Introduction
Glioma progression can lead to glioma-associated
brain edema, which is a significant source of morbidity and
mortality (1). In-depth studies of
molecular mechanisms of glioma-associated edema have implicated
vascular endothelial growth factor (VEGF), aquaporin-4 (AQP4),
cyclooxygenase-2, zonula occludens (ZO), occludins, claudins, and
junction associated molecules (JAM) in the process (2–6). VEGF
(one of the most important factors promoting angiogenesis) is also
responsible for plasma extravasation leading to peritumoral tissue
edema, increased vessel permeability, and increase in the water
content of glioma tissue (7,8).
Although some evidence is contradictory (9,10),
most accumulating evidence suggests the involvement of AQPs in the
dynamics of brain edema formation or resolution (11). Mou et al found that the
degree of peritumoral edema correlates with peritumoral AQP4
protein expression and that AQP4 expression correlates with VEGF
and HIF-1α expression (12).
Another study showed that intracerebral VEGF injection dramatically
upregulates AQP4 mRNA and protein in the perivascular space and
glia limitans externa (13).
Although there is a significant correlation between aquaporin-4
expression and the degree of cerebral edema, it is not clear
whether increased aquaporin-4 expression enhances edema formation
or clearance. The effects of VEGF on AQP4 expression may be
important for understanding the molecular mechanism of edema.
However, to our knowledge, there are no published reports on the
effects of VEGF on AQP4 expression in glioma. The goal of the
present study was to assess these effects and possibly provide a
basis for developing novel therapeutic approaches for
glioma-associated edema.
Materials and methods
Cell culture
Rat C6 glioma cells (Cell Biology Research Institute
of Shanghai, Shanghai, China) and C6 cells with expression vectors
containing antisense (C6/VEGF−) VEGF164 cDNA or an empty
vector (C6/vec) which were confirmed by assays for VEGF protein in
cell culture supernatants and saved in our laboratory (7,8) were
cultured in RPMI-1640 medium (1640M) (Invitrogen, Carlsbad, CA,
USA) supplemented with fetal calf serum (10%).
For cell proliferation assay, 2×104 cells
were placed in a 6-well plate and were counted after 24, 48, 72,
96, 120 and 144 h culture by hemocytometer.
To measure VEGF secretion in vitro,
5×105 cells were placed in 6-well plates and treated
with serum-free 1640M. Medium was collected after 48 h of
culturing. Debris was removed by centrifugation at 2000 × g for 5
min and supernatant was collected for enzyme-linked immunosorbent
assay (ELISA). A commercially available ELISA kit (R&D Systems,
Minneapolis, MN, USA) was used to detect mouse VEGF according to
the manufacturer’s recommendations. Each experiment was performed a
minimum of three times.
VEGF activity assay
Confluence (80–90%) of C6 cells, C6/vec cells and
C6/VEGF− cells were treated with serum-free 1640M for 48
h. Media were collected and VEGF concentration were measured by
ELISA. Human umbilical vein endothelial cells (HUVEC)
(2×104, Cell Biology Research Institute of Shanghai)
were placed in a 6-well plate and treated with 100 ng of VEGF
secreted from three C6 cell lines. The HUVEC cell growth curve was
monitored by cell count.
Xenograft glioma animals
Male 4–6-week-old BALB/c (nu/nu) mice (SLAC,
Shanghai, China) (n=33) were randomized into three groups (n=11).
Two individual clones of stable transfected C6 cells in the
logarithmic growth phase were used for each construct (vector only,
VEGF antisense), and the parental cell line was also studied. Two
hundred microliters of cells (in serum-free medium with a final
concentration of 7.5×106 cells/ml) were injected
subcutaneously into the right inguinal area of the mice.
Anesthetized mice were sacrificed with decapitation and tumors were
removed from the athymic (nu/nu) mice at 20 days post-implantation.
The animals were sacrificed and the tumors were removed and then
quickly frozen in liquid nitrogen for further analysis. All
procedures met the national guidelines for the care and use of
laboratory animals and were approved by the Institutional Animal
Care and Use Committee of the Fujian Medical University, Fujian,
China.
Quantitative polymerase chain reaction
(Q-PCR)
Total RNA was isolated and cDNA was synthesized as
has been described (7,8). The sequences of primer sets were VEGF,
forward: 5′-CCCAAGCTTATGAACTTTCTGCTCTCTTG-3′, reverse:
5′-CGCGGATCCTCACCGCCTTGGCTTGTC-3′; AQP4, forward:
5′-GCATGAATCCAGCTCGATCCTTTGG-3′ revese:
5′-AATGGGTGGCAGGAAATCTGAGGC-3′; β-actin, forward:
5′-GAGGCATCCTGACCCTGAAG-3′, reverse: 5′-CATCACAATGCCAGTGGTACG-3′.
