Introduction
Prevalence of small solid renal masses in adult
population recently increased probably due to the large number of
people that daily undergo preventive diagnostic investigations such
as ultrasonography and computed tomography (CT) scan for unrelated
symptoms, resulting in incidental tumor detection (1). Patients’ comorbidities, such as
cardiovascular or respiratory diseases, could represent limiting
factors for planning adequate subsequent surgical approaches
although conservative laparoscopy surgery is now considered the
gold standard treatment, particularly for tumors <3–4 cm
(2). Minimally invasive procedures,
such as cryoablation (CryA) and radiofrequency ablation (RFA), have
been recently proposed by different authors as alternative methods
of treatment to conservative surgery with satisfactory results in
terms of perioperative complications such as hemorrhage episodes or
metabolic problems related to prolonged period of general
anaesthesia (3). Some doubts remain
on the efficacy of these methods on tumor complete eradication due
to the high prevalence of tumor local recurrences due to persistent
vital tumor cells in the context of the ablated tissue (tumor
skipping) (4). Both CryA and RFA
are based on the application of physical means (cold and heat
respectively) at the centre of the lesion to induce tumor tissue
necrosis. Both of them are suitable for clinical application either
through laparoscopic trocars or ultrasound/CT-guided percutaneous
approaches (5). Microwaves are a
source of electromagnetic energy which, similarly to radiofrequency
or cryoablation, could be considered in the future for treating
small solid renal masses percutaneously or laparoscopically
(5). Indeed, with respect to RFA
and CryA, microwave thermal ablation (MWTA) allows for a more rapid
and homogeneous ablation, due to its reduced sensitivity to local
variations of tissue physical properties (6,7). We
previously studied the in vivo effects of MWTA on porcine
kidney, proving that MWTA is a safe and effective procedure. In
particular, we demonstrated the absence of viable cells inside the
ablation volume and the absence of any damage to the renal
parenchyma outside it (8). Although
empirical studies on the effects of MWTA in the treatment of small
renal masses were previously reported by other authors on small
series of patients, none of them reported adequate information on
patients tolerability and pathological effects on ablated tissues
(4,5). The aim of this phase I prospective
multicentre study was to determine both the tolerability of the new
Amica-probe V4 applicator-induced MWTA administered in vivo
on patients with solid renal masses and the effects of heating on
the renal tumors and normal renal parenchyma.
Materials and methods
Study design
From February 2009 to June 2010, 14 consecutive
patients affected by solid renal masses eligible for open radical
surgical treatment were enrolled in three different Italian
Urological Centers. We considered for enrolling only patients with
renal masses who were candidates to open radical nephrectomy in
order to obtain useful adjunctive information on both the
anatomical extent and the distribution of MWTA on tumor and
peritumoral healthy renal tissues. The patients underwent standard
laboratory examinations and radiological evaluations before the
treatment. The effects of MWTA on patients’ coagulation,
tumor/renal parenchyma and tumor skipping were assessed. Patients
were informed on the risk of a new therapy with no long-term
follow-up data and agreed to participate in the study. The present
study was approved by the Research Ethics Committee in each of the
Centers participating in the study (IRB Coordinator Center no.
URO/01/2008-322/2008). Written informed consent was obtained from
all patients before treatment. The study was conducted in line with
Good Clinical Practice guidelines and with the Ethical Principles
laid down in the latest version of the Declaration of Helsinki.
Inclusion and exclusion criteria
Requirements for inclusion were the presence of a
single, solid, contrast-enhanced parenchymal renal mass
(attenuation increase, >15 H on contrast-enhanced CT or >15%
on gadolinium-enhanced MRI) consistent with renal cell carcinoma on
preoperative imaging and to be scheduled for radical nephrectomy
through an open approach (9).
Patients affected by major concomitant diseases that precluded the
surgical treatment, ASA score ≤3, poor performance status (ECOG,
3–4), previous abdominal major surgery, severe medical or
psychiatric illness that precluded adequate informed consent,
<18 or >85 years, who had undergone radiation therapy to the
retroperitoneum were excluded.
