Introduction
Colorectal cancer (CRC) is the third most common
type of cancer. About 608,700 deaths from CRC occurred in 2008,
accounting for 8% of all cancer deaths (1). About 30% of recurrent CRC patients
have liver metastasis, and >70% of them are not candidates for
curative resection (2). In the past
few years, accumulating evidence has suggested that microRNAs
(miRNAs) are involved in the pathogenesis of CRC (3). The pattern of miRNA expression is
related with cancer type, stage and other clinical variables, which
makes miRNA a promising prognostic and therapeutic tool (4,5).
In order to support high rates of proliferation,
cancer cells consume additional nutrients and divert those
nutrients into macromolecular synthesis pathways. Cancer cells
prefer to metabolize glucose by glycolysis, intentionally avoid
oxidative phosphorylation even when oxygen is abundant - the
Warburg effect (6). Therefore, many
cancer cells are characterized with increased lactate production
and decreased O2 consumption (7). Active glycolysis coupled with
increased glucose intake will provide sufficient intermediate
metabolites, such as NADPH, acetyl-CoA and ribose, for biosynthetic
process (8,9).
Pyruvate kinase (PK), which converts
phosphoenolpyruvate into pyruvate, is one of rate-limiting enzymes
in glycolytic pathway. PKM is alternatively spliced to either M1
(PKM1) or M2 (PKM2) isoform, which contains exon 9 or exon 10,
respectively (10,11). The single exon difference endows the
enzymes with distinct expression patterns and functions. PKM2 is
exclusively expressed in embryonic, proliferating and cancer cells,
which promotes glycolysis even in an aerobic environment. PKM1 is
expressed in normal differentiated tissues, which promotes
oxidative phosphorylation (12,13).
The expression PKM2 is critical for cancer cell growth. Switching
PKM expression from PKM2 to PKM1 leads to inhibition of the Warburg
effect and cancer growth (14).
Three heterogeneous nuclear ribonucleoproteins
(hnRNPs) proteins, including polypyrimidine tract binding protein
(PTB, also known as hnRNPI), hnRNPA1 and hnRNPA2, bind repressively
to sequences flanking exon 9. In the presence of PKM alternative
splicing proteins (PTB1/hnRNAPA1/hnRNAPA2), exon 10 is included in
PKM transcript. To ensure high ratio of PKM2/PKM1 in cancer cells,
expression of PTB1, hnRNAPA1 and hnRNAPA2 are tightly controlled by
oncogenes, such as c-Myc. Importantly, the alternative splicing of
PKM gene is important to sustain cancer cell growth through
regulating glucose metabolism (15).
In this study, we found that miR-124, miR-137 and
miR-340 were associated with survival of CRC. Restoring the miRNA
expression significantly inhibited growth of colorectal cancer
cells. PKM alternative splicing proteins (PTB1/hnRNAPA1/hnRNAPA2)
were common targets of these miRNAs. miR-124, miR-137 and miR-340
promoted the alternative splicing of PKM gene to shift from PKM2 to
PKM1. Therefore glucose was mainly metabolized through oxidative
phosphorylation rather than glycolysis in the presence of miR-124,
miR-137 and miR-340.
Materials and methods
Cell lines, reagents and tissue
specimens
The colorectal cell lines HCT116, DLD1, SW480 and
HT29, were purchased from ATCC. Cells were cultured in a humidified
atmosphere of 95% air, 5% CO2, using recommended medium
and 10% FBS. Formalin-fixed paraffin-embedded (FFPE) colorectal
cancer samples were collected from Zhongshan Hospital, Shanghai,
China. The study protocol was reviewed and approved by the ethics
committee of Zhongshan Hospital. miRIDIAN shMIMIC lentiviral
microRNAs (OpenBiosystems) were used to express miRNAs in
colorectal cancer cells. For miRNA activity assay, pmirGLO
Dual-Luciferase miRNA target expression vector (Promega) was
used.
miRNA profiling
Small RNA was extracted from FFPE tumor samples
using High Pure miRNA isolation kit (Roche). RNA quality was
assessed by absorbance spectrometry and electrophoresis on NanoDrop
and Bioanalyzer 2100 instruments (Agilent). Agilent human genome
microRNA microarray was used for miRNA profiling. Raw mean signal,
total probe intensities and total gene intensities were uploaded
into GeneSpring GX 10.0 software. Differences were considered
statistically significant when the Benjamini-Hochberg false
discovery rate-corrected p<0.05.
Real-time RT-PCR
For microRNA, we used stem-loop quantitative RT-PCR.
Briefly, 25 ng of total RNA was reverse transcribed using the
miRCURY First-strand cDNA kit and the miRCURY microRNA primer sets.
QPCR was performed with the Sequence Detection System 7900HT
(Applied Biosystems) using the miRCURY LNA™ SYBR® Green
Master mix. The comparative Ct (ΔΔCt) method was used to determine
the expression level of miRNA, and 5S RNA and U6B as endogenous
controls (16–18).
Metabolism assay
Glycolysis rates were measured by following the
conversion of 5-3H-glucose to 3H2O
as described previously (19).
Briefly, cells were washed once in PBS before incubation in Krebs
buffer without glucose for 30 min at 37°C. The Krebs buffer was
then replaced with Krebs buffer containing 10 mM glucose spiked
with 10 μCi of 5-3H-glucose. After 1 h, triplicate
samples of media were transferred to PCR tubes containing 0.2 N HCl
and the amount of 3H2O generated which was
determined by diffusion. Lactate was measured with a colorimetric
kit according to the manufacturer’s instructions (Biovision).
