Introduction
Malignant glioma is one of the most common and fatal
types of brain tumors in humans, which are notoriously difficult to
treat and recurrences arise virtually in every case (1,2).
Despite new biological insights and advances in therapy, the
prognosis of patients with gliomas still remains poor in the last
four decades (3,4). Recent studies have developed a novel,
molecularly-targeted, multimodal therapeutic approach with the use
of antisense ‘antagomir’ oligonucleotides (5), which may enable development of novel
cancer therapies.
miRNAs are small, evolutionary conserved RNA
molecules that control gene expression through binding to the seed
sequence at the 3′UTR of target mRNAs, resulting in translational
repression or mRNA degradation (6,7). They
are involved in diverse biological processes, such as development,
differentiation, cell proliferation and apoptosis (8,9).
Recent studies have shown that the expression of many miRNAs are
altered in various human tumors and some miRNAs may function as
oncogenes or tumor suppressor genes (10–13).
Recent studies showed that miR-92a, a member of miR-17-92 cluster
was frequent deregulated in a variety of cancer types (14–17).
So far, there are few reports on miR-92a in glioma.
In our study, we analyzed the expression of miR-92a
both in glioma cell lines and samples. We demonstrated that
downregulation of miR-92a significantly induced apoptosis. In
addition, we demonstrated that Bim was the direct target of miR-92a
and played an important role in the miR-92a induced apoptosis.
Furthermore, we also showed that miR-92a inversely correlated with
Bim expression in glioma tissues. These results identify a critical
role for miR-92a in regulation of proliferation and apoptosis in
glioma, suggesting that miR-92a could be critical therapeutic
target for glioma intervention.
Materials and methods
Cell culture and culture conditions
The human U251, U87, LN229, U87 and A172
glioblastoma cell lines were purchased from the Institute of
Biochemistry and Cell Biology, Chinese Academy of Science. All
cells were maintained in a 37°C, 5% CO2 incubator in
Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Carlsbad, CA,
USA) supplemented with 10% fetal bovine serum (Invitrogen,
Carlsbad, CA, USA).
Human glioma samples
Human glioma samples were obtained from the
Department of Neurosurgery, Sir Run Run Shaw Hospital, Medical
College, Zhejiang University after informed consent from adult
patients diagnosed with glioblastoma, freshly resected during
surgery and immediately frozen in liquid nitrogen for subsequent
total RNA extraction.
RNA extraction and quantitative real-time
PCR
Total RNA was isolated from cultured cells, human
glioma specimens, or NAT (normal adjacent tissue)s using TRIzol®
reagent (Invitrogen) according to the manufacturer’s instructions.
Quantitative real-time polymerase chain reaction (qRT-PCR) was
performed in triplicate in ABI 7500HT fast real-time PCR system
(Applied Biosystems, Foster City, CA, USA) and normalized with U6
and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) endogenous
control. Total RNA from NATs was used as a control. miR-92a levels
were measured with the TaqMan microRNA assay kit, and endogenous
mRNA levels of Bim were detected using SYBR-Green PCR Master Mix
kit in accordance with the manufacturer’s instructions (Applied
Biosystems).
Oligonucleotide, Bim expression plasmid
synthesis and transfection
miR-92a: 5′-UAUUGCACUUGUCCCGGCCUGU-3′, AS-miR-92a:
5′-ACAGGCCGGGACAAGUGCAAUA-3′ and scrambled control oligonucleotides
were purchased from Shanghai GenePharma Co., Ltd. (Shanghai,
China). Bim expression plasmid was preserved in our laboratory.
miRNA sequences/Bim expression plasmid were transfected into
cultured cells using Lipofectamine 2000 reagent (Invitrogen)
following the manufacturer’s instructions.
Cell growth assays
The MTT assay was used to determine relative cell
growth as follows. U251 and U87 cells were plated at 104
cells/well in 96-well plates with 6 replicate wells for each
condition, transfected with oligonucleotides, and assayed 48-h
post-transfection. Cell growth assay was performed by MTT (Sigma,
St. Louis, MO, USA) as previously described (18). The cell viability was determined at
540-nm absorbance using an enzyme-linked immunosorbent assay plate
reader. All data points represent the mean of a minimum of
6-wells.
Apoptosis assay
Forty-eight hours after transfection, apoptosis in
cultured cells was evaluated with Annexin V labeling, caspase-3/7
activity and mitochondrial membrane potential. For the Annexin V
assay, an Annexin V-FITC labeled Apoptosis Detection kit (Abcam)
was used according to the manufacturer’s protocol. Caspase-3/7
activity was measured using caspase-Glo-3/7 reagent (Promega,
Madison, WI, USA). Mitochondrial membrane potential was determined
with cationic dye JC-1
(5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzi-midazolylcarbocyanine-chloride/C25H27Cl3N4)
staining (19). Briefly the cells
were harvested and first stained with PI. Following wash twice with
PBS, the cells were incubated with 10 mg/ml JC-1 for 20 min at room
temperature and then analyzed with FACSCalibur to detect green
fluorescence at excitation/emission wavelengths of 485/530 nm and
red fluorescence at excitation/emission wavelengths of 485/590 nm.
TUNEL assay was used to detect the apoptosis in tumor specimens and
was performed as previously described (20).