RENAL score and Charlson comorbidity
index
The RENAL nephrometry score was calculated in
accordance with Kutikov and Uzzo (10). In brief, standardized points (1–3
points/descriptor) were assigned based on tumor size,
endophytic/exophytic properties, nearness to collecting system and
lesion location relative to polar lines. Moreover, the Charlson
comorbidity index was calculated by using the software purposed in
the website of the Institute for Algorithmic Medicine (Texas
non-profit Corporation) (http://www.medal.org/OnlineCalculators/ch1/ch1.13/ch1.13.01.php)
(11).
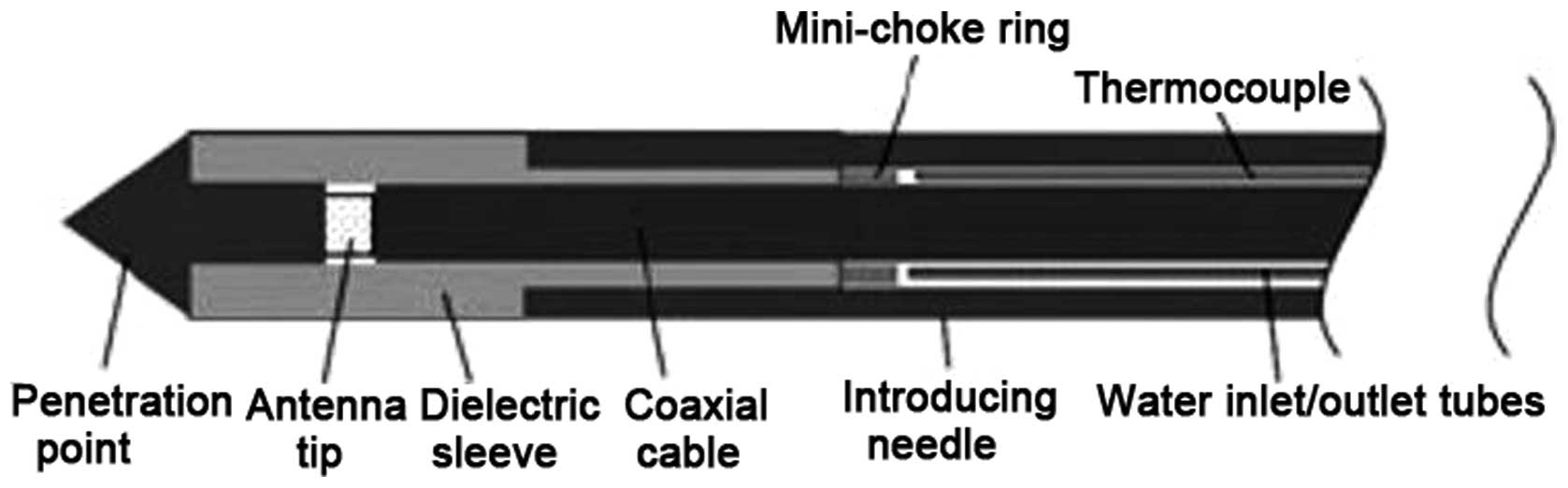
Microwave probe applicator
The system used for MWTA was HS AMICA, with the
Amica-probe V4 applicator (Hospital Services SpA, Aprilia, Italy),
which is a technical development of a previously described
applicator (Amica-probe V3) (12).
Amica-probe V4 includes a coaxial antenna and an integrated
hydraulic circuit to cool the coaxial feeding line. The antenna is
provided with a patented device (minichoke) for the entrapment of
back-propagating waves and is terminated by a sharp point for easy
penetration into the tissues (Fig.
1). Control over the coagulative lesion size is obtained by
setting the microwave power level and delivery time.