The calculation of expression levels of VEGF and AQP4 was
normalized by β-actin.
Immunohistochemistry
To detect VEGF and AQP4 expression in vitro,
cells were fixed in 4% paraformaldehyde and blocked with 3% normal
goat serum for 2 h at room temperature for immunocytochemistry
analysis. To determine VEGF and AQP4 expression in vivo,
anesthetized mice were decapitated, and tumor tissues were removed
and quickly fixed in 10% formalin. For immunohistochemistry,
4-μm-thick sections were cut and rehydrated, treated with 0.3%
hydrogen peroxide in methanol for 30 min to inactivate endogenous
peroxidase, rinsed with 0.1 M phosphate buffer (PB) for 10 min, and
exposed to blocking serum (3% normal goat serum) for 2 h at room
temperature.
Immunoreactions were performed as previously
described (14). After incubation
with anti-VEGF (1:150 dilution, United States Biological,
Swampscott, MA, USA), and anti-AQP4 (1:150 dilution, Oncogene,
Cambridge, MA, USA), the slices were rinsed with 0.1 M PB and
exposed to anti-rabbit IgG HRP (1:500, Maixin, Fuzhou, China).
After an additional 10-min rinse, the slices were treated with
Vectastain® Elite ABC reagent (Maixin) for 30 min and
developed with DAB detection kit (Maixin). The slices were
counterstained by hematoxylin and mounted by Permount (Maixin).
Enzyme-linked immunosorbent assay
(ELISA)
To measure VEGF secretion in vitro,
5×105 cells were placed in 6-well plates and treated
with serum-free 1640M. Medium was collected after 48 h of culture.
Debris was removed by centrifugation at 2,000 × g for 5 min and the
supernatant collected for ELISA assay.
To measure VEGF levels in vivo, tumor tissues
(0.1 g) were homogenized in Tris-HCl buffer (25 mM, pH 7.6)
containing 100 mM NaCl, 1 mM EDTA, and 1 mM phenylmethanesulfonyl
fluoride (PMSF). Debris was removed by centrifugation at 2,000 × g
for 5 min, followed by centrifugation at 20,000 × g for 20 min, and
supernatants were collected for ELISA assay. Protein concentrations
were measured using Protein assay kit (Bio-Rad Laboratories,
Hercules, CA, USA), and was normalized to a concentration of 1
mg/ml. Series dilutions of samples with the highest and the lowest
expected values were performed to determine VEGF expression level
using commercial VEGF ELISA kit (R&D Systems, Minneapolis, MN,
USA) following the manufacturer’s instructions. VEGF expression
levels were calculated by a standard curve available from R&D
Systems. All experiments were performed in triplicate.
The water contents of tumor tissue
assays
Referring to previously described studies (7,8), the
water contents of the tumor samples were measured and taken to
represent the degree of edema. Tumor tissues from the same sample
which was also sampled for assays of VEGF expression were
immediately weighed on an electronic analytical balance to obtain
the wet weight (WW). The samples were then dried in a gravity oven
at 100°C for 24 h to obtain the dry weight (DW). Water content was
expressed as a percentage of wet weight; the formula for
calculation was (WW-DW)/WW × 100%.
Tumor vessel permeability
Tumor-bearing mice received a 0.1-ml/g i.v.
injection of Evans blue dye (1% in saline; Sigma-Aldrich, St.
Louis, MO, USA). After 6 h the animals were sacrificed and Evans
blue was extracted from tumor as described (7). Briefly, tumors were removed and
homogenized with 3 ml of N,N-dimethylformamide
(Sigma-Aldrich), and incubated at 57°C for 12 h. The solutions were
vortexed, then 2 ml of 1 N hydrochloric acid (HCl) were added, and
the solutions were vortexed again, and then centrifuged at 2,500
rpm for 15 min. Supernatant was collected and measured at 620 nm
with a spectrophotometer (Beckman Coulter, Fullerton, CA, USA).
Concentrations were calculated by using a standard curve for Evans
blue dye.
Protein analysis
To analyze AQP4 expression in vivo, tumor
tissues were homogenized in Tris-HCl buffer (50 mM, pH 8.0)
containing the protease inhibitor cocktail V (Calbiochem, San
Diego, CA, USA). Homogenate (20 μg) proteins were separated by
electrophoresis on 4–20% SDS-PAGE gel and transferred onto
Immobilon membranes (Millipore, Billerica, MA, USA). Western blot
analyses were conducted using antibodies against AQP4 (1:100), and
β-actin (1:2000, Neomarker, Fremont, CA, USA). Bands were
visualized using an electrochemiluminescence (ECL) kit (Amersham
Biosciences, Piscataway, NJ, USA).