Western blot analysis, miRNA locked
nucleic acid (LNA) in situ hybridization, immunohistochemistry and
luciferase reporter assay
Western blot analysis, miRNA-LNA in situ
hybridization and immunohistochemistry were performed as previously
described (21). For reporter
assay, the pGL3-WT-Bim-3′UTR-Luc reporter (WT Bim) was created by
the ligation of Bim 3′UTR PCR products into the XbaI site of
the pGL3 control vector (Promega). The pGL3-MUT-Bim-3′UTR-Luc
reporter (MUT Bim) was generated from pGL3-WT-Bim-3′UTR-Luc by
replacing the binding site of miR-92a with restriction enzyme
cutting site CGGATCCG. For the reporter assay, cells were cultured
in 96-well plates and transfected with WT Bim/MUT Bim and
AS-miR-92a. Luciferase activity was measured 48 h after
transfection with the Dual-luciferase reporter assay system. The
Renilla luciferase activity was utilized as an internal
control.
Nude mouse tumor xenograft model and
AS-miR-92a treatment
U251 glioma cells were subcutaneously injected to
5-week-old female nude mice (Cancer Institute of The Chinese
Academy of Medical Science). When the tumor volume reached 100
mm3, the mice were randomly divided into two groups (10
mice per group) which were treated with 200 pmol scramble oligo or
AS-miR-92a in 10 μl Lipofectamine 2000 through local injection of
xenograft tumor in multiple sites. The treatment was performed once
every 2 days for 14 days. The tumor volume was measured with a
caliper every 2 days, using the formula: volume = length ×
width2/2.
Statistical analysis
All tests were done using SPSS Graduate Pack 11.0
statistical software (SPSS Inc., Chicago, IL, USA). Statistical
evaluation for data analysis was determined by one-way ANOVAs, the
Student’s t-test and the Chi-square test. Differences with
P<0.05 were considered statistically significant.
Results
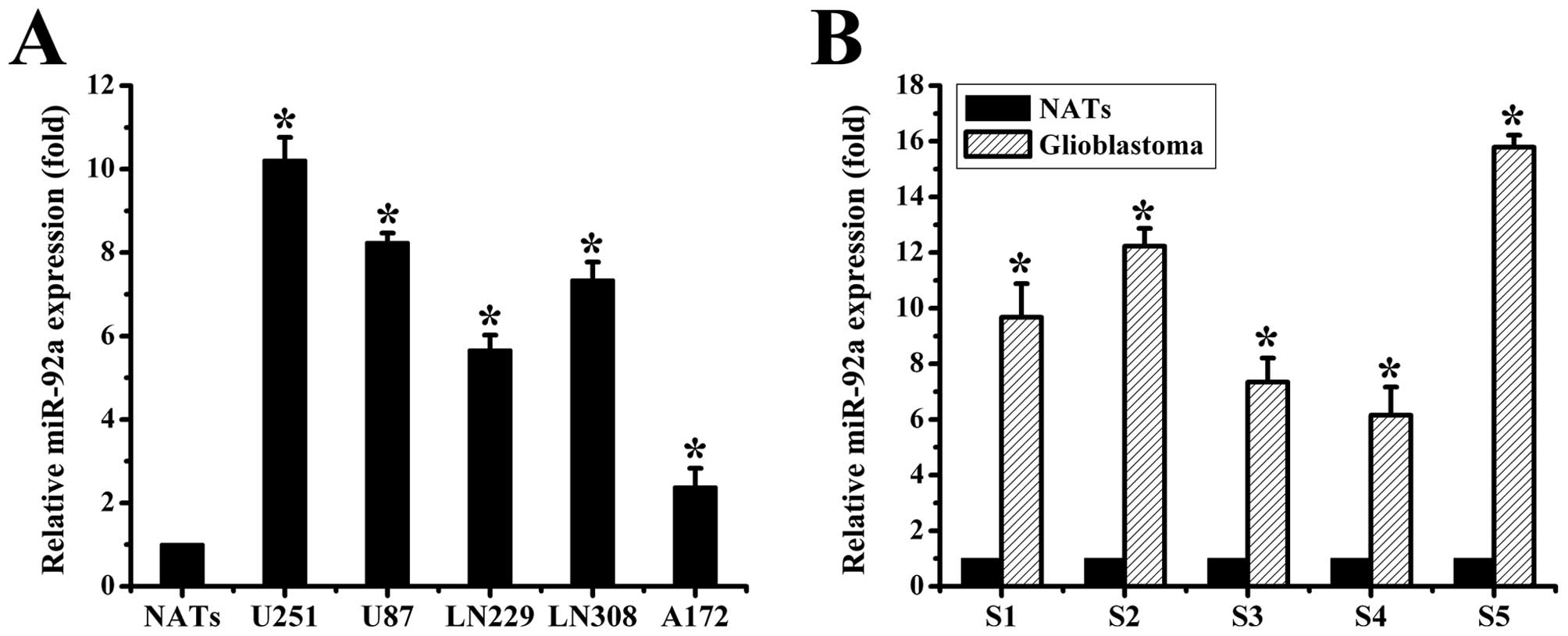
miR-92a is overexpressed in human
glioma
To explore the expression of miR-92a in human glioma
samples and cell lines, we performed the TaqMan-based real-time
stem-loop RT-PCR analyses. Our data showed that miR-92a was
obviously upregulated in glioma cell lines vs. NATs (Fig. 1A). Similar results were observed in
the glioma tissues (Fig. 1B). Taken
together, our results demonstrated that miR-92a was abnormally
overexpressed both in human glioma samples and cell lines.