Lactate production was normalized to the number of cells. Cellular
oxygen consumption rates were measured using Biological oxygen
consumption analysis system (Teket).
Statistical analysis
All statistical analysis was carried out using SPSS
version 18.0 statistical software (SPSS). Two-tailed test and
p-values <0.05 for significance were used.
Results
miRNA profiling in stage III colorectal
cancer patients
Survival in patients with stage III colorectal
cancer is totally different after standard treatment. Therefore,
this cohort is quite suitable for prognostic analysis. miRNA
expression profiles were evaluated in 12 subjects with stage III
colorectal cancer. Thirty-two miRNAs showed >1.5-fold
(p<0.05) difference. Compared with patients with <5 years
survival, 21 miRNAs were up-regulated and 11 miRNAs were
down-regulated in patients with ≥5-year survival. Expression map
through unsupervised hierarchical clustering showed a clear
separation of the two groups (Fig.
1A). To pursue underlying mechanisms, miRNA targets were
analyzed by multiple databases (miRanda, TargetScanHuman and
PicTar). Of 21 protective miRNAs, miR-124, miR-137 and miR-340
targeted the alternative splicing proteins of PKM:
PTB1/hnRNAPA1/hnRNAPA2. This group of proteins has recently been
found to regulate the Warburg effect, which is critical for cancer
growth (20). To better understand
the role of miRNA in cancer metabolism, miR-124, miR-137 and
miR-340 were chosen for further study.
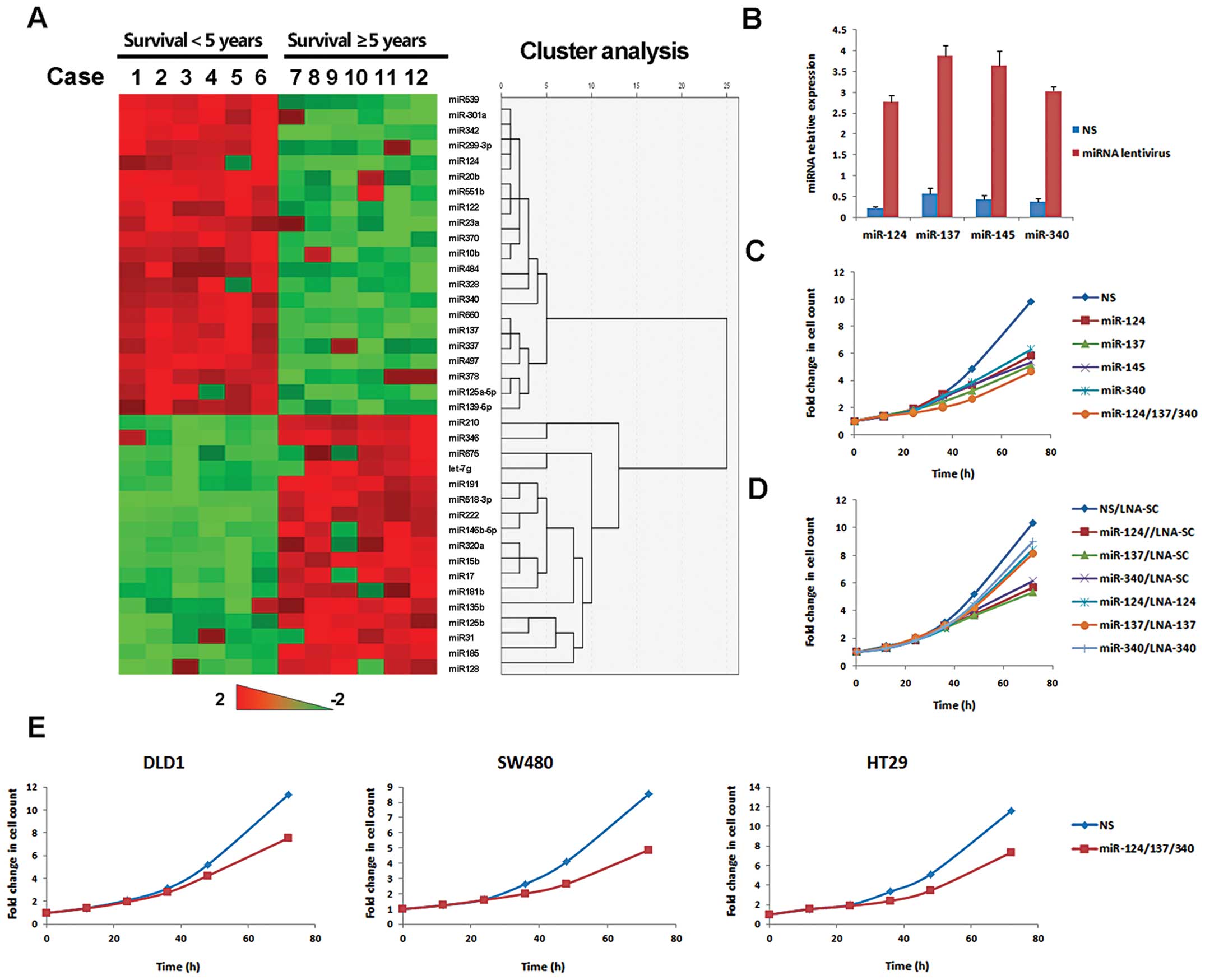 | Figure 1miR-124, miR-137 and miR-340 inhibited
proliferation of colorectal cancer cells. (A) Heatmap and
hierarchical clustering of significantly differentially expressed
miRNAs, patients in columns and miRNA in rows. miRNA expression
data are shown relative to the average value of normal colorectal
tissue. The expression values ranged from +2 log10 to −2
log10. (B) HCT116 cells were infected by lentivirus with
miR-124, miR-137, miR-145 or miR-340, respectively, NS as a
control. miRNA levels were assessed by QPCR, 5s rRNA, 18s rRNA and
U6 as global median-normalization references. (C) HCT116 cells were
treated by lentivirus with indicated miRNA or NS for proliferation
assay. Cell counts were performed every 12 h for 3–5 days. Time
zero was defined as 12 h after seeding. Proliferation curves were
drawn according to the fold of cell counts compared with time zero
point. (D) miRNA stable expressing HCT116 cells were treated with
LNA oligonucleotide complementary to the specific miRNA (LNA-124,
LNA -370 or LNA -137) or control LNA oligonucleotide (LNA-SC). Cell
proliferation was then analyzed by cell counting. (E) Cell
proliferation was analyzed in DLD1, SW480 and HT29 cells
transfected with miR-124/137/340 or NS. |
Growth inhibition of colorectal cancer
cells by miR-124, miR-137 and miR-340
Poor prognosis is related with multiple aspects of
colorectal cancer, including proliferation, survival and invasion.
