Introduction
Breast cancer is one of the most common types of
cancer in women and accounts for 7–10% of all diseases.
Approximately 40 million people succumb to the disease each year
(1). The incidence of breast cancer
is rising in China (2) and is
threatening to become a severe public health problem. Of patients
with breast cancer 60–80% will develop bone metastases during the
course of the disease (3). More
than half of these patients will experience persistent and
increasing bone pain, which seriously impairs the patient’s quality
of life.
Radiation therapy is a common option for the
treatment of breast cancer. Such therapeutic options include
external beam radiation therapy and internal radionuclide therapy.
In most breast cancer patients with bone metastases, pain in the
bone can be relieved through radiation therapy. However, the
benefits of radiation therapy can be limited by damage repair
mechanisms, cell repopulation, and radiation-resistant hypoxic
tumor cells. The radiosensitivity of tumor cells is an important
factor that affects the efficacy of radiotherapy. In 1963, Adams
et al (4) used
nitroacetophenone to radiosensitize rat hypoxic cells. Following
this initial report, many researchers have been developing
radiation-sensitizing drugs to improve the efficacy of
radiotherapy.
Radiation sensitizing agents are defined as any
chemicals or biological reagents that selectively enhance the
lethality of radiation on tumor cells or that reduce the radiation
resistance of tumor cells. Thus, they improve the effect of
radiation therapy. The ideal radiation sensitizer should meet the
following criteria (5): (i) the
agent should be stable and should not easily react with other
substances; (ii) the effective dose should not be toxic or at least
tolerable; (iii) the agent should be soluble in water for easy
delivery; (iv) even at low doses, the drug should have radiation
sensitizing effects in conventional fractionated treatment.
However, it is difficult to find radiation sensitizers that fully
meet all these criteria. In practice, drugs that enhance
radiotherapy and show limited toxicity are often used as radiation
sensitizers.
Chemotherapy drugs are the best studied agents for
promoting radiosensitivity. However, chemotherapeutic drugs are
toxic to normal cells and can have synergistic effects with
radiation, often making them intolerable for patients. Therefore,
there is still a clear need for novel radiation sensitizers in the
clinic.
Arsenic trioxide (As2O3) was
first used for the treatment of acute promyelocytic leukemia (APL)
in 1970, and it was approved by the U.S. Federal Food and Drug
Administration (FDA) in 2000. Previous studies (6-8) found
that, in addition to APL, As2O3 is also
effective against a number of solid tumors, including esophageal,
liver, breast and gastric cancer. Although high doses of
As2O3 may show significant toxicity, low
doses can have notable antitumor effects, including changing tumor
cell cycle distribution, promoting cell differentiation and
apoptosis, reducing glutathione levels, directly damaging DNA, and
inhibiting tumor angiogenesis (9–12). It
is possible that As2O3 may also have
radiosensitizing effects. In the present study, we examined whether
As2O3 could sensitize human MCF-7 breast
cancer cells to 89SrCl2 β-ray irradiation
in vitro and we also investigated the underlying molecular
mechanisms.
Materials and methods
Cell line
Human breast cancer MCF-7 cell line was obtained
from the experimental center of clinical laboratory diagnostics at
Bengbu Medical College. They were cultured in RPMI-1640 medium
(Gibco, CA, USA) supplemented with l0% FBS (Gibco) in an incubator
with 5% CO2 and saturated humidity at 37°C.
MTT assay evaluation of the effects of
As2O3 on cell proliferation
MCF-7 cells (1.0×104 cells per well) were
seeded in 96-well plates and cultured for 24 h. The medium was then
replaced with the same medium containing
As2O3 (Sigma, MO, USA) at concentrations of
0, 0.5, 1, 2, 5, 10, 20, 50 and 100 μM for 24 h (6 wells at each
concentration). Following incubation, the medium was removed and
200 μl of fresh medium containing 50 μg/μl MTT (Sigma) was added
into each well and incubated for an additional 4 h. Medium was then
discarded and 150 μl dimethyl sulfoxide (DMSO, Sigma) was added.
Optical density (OD) at 570 nm was measured by DG3022A type
Microplate Reader (State-run East China Electronic Tube Factory).
The rate of cell proliferation was calculated as follows:
(experimental OD value/control OD value) ×100%. The experiment was
repeated 6 times. Growth curves were plotted; the ordinate and
abscissa represent the concentration of As2O3
and the rate of cell proliferation, respectively. According to the
curve, the 50% inhibiting concentration (IC50) was
calculated.
89SrCl2 irradiation
and the calculation of absorbed dose
Cells were cultured for 24 h, medium was removed,
and fresh medium containing 740, 1480 or 2960 kBq/ml of
89SrCl2 (Chengdu Gaotong Isotope Co., Ltd.)
was added to the wells for another 48 h. Cumulative absorbed doses
from 89Sr internal irradiation were computed according
to the formulation (13), D = AEt/m
(where A, E, m and t represent radioactivity of
89SrCl2, mean energy of β-ray from
89Sr, mass of irradiated cells and irradiating time,
respectively).
Cell grouping and treatment
MCF-7 (5×104) cells per well were seeded
in 6-well plates and incubated for 24 h. Cells were then randomly
divided into four groups: control, As2O3,
89SrCl2 and
As2O3+89SrCl2 group
(combination group). Each treatment was performed in triplicate.
The As2O3 and the combination group were
treated with 1 or 2 μmol/l of As2O3 for 24 h.
89SrCl2 was added into both the
89SrCl2 and the combination group for another
48 h. Medium was then removed and fresh medium was added into each
well. Cells were cultured for another 24 h. The experiment was
repeated 6 times.
Morphological observations
The morphological changes of MCF-7 cells in each
group were observed under an inverted microscope and images were
captured with a digital camera.
Colony formation assay to detect the
effects of 89SrCl2 on cell proliferation
Cells were detached from culture dishes with 0.25%
trypsin (Sigma) and adjusted to 5×104/ml with fresh
medium. Various treatments, including 0, 370, 740, 1480, 2960, 4440
and 5920 kBq/ml of 89SrCl2 were added to 100,
150, 200, 400, 1000, 2000 and 10000 cells. Cells were seeded in
triplicate in different wells of 6-well plates for 24 h. Cells in
the As2O3 group and the combination group
were treated with 1 or 2 μM of As2O3 for 24
h. 89SrCl2 was added into the
89SrCl2 group and the combination group for
another 48 h. Medium was then discarded and fresh medium was added
into each well. Cells were cultured for a total of 12 days.