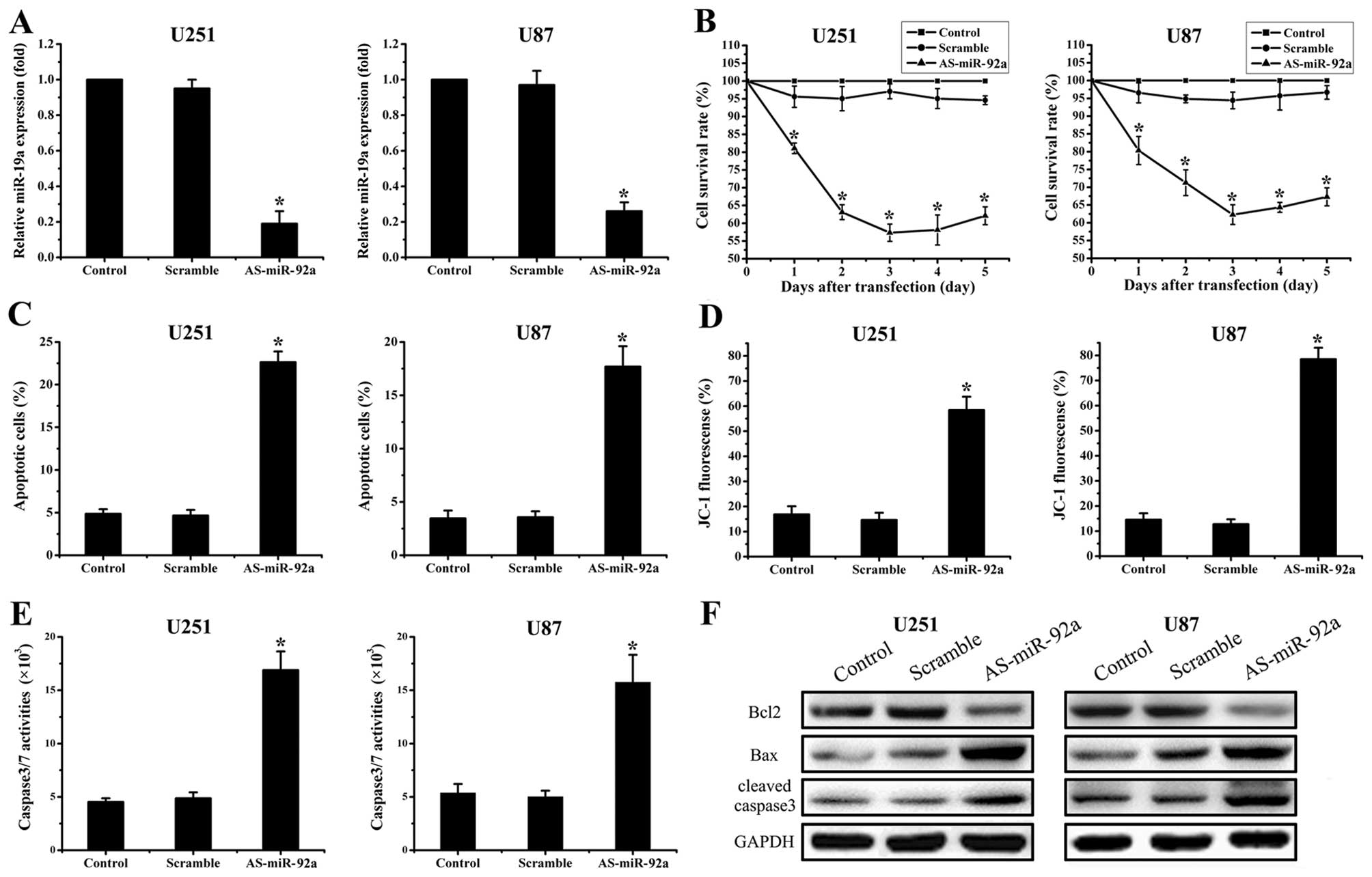
AS-miR-92a suppresses cell proliferation
and induces apoptosis in glioma cell lines
To examine biological significance of miR-92a in
glioma, U251 and U87 cells were treated with AS-miR-92a and
scramble oligonucleotides (Fig.
1B). First of all, real-time PCR showed that AS-miR-92a reduced
miR-92a levels by 81% in U251 cells and 74% in U87 cells
(P<0.05) (Fig. 2A). MTT assay
showed that proliferation was inhibited in the miR-92a abrogation
glioma cells, which was the most apparent on Day 3 (Fig. 2B). We further explored effects of
AS-miR-92a on apoptosis. Annexin V labeling revealed that knockdown
of miR-92a significantly increased cell apoptosis compared to the
cells treated with scramble oligonucleotide and control group
(Fig. 2C). Moreover, western blot
assay displayed that pro-apoptotic protein Bax and
cleaved-caspase-3 expression were significantly upregulated while
Bcl-2 expression was downregulated in As-miR-92a group (Fig. 2F). In addition, caspase-3/7 activity
was also considerably elevated in miR-92a deleted cells (Fig. 2E). Since collapse of the
mitochondrial membrane potential is one of the early events in
apoptosis (22), we next examined
if miR-92a regulates mitochondrial membrane potential. The cells
with suppression of miR-92a were stained with cationic dye JC-1.
FACSCalibur analysis showed that the mitochondrial membrane
potential was largely damaged when miR-92a were downregulated
(Fig. 2D). These findings indicate
that miR-92a plays an important role in regulation of cell
apoptosis.