miRNA target prediction suggested that miR-124, miR-137 and miR-340
were probably involved in cancer metabolic reprogramming. The main
aim of metabolic reprogramming is to sustain rapid cancer growth.
Then we asked whether growth of colorectal cancer cells was
regulated by these miRNAs. HCT116 cells were infected with
lentivirus containing the precursor of miRNA or non-silencing
control sequence (NS) (Fig. 1B).
MiR-145, as a positive control, was known to have potent
antiproliferative activity in colorectal cancer (21–23).
miR-124, miR-137 or miR-145 inhibited the growth of HCT116 cells.
Growth inhibition was more significant in cells treated with three
miRNAs simultaneously (Fig. 1C).
Consistent results were obtained in other colorectal cancer cell
lines (Fig. 1E). To further
corroborate the anti-proliferative role of miR-124, miR-137 and
miR-340 we used the miRNA specific inhibitor LNA (locked nucleic
acid), to reverse miRNA-mediated growth inhibition. After 2 weeks
of puromycin selection, miRNA stably expressed HCT116 cells were
obtained. Then these cells were transfected with specific LNAs,
respectively. LNA rescued cells from miRNA-induced growth
inhibition (Fig. 1D). Therefore,
miR-124, miR-137 and miR-340 had important roles of regulating
colorectal cancer cell growth.
PTB1, hnRNAPA1 and hnRNAPA2 are targeted
by miR-124, miR-137 and miR-340
miRNA target prediction indicated that PTB1,
hnRNAPA1 and hnRNAPA2 contained binding sites of these miRNAs. The
protein level of PTB1 was significantly reduced in HCT116 cells
transfected by miR-124, miR-137 or miR-340. miR-124 or miR-340
repressed expression of hnRNAP2 protein, and hnRNAP1 was targeted