Statistical analysis
SPSS 12.0 software (SPSS, Chicago, IL, USA) was
applied for statistical data analysis. Data were analyzed by using
one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc
test for multiple comparisons to the control groups. Differences
were considered significant at P<0.05.
Results
Expression of VEGF in glioma cells in
vitro
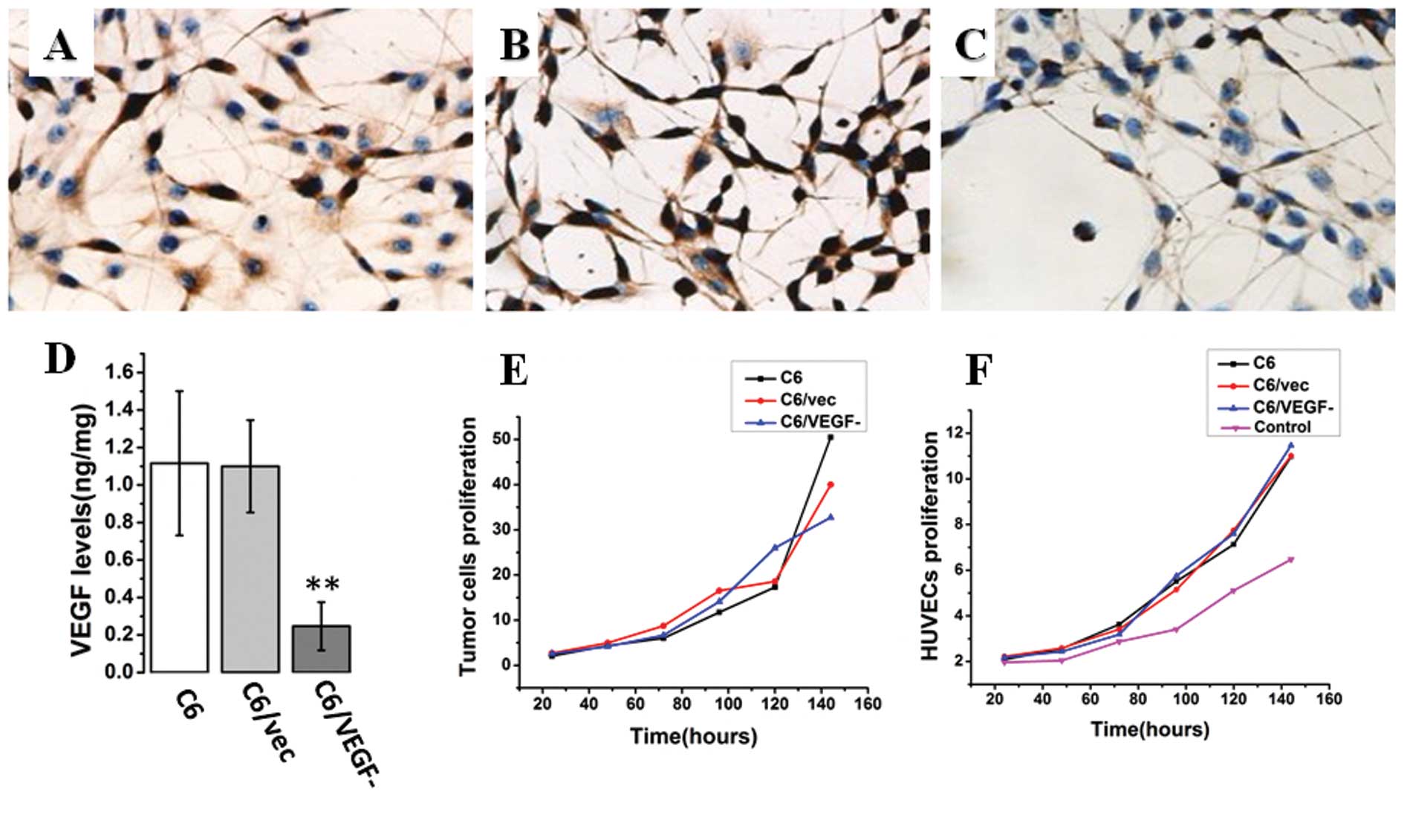
Immunostaining with VEGF antibody showed that
endogenous VEGF level was lower in C6/VEGF− cells than
in C6/vec and C6 cells after 48 h of serum deprivation (Fig. 1A-C). Similarly, the level of VEGF
protein in the medium from C6/VEGF− cells was
significantly lower than that from C6/vec and C6 cells (Fig. 1D). To investigate the effect of
antisense VEGF on tumor cell proliferation, the number of
C6/VEGF−, C6/vec, and C6 cells, respectively, placed
into 6-well plates (2×104 cells/well) were counted after
24, 48, 72, 96, 120 and 144 h in culture. Proliferation of
C6/VEGF− cells was found to be slower than that of the
two control cells (C6/vec and C6; Fig.