Subsequently, cells were rinsed with phosphate-buffered saline
(PBS), and colonies were fixed with 95% ethanol for 15 min. They
were then stained with crystal violet for 20 min and counted under
the microscope. Surviva1 fraction, SF = no. of colonies formed/(no.
of cells seeded × plating efficiency of the control). The cell
survival curve was plotted by the multi-target one hit model
(14). Mean lethal dose
(D0), quasi-threshold dose (Dq),
extrapolation number (N) and radiosensitivity enhancing ratio (SER)
were calculated.
Cell cycle analysis
Cells were harvested and rinsed with PBS. They were
fixed with 70% ethanol at 4°C overnight. The following day, cells
were centrifuged and the supernatant was discarded. Pellets were
rinsed twice with PBS and incubated with 1.5 μl 25 mg/ml RNase A
(Sigma) at 37°C for 30 min. They were then stained with 12 μl 50
μg/ml PI and protected from light at 4°C for 30 min. Cell cycle
distribution was measured by FACSCalibur type flow cytometer
(Becton-Dickinson).
Cell apoptosis analysis
Buffer was prepared according to instructions
provided for the Annexin V-FITC/PI apoptosis detection kit (BD
Biosciences, USA). Cells were harvested, mixed with 300 μl buffer,
incubated in the dark with 5 μl Annexin V-FITC for 15 min, and then
mixed with 5 μl PI for 5 min. Cell apoptosis was analyzed by flow
cytometry (FCM).
Expression of Bcl-2 and Bax mRNA measured
by RT-PCR
Total RNA was isolated with TRIzol reagent
(Invitrogen, CA, USA) according to the manufacturer’s instructions.
cDNA was synthesized at 42°C for 1 h and at 70°C for 10 min from 4
μg of total RNA using the SuperScript II Reverse Transcriptase
(Invitrogen) following the manufacturer’s protocol. cDNA was
subsequently amplified using Hot-StarTaq DNA Polymerase (Qiagen,
Australia) and primers at MyCycler™ type PCR (Bio-Rad, CA, USA).
Sequences of the primers are as follows (Shanghai Sangon Biological
Engineering Technology and Services Co., Ltd.): GAPDH:
5′-GGGAAGGTGAAGGTCGGAGTC-3′ (sense primer),
5′-AGCAGAGGGGGCAGAGATGAT-3′ (antisense primer); Bcl-2:
5′-CAGCTGCACCTGACGCCCTT-3′ (sense primer),
5′-GCCTCCGTTATCCTGGATCC-3′ (antisense primer); Bax:
5′-ACCAAGAAGCTGAGCGAGTGTC-3′ (sense primer),
5′-TGTCCAGCCCATGATGGTTC-3′ (antisense primer).
The PCR program consisted of initial denaturation at
95°C for 3 min, followed by 30 cycles of denaturation at 95°C for
45 sec, annealing for 45 sec at 55°C (Bcl-2), 58°C (Bax) or 63°C
(GAPDH) and extension at 72°C for 1 min. A final extension step at
72°C for 10 min was included for all primers. PCR products were
stored at 4°C and electrophoresed in agarose gel buffered by 1X TBE
at 100 V. Images were captured with an ultraviolet analyzer. The
electrophoretic pattern was semi-quantitatively analyzed with
SmartView image software, and the ratio of sample intensity to
intensity of GAPDH was determined.
Western blot analysis
Protein was extracted from cultured cells and
subjected to western blot analysis using specific antibodies to
Bcl-2 and Bax. The cells were harvested and rinsed with PBS. Cell
extracts were prepared with pre-chilled lysis buffer (50 mM
Tris-HCl, 150 mM NaCl, 1% Triton X-100, 0.5% deoxycholate, 1 mM
EDTA, 1 mM Na3VO4, 1 mM NaF, 2% Cocktail) and
cleared by centrifugation at 12,000 g for 30 min at 4°C. The
supernatant was collected and total protein concentration was
measured using the BCA assay kit (Sigma) according to the
manufacturer’s instructions. Cellular extract containing 30 μg of
total protein was separated by 12% sodium dodecylbenzene
sulfonate-polyacrylamide gel electrophoresis (SDS-PAGE), and the
protein was transferred to PVDF membrane (Millipore, MA, USA). The
membrane was then blocked with TBST (10 mM Tris-HCl, pH 7.4, 150 mM
NaCl, 0.1% Tween-20) containing 5% w/v non-fat dry milk at 37°C for
1 h. It was then incubated with mouse anti-human Bcl-2 or Bax
antibodies (1:200; Santa Cruz Biotechnology, CA, USA) or β-actin
antibody (1:800; Santa Cruz Biotechnology) in TBST at 4°C
overnight. The membrane was washed three times and hybridized with
horseradish peroxidase-conjugated sheep anti-mouse IgG (1:2000;
Millipore) for 2 h at room temperature. After washing three times
for 10 min each with 15 ml TBST, protein bands specific for the
antibodies were visualized by enhanced chemiluminescence (Amersham
Pharmacia Biotech, NJ, USA) associated with fluorography. The
intensity of bands for Bcl-2 and Bax proteins were analyzed with
Smart view image software and the relative values of Bcl-2/Bax in
different groups were calculated.
Statistics
Data are presented as the mean ± SD. Differences
between two groups were determined by a t-test between independent
samples using SPSS 11.5 software. The relationship between cell
viability and As2O3 concentration was assayed
using curvilinear regression and correlation analysis.
Results
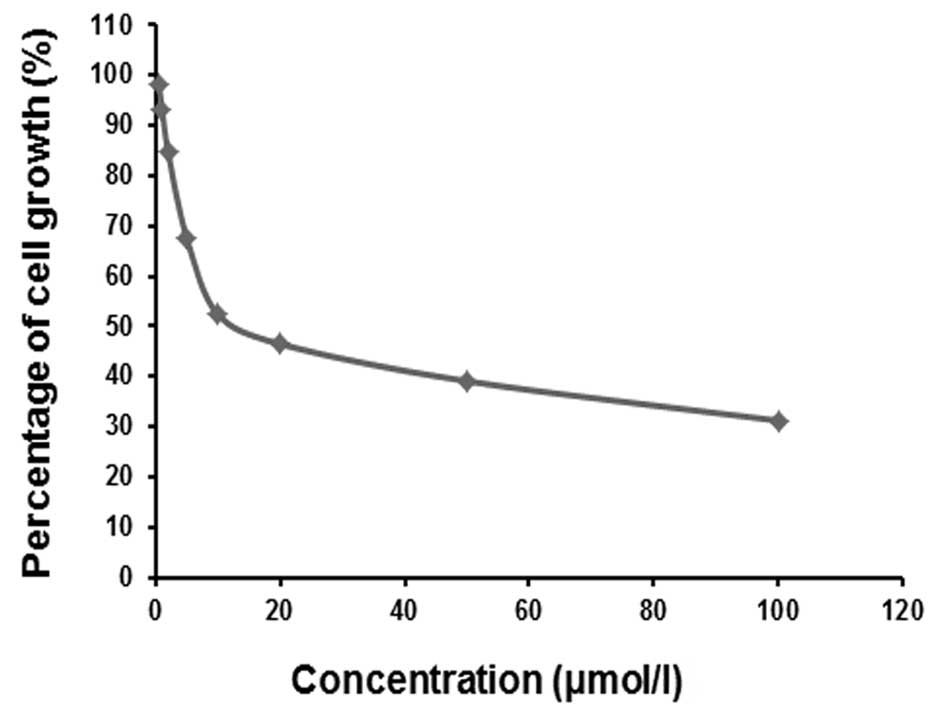
Suppression of MCF-7 cell proliferation
by As2O3
We measured MCF-7 cell proliferation 24 h after
treatment with multiple concentrations of
As2O3 and found that the proliferation of
cells was suppressed to varying degrees (Fig. 1). As2O3 doses
below 2 μM slightly inhibited (reduced by only 20%) cell
proliferation; at As2O3 concentrations above
5 μM, proliferation was much more significantly inhibited.