by miR-137 (Fig. 2A). Consistent
results were also found in mRNA level of target genes (Fig. 2B). To verify these transcripts
targeted by miRNAs, 3′UTR luciferase reporter plasmids were
constructed. Mutation sites were selected according to their
evolutionary conservation. Constructs with intact miRNA binding
sites of PTB1 were expressed at significantly lower levels of
luciferase activity in the presence of these miRNAs. miR-124 and
miR-340 repressed luciferase activity of wild type reporter of
hnRNAP2. Only miR-137 repressed luciferase activity of wild-type
reporter of hnRNAP1 (Fig. 2C).
Moreover, expression of PTB1, hnRNAP1 or hnRNAP2 compromised the
growth inhibition induced by these miRNAs (Fig. 2D). These data indicated that the PKM
alternative splicing proteins were required for miRNA-mediated
growth inhibition of colorectal cancer cells.
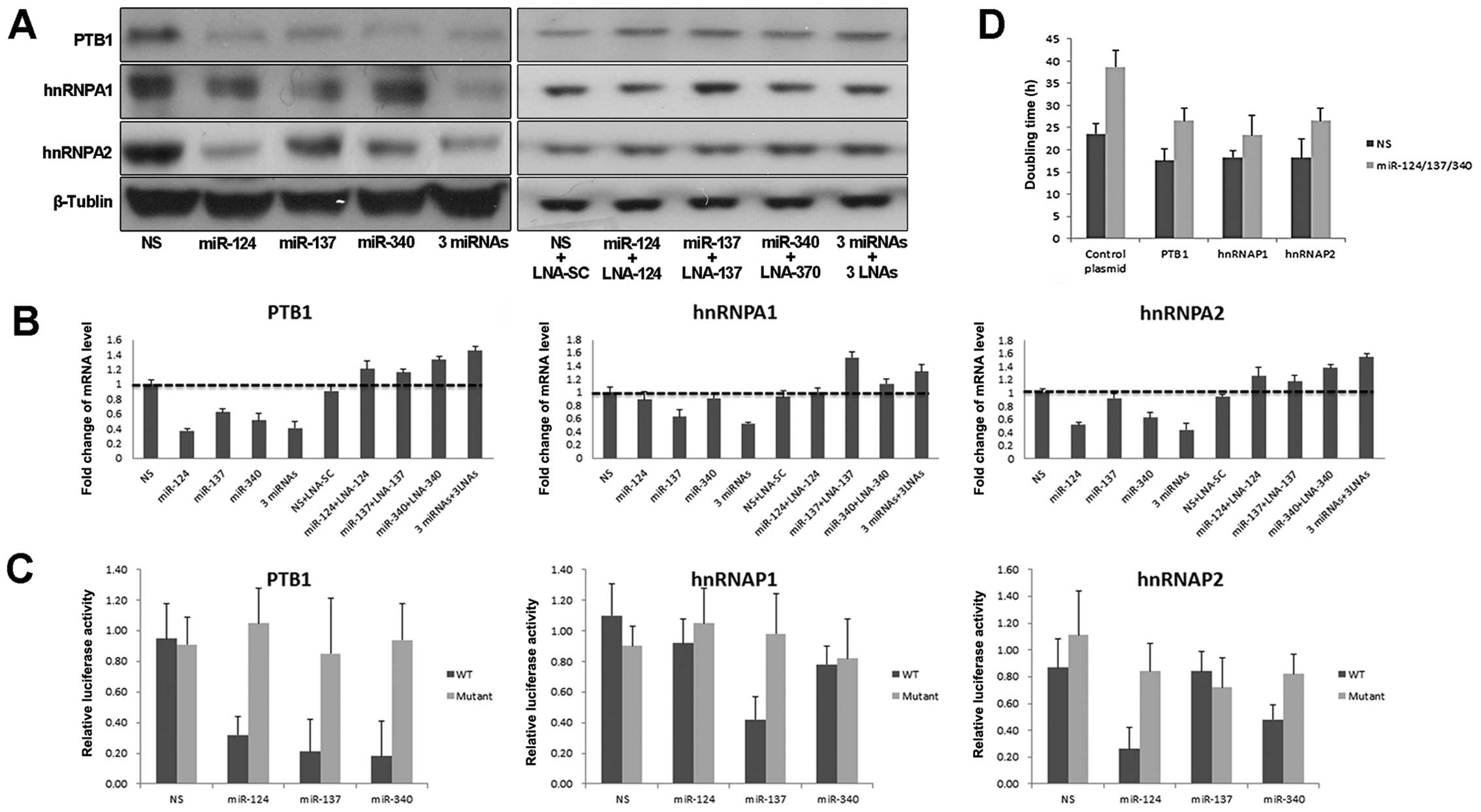 | Figure 2PTB1, hnRNAPA1 and hnRNAPA2 are
targets of miR-124, miR-137 and miR-340. (A) Immunoblotting of
miRNA stably expressing HCT116 cells with PTB1, hnRNAPA1, hnRNAPA2
and β-tublin antibodies. (B) mRNA levels of PTB1, hnRNAP1 or
hnRNAP2 were analyzed in cells with indicated treatment. Expression
value was calculated with the ΔΔCT method. (C) Relative firefly
luciferase activity derived from PTB1, hnRNAP1, hnRNAP2 3′UTR
wild-type or mutant reporter constructs was analyzed in miRNA
stably expressing HCT116 cells. All values were normalized to
renilla luciferase activity. (D) HCT116 cells were treated with
mixture of miR-124/137/340 or NS control for 24 h, then transfected
with PTB1, hnRNAP1, hnRNAP2 or control plasmid, respectively.
Doubling time was calculated by cell counting. Error bars represent
