Introduction
The insulin-like growth factor receptor-I (IGF-IR)
is a membrane-bound tyrosine kinase receptor that plays an
important role in tumor cell proliferation, differentiation,
apoptosis and metastasis (1,2).
IGF-IR can bind with both IGF-I and IGF-II and is overexpressed in
some cancers (3–8). The expression of IGF-IR was
constitutively low in normal hepatocytes, but highly expressed in
HCC and HCC cell lines (9).
Epidemiologic data have shown that elevated plasma IGF-I level is
linked with prostate, breast, lung and colon cancer risk (10,11).
IGF-II is frequently overexpressed in liver cancer (12–17).
The level of IGF-II is highly expressed in many human malignancies,
such as breast cancer, pediatric tumors, colon cancer and
hepatocellular carcinoma (HCC) (18,19).
Binding of IGF-II with IGF-IR has been associated with increased
tumor cell mitosis and anti-apoptosis as well as enhanced
angiogenesis (20). Therefore,
IGF-IR is an attractive antitumor target for HCC.
Mitogen-activated protein kinase (MAPK) and
phosphatidylinositol 3/-kinase/AKT are the principle pathways for
transduction of the IGF signal (21,22).
After ligand-dependent receptor autophosphorylation, IGF-IR
phosphorylates a series of adaptor proteins, such as insulin
receptor substrate-1 (IRS-1), to activate intracellular signaling
pathways. The MAPK pathway plays an important role in the mitogenic
signal elicited after IGF stimulation but may also function in cell
survival in cells overexpressing the IGF-IR (23,24).
After IGF stimulation the phosphatidylinositol 3/-kinase elicits
survival processes including the activation of the Akt and, as a
result, has been shown to protect cells from damage-induced
apoptosis (25).
The development of specific small molecule
inhibitors of IGF-IR tyrosine kinase activity was challenging
because of the high degree of homology to insulin receptor.
Recently, many neutralizing antibodies specific for IGF-IR have
been developed such as α-IR3 (26),
scFv-FC (27), CP-751,871 (28) and IMC-A12 (29). Although the antitumor effects of
these antibodies were tested for several cell types in preclinical
studies, no comprehensive research regarding the antitumorigenic
impact on HCC cells have been reported to date. Here, we generated
a murine anti-IGF-IR antibody to test our hypothesis both in
vitro and in vivo by treating HCC tumor cells with the
4F2 antibody alone and in combination with the cytotoxic
chemotherapeutic drugs. The mechanism underlying the antitumor
effect of 4F2 was also elucidated.
Materials and methods
Cell lines and culture
The human HCC cell lines SMMC-7721, 7402 and breast
cancer cell lines MCF-7, MDA-MB-468 were cultured in DMEM medium
containing 10% fetal bovine serum, NIH3T3 cells overexpressing
IGF-1R, NIH3T3/IGF-1R were constructed by our laboratory and grown
in DMEM supplemented with 10% fetal bovine serum.
Reagents
Human recombinant IGF-I and IGF-II were purchased
from Calbiochem. Annexin V-FITC apoptosis detection kit I was from
BD Biosciences. Specific antibodies against the following antigens
were used: IGF-IRβ (C20), insulin receptor (C19), IGF-IRα (3B7),
phospho-Y1158/1162/1163 IGF-IR, ERK2, AKT, phospho-S473 AKT,
phospho-p42/p44 extracellular signal-regulated kinase (ERK), bcl-2,
p27, CycineD1 (Santa Cruz Biotechnology); IRS-1, phospho-Y896,
IRS-1, Bax, Bad (Epitomics); 4G10 (Up-state Biotechnology).
Anti-mouse and anti-rabbit horseradish peroxidase conjugates were
from Amersham. Cell Counting Kit-8 was from Dojindo
Laboratories.
Generation of anti-IGF-IR antibodies
BALB/c mice were immunized i.p. with human IGF-IR
overexpressing 3T3-IGF-1R cells (5×105 cells, suspended
in 0.2 ml of PBS) with Freund’s adjuvant. Some of the animals were
boosted with 106–107 cells several times
before fusion. The splenocytes from immunized mice were isolated
and used to generate hybridoma according to standard protocols
(30). The hybridoma supernatants
were screened by ELISA for specific binding to the 3T3-IGF-IR cells
used for immunization and for the absence of binding to 3T3 cells.
Immulon-2HB plates (Dynatech) precoated with 100 μl of
phytohemagglutinin lectin (20 μg/ml; Sigma) were charged with
trypsin/EDTA treated cells (1–3×106 cells/100 μl),
centrifuged, then kept at ambient temperature for 10 min and
finally dried overnight at 37°C. The wells were blocked with 5
mg/ml BSA in PBS (blocking solution) for 1 h at 37°C, washed gently
with PBS, and then incubated with supernatants from hybridoma
clones (diluted in blocking solution) for 1 h. The wells were
washed with PBS, incubated with goat anti-mouse-IgG-Fc-antibody-HRP
conjugate (0.8 μg/ml; in blocking solution) for 1 h, washed, and
the binding was detected using ABTS/H2O2
substrate [0.5 mg/ml ABTS, 0.03% H2O2, 0.1 M
citrate buffer (pH 4.2); 405 nm]. A hybridoma supernatant was
identified, which showed strong binding to 3T3-IGF-IR cells and not
to 3T3 cells. Using protein A affinity chromatography, a murine
IgG2a antibody was isolated from the hybridoma supernatant and
designated 4F2.
RT-PCR
Total RNA was extracted from cultured cell lines
with RNA Clean following the recommendation of the manufacturer
(Hybaid, London, UK). To eliminate any possible contamination with
genomic DNA, RNAs were treated with 1 unit DNase per mg RNA for 15
min at room temperature and were then reverse transcribed into cDNA
using oligo(dt) primers and the SuperScript Preamplification-Kit
following the manufacturer’s instructions (Gibco). PCR reactions
were carried out in a total volume of 50 μl containing 400 nM of
each primer, 200 mM of each dNTP (Pharmacia, Uppsala, Sweden), 50
mM KCl, 1.5 mM MgCl2, 10 mM Tris and 1 unit
Taq-polymerase (Pharmacia). The primers for IGF-IR were
5′-GGGAATGGAGTGCTGTATG-3′ (forward) and 5′-CACAGAAGCTTCGTTGAGAA-3′
(reverse). Amplification of human β-actin served as an internal
control. The primers used were 5′-GGACCTGACTGACTACCTC-3′ (forward)
and 5′-TCATACTCCTGCTTGCTG-3′ (reverse).
Immunocytochemistry labeling and
microscopy
The cellular localization of proteins of interest
was accomplished by indirect immunocytochemistry. Briefly,
SMMC-7721, Bel-7402 and 3T3-IGF-1R cells were plated on sterile
glass cover slips in 6-well plates and allowed to attach overnight.
Cells were then rinsed twice in PBS, fixed in 4% phosphate-buffered
paraformaldehyde for 15 min, and permeabilized in acetone at −20°C
for 4 min. Following permeabilization, cells were blocked in 5%
normal goat serum-PBS for 30 min, incubated with a primary antibody
against IGF-IR for 1 h at room temperature, washed thrice in PBS,
and then incubated with goat anti-mouse secondary antibodies. Cells
were then incubated 1 h with peroxidase-anti-peroxidase mix and
rinse with buffer as before. Then cells were incubated in fresh DAB
solution (10 mg DAB + 20 μl 38% H2O2 in 20 ml
0.1 M Tris pH 7.2; 200 μl 1 M imidazole), The reaction was stopped
by washing in water when a uniform brown color first became visible
on the cells. Microscopic analyses were done using a Leica 4000B
microscope in accordance with established methods.