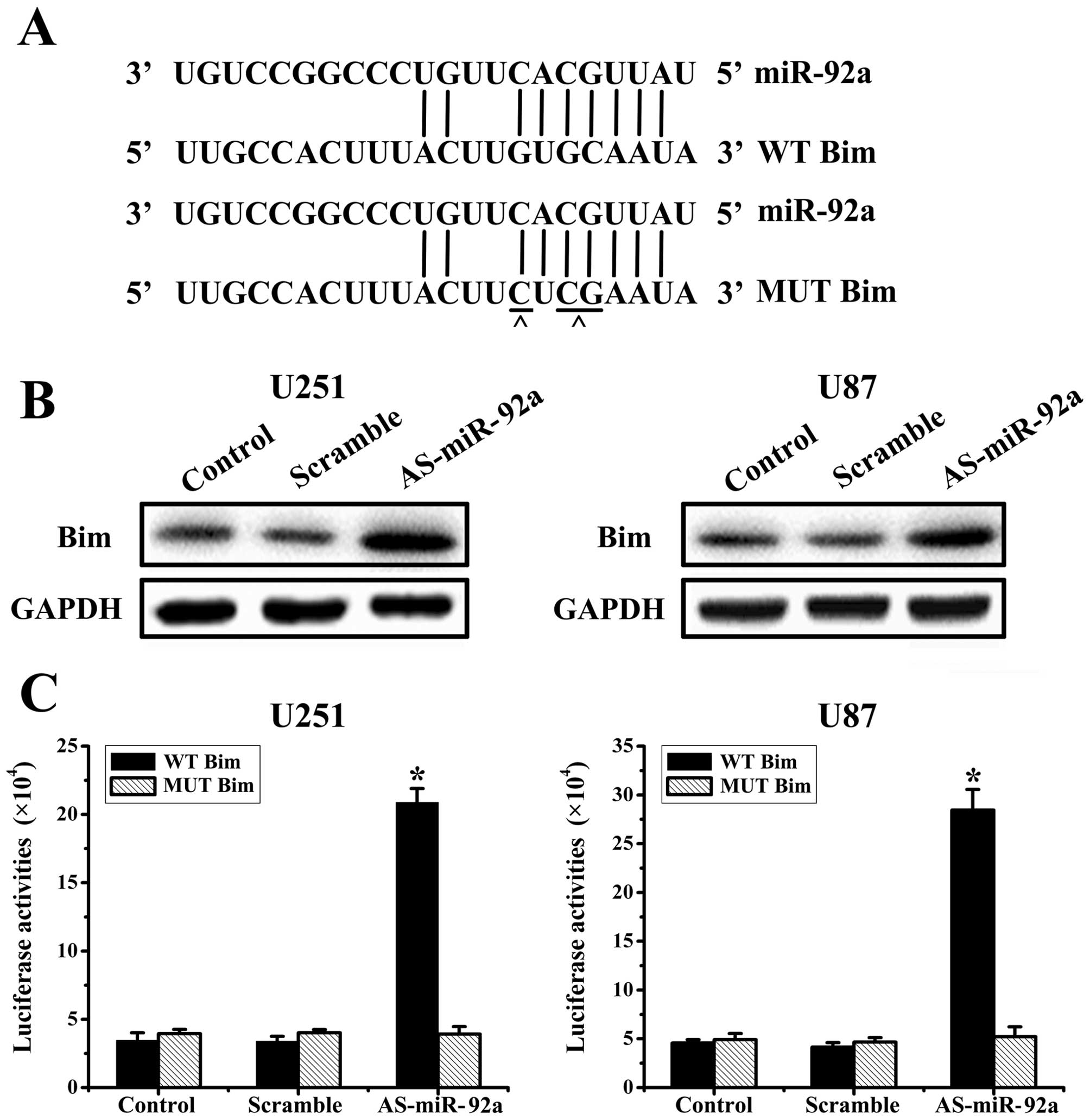
miR-92a directly targets Bim
Based on the analysis of databases miRanda
(http://www.cbio.mskcc.org/mirnaviewer), PicTar
(http://www.pictar.bio.nyu.edu), and
TargetScan (http://www.targetscan.org), we
predicted that Bim may be a target gene for miR-92a (Fig. 3A). To determine whether Bim is
regulated by miR-92a, we knocked-down miR-92a in U251 and U87
cells, and evaluated Bim protein levels at 48 h post-transfection.
And western blot analysis showed that AS-miR-92a obviously
increased Bim protein levels in U251 and U87 glioma cells (Fig. 3B).
To assess whether Bim is a direct target of miR-92a,
we created WT Bim and MUT Bim plasmids, which were co-transfected
with AS-miR-92a or scrambled oligonucleotide respectively into
glioma cells for 48 h, followed by measurement of luciferase
activity in transfected cells. Our results showed that the reporter
plasmid with wild-type 3′UTR of Bim caused a significant increase
in luciferase activity in cells transfected with AS-miR-92a, while
MUT reporter plasmid produced no change in luciferase activity
(Fig. 3C). Taken together, these
data indicated that miR-92a directly modulates Bim expression by
binding to 3′UTR of Bim.