standard deviations from 3 independent experiments. 3-miRNAs
indicate combination of miR-124, miR-137 and miR-340. 3-LNAs
indicate combination of three LNA miRNA inhibitors. |
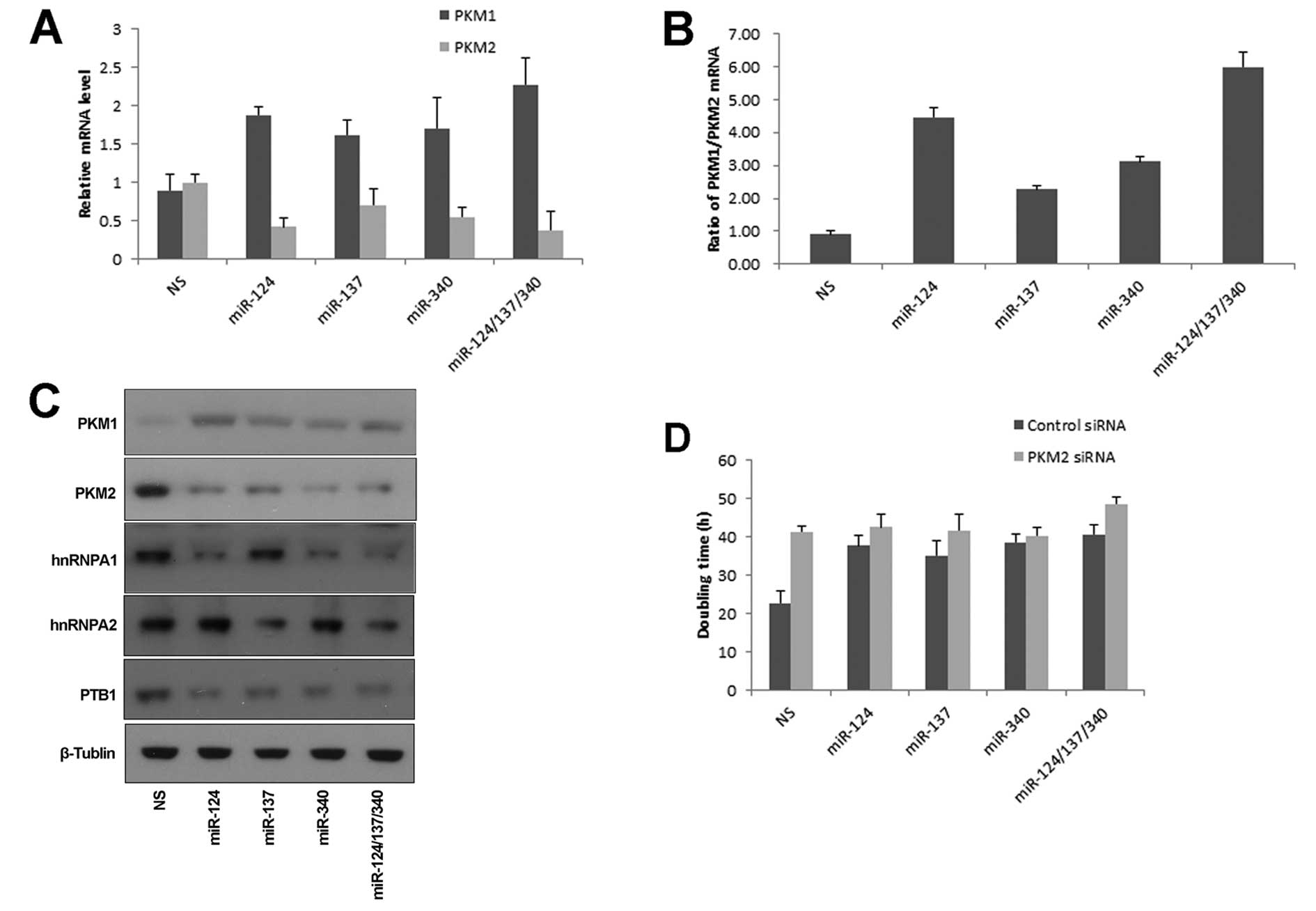
miR-124, miR-137 and miR-340 regulate PKM
alternative splicing
The two isoforms, PKM1 and PKM2, were produced from
mutually exclusive alternative splicing of PKM gene. This process
is mainly regulated by PTB1, hnRNAPA1 and hnRNAPA2 (15). Cancer cells were characterized by
switching PKM1 to PKM2 expression. PKM1 or PKM2 was assayed by
specific exon-overlapping primers, respectively. miR-124, miR-137
and miR-340 induced an increase of PKM1 mRNA, and concurrent
decrease of PKM2 mRNA (Fig. 3A).
The ratio of PKM1/PKM2 was significantly increased in cells treated
by three miRNAs simultaneously (Fig.
3B). Consistent results were obtained by analyzing their
protein levels (Fig. 3C). Next we
asked whether the switch from PKM2 to PKM1 was essential for
miRNA-mediated growth regulation. Knockdown of PKM2 by siRNA in
HCT116 cells resulted in growth inhibition, which was consistent
with published reports (10,14).
In the absence of PKM2, these miRNAs had no effect on cell growth
(Fig. 3D). These data indicated
that miR-124, miR-137 and miR-340 inhibited colorectal cancer
growth through regulating PKM alternative splicing.
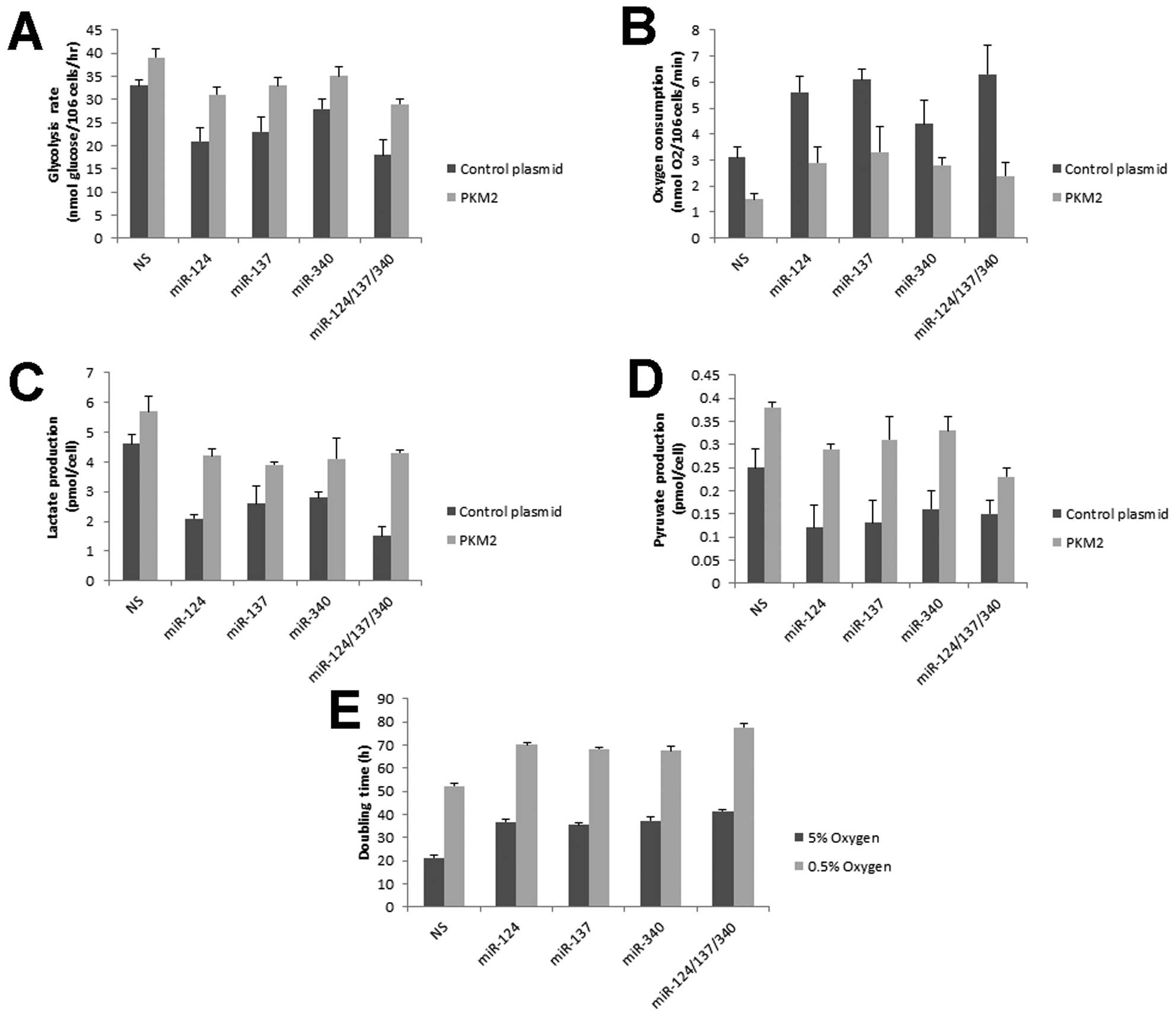
Aerobic glycolysis is inhibited by
miR-124, miR-137 and miR-340
PKM2, as a key enzyme for glycolysis, is selectively
expressed in cancer cells and is critical for cell proliferation
(9,24). Cellular glycolytic rate was measured
by conversion of 5-3H-glucose to
3H2O (14,25,26).