Immunoprecipitation and western blot
analysis
Cells were plated into 6-well culture dishes and
grown to 70–80% confluence. Monolayers were washed twice in PBS and
cultured overnight in serum-free medium. Antibody was then added in
fresh serum-free medium and incubated at 37°C for 2 h. Cells were
incubated with ligand for 15 min and then placed on ice and washed
with ice-cold PBS. For immunoprecipitation of pure IRs,
IGF-IR-depleted supernatant from an IGF-IR immunoprecipitate was
immunoprecipitated with anti-insulin antibody C19 or 4F2. To
isolate pure IGF-IRs, IR-depleted supernatant from an IR
immunoprecipitate was immunoprecipitated with anti-IGF-IR antibody
C-20 or 4F2. Immunoprecipitates bound to the protein A-agarose
beads were stripped into denaturing gel sample buffer. Lysates or
immunoprecipitates were processed for denaturing gel
electrophoresis and run on a 10% SDS-PAGE, and blotted to
nitrocellulose membrane by western blotting.
Cell proliferation/survival assays
The effect of 4F2 treatment on the growth of human
HCC cell lines SMMC-7721, Bel-7402 upon stimulation by IGF-I,
IGF-II or serum was measured using the CCK-8 assay after 3 days.
Typically, 1500–3000 cells/well were plated in a 96-well plate in
regular growth medium with serum, which was replaced with
serum-free medium the next day. After 1 day of incubation in
serum-free medium, the cells were washed gently with serum-free
medium and then incubated with 4F2 antibody and doxorubicin alone
or combination in serum-free medium for 2 h, which was followed by
the addition of IGF-II solution (or IGF-I solution or serum) to
obtain a final concentration of 5–50 μg/ml 4F2 antibody and 20
ng/ml IGF-II (or 20 ng/ml IGF-I or 2% serum). The cells were then
allowed to grow for 3 days. Of the CCK-8 solution 10 μl was added
to cells cultured for the designated time. The plates were
incubated for 1–4 h in the incubator. The resulting color was
assayed at 450 nm using a microplate absorbance reader (Tecan,
Safire II, Switzerland).
Inhibition of IGF-IR-mediated cell
signaling by 4F2
The potential of 4F2 to inhibit the IGF-I-stimulated
autophosphorylation of IGF-IR and the phosphorylation of downstream
effectors, IRS-1, Akt and ERK, was studied in SMMC-7721 cells.
Antibodies were added to cells for 2 h. Cells were then stimulated
with 20 ng/ml IGF-I or IGF-II for 15 min at 37°C, washed twice with
cold PBS containing 0.1 mmol/l sodium vanadate and lysed in lysis
buffer [50 mmol/l HEPES (pH 7.4), 150 mmol/l NaCl, 10% glycerol, 1%
Triton X-100, 1.5 mmol/l MgCl2, protease inhibitors, 2
mmol/l sodium vanadate]. Lysates were incubated on ice for 30 min
and then centrifuged at 13000 rpm for 10 min at 4°C. Protein
concentrations of the lysates were measured with BCA kit (Pierce).
Lysates were then subjected to immunoprecipitation and western blot
analysis.
Receptor degradation analysis
SMMC-7721 cells were plated in regular culture
medium followed by overnight incubation in serum-free medium.
IGF-I, IGF-II (20 ng/ml) or 4F2 was then added and cells were
incubated at 37°C for up to 24 h. Cells were washed in ice-cold
PBS, lysed in immunoprecipitation assay buffer, and quantitated by
BCA kit (Pierce). Equal amount of cell lysates was separated on 10%
SDS-PAGE, transferred to nitrocellulose filters, probed with an
anti-IGF-IR rabbit polyclonal IgG and revealed with an anti-rabbit
IgG coupled to the HRP and visualized by ECL.
Apoptosis assays
SMMC-7721 cells were treated with 4F2 or doxorubicin
in the presence of IGF-II (or serum) for 24 h. Apoptotic and
necrotic index were assessed by flow cytometry, using fluorescein
isothiocyanate (FITC) labeled Annexin V, and simultaneously with PI
stain. Cells were washed twice with ice-cold PBS and incubated for
30 min in a binding buffer (1 μg/ml PI and 1 μg/ml FITC labeled
Annexin V), respectively. FACS analysis for Annexin V and PI
staining was performed by flow cytometry. All experiments were
performed in triplicate.
Statistical analysis
Student’s t-test and One-way ANOVA analysis were
performed for continuous variables. The χ2 test or
Fisher’s exact test were used for categorical variables. The error
bars represent the standard error of the mean. Statistical
significance for all the tests, assessed by calculating P-value,
was <0.05 from two-sided tests. The statistical analyses were
performed using SAS 9.0 software (SAS Inc., Cary, NC, USA).
Results
Identification of an inhibitory
anti-IGF-IR monoclonal antibody through a rapid biological
screen
To generate monoclonal antibodies, BALB/c mice were
immunized and subsequently boosted with 3T3-IGF-IR cells that
overexpress human IGF-IR. The generated hybridoma supernatants were
screened by cell-based ELISA methods for specificity of binding and
biological activity. The hybridoma clones that bound to the
3T3-IGF-IR cells but not to the NIH-3T3 cells were selected. The
selected antibody clones were further screened by their growth,
inhibiting activity on HCC cells using CCK-8 assay (data not
shown). As the most effective inhibitory antibody clone, 4F2 was
picked out for further study.
Expression of IGF-1R in hepatocellular
carcinoma
mRNA expression of the insulin-like growth factor
receptor 1 (IGF-1R) was investigated in the human hepatoblastoma
and hepatocelluar carcinoma cell lines. Robust expression of mRNAs
specific for IGF-1R was detected in all cell lines (Fig. 1A). To evaluate the protein
expression of IGF-1R, western blot analysis was performed. Both the
IGF-1R precursor and the β-chain of the mature form of IGF-1R were
detected in HCC cells (Fig. 1B).
Immunocytochemistry results show that most IGF-IR located in the
cell membrane and cytoplasm of SMMC-7721 and Bel-7402 cells.