Markedly, the degree of inhibition increased in a dose-dependent
manner. The curvilinear regression equation
Y=91.7C−0.225 (r2=0.983, P<0.05) between
the percentage of cell proliferation (Y, %) and
As2O3 concentration (C, μM) was plotted, and
the IC50 at 24 h was calculated to be 11.7 μM. To
prevent the killing of MCF-7 cells by As2O3,
only 1 and 2 μM doses of compound were used to investigate the
radiosensitizing effects of As2O3 on cells
treated with 89SrCl2.
Cumulative absorbed dose of MCF-7 cells
exposed to 89SrCl2
MCF-7 cells were exposed for 48 h to 370, 740, 1480,
2960, 4440 and 5920 kBq/ml of 89SrCl2.
Cumulative absorbed doses of 89SrCl2 were
0.5, 1, 2, 4, 6 and 8 Gy, respectively.
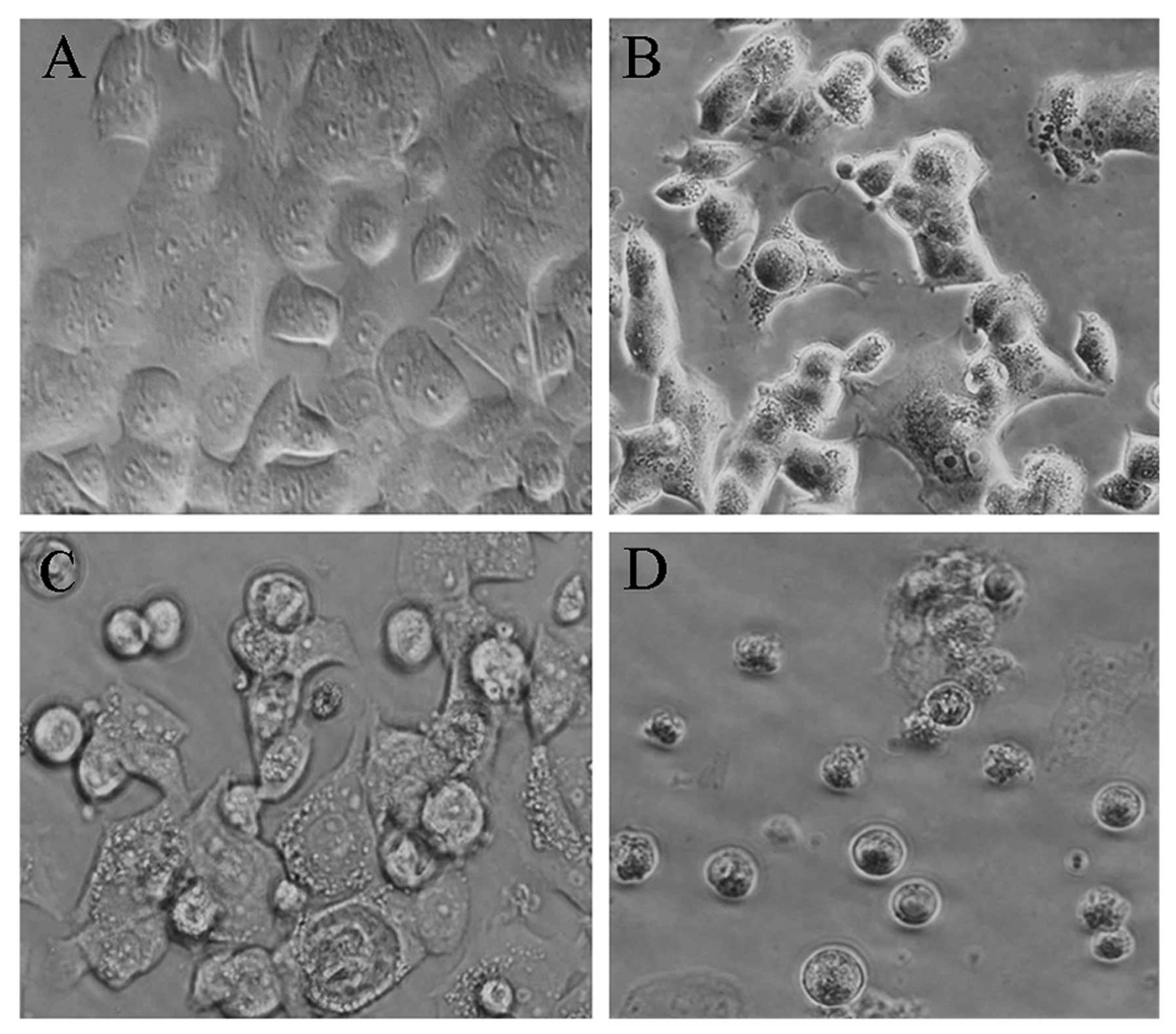
Morphological changes of MCF-7 cells
treated with As2O3 and
89SrCl2
MCF-7 cells were observed under an inverted
microscope. Cells in the control group were adhered to the culture
dish and grew normally. They displayed a typical polygon or spindle
shape, clear cell profile, and intact nuclei. However, cells in the
As2O3 group experienced morphological
changes, including partly condensed chromatin and the appearance of
vacuoles. Morphological changes of cells in the
89SrCl2 group were also apparent. With the
increase of 89SrCl2 concentration, cells
gradually became round and unattached. They showed decreased
refractive indexes, increased particles in crystal, vacuole-like
structures and partly ruptured nuclear membranes. Additionally,
debris appeared around cells, some dead cells were found floating
in the medium, soma became round in shape, the chromatin condensed
and karyopyknosis and fragmentation occurred. The proliferation of
cells in the combination group was significantly suppressed, with
increased cell debris, incomplete nuclear membranes and smaller
shapes and sizes (Fig. 2).