Surgical treatment and MWTA
After the surgical exposure of the kidney and before
vascular renal pedicle clamping, a Tru-cut biopsy specimen was
taken from the tumor, in order to obtain a tumor sample for
histopathological analysis. Then, the Amica-probe V4 applicator was
inserted in the bed of Tru-cut needle into the tumor. The
applicator was placed at least 3 cm deep into the tumor, for tumor
size >4–5 cm (calculated on the basis of CT scan resolution),
and at least 1 cm beyond the tumor, in the case of tumor size <4
cm. Such different applicator insertion methods were used in order
to verify the complete tumor ablation in subjects with tumor size
<4 cm. For each patient, 50 W microwave power was delivered into
the renal tissue for 5 min. The selection of power and time of
exposure to MWTA was determined on the basis of previous studies on
porcine kidney where these two parameters were standardized to
obtain spheroidal shaped repeteable lesions (8). The probe was removed, the renal blood
vessels were promptly legated and the surgeon proceeded to classic
radical nephrectomy. The surgical specimen, consisting of the
kidney and tumor within the Gerota fascia, was removed en bloc and
underwent pathological analysis for standard hystopathological
diagnosis, microwave thermo-induced effects in the context of both
tumor and healthy renal tissue and tumor skipping evaluation.
Heating-induced lesion measurement and
histopathological analysis
Pathological samples were collected from the ablated
tissues immediately after surgery to investigate neoplastic vital
cell skipping. Small tumor samples were collected from the tumor
bed after adequate sagittal and horizontal line kidney sections
useful for both the tumor and the microwave thermo-induced lesion
measurements. These small samples were immediately sectioned and
stained with trypan blue according to the procedure described by
Allison and Ridolpho (13), in
order to test cell viability. Pathologist thus provided for tumor
and lesion measurements and tissues were stored in formalin for
subsequent staining with haematoxylin and eosin (H&E) and
microscopical analysis. Pathological evaluation was carried out by
one dedicated pathologist in each center, in accordance with
Bartoletti et al (8). All
samples were analyzed to evaluate the effect of microwave energy
delivered to renal tissue (no skipping) and the effect of energy on
the collecting system or peritumoral healthy renal tissue, as
previously described (8). All
ablation lesions were assessed using callipers for both the
short-axis diameter (r1, perpendicular to the applicator) and the
long-axis diameter (r2, along the applicator). In addition, both
diameters were used to calculate the sphericity of the ablation.
Sphericity index (SI) was defined as the volume of ablation divided
by the volume sphere, using only the greatest diameter. According
to Hines-Peralta et al (14), the volume of ablation was defined as
the calculated volume of an ellipsoid, obtained by using r1 and r2
radii. As suggested by these authors, SI was simplified to
r12/r2 (2); a perfect sphere has an index of 1.0 and an
ellipsoid <1.0 (14).
Trypan blue exclusion test
Trypan blue exclusion test (Life Technologies) is
able to accurately determine cell viability. In fact, if cells
take-up trypan blue, they are considered non-viable. Live cells or
tissues with intact cell membranes are not colored. After the
Amica-probe application, a sample of the renal lesion was collected
and immediately sent to laboratory in isotonic salt solution under
refrigerate conditions. Renal cells were added to a solution 0.4%
of trypan blue in buffered isotonic salt solution, pH 7.2–7.3, such
as phosphate-buffered saline and were examined immediately under a
microscope at low magnification. In order to obtain reproducible
results, the sample collection and tissue processing was performed
according to Wu et al (15).
Preoperative complication evaluation
The classification of perioperative complications
was performed using the Clavien-Dindo classification (16).
Statistical analysis
All ablation diameters are reported as the mean
(±SD) and were compared using ANOVA, followed by Bonferroni test,
when appropriate, with P<0.05 (two-sided) considered to indicate
significance. All statistical calculations were performed with the
Statistical Package for Social Sciences 11.0 (SPSS, Inc., Chicago,
IL, USA).
For the ablation equipment, materials, support was
provided by Hospital Services SpA, and the results were analyzed
and discussed independently of any influence of the Sponsor
Company.
Results
Fourteen patients (12 men and 2 women; mean age,
70.9±9.8; range, 53–88 years) underwent open radical nephrectomy.