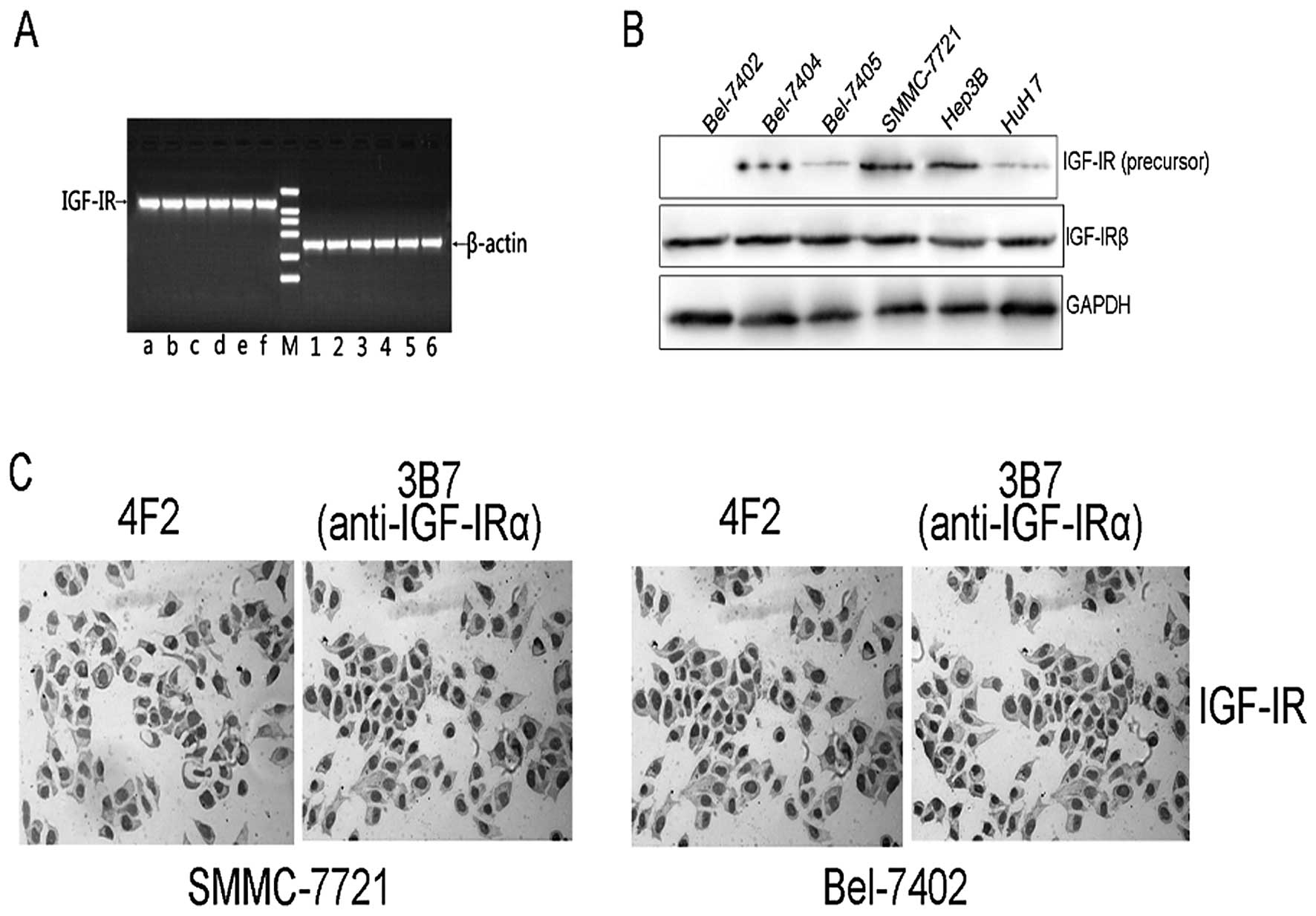 | Figure 1Expression of IGF-IR in
hepatocellular carcinoma. (A) mRNA expression of IGF-IR (amplicon
size, 541 bp) in Bel-7402 (lane a), Bel-7404 (lane b), Bel-7405
(lane c), SMMC-7721 (lane d), HuH7 (lane e) and Hep3B (lane f)
cells. The expression of the housekeeping gene β-actin (amplicon
size, 260 bp) in Bel-7402 (lane 1), Bel-7404 (lane 2), Bel-7405
(lane 3), SMMC-7721 (lane 4), HuH7 (lane 5) and Hep3B (lane 6)
cells was analyzed for standardization. M, DL2000 DNA ladder. (B)
Expression of IGF-IR and IGF-IR-precursor protein was evaluated by
western blot analysis. (C) The localization of IGF-IR protein in
SMMC-7721 and Bel-7402 cells were investigated by
immunocytochemistry detection with 4F2 or anti-IGF-IR (3B7)
antibody. |
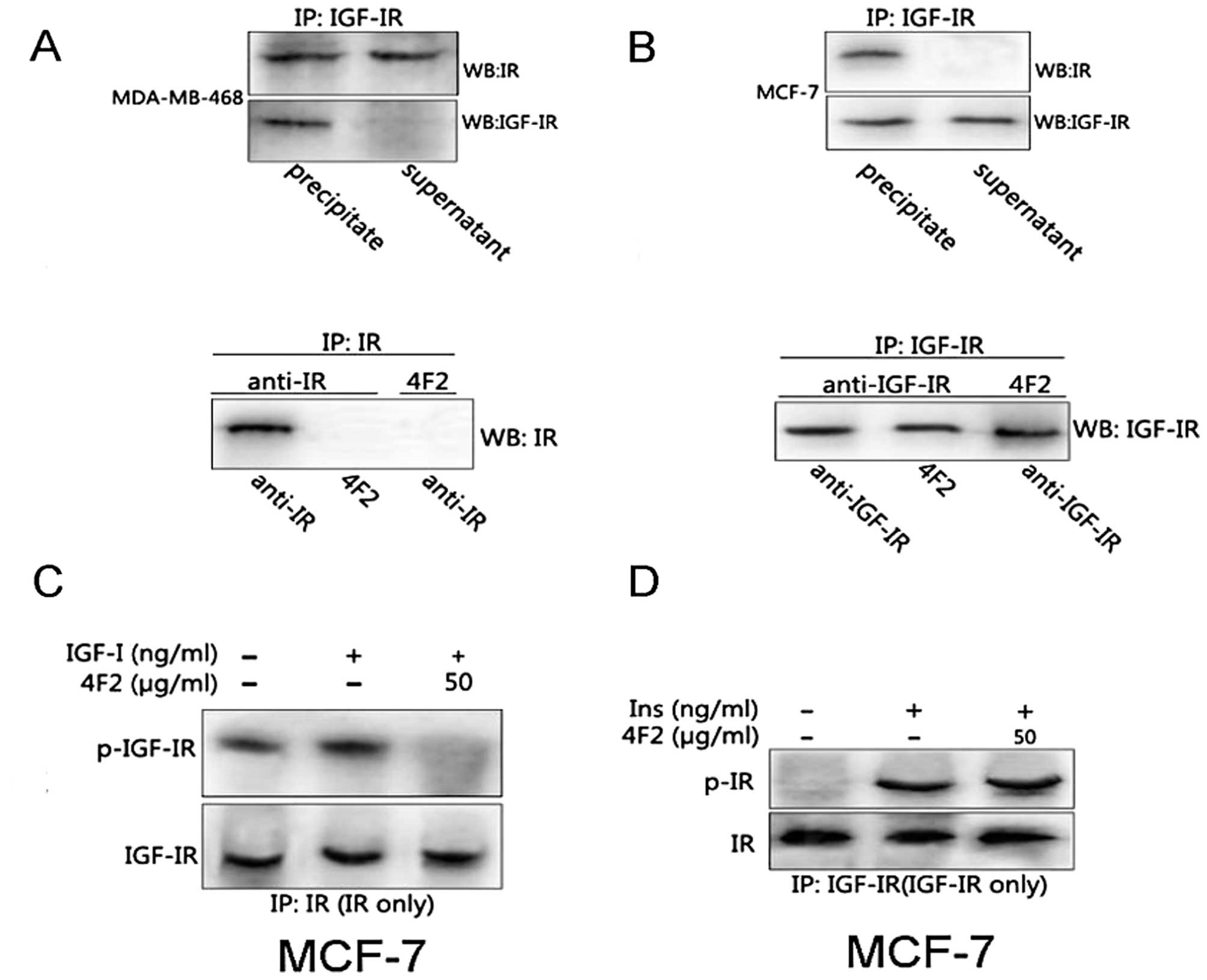
Specificity identification of 4F2
Since IGF-IR shares considerable homology with the
IR (34), it is necessary to
demonstrate the selectivity of 4F2 for the IGF-IR. We performed
successive immunoprecipitation of IGF-IR and IR in MDA-MB-468 and
MCF-7 cell lines, which possess a greater IGF-IR:IR and IR:IGF-IR
ratio, respectively (31), to
obtain purified classical IGF-IR and IR homodimers.