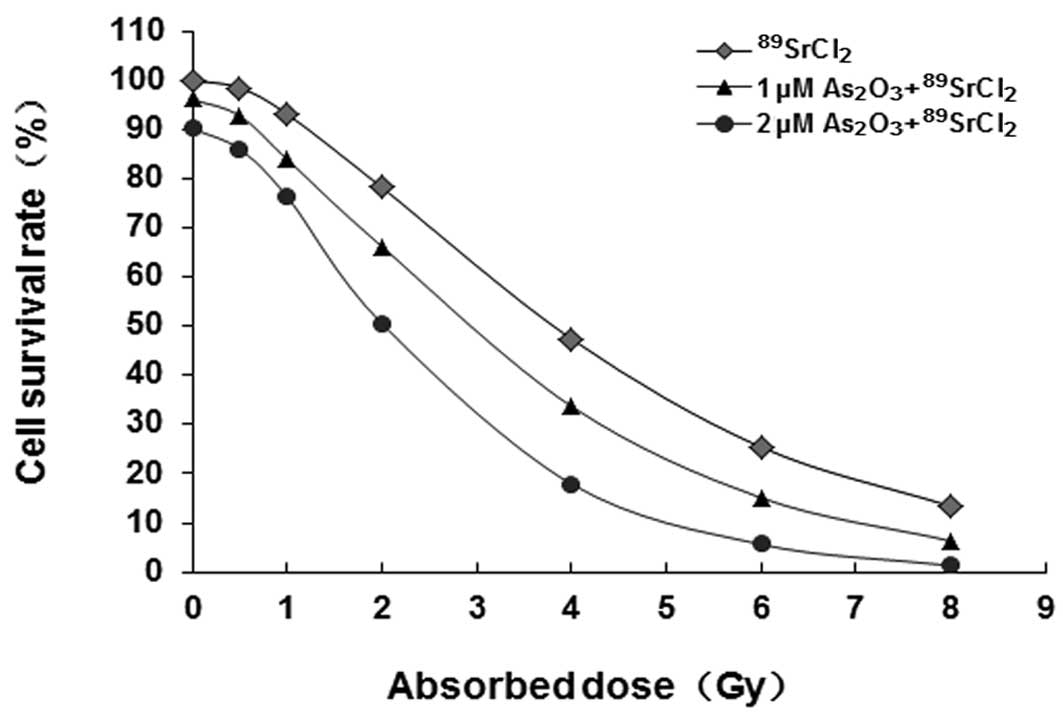
Clone formation assay evaluating the
radiosensitizing effects of As2O3 on MCF-7
cells treated with 89SrCl2
MCF-7 cell survival curves in different groups are
shown in Fig. 3. Curve equations
were as follows: 89SrCl2 group,
SF1=1−(1−e−D/2.89)2.18;
1 μM As2O3+89SrCl2
group, SF2=1−
(1−e−D/2.32)2.10; 2
μM As2O3+89SrCl2 group,
SF3=1−
(1−e−D/1.61)1.95.
For the formulas, SF represents cell survival rate and D is
irradiated dose. The shoulder zone of the cell survival curve in
the combination group became narrow, with an increased slope of the
linear part, reduced mean lethal dose (D0) and
quasi-threshold dose (Dq) (compared with those in the
89SrCl2 group, P<0.05). It showed that
As2O3 may radiosensitize MCF-7 cells exposed
to β-rays from 89SrCl2. Radiosensitization
effects were enhanced with increased As2O3
concentrations in the low level range. Clones are shown in Fig. 4.
Effects of As2O3
and 89SrCl2 on the distribution of MCF-7 cell
cycle
Cells in the control group were found mostly in
phases G0/G1 or S of the cell cycle. Cells in
the treatment groups were mostly found to be in the G2/M
phase. In both the As2O3-treated and
89SrCl2-treated groups, the percentage of
cells in G2/M phase increased in a dose-dependent manner
(compared with control, P<0.05). The percentage of cells in
G2/M phase in the combination group increased
dramatically. This increase was most apparent in the 2 μM
As2O3 and 4 Gy 89SrCl2
group; 45.8% of the cell population was in G2/M. Of
note, cells in S phase decreased compared with those in the
89SrCl2 group (P<0.05) (Table I).
 | Table IThe distribution of MCF-7 cell cycle
after exposure to As2O3 and
89SrCl2 (mean ± SD, n=6). |
Table I
The distribution of MCF-7 cell cycle
after exposure to As2O3 and
89SrCl2 (mean ± SD, n=6).
| Group |
G0/G1 (%) | S (%) | G2/M
(%) |
|---|
| Control | 65.6±10.6 | 25.0±4.8 | 9.4±5.3 |
| 1 μM
As2O3 | 60.9±8.4 | 23.6±6.0 | 15.5±4.7a |
| 2 μM
As2O3 | 55.9±6.6 | 25.8±6.5 | 18.3±5.9a |
| 1 Gy
89SrCl2 | 64.2±7.8 | 21.8±5.4 | 14.0±5.1a |
| 2 Gy
89SrCl2 | 59.1±6.8 | 19.5±4.9 | 21.4±6.1b |
| 4 Gy
89SrCl2 | 55.5±7.0 | 16.5±5.4 | 28.0±6.7b |
