et al:
Large-scale meta-analysis of cancer microarray data identifies
common transcriptional profiles of neoplastic transformation and
progression. Proc Natl Acad Sci USA. 101:9309–9314. 2004.
View Article : Google Scholar
|
|
7
|
Jung CR, Hwang KS, Yoo J, et al: E2-EPF
UCP targets pVHL for degradation and associates with tumor growth
and metastasis. Nat Med. 12:809–816. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harada H, Kizaka-Kondoh S, Li G, et al:
Significance of HIF-1-active cells in angiogenesis and
radioresistance. Oncogene. 26:7508–7516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ohh M: pVHL’s kryptonite: E2-EPF UCP.
Cancer Cell. 10:95–97. 2006.
|
|
10
|
Tedesco D, Zhang J, Trinh L, et al: The
ubiquitin-conjugating enzyme E2-EPF is overexpressed in primary
breast cancer and modulates sensitivity to topoisomerase II
inhibition. Neoplasia. 9:601–613. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen MF, Lee KD, Lu MS, et al: The
predictive role of E2-EPF ubiquitin carrier protein in esophageal
squamous cell carcinoma. J Mol Med. 87:307–320. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lim JH, Jung CR, Lee CH and Im DS: Egr-1
and serum response factor are involved in growth factors- and
serum-mediated induction of E2-EPF UCP expression that regulates
the VHL-HIF pathway. J Cell Biochem. 105:1117–1127. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roos FC, Evans AJ, Brenner W, et al:
Deregulation of E2-EPF ubiquitin carrier protein in papillary renal
cell carcinoma. Am J Pathol. 178:853–860. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Birle DC and Hedley DW: Suppression of the
hypoxia-inducible factor-1 response in cervical carcinoma
xenografts by proteasome inhibitors. Cancer Res. 67:1735–1743.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu T, Merbl Y, Huo Y, Gallop JL, Tzur A
and Kirschner MW: UBE2S drives elongation of K11-linked ubiquitin
chains by the anaphase-promoting complex. Proc Natl Acad Sci USA.
107:1355–1360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garnett MJ, Mansfeld J, Godwin C, et al:
UBE2S elongates ubiquitin chains on APC/C substrates to promote
mitotic exit. Nat Cell Biol. 11:1363–1369. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pugh CW and Ratcliffe PJ: Regulation of
angiogenesis by hypoxia: role of the HIF system. Nat Med.
9:677–684. 2003. View Article : Google Scholar : PubMed/NCBI
|