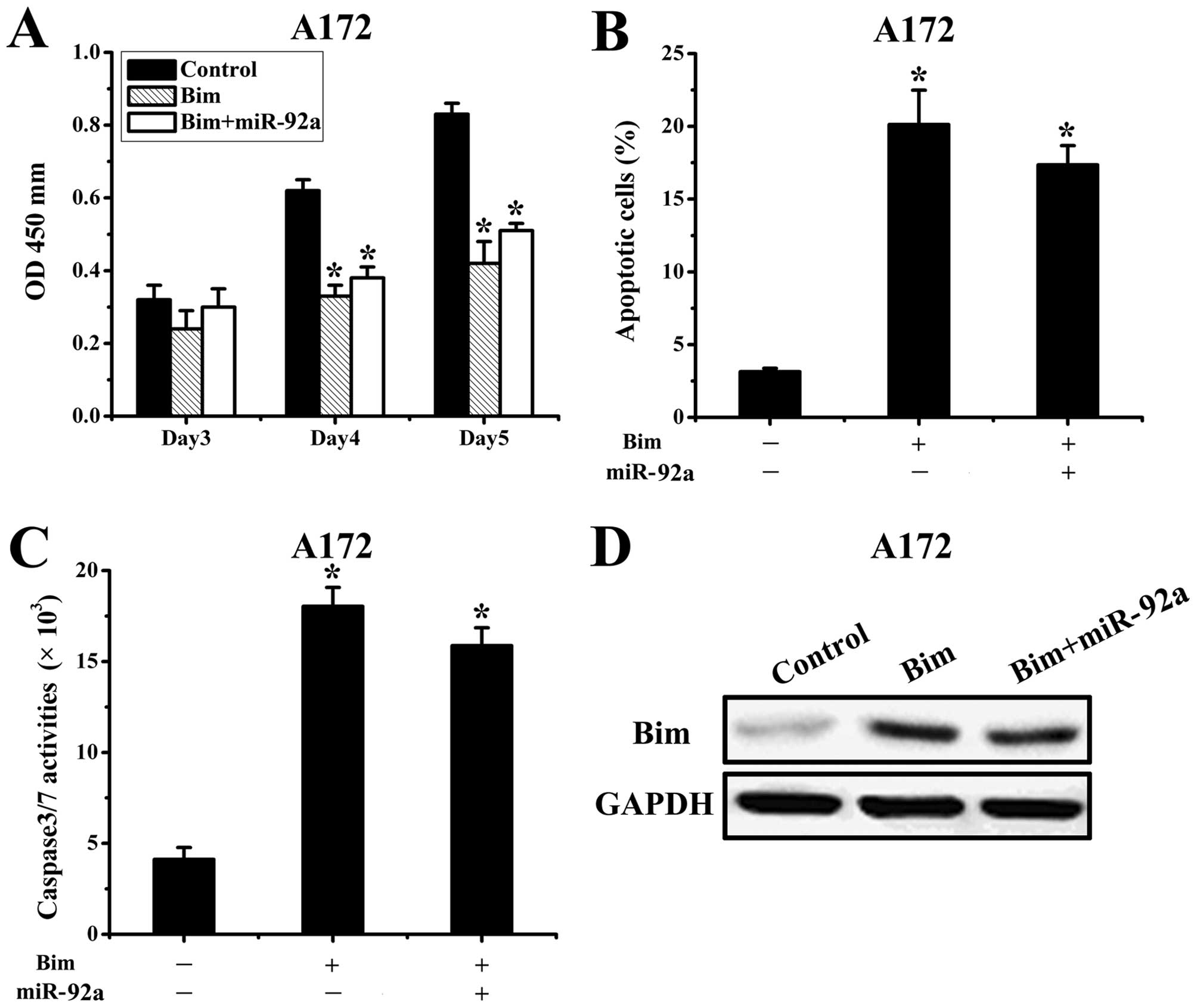
Bim is a functional target of
miR-92a
We next assessed the importance of Bim in
miR-92a-mediated cell survival. Since A172 cells had relatively low
expression of miR-92a, we transfected AS-miR-92a or scramble
oligonucleotide 24 h after transfection with Bim expression plasmid
lacking 3′UTR. The proliferation and apoptosis were assessed in
co-transfected A172 cells, which showed that ectopic expression of
Bim abrogated miR-92a effects on cell proliferation and apoptosis
(Fig. 4A-C). Subsequently, western
blot results demonstrated the upregulation of Bim protein levels
was significantly prevented by miR-92a (Fig. 4D). Taken together, these results
suggested that Bim is a critical target in miR-92a-mediated glioma
apoptosis.