| 1 μM
As2O3+1 Gy 89SrCl2 | 59.7±8.3 | 20.7±5.3 | 19.6±5.7c |
| 1 μM
As2O3+2 Gy 89SrCl2 | 54.9±9.9 | 16.4±7.1 | 28.7±7.9c |
| 1 μM
As2O3+4 Gy 89SrCl2 | 52.6±7.5 | 9.9±2.1c | 37.5±8.7c |
| 2 μM
As2O3+1 Gy 89SrCl2 | 57.9±11.6 | 18.6±6.2 | 23.5±5.3c |
| 2 μM
As2O3+2 Gy 89SrCl2 | 52.7±8.1 | 10.5±5.2d | 36.8±8.1d |
| 2 μM
As2O3+4 Gy 89SrCl2 | 49.0±7.4 | 5.2±4.1d | 45.8±9.0d |
Effects of As2O3
and 89SrCl2 on MCF-7 cell apoptosis
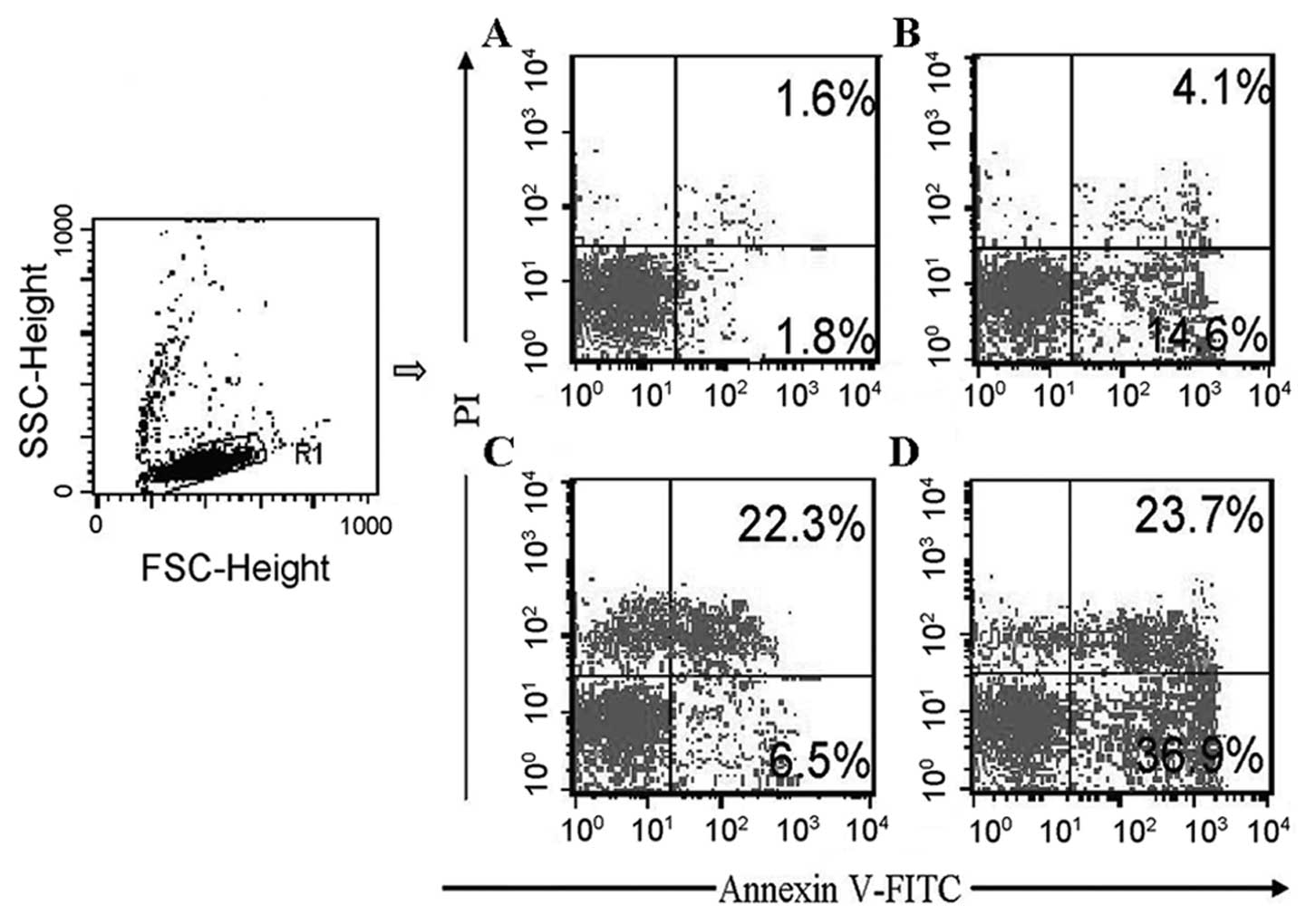
Annexin V-FITC/PI double staining flow cytometry was
used to discriminate among live cells, dead cells, and cells during
early or late apoptotis (Fig. 5).
Cells were divided into four subpopulations: live cells with low
levels of Annexin V and PI (LL zone), cells in the early apoptotic
phase with high levels of Annexin V and low levels of PI (LR zone),
cells in the late apoptotic phase, and dead cells with high levels
of Annexin V and PI (UR zone). We were also able to identify cells
that had experienced mechanical injury during the experiment as
having low levels of Annexin V and high levels of PI (UL zone). The
spontaneous apoptotic rate of MCF-7 cells in the control was low.
MCF-7 cells in the early apoptotic phase could be increased by
As2O3. 89SrCl2
increased the percentage of dead MCF-7 cells and increased the
number of cells in the late apoptotic phase from (2.1±0.7%) in the
control to (20.5±4.3%) in the 4 Gy 89SrCl2
group. There was only a limited effect in the early apoptotic
phase. In the combination groups, cells in both the early and late
apoptotic phases as well as dead cells were all significantly
increased. The rate of cells in the early apoptotic phase increased
from (6.7±1.8%) in the 4 Gy 89SrCl2 group to
(32.6±4.5%) in the 2 μM As2O3 and 4 Gy
89SrCl2 group (P<0.01). The number of
cells in the late apoptotic phase and dead cell groups increased
from (20.5±4.3%) in the 4 Gy 89SrCl2 group to
(25.7±6.2%) in the 2 μM As2O3 and 4 Gy
89SrCl2 group (P<0.05) (Fig. 5, Table
II).
 | Table IIThe apoptosis of MCF-7 cells exposed
to As2O3 and 89SrCl2
(mean ± SD, n=6). |
Table II
The apoptosis of MCF-7 cells exposed
to As2O3 and 89SrCl2
(mean ± SD, n=6).