miR-124, miR-137 and miR-340 showed a decrease of the glycolytic
rate (Fig. 4A). Switching to PKM1
expression resulted in cells preferentially metabolizing glucose by
oxidative phosphorylation rather than glycolysis. Oxygen
consumption was increased in the presence of miR-124, miR-137 and
miR-340 (Fig. 4B). Consistently,
these miRNAs promoted cells to convert less pyruvate to lactate
(Fig. 4C). Pyruvate was also
decreased in these miRNA-treated cells (Fig. 4D) due to higher activity of PKM1
than PKM2 (12). On the other hand,
recovering PKM2 expression compromised the miRNA-mediated
glycolytic reprogramming (Fig.
4A-D). In lower oxygen condition, energy generation is strongly
dependent on glycolysis. Compared with normoxia, miRNAs induced
more apparent growth inhibition in hypoxia (Fig. 4E). Taken together, these data
suggested that glucose metabolism of colorectal cells were
regulated by miR-124, miR-137 and miR-340.
Discussion
Currently, it is still hard to predict the outcome
of colorectal cancer depending on traditional pathological
variables, including tumor size, lymph node status and tumor grade.
Survival rates are totally different among stage III patients.
After curative surgery, stage III patients experience 50–60% chance
of recurrence (27). Therefore,
analysis of genetic and epigenetic changes of stage III CRC was
very informative. In the present study, miRNA expression profiling
of stage III CRC was performed using a microarray approach.
Aberrantly expressed miRNAs associated with survival were
identified. Twenty-one miRNAs, as anti-oncomirs, were decreased
>1.5-fold in ≥5 years in CRC patients. Using miRNA target
predication databases, 3 anti-oncomirs (miR-124, miR-137 and
miR-340) were shown to target the PKM alternative splicing proteins
(PTB1/hnRNAPA1/hnRNAPA2).
Luciferase reporter and protein level analysis
demonstrated that miR-124, miR-137 and miR-340 regulated the
expression of PKM alternative splicing proteins. PTB1 was a target
of miR-124, miR-137 and miR-340. Also, miR-124 and miR-340 directly
regulated expression of hnRNAP2. HnRNAP1 was only targeted by
miR-137. miRNA targets were further validated by mutating conserved
binding sites for miRNA. PTB1 expression is under control of
miR-124, which is important for development of the nervous system
(28). In the same manner, miR-124
regulates amyloid precursor protein expression via targeting
PTB1-mediated alternative splicing (29). Trans-β-nitrostyrene (TBNS), as a
potent inhibitor of protein phosphatase PTB1, displays an
antiproliferative effect in colorectal cancer (30). Our data indicated that expression of
PTB1, hnRNAPA1 or hnRNAPA2 abolished the miRNA-induced growth
inhibition of colorectal cancer. Besides miRNA regulation, the
expression of PKM alternative splicing proteins are also regulated
by the upstream oncogene c-Myc (15). Therefore, c-Myc could regulate
cancer growth directly through PKM alternative splicing proteins or
indirectly through miR-124, miR-137 and miR-340.
Alternative splicing of PKM is involved in
determining the metabolic phenotype of mammalian cells. PKM2
expression in cancer cells has an important role in maintaining
sufficient materials for rapid proliferation. Here we found that
miR-124, miR-137 and miR-340 switched PKM expression from PKM2 to
PKM1 isoform. Ratio of PKM1/PKM2 was more dramatically increased
with miR-124/137/340 co-transfection, compared to single miRNA
treatment. Growth regulation induced by these miRNAs was dependent
on PKM2 expression. Knockdown of PKM2 compromised miRNA-induced
growth inhibition. For cancer cells, PKM2 not only supports cell
growth via Warburg effect as a metabolic enzyme, but also promotes
transactivation of HIF-1 target genes as a transcription
coactivator (31). It implied that
other mechanisms were probably also involved. PKM2, as a
transcription cofactor, might regulate miR-124, miR-137 and miR-340
expression in the transcription level.