AS-miR-92a suppresses glioblastoma
xenograft growth accompanying Bim upregulation
Since miR-92a are frequently elevated in
glioblastoma and play an important role in cell survival, we
further examined the effects of knockdown of miR-92a on glioma
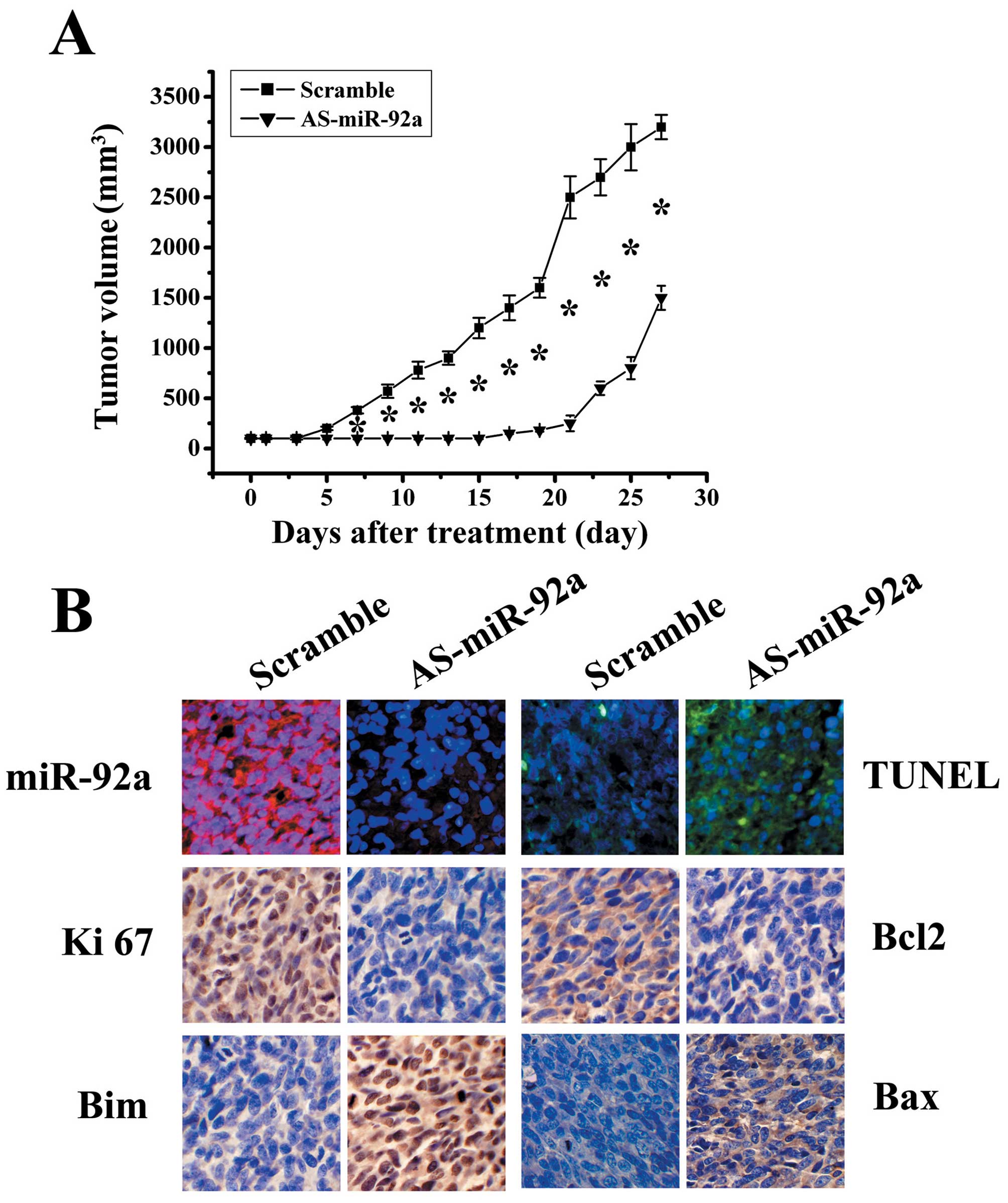
growth in vivo. As shown in Fig.
5A, AS-miR-92a significantly reduced tumor growth compared with
scramble group (P<0.05). LNA-ISH analysis confirmed that miR-92a
levels were considerably reduced in AS-miR-92a group (Fig. 5B). TUNEL assay analysis of xenograft
tumor taken at 28 days after treatment revealed much more apoptosis
in AS-miR-92a group when compared to tumors from scramble groups
(Fig. 5B). In addition, Ki-67
staining shows that AS-miR-92a treated tumors had a lower
proliferation index compared with the control groups (Fig. 5B). Immunohistochemical stanining
analysis revealed that Bim levels were upregulated in AS-miR-92a
group (Fig. 5B), confirming the
data in vitro that BIM as a direct target of miR-92a.
Additionally, Bax expression was increased, whereas Bcl-2
expression was decreased in xenograft tumor sections (Fig. 5B). These findings further indicate
that miR-92a targets Bim and that AS-miR-92a could be therapeutic
means for glioblastoma intervention.
Inverse correlation of expression of
miR-92a and Bim in glioma tissues
Having demonstrated Bim as a major target of
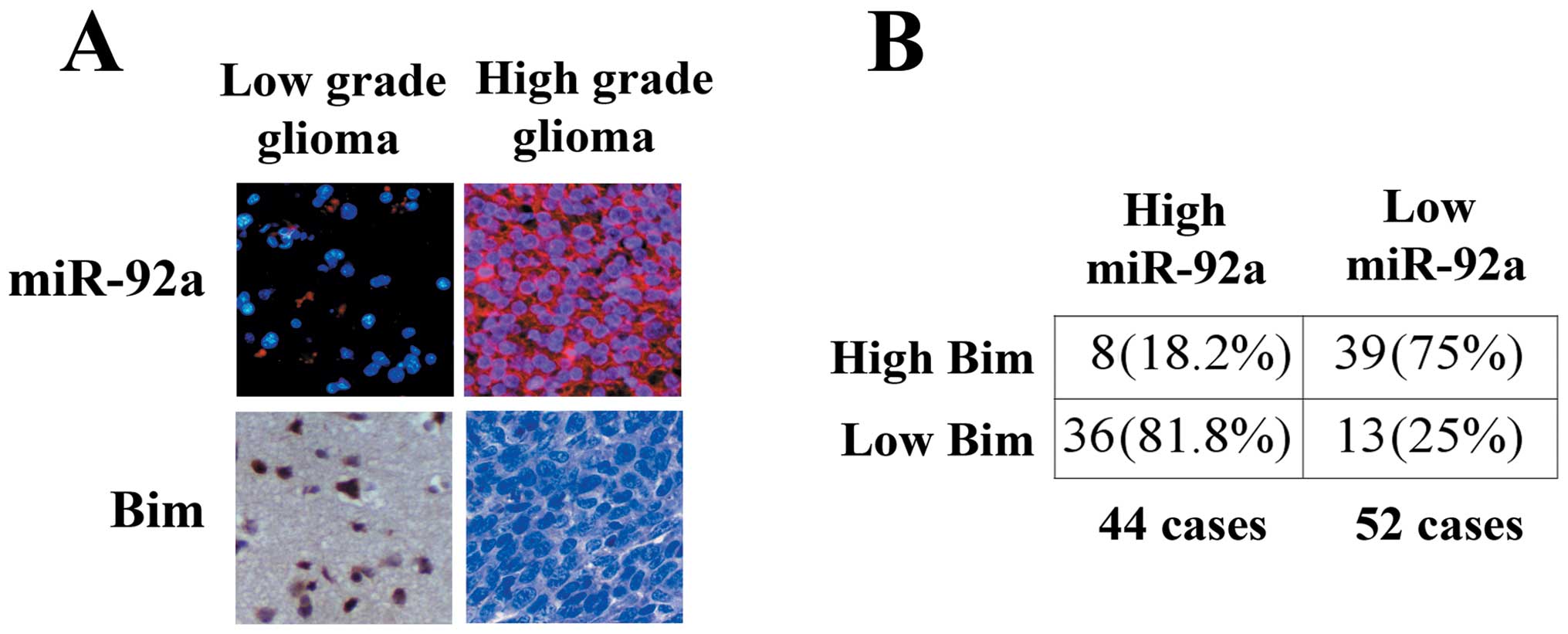
miR-92a, we further investigated the correlation of between miR-92a
and Bim expression in gliomas. We examined 96 human glioma
specimens with LNA-ISH and immunohistochemical staining.
Representative images of miR-92a and Bim are shown in Fig. 6A. Upregulation of miR-92a was
detected in 44 gliomas (Fig. 6B).
Of the 44 tumors with elevated miR-92a, 36 (81.8%) had low levels
of Bim (P<0.05). Thirty-nine of 52 (75%) specimens with
downregulated miR-92a presented high levels of Bim. In addition, we
found that miR-92a expression increased significantly in high grade
gliomas compared with low grade gliomas.
Discussion
Glioblastomas are the most malignant brain tumors of
glial origin (23,24). They are also among the most
resistant of all human tumors to available modalities of treatment
(24). Exploring the signaling
pathways involved in surviving and inducing cell death of glioma
cells is important for the development of more effective tumor
therapies.
miRNAs have recently been described as important
players in human cancer and the role of microRNA as therapeutic
target has been proposed (25–28).
In the present study, we focued on the upregulated miR-92a in
glioma, which is a member of miR-17-92 cluster and exerts potential
proliferative, anti-apoptotic, invasion-promoting effects in a
variety of cancer types. Shigoka et al (14) found that miR-92a is highly expressed
in hepatocellular carcinoma (HCC). In addition, the proliferation
of HCC-derived cell lines was enhanced by miR-92a and inhibited by
the anti-miR-92a antagomir. Chen et al (15) reported that miR-92a promotes
esophageal squamous cell carcinoma (ESCC) cell migration and
invasion at least partially via suppression of CDH1 expression, and
patients with upregulated miR-92a are prone to lymph node
metastasis and thus have poor prognosis. Haug et al
(16) demonstrated that miRNA-92 is
regulated by MYCN, and inhibits secretion of the tumor suppressor
DICKKOPF-3 (DKK3) in neuroblastoma. Tsuchida et al (17) proved that miR-92a plays a pivotal
role in the development of colorectal carcinoma. However, the
underlying functional mechanisms in glioma remain largely unknown.
In this study, we revealed that miR-92a was upregulated in
specimens of human gliomas of different grades and in glioma cell
lines, and high levels of miR-92a in human glioma specimens were
significantly correlated with low levels of Bim protein and high
histological grade. We further presented that miR-92a abgoration
results in growth inhibition and apoptosis induction with
upregulation of Bim in vitro and in vivo.
A mitochondrial-dependent step in apoptosis,
involving mitochondrial outer membrane permeabilization (MOMP), is
associated with most pro-apoptotic stimuli (29). This process is controlled by both
pro- and anti-apoptotic members of the Bcl-2 family, including Bim,
Bcl-2 and Bax. Reportedly, Bim can trigger apoptosis by at least
two different mechanisms, by the interaction and neutralization of
Bcl-2-like molecules and/or direct activation of Bax, and that the
decision on which mode of action ensues perhaps depends on the
nature of the incoming apoptosis signal. Low expression of Bim has
been shown for tumor entities, including melanoma and renal cell
carcinoma (30). The mechanism
suppressing Bim in these cancer types has not been clarifed yet. In
our study, we found that knockdown miR-92a could downregulate Bcl-2
and upregulate Bim and Bax by western blot assay. Furthermore,
bioinformatics analysis showed that 3′UTR of Bim mRNA existed the
highly conserved putative miR-92a binding sites. Luciferase
reporter assay validated that Bim was a direct target of miR-92a.