| Group | Early apoptotic
cells (%) | Dead and late
apototic cells (%) |
|---|
| Control | 1.4±0.6 | 2.1±0.7 |
| 1 μM
As2O3 | 8.6±2.1a | 3.7±1.4 |
| 2 μM
As2O3 | 13.9±2.5a | 4.2±0.9 |
| 1 Gy
89SrCl2 | 3.1±0.5 | 6.3±1.4a |
| 2 Gy
89SrCl2 | 5.1±1.1 | 9.3±0.8a |
| 4 Gy
89SrCl2 | 6.7±1.8 | 20.5±4.3a |
| 1 μM
As2O3+1 Gy 89SrCl2 | 8.8±0.9 | 9.2±1.0d |
| 1 μM
As2O3+2 Gy 89SrCl2 | 13.1±2.6b | 14.9±2.3d |
| 1 μM
As2O3+4 Gy 89SrCl2 | 14.5±3.8b | 23.3±4.0 |
| 2 μM
As2O3+1 Gy 89SrCl2 | 19.2±5.2c | 9.6±2.4d |
| 2 μM
As2O3+2 Gy 89SrCl2 | 23.7±5.6b | 17.3±3.1d |
| 2 μM
As2O3+4 Gy 89SrCl2 | 32.6±4.5b | 25.7±6.2e |
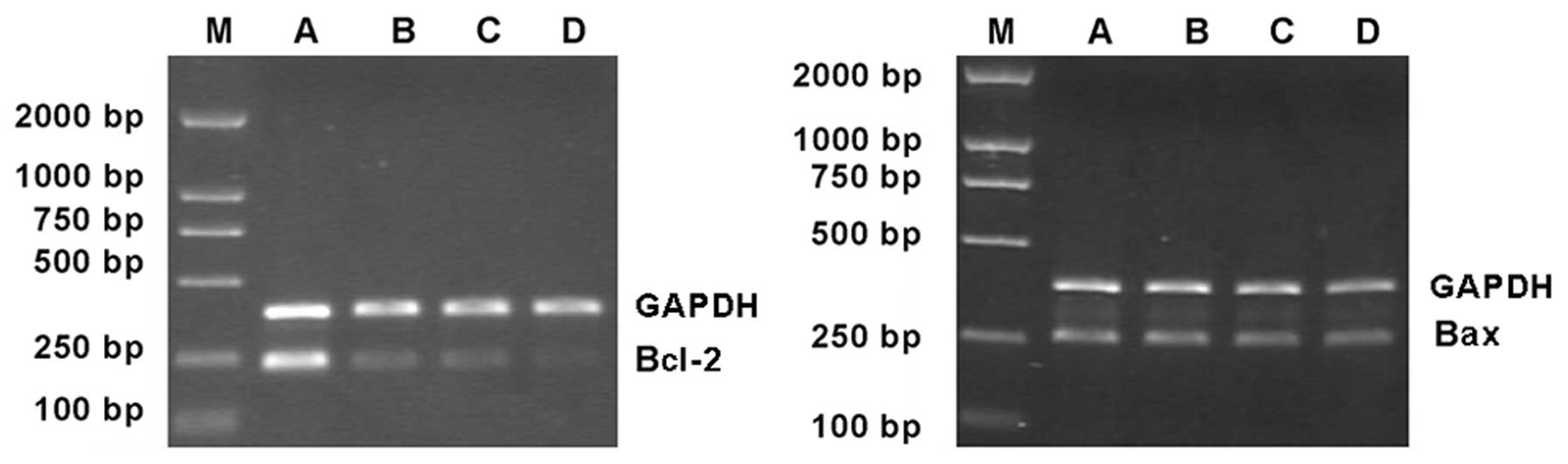
Effects of As2O3
and 89SrCl2 on the expression of Bcl-2 and
Bax mRNA in MCF-7 cells
The expression of Bcl-2 mRNA was lower in the 2 μM
As2O3 group and the 4 Gy
89SrCl2 group, compared to control. However,
expression of Bax mRNA was unchanged by treatment (Fig. 6).
Data was analysed by Smart view image software, and
the relative values (the ratio of the sample intensity to the
intensity of GAPDH) of expression of Bcl-2 mRNA in the 4 Gy
89SrCl2 group and in the combination group
were (27.25±3.56%) and (11.47±2.32%) (P<0.05), respectively.
There were no differences in Bax mRNA (P>0.05). Despite this,
the ratio of Bcl-2/Bax was significantly altered compared to
control (P<0.05). Thus, As2O3 represses
Bcl-2 mRNA expression caused by 89SrCl2
without affecting the expression of Bax mRNA (Fig. 6, Table
III).
 | Table IIIComparison between the expressions of
Bcl-2 and Bax mRNA in MCF-7 cells in different groups (%, mean ±
SD, n=3). |
Table III
Comparison between the expressions of
Bcl-2 and Bax mRNA in MCF-7 cells in different groups (%, mean ±
SD, n=3).
| Group | Bcl-2 | Bax | Bcl-2/Bax |
|---|
| Control | 93.41±8.79 | 49.36±6.23 | 1.84±0.21 |
| 2 μM
As2O3 | 46.52±5.24a | 52.14±5.71c | 0.93±0.12a |
| 4 Gy
89SrCl2 | 27.25±3.56a | 47.73±5.62c | 0.53±0.07a |
| Combination
group | 11.47±2.32b,d | 49.67±6.07c,e | 0.20±0.04b,d |
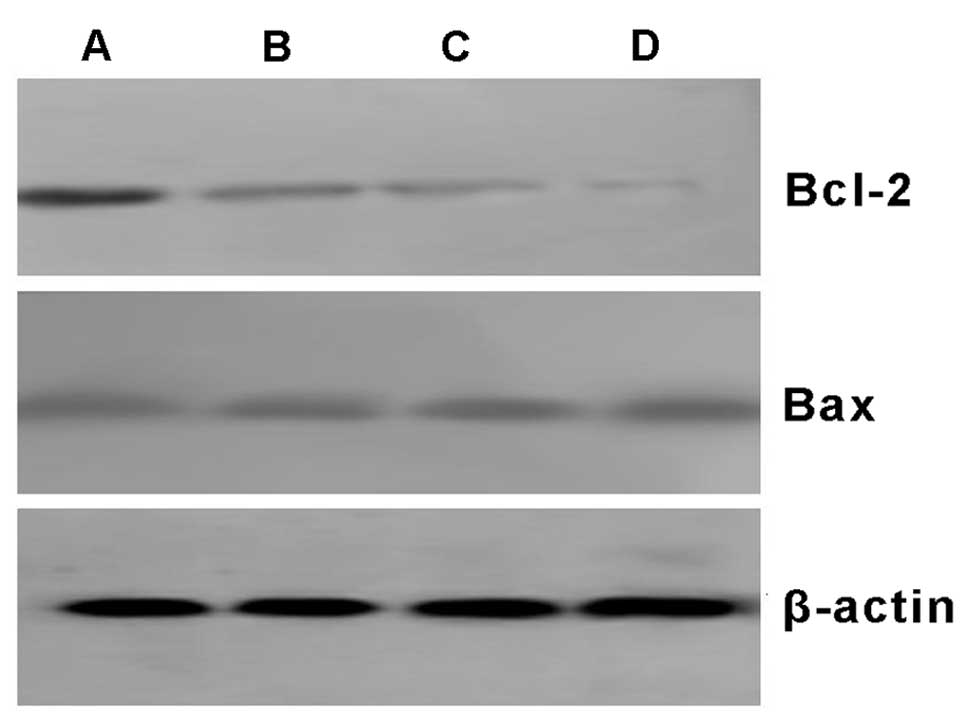
Effects of As2O3
and 89SrCl2 on the expression of Bcl-2 and
Bax proteins in MCF-7 cells
Bcl-2 and Bax proteins were both expressed in all
MCF-7 control cells. Bcl-2 protein levels decreased in the 2 μM
As2O3, the 4 Gy 89SrCl2
and the combination group. However, Bax protein was unchanged by
treatment. The relative expression of Bcl-2 protein in the
combination group was less than that in the 4 Gy
89SrCl2 group (P<0.05). There was no
significant difference between the relative expression of Bax
protein in each group (P>0.05) when analyzed by Smart view image
software. It showed that As2O3 can inhibit
the expression of Bcl-2 protein, without evident effects on the
expression of Bax protein. Thus, the ratio of Bcl-2/Bax was
decreased, which is in accordance with the effects of
89SrCl2 (Fig.