Immunoprecipitation and immunoblot analysis showed that 4F2 bound
selectively to IGF-IR but not to IR (Fig. 2A and B). To demonstrate selective
blocking effect on IGF-IR signaling but not IR signaling by 4F2, we
performed successive immunoprecipitation after cells were treated
with IGF-I and insulin stimulation. Immunoblot analysis showed that
4F2 completely inhibited the phosphorylation of IGF-IR but not that
of IR (Fig. 2C and D).
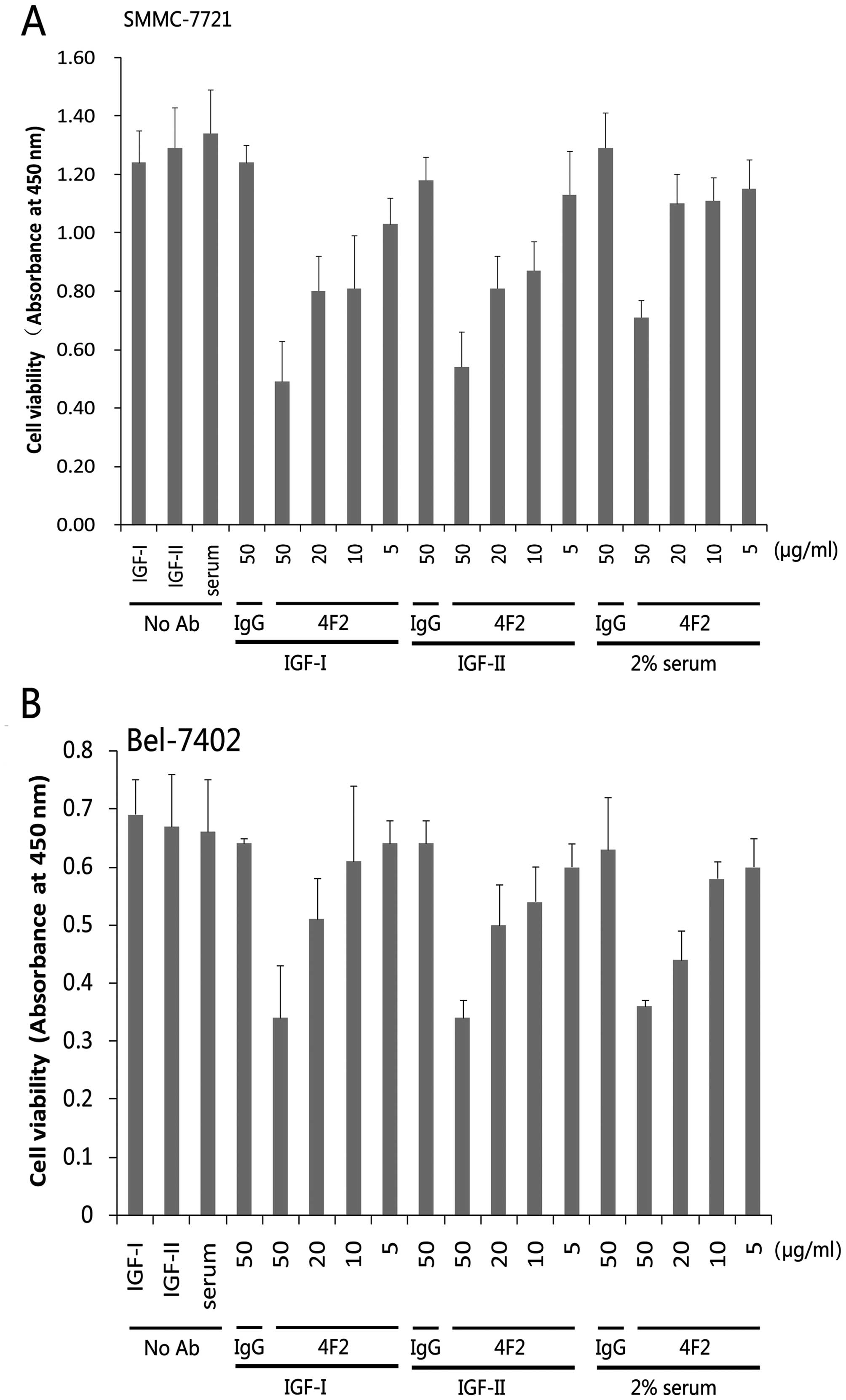
4F2 inhibits the proliferation of HCC
cells
To characterize the in vitro
antiproliferative effect of 4F2, HCC cell lines were grown in
serum-free medium with exogenously added IGF-I/IGF-II in the
presence or absence of 4F2, typically for 3 days, and the cell
viability was then measured by CCK-8 assay. We found a
dose-dependent reduction in the number of viable cells (Fig. 3). In similar experiments based on
the CCK-8 assay after 3 days of growth, 4F2 treatment caused
dose-dependent inhibition of the serum (2%)-stimulated
proliferation and survival of SMMC-7721 and Bel-7402 cells,
implicating the essential role of IGF-I as a growth factor present
in serum (Fig. 3).