1E), the morphology of all three cell lines was similar and
remained unchanged (data not shown). However, the biological
activity of VEGF released from different cell lines was not
altered. Monitoring the growth of HUVEC cells treated with the same
amount of VEGF secreted from our three C6 cell lines revealed
similar levels of HUVEC cell growth, regardless of the source of
VEGF, and slower growth in the absence of VEGF (Fig. 1F).
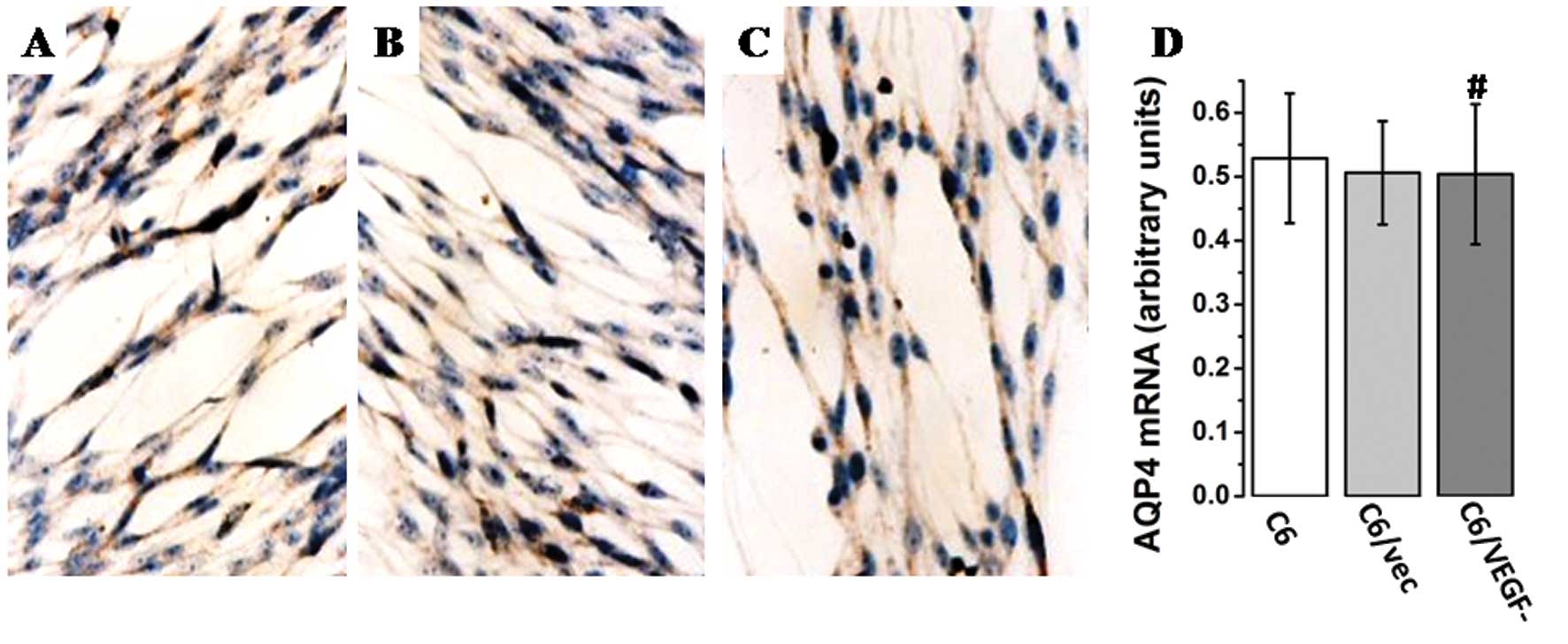
Effect of glioma-derived VEGF on AQP4
expression in glioma cells in vitro
AQP4 expression in the three cell lines was assayed
by RT-PCR and immunostaining. Similar AQP4 mRNA levels (Fig. 2A) were found in all three cell
lines. In addition, the intensity of AQP4 immunoreactivity was
similar in all lines after 48 h of serum deprivation (Fig. 2B-D). Thus, VEGF does not appear to
have a direct role in AQP4 expression in glioma tumor cells.