The mean age-adjusted Charlson comorbidity index was 4.8 (range,
3–7). The mean RENAL score was 9.4 (range, 8–12). Table I shows the clinical and laboratory
characteristics of enrolled patients.
 | Table IPatient characteristics at
baseline. |
Table I
Patient characteristics at
baseline.
| Characteristics | Mean | SD | Median | Range |
|---|
| Age (years) | 70.9 | 9.8 | 70 | 53–88 |
| Charlson CI | 4.8 | 1.2 | 4 | 4–7 |
| Lesion diameter
(mm) | 47.00 | 18.7 | 44 | 40–92 |
| RENAL score | 9.4 | 1.1 | 9 | 8–12 |
| D-dimer (mg/l) | 226.8 | 87 | 225.5 | 102.5–433.3 |
| INR | 1.01 | 0.08 | 0.95 | 0.89–1.11 |
| aPTT | 31 | 3.9 | 30.5 | 23–37.5 |
Clinical and laboratory results
No significant differences between D-dimer, aPPT and
INR values were found before and after MWTA and surgery treatment
independently from previous anti-platelet medical treatments. Two
patients (14.2%) with tumor extension to vena cava (pT3b), showed
low grade complications (Clavien II) that required blood
transfusions. This procedure was, however, independent from the
parenchyma MWTA treatment. All patients had a normal postoperative
time of hospitalization (mean, 3.1 days; range, 3–5) without
relevant complications concerning both the wound healing and the