7, Table IV).
 | Table IVComparison between the expressions of
Bcl-2 and Bax proteins in MCF-7 cells (%, mean ± SD, n=3). |
Table IV
Comparison between the expressions of
Bcl-2 and Bax proteins in MCF-7 cells (%, mean ± SD, n=3).
| Group | Bcl-2 | Bax | Bcl-2/Bax |
|---|
| Control | 86.52±9.23 | 42.53±5.23 | 1.98±0.23 |
| 2 μM
As2O3 | 41.35±6.41a | 45.94±5.92c | 0.97±0.16a |
| 4 Gy
89SrCl2 | 33.58±4.54a | 49.68±4.85c | 0.64±0.09a |
| Combination
group | 12.72±2.16b,d | 52.15±6.34c,e | 0.22±0.05b,d |
Discussion
Breast cancer is one of the most common types of
cancer in women and it is often associated with bone metastases
(2,3). Currently, the common approach for the
treatment of bone metastasis is external beam radiation therapy.
While this method is relatively effective for a single large
metastasis, it is quite poor for the treatment of multiple lesions.
Systemic medication (analgesic drug therapy and chemotherapy) has
some advantages for patients with multiple bone metastases, but
there are other toxic side-effects associated with such therapies.
Systemic radionuclide therapy has drawn much attention in the
clinic. It is a relatively simple and economical procedure that
effectively relieves pain and can target multiple bone metastases
with few side-effects.
89SrCl2 has been widely and
successfully used for the treatment of bone metastases (15–17).
Similar to calcium, 89Sr is a nuclide that is commonly
found in bone. It is estimated that 30–80% of intravenously
injected 89Sr will aggregate in the bone, especially at
sites of active bone formation. Thus, its accumulation within bone
metastases is about 2–25 times higher than it is in normal bone
(18). The physical half-life of
89Sr is 50.56 days, and its biological half-life in
normal bone is 14 days. However, 12–90% of 89Sr is still
retained within bone metastases 3 months after 89Sr
injection. The long-term accumulation of 89Sr within the
bone metastatic lesion is a significant advantage for
89Sr to treat bone metastases. Physically,
89Sr emits pure β-rays with an average energy of 1.46
MeV, producing an average range in the bone of only 3 mm.
Therefore, 89Sr can effectively kill tumor cells while
limiting its effect on the surrounding bone. This results in the
shrinkage or elimination of bone metastases and relief of bone
pain. Nevertheless, there are still some patients who do not
respond due to radioresistance of tumor cells (19,20).
Thus, searching for safe and effective radiation sensitizers to
improve the efficacy of radiation therapy has become a hot topic in
radiation biology.
In recent years, arsenic and some Chinese herbs
have been found to have radiosensitizing effects.
As2O3 is the main component of Chinese
medicine arsenic, which was first used for the treatment of
patients with APL; in these patients, the treatment had a
beneficial effect. In addition, other studies (21–24)
have shown that As2O3 can induce tumor cell
apoptosis and inhibit the growth of some solid tumors, including
esophageal and liver cancers. This effect is partly due to
regulation of cell cycle progression, induction of apoptosis and
differentiation, blockage of tumor cell sub-lethal DNA damage
repair, reduction of telomerase activity and glutathione content in
tumor cells, and inhibition of angiogenesis (25). Such mechanisms can also enhance the
radiosensitivity of tumor cells (26), providing the theoretical basis for
the clinical application of As2O3 as a
radiation sensitizer. In this study, we demonstrated that
As2O3 dose-dependently inhibits the
proliferation of MCF-7 cells in vitro (with IC50
at 11.7 μM, Fig. 1). In our
radiosensitizing experiment, we found that there were no
significant morphological changes of MCF-7 cells treated with low
doses of As2O3, but obvious changes were
observed for the cells exposed to 89SrCl2 for
48 h, which was accompanied by cell growth inhibition. When cells
were treated with the combination of As2O3
and 89SrCl2 treatment, significant cell
death, apoptosis and growth inhibition were observed.
Radiation dose-survival curves reflect irradiated
cell survival in vitro and accurately determine
proliferation and cell death. The parameters D0,
Dq and N can be calculated from the radiation
dose-survival curve. The D0 is the average lethal dose
needed to cause cell death; this reflects the degree of cell
sensitivity to radiation. The greater the D0 value, the
lower the cell sensitivity to radiation. The threshold dose
Dq value reflects the repair capacity of cells to
sublethal damage and negatively correlates with radiation
sensitivity. The N is the extrapolation number, reflecting the
number of radiation-sensitive areas within the cell or required
number of target hits. Any two of the three parameters can be used
to reflect the extent of radiation sensitivity of cells (14).
In our study, we found that compared to
89SrCl2 radiation alone, the addition of
As2O3 significantly decreased the
D0 and Dq values of MCF-7 cells (P<0.05).
This translated into radiosensitization ratios of 1.25 and 1.79,
respectively, indicating that pretreatment with
As2O3 significantly reduces the average
lethal dose of MCF-7 cells to 89SrCl2
irradiation. Thus, As2O3 significantly
increases the radiosensitivity of MCF-7 cells to
89SrCl2 irradiation in a dose-dependent
manner.
Uncontrolled cell proliferation is one of the
prominent features of cancer cells, which may result from
deregulated cell cycle control. There are a few key phase
regulatory sites that play key roles in the regulation of cell
cycle progression. Of these, the regulatory sites at the
G1/S and G2/M phases are the most important
(27,28). Many anticancer drugs cause cell
cycle arrest in the G1/S or G2/M phases to
inhibit tumor proliferation (29).
The radiosensitivity of tumor cells is closely related to both
their capacity to repair DNA and their phase in the cell cycle.
Cells in the G2/M phase are most sensitive to radiation;
cells in early S phase and G1 phase are moderately
sensitive, and cells in late S phase are least sensitive to
radiation (30). Therefore, an
important consideration to improving radiosensitivity and
radiotherapy efficacy involves promoting tumor cells to enter the
cell cycle but to remain in the G2/M phase. In our
study, we found that 89SrCl2 or
As2O3 treatment dose-dependently increased
the percentage of MCF-7 cells in G2/M phase, but there
was no significant effect on G0/G1 phase.