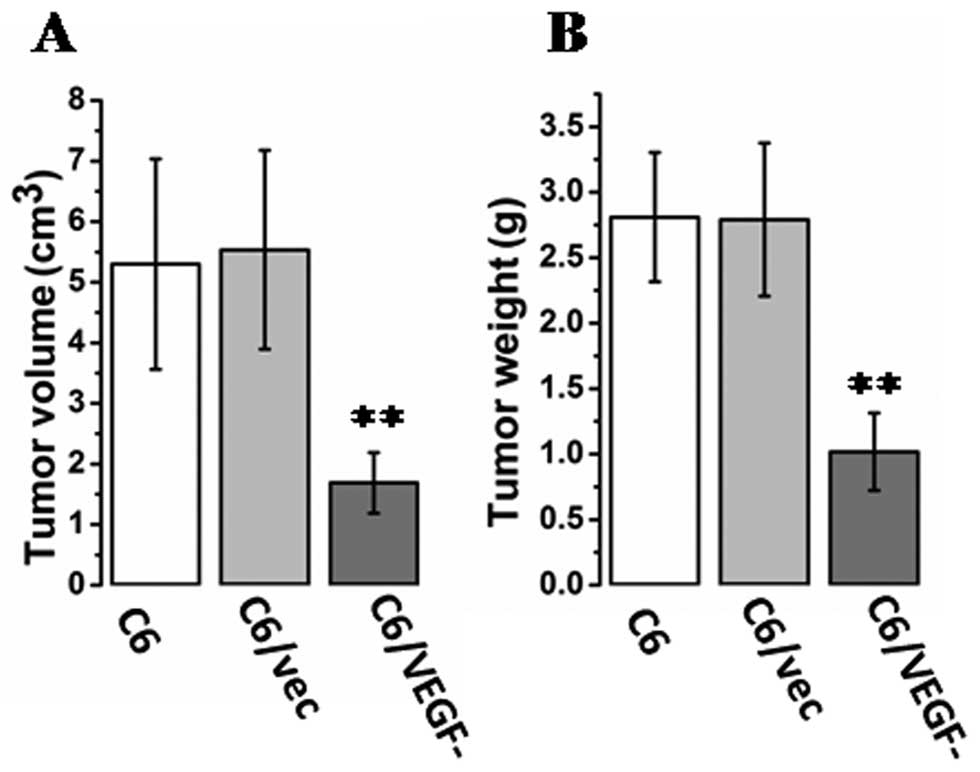
Effect of VEGF on tumorigenesis
To evaluate the possible role of VEGF in
tumorigenesis, mice were injected with glioma cells (C6, C6/vec, or
C6/VEGF− cells) directly into the right inguinal area,
and tumor size was measured. At 20 days after inoculation, tumor
size (Fig. 3A) and tumor weight
(Fig. 3B) were notably smaller in
C6/VEGF− mice.
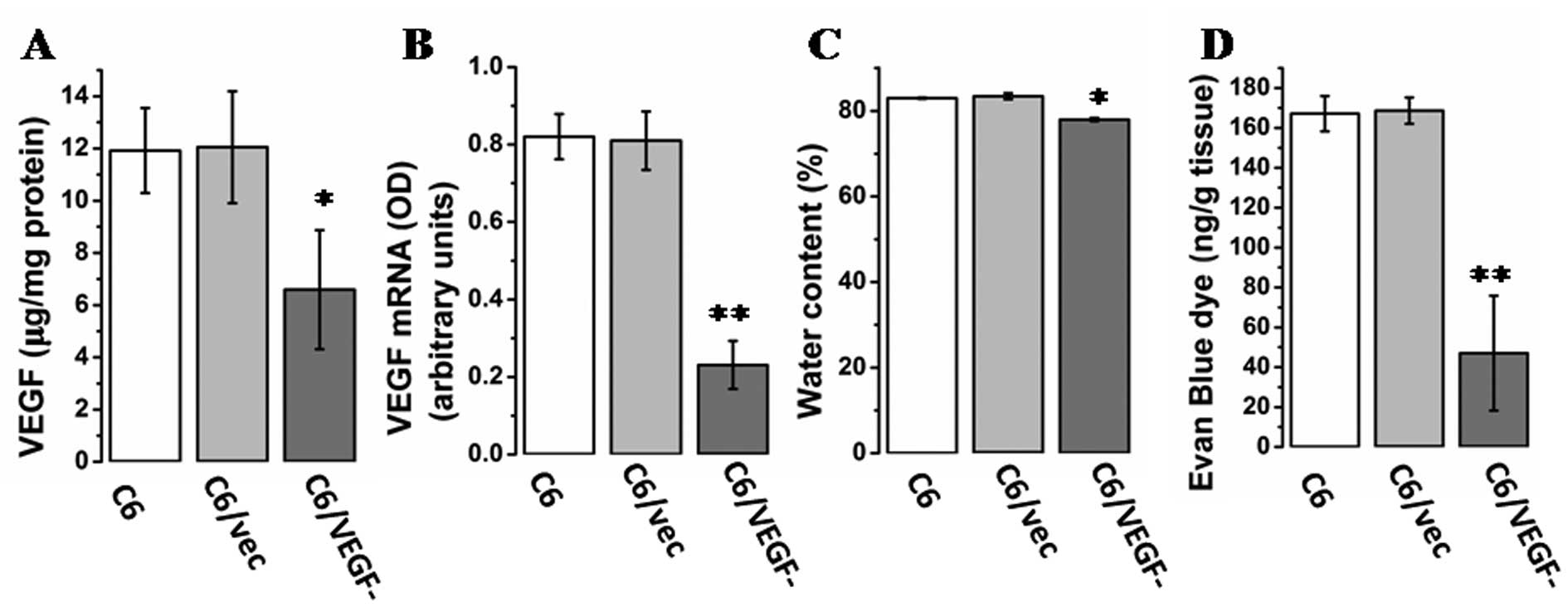
Expression of VEGF in tumors and the
water content of tumor tissue
To confirm the expression of VEGF in vivo as
well as in vitro, VEGF levels were determined in genetically
modified C6 cells by RT-PCR and ELISA. ELISA analysis (Fig. 4A) and RT-PCR (Fig. 4B) showed markedly lower level of
VEGF mRNA and protein, respectively, in tumors from
C6/VEGF−mice than tumors from C6/vec and C6 mice. A
common feature of malignant brain tumors is increased capillary