laboratory analyses regularization. No short-term significant
bleeding was collected from draining tubes which were removed just
before patient discharge. No adverse events due to co-morbidities
were observed during hospitalization or after long-term follow-up
(27.4±4.2 months). Noticeable absence or reduction bleeding from
the tumor was described by different surgeons overall in patients
with smaller tumors, although this parameter could not be
scientifically quantifiable.
Histopathological results
Macroscopic evaluation
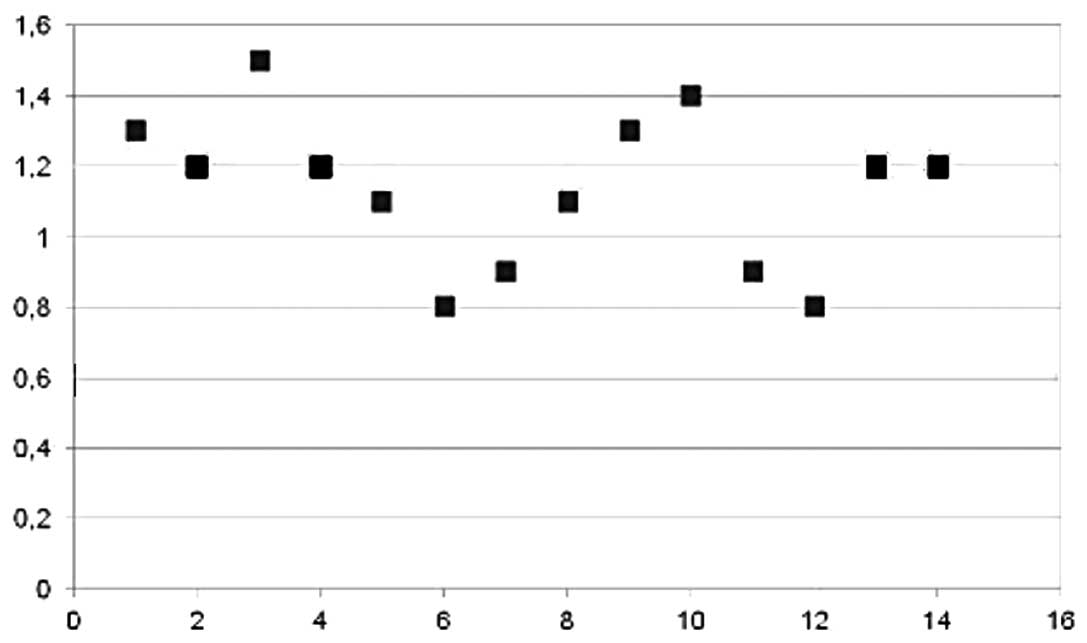
MW-induced lesion size was 44.14 mm (±22.59). All
removed kidneys were sectioned along three different planes
(sagittal, orizontal and transversal) in order to obtain precise
measurement of both tumor and MWTA-induced lesion diameters. Mean
SI of MWTA-induced lesions was 1.08 (±0.2). Fig. 2 shows the distribution of SI values
among all patients. All pathological and laboratory findings are
presented in Table II. The inner
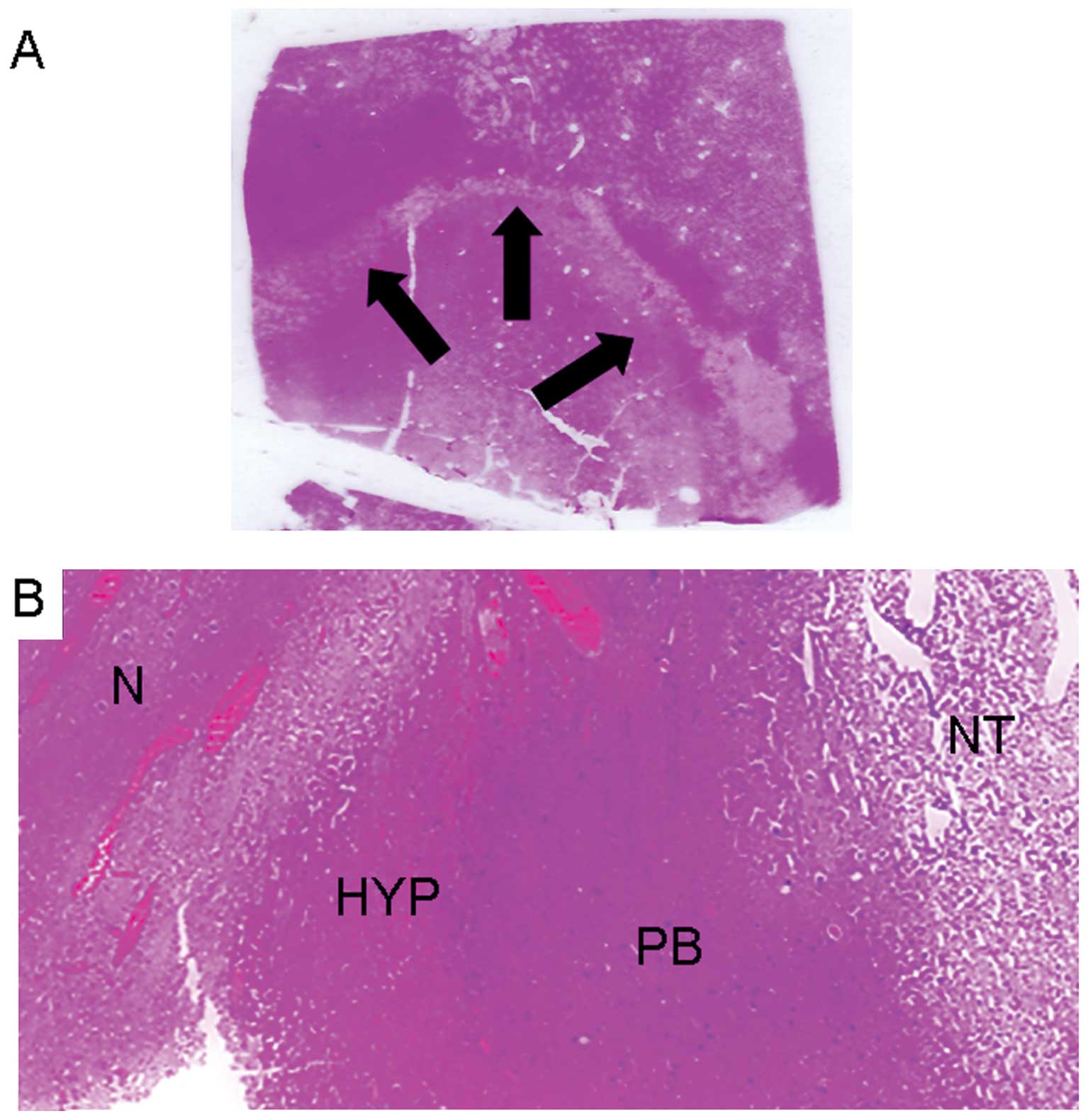
area of the MWTA-induced lesions appeared pale and necrotic, the
middle area hyperemic, while a purple border represented the outer
layer. H&E staining showed homogeneous coagulative necrosis at
the lesion core (inner area) surrounded by renal cells (Fig. 3A).
 | Table IIPathological and laboratory findings
after treatment. |
Table II
Pathological and laboratory findings
after treatment.
| Pathological
findings |
|---|
| Histological
findings | 13 RCC |
| 1 oncocytoma |
| Pathological
stage | 2 T1a |
| 5 T1b |
| 1 T2 |
| 3 T3a |
| 2 T3b |
| Fuhrman grade | − G1 |
| 4 G2 |
| 6 G3 |
| 3 G4 |
| Complications
(Clavien grade) | 2, grade II |
|
| Laboratory
findings | Mean | Difference
(P-value) |
|
| D-dimer pre
(mg/l) | 226.85 | 0.21 |
| D-dimer post
(mg/l) | 445.69 | |
| INR pre | 1.01 | 0.92 |
| INR post | 1.10 | |
| aPTT pre | 31 | 0.34 |
| aPTT post | 31.69 | |
Microscopic evaluation
Renal cells appear with irreversible cell death, as
shown by pyknotic nuclei and nuclear disruption. There were
variable amounts of granulation tissue, with immature fibroblasts
and inflammatory cells in the boundary region (middle and outer
areas). Beyond these, only healthy tissue was found and no skipping
was noted in any sample (Fig. 3B).
Moreover, histological review of the wide ablation zones showed the
inner zone to have uniform cell death by trypan blue exclusion
test. No residual vital tumor cells inside the MW-induced lesions
were found.
Discussion
The effects of MWTA on kidney tissue have been
recently evaluated and discussed by several authors with
conflicting results. Clark et al (9) demonstrated the feasibility of MWTA in
10 patients with large kidney tumors before radical nephrectomy and
demonstrated complete tumor cell kill inside the ablated lesions.
Liang et al (17) confirmed
the efficacy of MWTA on renal tumors by treating 12 patients with
small lesions (<4 cm in diameter) and no evidence of recurrence
after 20 months of follow-up, while Castle et al (18) reported poor oncologic outcomes and
significant complication rates in a series of 10 patients with
tumor diameter size ranging from 2.0 to 5.5 cm treated by
laparoscopic- or CT-guided percutaneous MWTA. Rational explanations
for such similar discrepancies could be easily found by evaluating
either the absence of a standardized and reproducible method for
microwave administration or the adequate selection of patients. Our
previous study on porcine kidney (8) demonstrated that a standardized method
of microwave administration could be obtained by using
pre-determined independent variables such as power of treatment and
time of exposure to energy. The Amica-probe antenna is a new
refrigerated probe able to determine approximately spherical
lesions with a maximum of 2.7±0.3 cm in the animal kidney tissue by
an exposure time of 5 min and 50 W power (8). The best results could be obtained by
using 15 min of time exposure and 50 W power with induced-lesion SI
~1.0 and diameters from 4.1 to 4.2 cm (±0.1). Moreover, the
thermocouple sensors placed ~4 cm radially far from the Amica-probe
antenna point of insertion showed temperature peaks of 41°C, thus,
confirming the safety of this method on healthy renal tissues.