In addition, the change of Bim, Bcl-2 and Bax expression in
xenograft study confirmed the data in vitro. These results
indicate that AS-miR-92a negatively regulate Bim which leads to
decrease Bcl-2 and increase Bax.
In conclusion, our experiments have shown a novel
oncogenic role for miR-92a through regulation of apoptosis
signaling pathways involving Bim. The resulting phenotype of an
upregulated miR-92a includes increased proliferation and decresed
apoptosis. Furthermore, these data raise the possibility that
miR-92a may serve as a potential therapeutic target for
gliomas.
References
|
1
|
Surawicz TS, McCarthy BJ, Kupelian V, et
al: Descriptive epidemiology of primary brain and CNS tumors:
results from the Central Brain Tumor Registry of the United States,
1990–1994. Neuro Oncol. 1:14–25. 1999.PubMed/NCBI
|
|
2
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hadjipanayis CG and Van Meir EG: Tumor
initiating cells in malignant gliomas: biology and implications for
therapy. J Mol Med. 87:363–374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Purow B and Schiff D: Advances in the
genetics of glioblastoma: are we reaching critical mass? Nat Rev
Neurol. 5:419–426. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Elmén J, Lindow M, Schütz S, et al:
LNA-mediated microRNA silencing in non-human primates. Nature.
452:896–899. 2008.PubMed/NCBI
|
|
6
|
Meister G and Tuschl T: Mechanisms of gene
silencing by double stranded RNA. Nature. 431:343–349. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tijsterman M and Plasterk RH: Dicers at
RISC; the mechanism of RNAi. Cell. 117:1–3. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jovanovic M and Hengartner MO: miRNAs and
apoptosis: RNAs to die for. Oncogene. 25:6176–6187. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He H, Jazdzewski K, Li W, et al: The role
of microRNA genes in papillary thyroid carcinoma. Proc Natl Acad
Sci USA. 102:19075–19080. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iorio MV, Ferracin M, Liu CG, et al:
MicroRNA gene expression deregulation in human breast cancer.
Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Metzler M, Wilda M, Busch K, et al: High
expression of precursor microRNA-155/BIC RNA in children with
Burkitt lymphoma. Genes Chromosomes Cancer. 39:167–169. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Murakami Y, Yasuda T, Saigo K, et al:
Comprehensive analysis of microRNA expression patterns in
hepatocellular carcinoma and non-tumorous tissues. Oncogene.
25:2537–2545. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shigoka M, Tsuchida A, Matsudo T, et al:
Deregulation of miR-92a expression is implicated in hepatocellular
carcinoma development. Pathol Int. 60:351–357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen ZL, Zhao XH, Wang JW, et al:
microRNA-92a promotes lymph node metastasis of human esophageal
squamous cell carcinoma via E-cadherin. J Biol Chem.
286:10725–10734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Haug BH, Henriksen JR, Buechner J, et al:
MYCN-regulated miRNA-92 inhibits secretion of the tumor suppressor
DICKKOPF-3 (DKK3) in neuroblastoma. Carcinogenesis. 32:1005–1012.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsuchida A, Ohno S, Wu W, et al: miR-92 is
a key oncogenic component of the miR-17-92 cluster in colon cancer.
Cancer Sci. 102:2264–2271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Spannuth WA, Sood AK and Coleman RL:
Angiogenesis as a strategic target for ovarian cancer therapy. Nat
Clin Pract Oncol. 5:194–204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arnoult D: Apoptosis-associated
mitochondrial outer membrane permeabilization assays. Methods.
44:229–234. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou X, Ren Y, Moore L, et al:
Downregulation of miR-21 inhibits EGFR pathway and suppresses the
growth of human glioblastoma cells independent of PTEN status. Lab
Invest. 90:144–155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamamichi N, Shimomura R, Inada K, et al:
Locked nucleic acid in situ hybridization analysis of miR-21
expression during colorectal cancer development. Clin Cancer Res.
15:4009–4016. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yee KS, Wilkinson S, James J, et al: PUMA-
and Bax-induced autophagy contributes to apoptosis. Cell Death
Differ. 16:1135–1145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Talotta F, Cimmino A, Matarazzo MR, et al:
An autoregulatory loop mediated by miR-21 and PDCD4 controls the
AP-1 activity in RAS transformation. Oncogene. 28:73–84. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thaker NG and Pollack IF: Molecularly
targeted therapies for malignant glioma: rationale for
combinatorial strategies. Expert Rev Neurother. 9:1815–1836. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nana-Sinkam SP and Croce CM: MicroRNAs as
therapeutic targets in cancer. Transl Res. 157:216–225. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Law PT and Wong N: Emerging roles of
microRNA in the intracellular signaling networks of hepatocellular
carcinoma. J Gastroenterol Hepatol. 26:437–449. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gandellini P, Profumo V, Folini M, et al:
MicroRNAs as new therapeutic targets and tools in cancer. Expert
Opin Ther Targets. 15:265–279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Farazi TA, Spitzer JI, Morozov P, et al:
miRNAs in human cancer. J Pathol. 223:102–115. 2011. View Article : Google Scholar
|
|
29
|
Zhang CZ, Zhang JX, Zhang AL, et al:
MiR-221 and miR-222 target PUMA to induce cell survival in
glioblastoma. Mol Cancer. 9:2292010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Piñon JD, Labi V, Egle A, et al: Bim and
Bmf in tissue homeostasis and malignant disease. Oncogene.
27:S41–S52. 2008.PubMed/NCBI
|