This suggested that 89SrCl2 or
As2O3 treatment could cause MCF-7 cells to
arrest in G2/M phase. Compared to
89SrCl2 treatment alone, addition of
As2O3 significantly increased the percentage
of cells in G2/M phase (and decreased in
G0/G1 phase). The percentage of cells in
radioresistant S phase reached its lowest proportion. These results
suggest that the G2/M phase arrest and decrease of cell
number in S phase might be one mechanism by which
As2O3 sensitizes MCF-7 cells to
89SrCl2 irradiation. These results are
consistent with previous studies (31–33)
showing that As2O3 can upregulate cyclin B,
CDK-1 and p21, but downregulate CDK-6, CDC-2 and cyclin A
expression. This correlated with an effect on G2/M cell
cycle arrest.
Radiation-induced apoptosis is an important
mechanism to kill tumor cells. Thus, inducing apoptosis in tumor
cells has become a new strategy in radiation oncology. Apoptosis is
also the common pathway by which many antitumor drugs exert their
effects. FCM is a common method for studying apoptosis, and it can
sensitively and accurately distinguish between early and late
phases of apoptotic cells via double staining for Annexin V/PI
(34). In the present study, we
found that 89SrCl2 irradiation significantly
induced late phase apoptosis and cell death but had little effect
on early phase apoptosis of MCF-7 cells. However, addition of
As2O3 significantly induced not only late
phase apoptosis and cell death but also early phase apoptosis of
MCF-7 cells (P<0.01; P<0.05 vs. 89SrCl2
treatment only). These results suggest that increasing the
sensitivity of MCF-7 cells to 89SrCl2-induced
apoptosis might be one of the mechanisms by which
As2O3 sensitizes cells to
89SrCl2 irradiation.
Numerous factors affect the response of tumor cells
to radiation, including the ability to repair DNA double strand
breaks, transmembrane signal transduction and apoptosis-related
gene expression (35). Previous
studies (36–40) showed that Bcl-2 and Bax play
significant roles in the regulation of apoptosis after irradiation.
For example, it has been reported that knockdown of Bcl-2
expression significantly sensitized human PC-3 prostate cancer
cells to irradiation (38). In this
study, we found that the expression of Bcl-2 and Bax at both the
mRNA and protein levels can be detected in MCF-7 cells without any
treatment. Treatment with 2 μM As2O3 or 4 Gy
89SrCl2 irradiation reduced the antiapoptotic
gene Bcl-2 at both the mRNA and protein levels. This reduction was
more significant in the combination group (P<0.05 vs. 4 Gy
89SrCl2 group). We did not detect significant
changes in Bax expression, but the overall Bcl-2/Bax ration did
decrease in the individual experimental groups compared to control.
This suggests that the radiosensitizing effect of
As2O3 might be due to inhibition of Bcl-2
expression and subsequent reduction of the ratio of Bcl-2/Bax,
which increases the sensitivity of MCF-7 cells to
89SrCl2 irradiation. Another study showed
that with the reduction of the Bcl-2/Bax ratio, the formation of
Bax homodimers increases, resulting in an increase of the
permeability of the mitochondrial membrane in tumor cells (41). This leads to the release of
cytochrome c, activation of downstream signaling pathways,
and activation of caspase family members and apoptosis. Kumagai
et al (42) have
consistently found that As2O3-induced
apoptosis is correlated with downregulation of Bcl-2 and Bcl-xL
expressions in NB4 cells.
In summary, we found that low doses of
As2O3 (below 20% of the IC50)
significantly enhance the sensitivity of MCF-7 cells to
89SrCl2 β-ray irradiation in vitro,
which is, at least in part, through inducing G2/M cell
cycle arrest, downregulating Bcl-2 gene expression, and reducing
the ratio of Bcl-2/Bax. Consequently, the overall effect is an
increase in cell apoptosis. Further understanding of the underlying
molecular mechanisms and exploring the possibility of
As2O3 radiosensitization in vivo may
have significant clinical implications and provide experimental
evidence to improve the radionuclide therapeutic efficacy on
malignant bone metastasis.
In conclusion, we demonstrated that 1–2 μM of
As2O3 could increase the radiosensitivity of
human breast cancer MCF-7 cell line by inducing G2 phase
delay. The lethal effect of 89SrCl2 on MCF-7
cells thus was enhanced concomitantly with
As2O3 pretreatment. The mechanism could be
involved in the raising of the apoptosis rate of cells owing to the
reduction of the Bcl-2/Bax rate.
Acknowledgements
This study was supported by the Anhui Province
Natural Science Foundation (1208085MH162), Anhui Province
Department of Education Fund (20101941, KJ2011Z162), Anhui Province
Department of Health Medical Science Fund (2010C081) and the Key
Laboratory Program of New Thin Solar Cell, Chinese Academy of
Science (2010007). We also thank the experimental center of
clinical laboratory diagnostics in Bengbu Medical College for
providing us the MCF-7 cell line.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Yang L, Parkin DM, Li L and Chen Y: Time
trends in cancer mortality in China: 1987–1999. Int J Cancer.
106:771–783. 2003.
|
|
3
|
Baczyk M, Czepczynski R, Milecki P,
Pisarek M, Oleksa R and Sowinski J: 89Sr versus 153Sm-EDTMP:
comparison of treatment efficacy of painful bone metastases in
prostate and breast carcinoma. Nucl Med Commun. 28:245–250. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adams GE, Ahmed I, Sheldon PW and
Stratford IJ: RSU 1069, a 2-nitroimidazole containing an alkylating
group: high efficiency as a radio- and chemosensitizer in vitro and
in vivo. Int J Radiat Oncol Biol Phys. 10:1653–1656. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ni X, Zhang Y, Ribas J, et al:
Prostate-targeted radiosensitization via aptamer-shRNA chimeras in
human tumor xenografts. J Clin Invest. 121:2383–2390. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baj G, Arnulfo A, Deaglio S, et al:
Arsenic trioxide and breast cancer: analysis of the apoptotic,
differentiative and immunomodulatory effects. Breast Cancer Res
Treat. 73:61–73. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Qu X, Qu J, et al: Arsenic trioxide
induces apoptosis and G2/M phase arrest by inducing Cbl to inhibit
PI3K/Akt signaling and thereby regulate p53 activation. Cancer
Lett. 284:208–215. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Siu KP, Chan JY and Fung KP: Effect of
arsenic trioxide on human hepatocellular carcinoma HepG2 cells:
inhibition of proliferation and induction of apoptosis. Life Sci.
71:275–285. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ling YH, Jiang JD, Holland JF and
Perez-Soler R: Arsenic trioxide produces polymerization of
microtubules and mitotic arrest before apoptosis in human tumor
cell lines. Mol Pharmacol. 62:529–538. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Davison K, Mann KK and Miller WH Jr:
Arsenic trioxide: mechanisms of action. Semin Hematol. 39:3–7.
2002. View Article : Google Scholar
|
|
11
|
Ai Z, