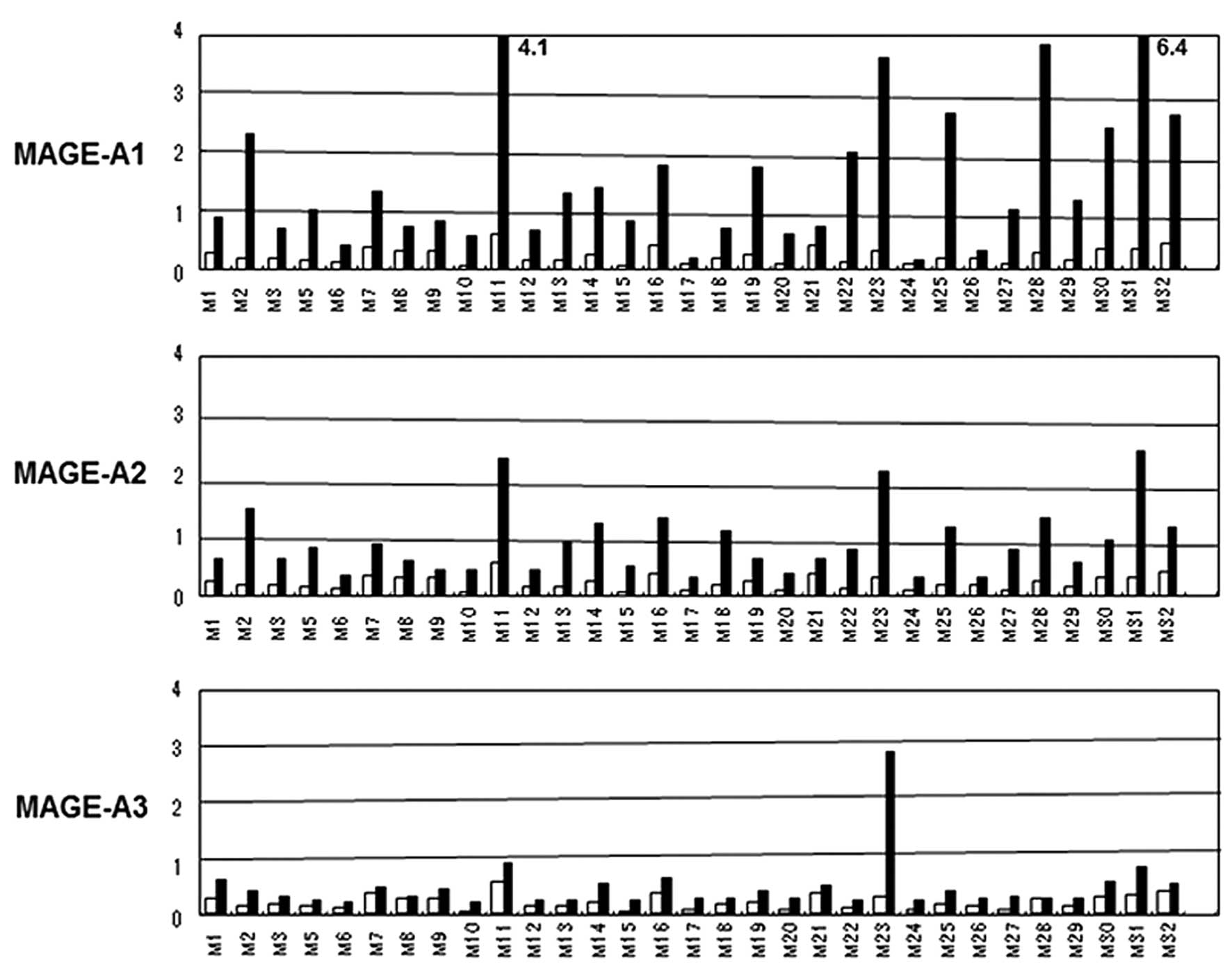
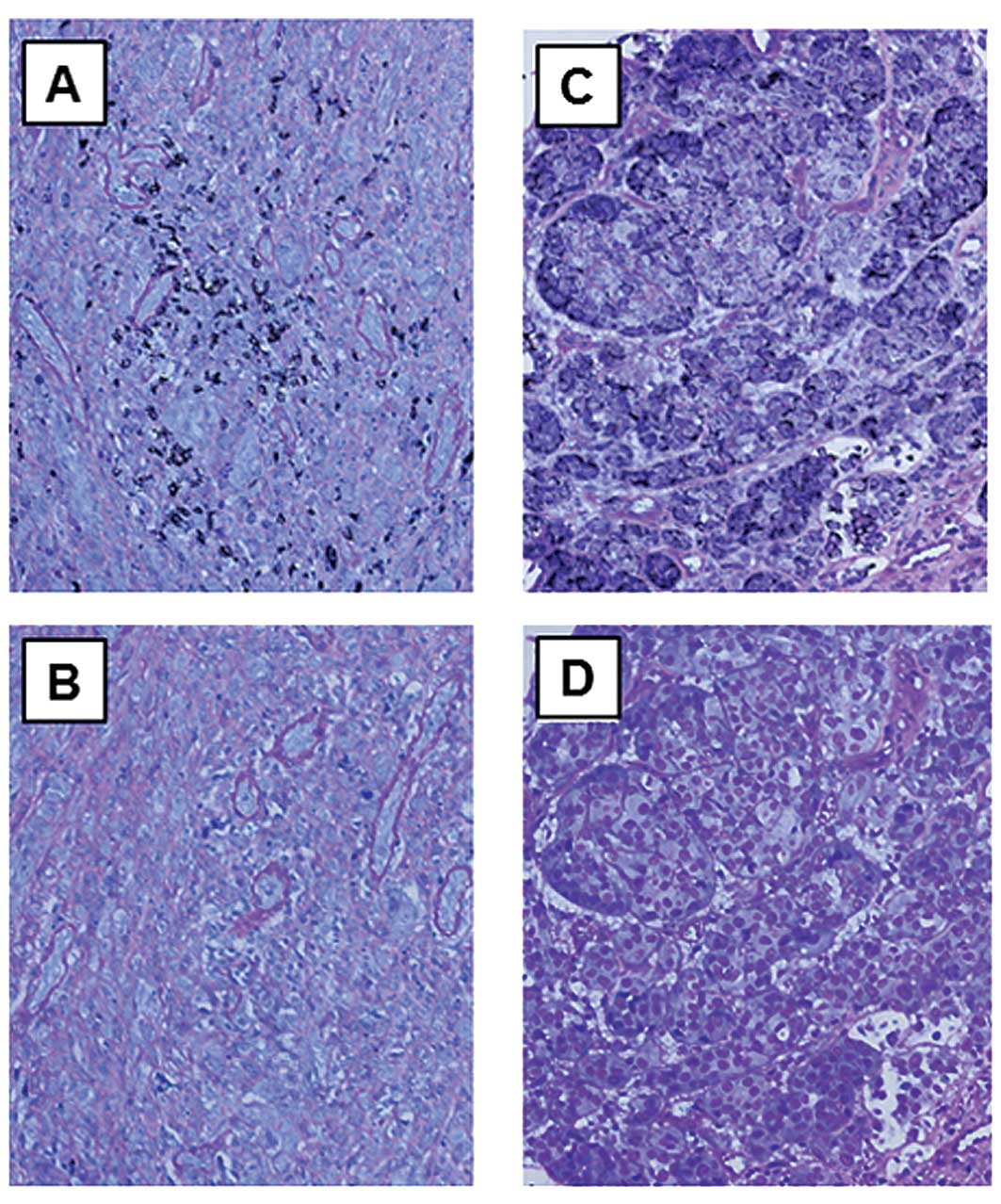
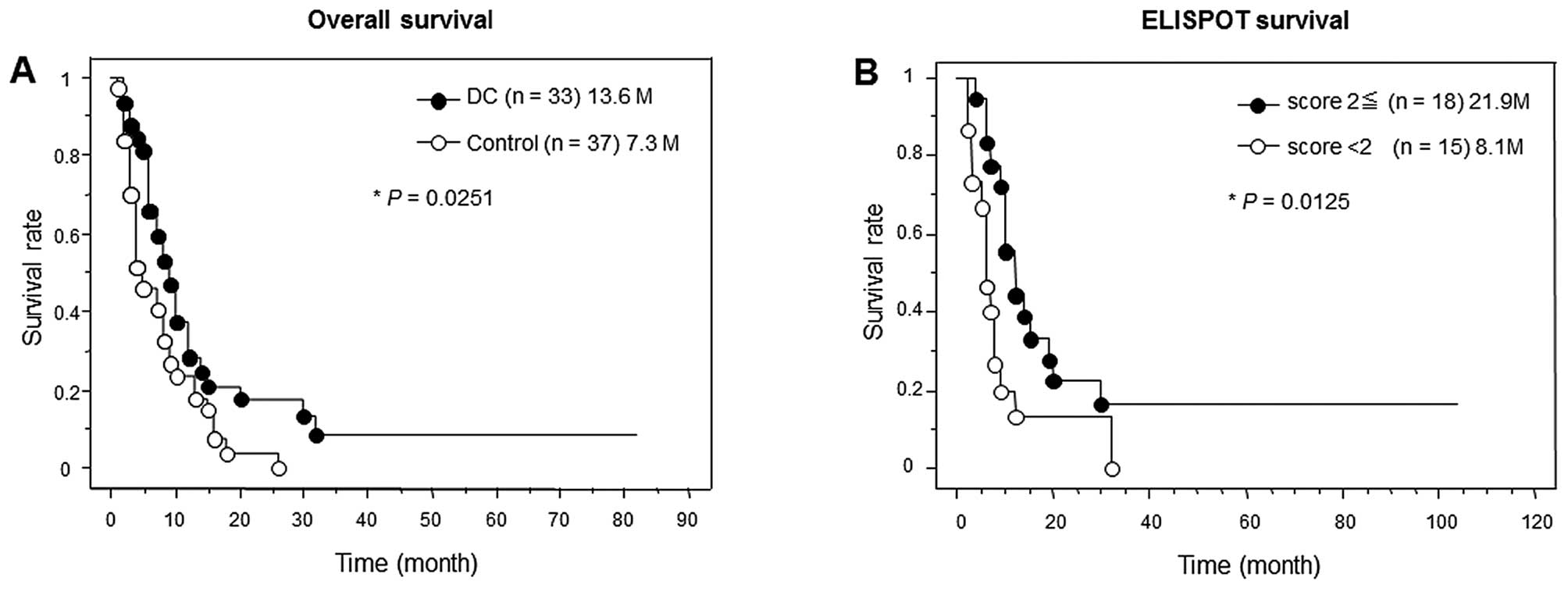
|
1
|
Mayordomo JI, Zorina T, Storkus WJ,
Zitvogel L, Celluzzi C, Falo LD, Melief CJ, Ildstad ST, Kast WM and
Deleo AB: Bone marrow-derived dendritic cells pulsed with synthetic
tumor peptides elicit protective and therapeutic antitumor
immunity. Nat Med. 1:1297–1302. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zitvogel L, Mayordomo JI, Tjandrawan T,
DeLeo AB, Clarke MR, Lotze MT and Storkus WJ: Therapy of murine
tumors with tumor peptide-pulsed dendritic cells: dependence on T
cells, B7 costimulation, and T helper cell 1-associated cytokines.
J Exp Med. 183:87–97. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nestle FO, Alijagic S, Gilliet M, Sun Y,
Grabbe S, Dummer R, Burg G and Schadendorf D: Vaccination of
melanoma patients with peptide-or tumor lysate-pulsed dendritic
cells. Nat Med. 4:328–332. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ranieri E, Kierstead LS, Zarour H,
Kirkwood JM, Lotze MT, Whiteside T and Storkus WJ: Dendritic
cell/peptide cancer vaccine: clinical responsiveness and epitope
spreading. Immunol Invest. 29:121–125. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thurner B, Haendle I, Roder C, Dieckmann
D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, von
den Driesch P, et al: Vaccination with Mage-3a1 peptide-pulsed
mature, monocyte-derived dendritic cells expands specific cytotoxic
T cells and induces regression of some metastases in advanced stage
IV melanoma. J Exp Med. 190:1669–1678. 1999. View Article : Google Scholar
|
|
6
|
Panelli MC, Wunderlich J, Jeffries J, Wang
E, Mixon A, Rosenberg SA and Marincola FM: Phase 1 study in
patients with metastatic melanoma of immunization with dendritic
cells presenting epitopes derived from the melanoma-associated
antigens MART-1 and gp100. J Immunother. 23:487–498. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jonuleit H, Giesecke-Tuettenberg A, Tuting
T, Thurner-Schuler B, Stuge TB, Paragnik L, Kandemir A, Lee PP,
Schuler G, Knop J and Enk AH: A comparison of two types of
dendritic cell as adjuvants for the induction of melanoma-specific
T-cell responses in humans following intranodal injection. Int J
Cancer. 93:243–251. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Banchereau J, Palucka AK, Dhodapkar M,
Burkeholder S, Taquet N, Rolland A, Taquet S, Coquery S, Wittkowski
KM, Bhardwaj N, et al: Immune and clinical responses in patients
with metastatic melanoma to CD34+ progenitor-derived
dendritic cell vaccine. Cancer Res. 61:6451–6458. 2001.PubMed/NCBI
|
|
9
|
Lau R, Wang F, Jeffery G, Marty V,
Kuniyoshi J, Bade E, Ryback ME and Weber J: Phase I trial of
intravenous peptide-pulsed dendritic cells in patients with
metastatic melanoma. J Immunother. 24:66–78. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chakraborty NG, Sporn JR, Tortora AF,
Kurtzman SH, Yamase H, Ergin MT and Mukherji B: Immunization with a
tumor-cell-lysate-loaded autologous-antigen-presenting-cell-based
vaccine in melanoma. Cancer Immunol Immunother. 47:58–64. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smithers M, O’Connell K, MacFadyen S,
Chambers M, Greenwood K, Boyce A, Abdul-Jabbar I, Barker K,
Grimmett K, Walpole E and Thomas R: Clinical response after
intradermal immature dendritic cell vaccination in metastatic
melanoma is associated with immune response to particulate antigen.
Cancer Immunol Immunother. 52:41–52. 2003.
|
|
12
|
Chang AE, Redman BG, Whitfield JR,
Nickoloff BJ, Braun TM, Lee PP, Geiger JD and Mule JJ: A phase I
trial of tumor-lysate-pulsed dendritic cells in the treatment of
advanced cancer. Clin Cancer Res. 8:1021–1032. 2002.PubMed/NCBI
|
|
13
|
Burch PA, Croghan GA, Gastineau DA, Jones
LA, Kaur JS, Kylstra JW, Richardson RL, Valone FH and Vuk-Pavlovic
S: Immunotherapy (APC8015, Provenge) targeting prostatic acid
phosphatase can induce durable remission of metastatic
androgen-independent prostate camcer: a Phase 2 trial. Prostate.
60:197–204. 2004. View Article : Google Scholar
|
|
14
|
Cheever MA and Higano CS: PROVENGE
(Sipuleucel-T) in prostate cancer: the first FDA-approved
therapeutic cancer vaccine. Clin Cancer Res. 17:3520–3526. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kantoff PW, Higano CS, Shore ND, Berger
ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims
RB, et al: IMPACT Study Investigators: Sipuleucel-T immunotherapy
for castration-resistant prostate cancer. N Engl J Med.
363:411–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Akiyama Y, Tanosaki R, Inoue N, Shimada M,
Hotate Y, Yamamoto A, Yamazaki N, Kawashima I, Nukaya I, Takesako
K, et al: Clinical response in Japanese metastatic melanoma
patients treated with peptide cocktail-pulsed dendritic cells. J
Transl Med. 3:42005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors European
Organization for Research and Treatment of Cancer, National Cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar
|
|
18
|
Iizuka A, Komiyama M, Tai S, Oshita C,
Kurusu A, Kume A, Ozawa K, Nakamuya Y, Ashizawa T, Yamamoto A, et
al: Identification of cytomegalovirus (CMV) pp65 antigen-specific
human monoclonal antibodies using single B cell-based antibody gene
cloning from melanoma partients. Immunol Lett. 135:64–73. 2011.
View Article : Google Scholar
|
|
19
|
Hersey P, Menzies SW, Halliday GM, Nguyen
T, Farrelly ML, DeSilva C and Lett M: Phase I/II study of treatment
with dendritic cell vaccines in patients with disseminated
melanoma. Cancer Immunol Immunother. 53:125–134. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Desmetz C, Maudelonde T, Mange A and
Solassol J: Identifying autoantibody signature in cancer: a
promising challenge. Expert Rev Proteomics. 6:377–386. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Desmetz C, Cortijo C, Mange A and Solassol
J: Humoral response to cancer as a tool for biomarker discovery. J
Proteomics. 72:982–988. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tan HT, Low J, Lim SG and Chung MC: Serum
autoantibodies as biomarkers for early cancer detection. FEBS J.
276:6880–6904. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Y, He J, Xie X, Su G, Teitz-Tennenbaum
S, Sabel MS and Lubman DM: Serum autoantibody profiling using a
natural glycoprotein microarray for the prognosis of early
melanoma. J Proteome Res. 9:6044–6051. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stockert E, Jäger E, Chen YT, Scanlan MJ,
Gout I, Karbach J, Arand M, Knuth A and Old LJ: A survey of the
humoral immune response of cancer patients to a panel of human
tumor antigens. J Exp Med. 187:1349–1354. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tosolini M, Kirilovsky A, Mlecnik B,
Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH,
Pages F and Galon J: Clinical impact of different classes of
infiltrating T cytotoxic and helper cells (Th1, Th2, Treg, Th17) in
patients with colorectal cancer. Cancer Res. 71:1263–1271. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Galon J, Costes A, Sanchez-Cabo F,
Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M,
Berger A, Wind P, et al: Type, density, and location of immune
cells within human colorectal tumors predict clinical outcome.
Science. 313:1960–1964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dieu-Nosjean MC, Antoine M, Danel C,
Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de
Chaisemartin L, et al: Long-term survival for patients with
non-small cell lung cancer with intratumoral lymphoid structures. J
Clin Oncol. 26:4410–4417. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rini BI, Weinberg V, Fong L, Conry S,
Hershberg RM and Small EJ: Combination immunotherapy with prostatic
acid phosphatase pulsed antigen-presenting cells (provenge) plus
bevacizumab in patients with serologic progression of prostate
cancer after definitive local therapy. Cancer. 107:67–74. 2006.
View Article : Google Scholar
|
|
29
|
Cheever MA: Twelve immunotherapy drugs
that could cure cancers. Immunol Rev. 222:357–368. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Scher HI, Halabi S, Tannock I, Morris M,
Sternberg CN, Carducci MA, Eisenberger MA, Higano C and Bubley GJ:
Dreicer R, et al; Prostate Cancer Clinical Trials Working
Group: Design amd end points of clinical trials for patients with
progressive prostate cancer and castrate levels of testosterone:
recommendations of the Prostate Cancer Clinical Trials Working
Group. J Clin Oncol. 26:1148–1159. 2008.PubMed/NCBI
|
|
31
|
Buonerba C, Ferro M and Di Lorenzo G:
Sipuleucel-T for prostate cancer: the immunotherapy era has
commenced. Expert Rev Anticancer Ther. 11:25–28. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Finke LH, Wentworth K, Blumenstein B,
Rudolph NS, Levitsky H and Hoos A: Lessons from randomized phase
III studies with active cancer immunotherapies-outcomes from the
2006 meeting of the Cancer Vaccine Consortium (CVC). Vaccines.
25(Suppl 2): S97–S109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tyagi P and Mirakhur B: MAGRIT: the
largest-ever phase III lung cancer trial aims to establish a novel
tumor-specific approach to therapy. Clin Lung Cancer. 10:371–374.
2009. View Article : Google Scholar : PubMed/NCBI
|