These results could explain the importance of patient selection
(only patients with small renal masses could be treated) and
reliable technology (this refrigerated probe is able to induce a
satisfactory SI) to obtain reproducible methods of treatment and
evaluable results. This is the first study describing the use of
Amica-probe V4 microwave applicator on human kidney tumors, which
has been successfully tested. Although a subjective variability of
MWs induced lesion diameters due to both the applicator insertion
depth and the tumor water content, tumors until 2 cm could be
safely treated by using 50 W power energy and 5 min of exposure
time to MWs. Clinical studies on MWTA of small renal masses and
conservative surgery should be then conducted to obtain extensive
clinical clarifications and indications on this fascinating
technique. Microwaves create a thermal field that is absorbed by
surrounding tissues proportionally to the tissue water content
(19). Thus, tissues with greater
water content absorb more energy and produce heat, while tissues
with lower water content produce less heat and propagate microwaves
(20). Testing the Amica-probe
antenna on both healthy renal tissue and renal tumor tissue, we
found no substantial differences of diffusion of MWs by measuring
macroscopically MWTA-induced lesion diameters, although no
significant variations of SI were found. This could be related to
both the cellular type or the presence of necrosis in the context
of the tumor. Nevertheless, large tumors usually present necrotic
areas in their context while small tumors tends to be more compact
and homogeneous except oncocytomas. All patients selected for this
study presented large or complicated renal masses (mean RENAL
score, 9.4). This kind of selection aimed to obtain an accurate
evaluation of MWs diffusion and spread inside the tumor tissue,
thus, avoiding the risk of significant variations in heat-induced
lesion diameters due to different tissue resistance of tumor
pseudo-capsule. Nevertheless, this could justify future studies on
MWTA application on small solid renal masses. The potential tropism
of MWs to tumor and renal vasculature represented one of the most
important parameters investigated in this study. No differences
were found in monitoring the patients haemo-coagulative set up
before surgery and after short- and long-term follow-up, showing a
limited diffusion of MWs through renal tissue independently from
the renal pedicle clamping during the MWTA treatment. All patients
underwent open radical nephrectomy after MWTA treatment and only
2/14 of them presented intraoperative bleeding with the need of
blood-transfusions due to the presence of a tumor thrombus in the
vena cava (Clavien II). The incidence of patients requiring
blood-transfusions during radical nephrectomy have been reported as
ranging from 18 to 44% in relation to tumor extension and the need
of extensive surgery (21). This
complication found in our series has not to be considered as
relevant and it was not related to the MWTA tumor treatment. No
evidence of tumor-cell skipping was found by using vital staining
analyisis in all cases. This could demonstrate that local
recurrence of disease, previously described by other authors, is
probably due to the incomplete ablation of large tumors where the
estimation of surgical results have been entrusted to the
macroscopically apparent evidence of ablation in case of both
laparoscopic- and CT-guided MWs application. The empirical
experience of Muto et al (22) confirmed our results demonstrating
that MWs application to small renal tumors (1.3–4.2 cm) provides
good results in terms of tumor follow-up and has to be considered
as optimal method for haemostasis making laparoscopic tumor
enucleation easier and possible without renal pedicle clamping and
haemostatic sutures subsequent to tumor removal. However, the
present study shows few limitations that should be taken into
account during interpretation of the findings. The MWs diffusion
and spread through tumor tissue may vary in relation to its liquid
content (water and/or necrosis), thus, large tumors could not be
considered as the optimal model although our findings demonstrated
satisfactory homogeneous results in terms of both heat-induced
lesion diameters and SI.
In conclusion, the Amica-probe V4 microwave antenna
is able to determine reproducible results in the treatment of small
kidney solid tumors. MWTA is a safe method and could be relatively
easily employed for future clinical efficacy and comparative
studies.
Acknowledgements
We are grateful to all members of the R&D Unit
of HS Hospital Service SpA (Aprilia, Italy) for their technical
assistance and to Professor John Denton for manuscript language
revision.
References
|
1
|
Novara G and Ficarra V: Is laparoscopic
cryoablation a less invasive and effective procedure to treat small
renal masses? Eur Urol. 60:444–447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Volpe A, Cadeddu JA, Cestari A, et al:
Contemporary management of small renal masses. Eur Urol.
60:501–515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clements T, Lin YK and Raman JD: Current
status of ablative techniques for small renal masses. Expert Rev
Anticancer Ther. 11:879–891. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kunkle DA and Uzzo RG: Cryoablation or
radiofrequency ablation of the small renal mass: a meta-analysis.