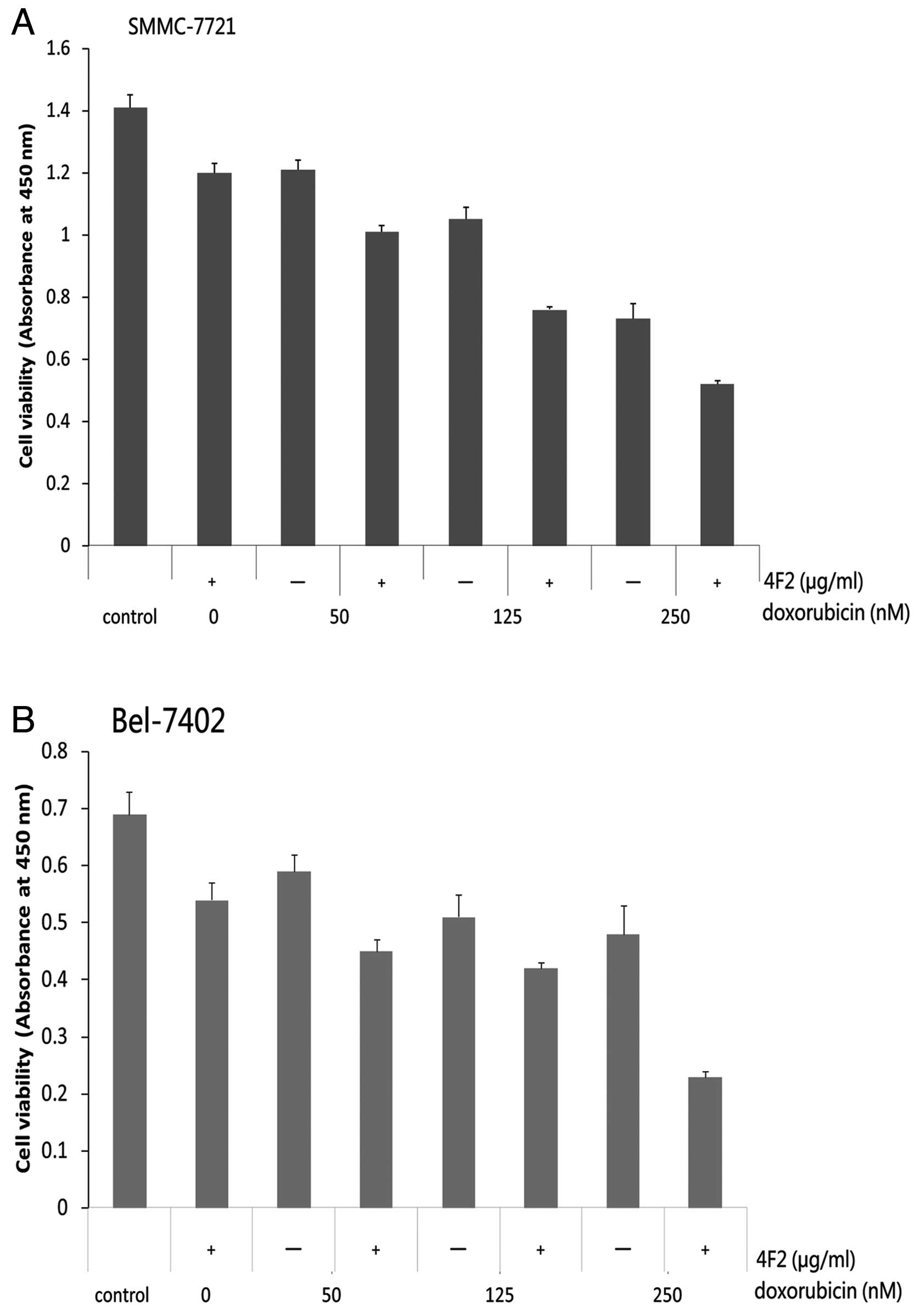
Antineoplastic potency of 4F2 in
combination with the doxorubicin
Several studies have reported that combinations of
doxorubicin and small molecular inhibitor of IGF-IR resulted in
additive growth inhibitory effects on HCC cells (36). We studied possible (over-) additive
antineoplastic effects of 4F2 plus doxorubicin in HCC cells. Cells
were treated with 4F2 (20 μg/ml) alone or in combination with
rising concentrations of the doxorubicin (50–250 nM) for 72 h. Upon
treatment with doxorubicin alone, a dose-dependent growth
inhibition was observed after 72 h in SMMC-7721 (Fig. 4A) and Bel-7402 cells (Fig. 4B), 250 nM of doxorubicin caused a 51
and 34% inhibition in SMMC-7721 and Bel-7402 cells, respectively
(P<0.01). The antineoplastic effects of doxorubicin increased
when the doxorubicin was combined, approximately 69 and 58%
inhibition was observed when 20 μg/ml 4F2 was combined with 250 nM
doxorubicin in SMMC-7721 and Bel-7402 cells (P<0.01).
Combinatorial treatment with 4F2 and doxorubicin resulted in
significantly higher inhibitory effects on SMMC-7721 and Bel-7402
cells than treatment with 4F2 (P<0.001) or doxorubicin
(P<0.05) alone.
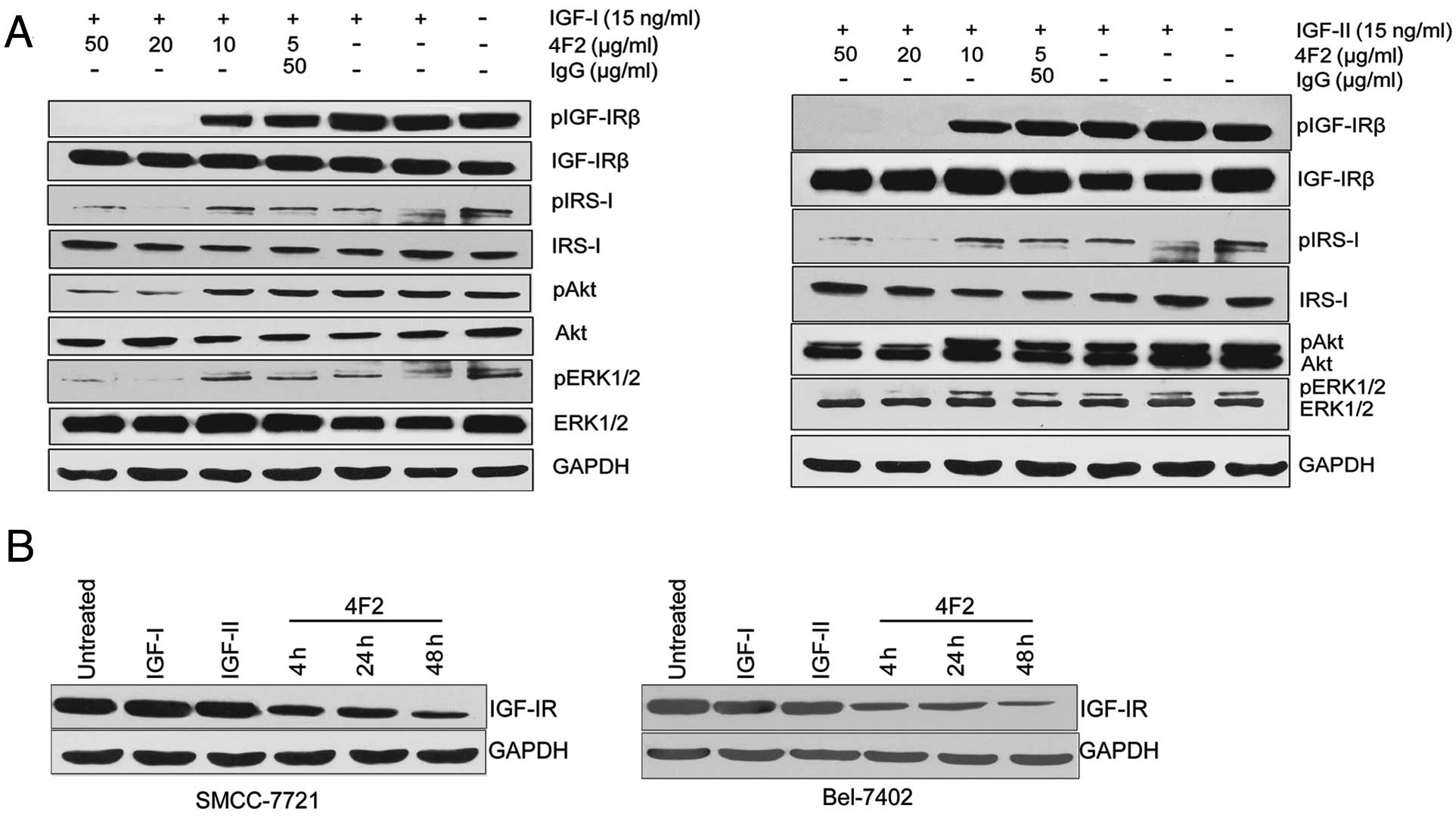
The anti-IGF-IR therapeutic antibody 4F2
blocks IGF-induced IGF-IR signaling and downregulates the
expression of IGF-IR in HCC cells
The effect of 4F2 on the IGF-I-stimulated or
IGF-II-stimulated activation of IGF-IR and the downstream signaling
were then measured. Serum-starved SMMC-7721 cells were treated for
2 h with various concentrations of 4F2 and then stimulated with
IGF-I or IGF-II in serum-free medium for 15 min. The cells were
then lysed and the lysates were analyzed for the phosphorylation
levels of effector proteins in the IGF-IR-signaling pathway,
including IGF-IR, IRS-1, Akt and MAPK. At a concentration of 50
μg/ml, 4F2 completely inhibited both IGF-I-induced and
IGF-II-induced phosphorylation of IGF-IR (Fig. 5A). In addition, at concentrations of
20 and 50 μg/ml, 4F2 inhibited IGF-I-induced and IGF-II-induced
phosphorylation of IRS-I, Akt and MAPK (Fig. 5A). To evaluate the potential
downregulation of IGF-IR by 4F2 treatment, the level of IGF-IRβ
chain in 4F2-treated SMMC-7721 cells was assessed by western
blotting. No detectable decrease in the amount of IGF-IRβ chain was
seen during the first 4 h of treatment with 4F2 in Bel-7402 and
SMMC-7721 cells, but a modest downregulation of the receptor was
observed after 48-h treatment (Fig.
5B). In contrast, no downregulation of IGF-IRβ was observed
upon treatment with the ligand IGF-I and IGF-II.
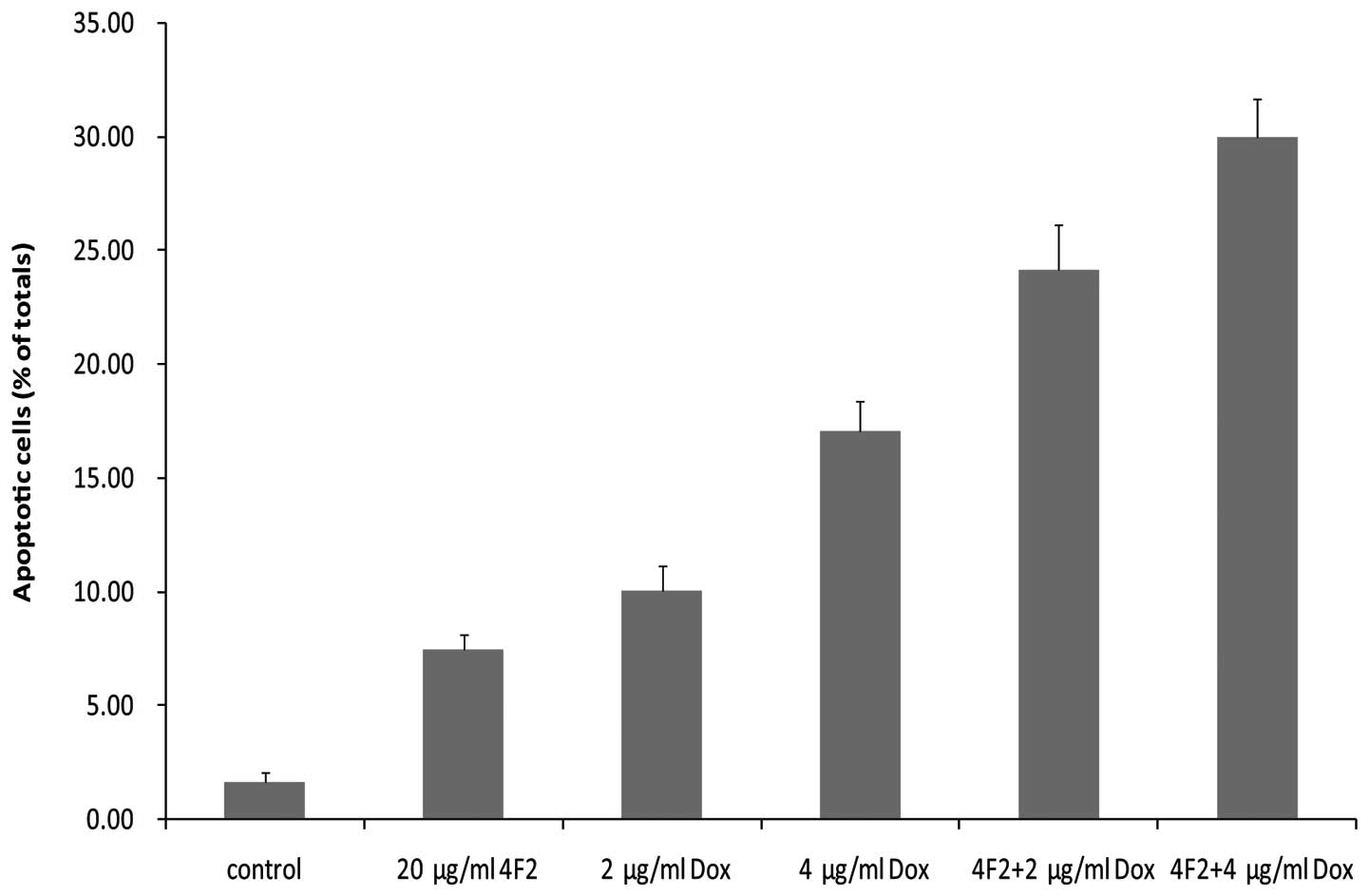
4F2 enhances the induction of apoptosis
by doxorubicin
The CCK-8 assays above show that 4F2 combined with
doxorubicin additively inhibits HCC cells growth but does not
characterize the mechanism of cell death. To examine whether 4F2
enhanced cytotoxicity of doxorubicin to cancer cells was mediated
through increased apoptosis, Annexin V/PI staining was used to
examine the apoptosis in SMMC-7721 cells. From the statistical
analysis of cytometry data, we found that doxorubicin combined with
20 μg/ml 4F2 induced more apoptotic cells (30.4±1.56%, P<0.01)
than doxorubicin alone (17.1±1.25%, Fig. 6). In order to determine whether the
increased cytotoxicity reflected induction of apoptosis, Annexin V
binding and propidium iodide staining were carried out using the
Annexin V-FITC Apoptosis Detection Kit 1 (BD Pharmingen) and flow
cytometry. As show in Fig. 6,
combination of 20 μg/ml 4F2 and doxorubicin resulted in a nearly
2-fold increase in apoptosis relative to control. The combination
seemed to result in additivity of the rates of apoptosis induced by
each single drug at the concentrations and time point tested.