permeability leading to edema. Assay of tumor water content showed
that C6/VEGF tumors had a lower water content than either of the
two control tumors (C6/vec and C6) (Fig. 4C). To confirm that the edema was
attributable to vascular hyperpermeability, vascular extravasation
was examined using a dye tracer. Vascular leakage was markedly
reduced in the C6/VEGF− tumors (Fig. 4D). Also, Pearson’s correlation
analysis found a correlation between water content and VEGF
expression (Pearson’s correlation, r=0.946 P=0.00).
Expression of AQP4 in tumors
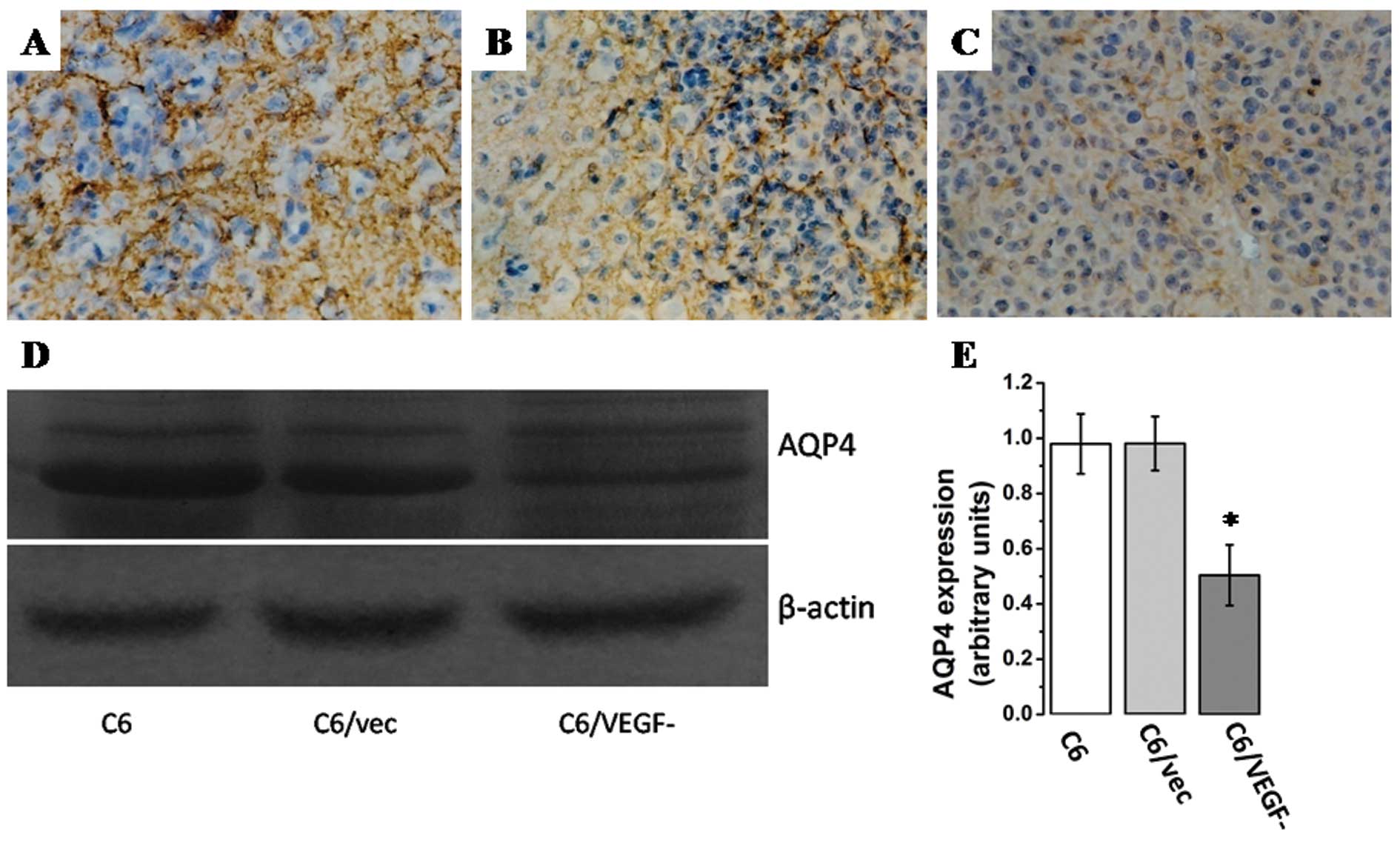
Levels of AQP-4 appeared to be lower in
C6/VEGF− tumors compared with C6G, C6/vecG tumors by
immunohistochemistry (Fig. 5A-C),
and western blot analysis (Fig. 5D and
E), and Pearson’s correlation analysis show that AQP4
expression paralleled the level of VEGF expression (r=0.883,
P=0.00) and the water content (r=0.912, P=0.00) of glioma tissue.