Cancer. 113:2671–2680. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zagoria RJ and Childs DD: Update on
thermal ablation of renal cell carcinoma: oncologic control,
technique comparison, renal function preservation, and new
modalities. Curr Urol Rep. 13:63–69. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bhardwaj N, Strickland AD, Ahmad F,
Atanesyan L, West K and Lloyd DM: A comparative histological
evaluation of the ablations produced by microwave, cryotherapy and
radiofrequency in the liver. Pathology. 41:168–172. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Itoh K, Suzuki Y, Miuru M, Tsukigi M,
Ichiyanagi O and Sasagawa I: Posterior retroperitoneoscopic partial
nephrectomy using microwave tissue coagulator for small renal
tumors. J Endourol. 16:367–371. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartoletti R, Cai T, Tosoratti N, et al:
In vivo microwave-induced porcine kidney thermoablation: results
and perspectives from a pilot study of a new probe. BJU Int.
106:1817–1821. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Clark PE, Woodruff RD, Zagoria RJ and Hall
MC: Microwave ablation of renal parenchymal tumors before
nephrectomy: phase I study. AJR Am J Roentgenol. 188:1212–1214.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kutikov A and Uzzo RG: The R.E.N.A.L.
nephrometry score: a comprehensive standardized system for
quantitating renal tumor size, location and depth. J Urol.
182:844–853. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Charlson ME, Pompei P, Ales KL and
MacKenzie CR: A new method of classifying prognostic comorbidity in
longitudinal studies: development and validation. J Chronic Dis.
40:373–383. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bartoletti R, Cai T, Tinacci G, et al:
Transperineal microwave thermoablation in patients with obstructive
benign prostatic hyperplasia: a phase I clinical study with a new
mini-choked microwave applicator. J Endourol. 22:1509–1517. 2008.
View Article : Google Scholar
|
|
13
|
Allison DC and Ridolpho P: Use of a trypan
blue assay to measure the deoxyribonucleic acid content and
radioactive labeling of viable cells. J Histochem Cytochem.
28:700–703. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hines-Peralta AU, Pirani N, Clegg P, et
al: Microwave ablation: results with a 2.45-GHz applicator in ex
vivo bovine and in vivo porcine liver. Radiology. 239:94–102. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu XX, Kakehi Y, Jin XH, Inui M and
Sugimoto M: Induction of apoptosis in human renal cell carcinoma
cells by vitamin E succinate in caspase-independent manner.
Urology. 73:193–199. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clavien PA, Barkun J, de Oliveira ML, et
al: The Clavien-Dindo classification of surgical complications:
five-year experience. Ann Surg. 250:187–196. 2009.PubMed/NCBI
|
|
17
|
Liang P, Wang Y, Zhang D, Yu X, Gao Y and
Ni X: Ultrasound guided percutaneous microwave ablation for small
renal cancer: initial experience. J Urol. 180:844–848. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Castle SM, Salas N and Leveillee RJ:
Initial experience using microwave ablation therapy for renal tumor
treatment: 18-month follow-up. Urology. 77:792–797. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carrafiello G, Laganà D, Ianniello A, et
al: Percutaneous radiofrequency thermal ablation of renal cell
carcinoma: is it possible a day-hospital treatment? Int J Surg.
6:31–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carey RI and Leveillee RJ: First prize:
direct real-time temperature monitoring for laparoscopic and
CT-guided radiofrequency ablation of renal tumors between 3 and 5
cm. J Endourol. 21:807–813. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shvarts O, Tsui KH, Smith RB, Kernion JB
and Belldegrun A: Blood loss and the need for transfusion in
patients who undergo partial or radical nephrectomy for renal cell
carcinoma. J Urol. 164:1160–1163. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Muto G, Castelli E, Migliari R, D’Urso L,
Coppola P and Collura D: Laparoscopic microwave ablation and
enucleation of small renal masses: preliminary experience. Eur
Urol. 60:173–176. 2011. View Article : Google Scholar : PubMed/NCBI
|