Warburg and colleagues discovered that many tumors
prefer glycolysis, even in normal oxygen level. Higher glycolysis
and glucose consumption support energy for proliferation and
provide catabolic intermediate to fuel lipid and nucleic acid
biosynthesis. Recently, several groups have found that the
mechanism of the Warburg effect is via PKM alternative splicing
mechanism (14,15,31).
Several indicators of glycolysis, including glycolytic rate, oxygen
consumption and intermediate metabolites, showed that Warburg
effect was inhibited by miR-124, miR-137 and miR-340. These miRNAs
diverted the glucose into oxidative phosphorylation rather than
glycolysis. In this case, short or intermediate metabolites for
anabolic processes impeded the colorectal cancer cell growth. Of
note, miRNA-mediated growth inhibition was more apparent under
hypoxic condition compared with normoxic condition.
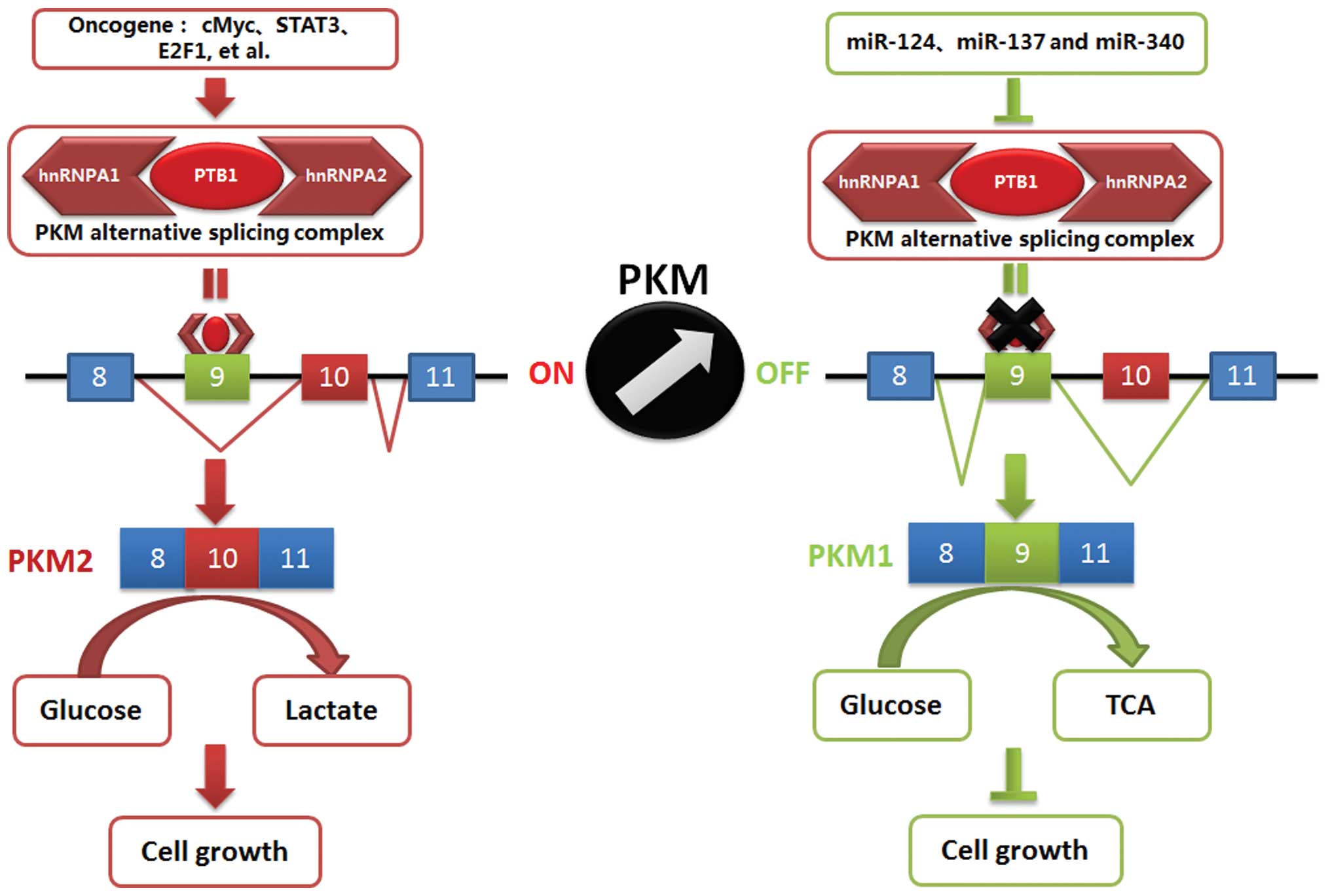
As shown in Fig. 5,
miR-124, miR-137 and miR-340 played important roles in regulating
colorectal cancer growth. The underlying mechanism was involved in
repressing the Warburg effect through switching PKM expression.
Acknowledgements
This work was supported by the National Natural
Science Foundation of China grant (no. 81001008).
Abbreviations:
|
CRC
|
colorectal cancer
|
|
miRNA
|
microRNA
|
|
PK
|
pyruvate kinase
|
|
hnRNP
|
heterogeneous nuclear
ribonucleoprotein
|
|
PTB
|
polypyrimidine tract binding
protein
|
References
|
1
|
American Cancer Society. NY: Global Cancer
Facts & Figures. 2nd edition. http://www.cancer.org/Research/CancerFactsFigures/GlobalCancerFactsFigures/index.
Accessed 2008
|
|
2
|
Malafosse R, Penna C, Sa Cunha A and
Nordlinger B: Surgical management of hepatic metastases from
colorectal malignancies. Ann Oncol. 12:887–894. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu J, Getz G, Miska EA, et al: MicroRNA
expression profiles classify human cancers. Nature. 435:834–838.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee YS and Dutta A: MicroRNAs in cancer.
Annu Rev Pathol. 4:199–227. 2009. View Article : Google Scholar
|
|
5
|
Schetter AJ, Leung SY, Sohn JJ, et al:
MicroRNA expression profiles associated with prognosis and
therapeutic outcome in colon adenocarcinoma. JAMA. 299:425–436.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gatenby RA and Gillies RJ: Why do cancers
have high aerobic glycolysis? Nat Rev Cancer. 4:891–899. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jones RG and Thompson CB: Tumor
suppressors and cell metabolism: a recipe for cancer growth. Gene
Dev. 23:537–548. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: the metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009.PubMed/NCBI
|
|
10
|
Mazurek S, Boschek CB, Hugo F and
Eigenbrodt E: Pyruvate kinase type M2 and its role in tumor growth
and spreading. Semin Cancer Biol. 15:300–308. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Noguchi T, Inoue H and Tanaka T: The M1-
and M2-type isozymes of rat pyruvate kinase are produced from the
same gene by alternative RNA splicing. J Biol Chem.
261:13807–13812. 1986.PubMed/NCBI
|
|
12
|
Christofk HR, Vander Heiden MG, Wu N,
Asara JM and Cantley LC: Pyruvate kinase M2 is a
phosphotyrosine-binding protein. Nature. 452:181–186. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vander Heiden MG, Locasale JW, Swanson KD,
et al: Evidence for an alternative glycolytic pathway in rapidly
proliferating cells. Science. 329:1492–1499. 2010.PubMed/NCBI
|
|
14
|
Christofk HR, Vander Heiden MG, Harris MH,
et al: The M2 splice isoform of pyruvate kinase is important for
cancer metabolism and tumour growth. Nature. 452:230–233. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
David CJ, Chen M, Assanah M, Canoll P and
Manley JL: HnRNP proteins controlled by c-Myc deregulate pyruvate
kinase mRNA splicing in cancer. Nature. 463:364–368. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang BD, Kline CL, Pastor DM, et al:
Prostate apoptosis response protein 4 sensitizes human colon cancer
cells to chemotherapeutic 5-FU through mediation of an NF kappaB
and microRNA network. Mol Cancer. 9:982010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Patnaik SK, Kannisto E, Knudsen S and
Yendamuri S: Evaluation of microRNA expression profiles that may
predict recurrence of localized stage I non-small cell lung cancer
after surgical resection. Cancer Res. 70:36–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Silber J, Lim DA, Petritsch C, et al:
miR-124 and miR-137 inhibit proliferation of glioblastoma
multiforme cells and induce differentiation of brain tumor stem
cells. BMC Med. 6:142008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vanderweele DJ and Rudin CM: Mammalian
target of rapamycin promotes vincristine resistance through
multiple mechanisms independent of maintained glycolytic rate. Mol
Cancer Res. 3:635–644. 2005. View Article : Google Scholar
|
|
20
|
Chen M, Zhang J and Manley JL: Turning on
a fuel switch of cancer: hnRNP proteins regulate alternative
splicing of pyruvate kinase mRNA. Cancer Res. 70:8977–8980. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, Guo H, Qian G, et al: MiR-145, a
new regulator of the DNA fragmentation factor-45 (DFF45)-mediated
apoptotic network. Mol Cancer. 9:2112010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Akao Y, Nakagawa Y, Hirata I, et al: Role
of anti-oncomirs miR-143 and -145 in human colorectal tumors.
Cancer Gene Ther. 17:398–408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gregersen LH, Jacobsen AB, Frankel LB, Wen
J, Krogh A and Lund AH: MicroRNA-145 targets YES and STAT1 in colon
cancer cells. PLoS One. 5:e88362010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaelin WG Jr and Thompson CB: Q&A:
Cancer: clues from cell metabolism. Nature. 465:562–564. 2010.
|
|
25
|
Vander Heiden MG, Plas DR, Rathmell JC,
Fox CJ, Harris MH and Thompson CB: Growth factors can influence
cell growth and survival through effects on glucose metabolism. Mol
Cell Biol. 21:5899–5912. 2001.PubMed/NCBI
|
|
26
|
Ashcroft SJ, Weerasinghe LC, Bassett JM
and Randle PJ: The pentose cycle and insulin release in mouse
pancreatic islets. Biochem J. 126:525–532. 1972.PubMed/NCBI
|
|
27
|
Center MM, Jemal A, Smith RA and Ward E:
Worldwide variations in colorectal cancer. CA Cancer J Clin.
59:366–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Makeyev EV, Zhang J, Carrasco MA and
Maniatis T: The MicroRNA miR-124 promotes neuronal differentiation
by triggering brain-specific alternative pre-mRNA splicing. Mol
Cell. 27:435–448. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Smith P, Al Hashimi A, Girard J, Delay C
and Hebert SS: In vivo regulation of amyloid precursor protein
neuronal splicing by microRNAs. J Neurochem. 116:240–247. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Werner JM, Eger K and Jurgen Steinfelder
H: Comparison of the rapid pro-apoptotic effect of
trans-beta-nitrostyrenes with delayed apoptosis induced by the
standard agent 5-fluorouracil in colon cancer cells. Apoptosis.
12:235–246. 2007. View Article : Google Scholar
|
|
31
|
Luo W, Hu H, Chang R, et al: Pyruvate
kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible
factor 1. Cell. 145:732–744. 2011. View Article : Google Scholar : PubMed/NCBI
|