Thus, aquaporin-4 expression in glioma tissue is suggested to be a
reaction to glioma-associated edema induced by VEGF.
Discussion
Aquaporins (AQPs) are a family of water channel
proteins that facilitate the flux of water through plasma
membranes. AQP4, a mercury-insensitive water channel protein, is
abundant in the central nervous system. It is localized in the
blood-brain barrier around blood vessels and luminal membrane of
ependymal cells, and its distribution in high density astrocytic
foot processes is polarized. AQP4 is speculated to maintain the
homeostasis of intracellular and extracellular water in the brain
(10,11,15).
In addition, chemotherapy and radiotherapy for glioblastoma
multiforme is reported to downregulate AQP4 expression, restoring
its perivascular rearrangement and suggesting the potential role of
AQP4 in the resolution of brain edema (16). Recent studies show that AQP4 is
involved in cell migration and cytoskeleton organization (15,17).
Taken together, these findings suggest that AQP4 has a critical
role in glioma malignancy.
Breakdown of the blood-brain barrier (BBB) has been
linked to upregulation of AQP4 expression. The increased AQP4
expression in high grade astrocytomas may facilitate the flow of
edema fluid (18). The pattern of
AQP4 expression in human gliomas, AQP4 overexpression in glioma
cells, and AQP4 localization on astrocytic end-feet are associated
with disturbance of the blood-brain barrier (19). The redistribution of AQP4 in
glioblastoma cells is believed to facilitate reabsorption of excess
fluid and to be a reaction to vasogenic edema stemming from the
breakdown of the BBB (20).
Mou et al hypothesized that AQP4 is
positively regulated by VEGF (12).
Rite et al (13) found that
intracerebral injection of VEGF induces an increase in AQP4, but in
our study, VEGF did not directly affect AQP-4 expression. The most
important factor regulating the function and expression of AQP4 is
osmotic pressure. Studies have shown that VEGF may alter vascular
permeability and affect osmotic pressure changes (21). VEGF can increase neovascular
permeability and promote the extravasation of plasma protein and
fibrinogen into intracellular spaces. In glioma, VEGF is one of the
most important factors promoting angiogenesis in the tumor. With
growth of the tumor, increase in vascular permeability due to
neovascularization causes extensive damage to the BBB integrity,
and a large number of macromolecules in plasma enter the
interstitial space, where they produce an obvious change in osmotic
pressure. Therefore, VEGF is an important factor affecting osmotic
pressure within glioma tissue. Therefore, although AQP4 was
associated with brain edema formation, we presume that upregulated
expression and redistribution of AQP4 in glioblastoma cells is a
reaction to VEGF-induced vasogenic edema and a response that
ameliorates or prevents cytotoxic brain edema by facilitating
reabsorption of excess fluid.
In summary, VEGF does not directly affect AQP-4
expression. The redistribution of AQP4 in glioblastoma cells is a
reaction to VEGF-induced vasogenic edema and facilitates
reabsorption of excess fluid. AQP4 induction might be a promising
approach in vasogenic brain edema prevention and treatment. Further
studies are needed to understand its functional role.
Acknowledgements
We thank Professor Lin Xu (Research Center of
Molecular Medicine, Fujian Medical University) for help in primer
design and Professor Tian Jun and Professor Hu Zhi-jian (Public
Health School, Fujian Medical University) for assistance in data
processing and statistical analysis. This study was supported by a
grant from National Natural Science Foundation of China (no.
30973083).