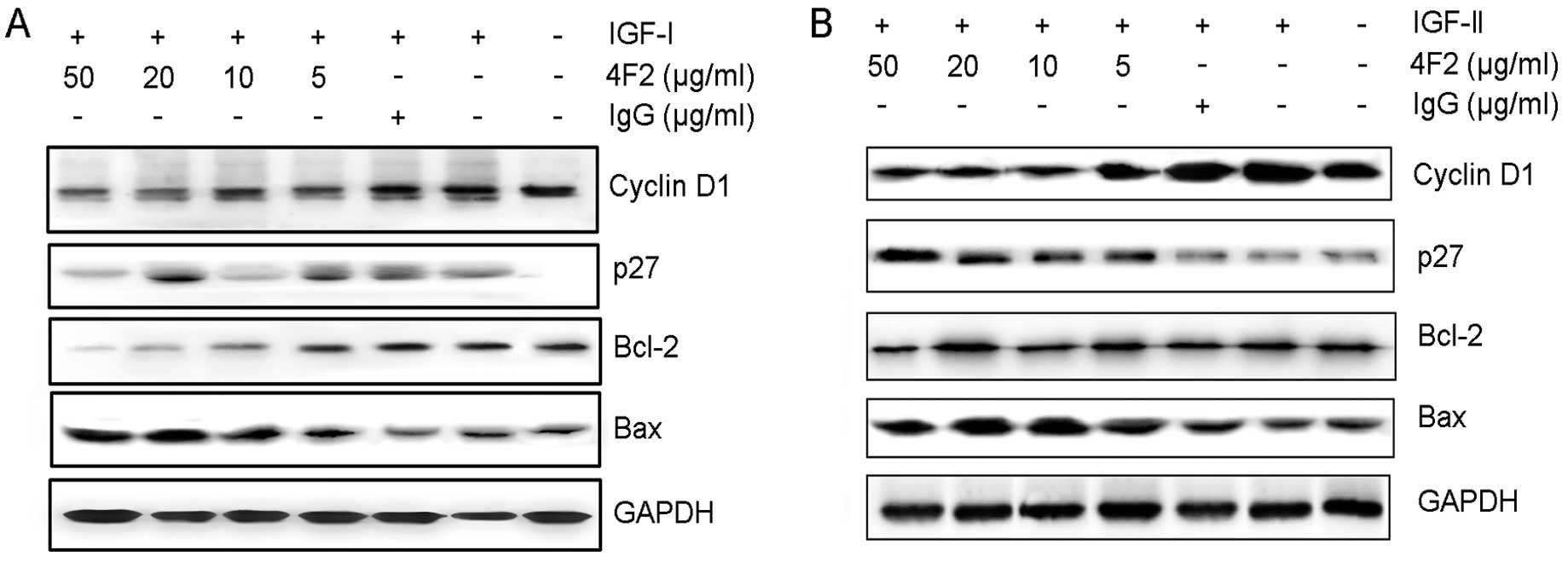
4F2 regulates expression of
apoptosis-specific and cell cycle regulating proteins
To elucidate the signaling pathways modulated by 4F2
inhibition in HCC cells, we investigated changes in the expression
of apoptosis-specific proteins and cell cycle regulating proteins.
Cells were incubated with IGF-I/II in the presence or absence of
4F2 for 48 h. 4F2 dose-dependently downregulated anti-apoptotic
Bcl-2 expression while upregulated pro-apoptotic Bax expression.
Moreover, 4F2 downregulated cyclin D1 expression while upregulated
p27 expression (Fig. 7).
Discussion
Several reports indicate that IGF-IRs are expressed
frequently in HCC (32), most
likely leading to the aggressive growth characteristics of tumors.
Consequently, the IGF-IR is a promising target for innovative
treatment strategies in HCC. In the present study, we have
generated a murine anti-IGF-1R antibody 4F2 which specifically
recognizes IGF-IR but not insulin receptor. This selectivity may be
an advantage over potential IGF-IR kinase inhibitors which may
partly inhibit insulin receptor and induce hyperglycemia. By
blocking IGF-IR activation, 4F2 can effectively inhibit its signal
transduction. The relative contribution of these effects to the
anticancer activity of 4F2 in different settings (including in
vivo models) remains to be determined. To elucidate the
underlying mechanisms of the 4F2 antiproliferative activity on HCC
cells, the expression of apopotosis and cell cycle related proteins
were also examined. Compared with untreated cells, 4F2
dose-dependently downregulated anti-apoptotic Bcl-2, pro-apoptotic
Bax was upregulated. Moreover, cell cycle promoting cyclin D1 was
downregulated, while the cell cycle arresting p27 was upregulated
by 4F2.
Since IGF-IR signaling has been shown to prevent
tumor cells from the cytotoxic effects of chemotherapy and may play
an important role in tumor cell drug resistance (33–35),
we supposed that anti-IGF-IR antibody 4F2 may be combined with
chemical drug for treating HCC. We then studied whether inhibition
of IGF signaling alters chemosensitivity, the results showed that
combined treatments using 4F2 with conventional cytotoxic agent
doxorubicin, significantly increase the cytotoxic effects,
suggesting that this combination might offer an alternative
strategy for treating HCC. As we all known, chemotherapeutic drugs
inhibit cancer cell growth mainly by inducing cell apoptosis. We
also know that activated IGF-IR is a powerful inhibitor of
apoptosis (36,37). For instance, Dunn et al
(38) reported that IGF-I could
induce a 20–40% increase in cell survival of breast cancer cells
treated with anticancer drugs. A study have proved that when the
novel IGF-1R tyrosine kinase inhibitor NVP-AEW541 was combined with
cytotoxic chemotherapy, additive antiproliferative effects were
observed in HCC, Furthermore, combinatorial treatment with IGF-1R
tyrosine kinase inhibitor NVP-AEW541 and cytotoxic drugs impose
additive antiproliferative effects on HCC. However, the development
of specific small molecule inhibitors of IGF-IR tyrosine kinase
activity was challenging because of high degree of homology to
insulin receptor. Because of the high specificity of 4F2 in
recognizing IGF-1R, we think it may be a new substitute of TKI in
combination with chemotherapeutic drug for treating HCC. We
observed that 4F2 actually sensitizes SMMC-7721 cell lines to
doxorubicin, mainly by inducing apoptosis as shown in Fig. 6.
IGF-IR is a promising anticancer therapy target
because of its defined role in establishing and maintaining the
cancer phenotype. Our study provides first evidence that the growth
of human hepatocellular SMMC-7721 cells can be potently suppressed
by IGF-IR inhibition with anti-IGF-1R antibodies. Evidence
suggesting a link between IGF-IR signaling and resistance to
cytotoxic therapies provides rationale for combining IGF-IR
inhibitors with chemotherapy. The present study is the first
published report showing a favorable interaction between
chemotherapy and IGF-IR blockade with an anti-IGF-IR monoclonal
antibody. Anti-IGF-IR antibodies are therefore promising agents as
monotherapy or in combination therapy for HCC.
Acknowledgements
This study is supported by the grant of Shandong
Tackle Key Problems in Science and Technology (2010GSF10245);
Shandong Excellent Young Scientist Research Award Fund Project
(BS2010YY013).
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
IGF-IR
|
insulin-like growth factor-I
receptor
|
|
MAPK
|
mitogen-activated protein kinase
|
|
IRS-1
|
insulin receptor substrate-1
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
FITC
|
fluorescein isothiocyanate
|
References
|
1
|
Baserga R, Peruzzi F and Reiss K: The
IGF-1 receptor in cancer biology. Int J Cancer. 107:873–877. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pollak M: The insulin and insulin-like
growth factor receptor family in neoplasia: an update. Nat Rev
Cancer. 12:159–169. 2012.PubMed/NCBI
|
|
3
|
Kaiser U, Schardt C, Brandscheidt D,
Wollmer E and Havemann K: Expression of insulin-like growth factor
receptors I and II in normal human lung and in lung cancer. J
Cancer Res Clin Oncol. 119:665–668. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parker AS, Cheville JC, Janney CA and
Cerhan JR: High expression levels of insulin-like growth factor-I
receptor predict poor survival among women with clear-cell renal
cell carcinomas. Hum Pathol. 33:801–805. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pekonen F, Nyman T, Ilvesmaki V and
Partanen S: Insulin-like growth factor binding proteins in human
breast cancer tissue. Cancer Res. 52:5204–5207. 1992.PubMed/NCBI
|
|
6
|
Resnik JL, Reichart DB, Huey K, Webster NJ
and Seely BL: Elevated insulin-like growth factor I receptor
autophosphorylation and kinase activity in human breast cancer.