Abbreviations:
|
AQP4
|
aquaporin-4
|
|
VEGF
|
vascular endothelial growth factor
|
|
ZO
|
zonula occludens
|
|
JAM
|
junction associated molecules
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
Q-PCR
|
quantitative polymerase chain
reaction
|
|
HUVEC
|
human umbilical vein endothelial
cells
|
References
|
1
|
Papadopoulos MC, Saadoun S, Davies DC and
Bell BA: Emerging molecular mechanisms of brain tumour oedema. Br J
Neurosurg. 15:101–108. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dobrogowska DH, Lossinsky AS, Tarnawski M
and Vorbrodt AW: Increased blood-brain barrier permeability and
endothelial abnormalities induced by vascular endothelial growth
factor. J Neurocytol. 27:163–173. 1998. View Article : Google Scholar
|
|
3
|
Wang W, Dentler WL and Borchardt RT: VEGF
increases BMEC monolayer permeability by affecting occludin
expression and tight junction assembly. Am J Physiol Heart Circ
Physiol. 280:H434–H440. 2001.PubMed/NCBI
|
|
4
|
Yool AJ, Brown EA and Flynn GA: Roles for
novel pharmacological blockers of aquaporins in the treatment of
brain oedema and cancer. Clin Exp Pharmacol Physiol. 37:403–409.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Badie B, Schartner JM, Hagar AR, et al:
Microglia cyclooxygenase-2 activity in experimental gliomas:
possible role in cerebral edema formation. Clin Cancer Res.
9:872–877. 2003.PubMed/NCBI
|
|
6
|
Vorbrodt AW and Dobrogowska DH: Molecular
anatomy of intercellular junctions in brain endothelial and
epithelial barriers: electron microscopist’s view. Brain Res Brain
Res Rev. 42:221–242. 2003.PubMed/NCBI
|
|
7
|
Lin ZX, Yang LJ, Huang Q, et al:
Inhibition of tumor-induced edema by antisense VEGF is mediated by
suppressive vesiculo-vacuolar organelles (VVO) formation. Cancer
Sci. 99:2540–2546. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang LJ, Lin ZX, Huang Q, et al: Effect of
vascular endothelial growth factor on remodeling of C6 glioma
tissue in vivo. J Neurooncol. 103:33–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Manley GT, Fujimura M, Ma T, et al:
Aquaporin-4 deletion in mice reduces brain edema after acute water
intoxication and ischemic stroke. Nat Med. 6:159–163. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Papadopoulos MC, Manley GT, Kfishna S and
Verkman AS: Aquaporin-4 facilitates reabsorption of excess fluid in
vasogenic brain edema. FASEB J. 18:1291–1293. 2004.PubMed/NCBI
|
|
11
|
Zador Z, Bloch O, Yao X and Manley GT:
Aquaporins: role in celebral edema and brain water balance. Prog
Brain Res. 161:185–194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mou K, Chen M, Mao Q, Wang P, Ni R, Xia X
and Liu Y: AQP-4 in peritumoral edematous tissue is correlated with
the degree of glioma and with expression of VEGF and HIF-alpha. J
Neurooncol. 100:375–383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rite I, Machado A, Cano J and Venero JL:
Intracerebral VEGF injection highly upregulates AQP4 mRNA and
protein in the perivascular space and glia limitans externa.
Neurochem Int. 52:897–903. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin ZX, Yang LJ, Huang Q and Fu J:
Activated vascular endothelia regulate invasion of glioma cells
through expression of fibronectin. Chin Med J. 123:1754–1761.
2010.PubMed/NCBI
|
|
15
|
Ding T, Gu F, Fu L and Ma YJ: Aquaporin-4
in glioma invasion and an analysis of molecular mechanisms. J Clin
Neurosci. 17:1359–1361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nico B, Mangieri D, Tamma R, et al:
Aquaporin-4 contributes to the resolution of peritumoural brain
oedema in human glioblastoma multiforme after combined chemotherapy
and radiotherapy. Eur J Cancer. 45:3315–3325. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McCoy E and Sontheimer H: Expression and
function of water channels (aquaporins) in migrating malignant
astrocytes. Glia. 55:1034–1043. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saadoun S, Papadopoulos MC, Davies DC,
Krishna S and Bell BA: Aquaporin-4 expression is increased in
oedematous human brain tumours. J Neurol Neurosurg Psychiatry.
72:262–265. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Davies DC: Blood-brain barrier breakdown
in septic encephalopathy and brain tumours. J Anat. 200:639–646.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Warth A, Mittelbronn M and Wolburg H:
Redistribution of the water channel protein aquaporin-4 and the
K+ channel protein Kir4.1 differs in low- and high-grade
human brain tumors. Acta Neuropathol. 109:418–426. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fu BM and Shen S: Structural mechanisms of
acute VEGF effect on microvessel permeability. Am J Physiol Heart
Circ Physiol. 284:H2124–H2135. 2003. View Article : Google Scholar : PubMed/NCBI
|