Cancer Res. 58:1159–1164. 1998.PubMed/NCBI
|
|
7
|
Steller MA, Delgado CH, Bartels CJ,
Woodworth CD and Zou Z: Overexpression of the insulin-like growth
factor-1 receptor and autocrine stimulation in human cervical
cancer cells. Cancer Res. 56:1761–1765. 1996.PubMed/NCBI
|
|
8
|
Werner H, Re GG, Drummond IA, et al:
Increased expression of the insulin-like growth factor I receptor
gene, IGF1R, in Wilms tumor is correlated with modulation of
IGF1R promoter activity by the WT1 Wilms tumor gene
product. Proc Natl Acad Sci USA. 90:5828–5832. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Breuhahn K and Schirmacher P: Reactivation
of the insulin-like growth factor-II signaling pathway in human
hepatocellular carcinoma. World J Gastroenterol. 14:1690–1698.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chan JM, Stampfer MJ, Giovannucci E, et
al: Plasma insulin-like growth factor-I and prostate cancer risk: a
prospective study. Science. 279:563–566. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vadgama JV, Wu Y, Datta G, Khan H and
Chillar R: Plasma insulin-like growth factor-I and serum
IGF-binding protein 3 can be associated with the progression of
breast cancer, and predict the risk of recurrence and the
probability of survival in African-American and Hispanic women.
Oncology. 57:330–340. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boulle N, Logie A, Gicquel C, Perin L and
Le Bouc Y: Increased levels of insulin-like growth factor II
(IGF-II) and IGF-binding protein-2 are associated with malignancy
in sporadic adrenocortical tumors. J Clin Endocrinol Metab.
83:1713–1720. 1998.PubMed/NCBI
|
|
13
|
Martin DM, Singleton JR, Meghani MA and
Feldman EL: IGF receptor function and regulation in autocrine human
neuroblastoma cell growth. Regul Pept. 48:225–232. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reeve AE, Eccles MR, Wilkins RJ, Bell GI
and Millow LJ: Expression of insulin-like growth factor-II
transcripts in Wilms’ tumour. Nature. 317:258–260. 1985.
|
|
15
|
Sohda T, Iwata K, Soejima H, Kamimura S,
Shijo H and Yun K: In situ detection of insulin-like growth factor
II (IGF2) and H19 gene expression in hepatocellular
carcinoma. J Hum Genet. 43:49–53. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takeda S, Kondo M, Kumada T, et al:
Allelic-expression imbalance of the insulin-like growth factor 2
gene in hepatocellular carcinoma and underlying disease. Oncogene.
12:1589–1592. 1996.PubMed/NCBI
|
|
17
|
Zhang L, Zhou W, Velculescu VE, et al:
Gene expression profiles in normal and cancer cells. Science.
276:1268–1272. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Breuhahn K, Longerich T and Schirmacher P:
Dysregulation of growth factor signaling in human hepatocellular
carcinoma. Oncogene. 25:3787–3800. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wilkin F, Gagne N, Paquette J, Oligny LL
and Deal C: Pediatric adrenocortical tumors: molecular events
leading to insulin-like growth factor II gene overexpression. J
Clin Endocrinol Metab. 85:2048–2056. 2000.PubMed/NCBI
|
|
20
|
Denley A, Brierley GV, Carroll JM, et al:
Differential activation of insulin receptor isoforms by
insulin-like growth factors is determined by the C domain.
Endocrinology. 147:1029–1036. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Adams TE, Epa VC, Garrett TP and Ward CW:
Structure and function of the type 1 insulin-like growth factor
receptor. Cell Mol Life Sci. 57:1050–1093. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Perer ES, Madan AK, Shurin A, et al:
Insulin-like growth factor I receptor antagonism augments response
to chemoradiation therapy in colon cancer cells. J Surg Res.
94:1–5. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Burtrum D, Zhu Z, Lu D, et al: A fully
human monoclonal antibody to the insulin-like growth factor I
receptor blocks ligand-dependent signaling and inhibits human tumor
growth in vivo. Cancer Res. 63:8912–8921. 2003.PubMed/NCBI
|
|
24
|
Heron-Milhavet L, Karas M, Goldsmith CM,
Baum BJ and LeRoith D: Insulin-like growth factor-I (IGF-I)
receptor activation rescues UV-damaged cells through a p38
signaling pathway. Potential role of the IGF-I receptor in DNA
repair. J Biol Chem. 276:18185–18192. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kulik G, Klippel A and Weber MJ:
Antiapoptotic signalling by the insulin-like growth factor I
receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol.
17:1595–1606. 1997.PubMed/NCBI
|
|
26
|
Jacobs S, Cook S, Svoboda ME and van Wyk
JJ: Interaction of the monoclonal antibodies αIR-1 and
αIR-3 with insulin and somatomedin-C receptors.
Endocrinology. 118:223–226. 1986.PubMed/NCBI
|
|
27
|
Sachdev D, Li SL, Hartell JS,
Fujita-Yamaguchi Y, Miller JS and Yee D: A chimeric humanized
single-chain antibody against the type I insulin-like growth factor
(IGF) receptor renders breast cancer cells refractory to the
mitogenic effects of IGF-I. Cancer Res. 63:627–635. 2003.
|
|
28
|
Cohen BD, Baker DA, Soderstrom C, et al:
Combination therapy enhances the inhibition of tumor growth with
the fully human anti-type 1 insulin-like growth factor receptor
monoclonal antibody CP-751,871. Clin Cancer Res. 11:2063–2073.
2005. View Article : Google Scholar
|
|
29
|
Lu D, Zhang H, Koo H, et al: A fully human
recombinant IgG-like bispecific antibody to both the epidermal
growth factor receptor and the insulin-like growth factor receptor
for enhanced antitumor activity. J Biol Chem. 280:19665–19672.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Langone JJ: Applications of immobilized
protein A in immunochemical techniques. J Immunol Methods.
55:277–296. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pandini G, Vigneri R, Costantino A, et al:
Insulin and insulin-like growth factor-I (IGF-I) receptor
overexpression in breast cancers leads to insulin/IGF-I hybrid
receptor overexpression: evidence for a second mechanism of IGF-I
signaling. Clin Cancer Res. 5:1935–1944. 1999.
|
|
32
|
Scharf JG and Braulke T: The role of the
IGF axis in hepatocarcinogenesis. Horm Metab Res. 35:685–693. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Benini S, Manara MC, Baldini N, et al:
Inhibition of insulin-like growth factor I receptor increases the
antitumor activity of doxorubicin and vincristine against Ewing’s
sarcoma cells. Clin Cancer Res. 7:1790–1797. 2001.PubMed/NCBI
|
|
34
|
Gooch JL, Tang Y, Ricono JM and Abboud HE:
Insulin-like growth factor-I induces renal cell hypertrophy via a
calcineurin-dependent mechanism. J Biol Chem. 276:42492–42500.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Turner BC, Haffty BG, Narayanan L, et al:
Insulin-like growth factor-I receptor overexpression mediates
cellular radioresistance and local breast cancer recurrence after
lumpectomy and radiation. Cancer Res. 57:3079–3083. 1997.
|
|
36
|
Nakamura S, Watanabe H, Miura M and Sasaki
T: Effect of the insulin-like growth factor I receptor on ionizing
radiation-induced cell death in mouse embryo fibroblasts. Exp Cell
Res. 235:287–294. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sell C, Baserga R and Rubin R:
Insulin-like growth factor I (IGF-I) and the IGF-I receptor prevent
etoposide-induced apoptosis. Cancer Res. 55:303–306.
1995.PubMed/NCBI
|
|
38
|
Dunn SE, Hardman RA, Kari FW and Barrett
JC: Insulin-like growth factor 1 (IGF-1) alters drug sensitivity of
HBL100 human breast cancer cells by inhibition of apoptosis induced
by diverse anticancer drugs. Cancer Res. 57:2687–2693.
1997.PubMed/NCBI
|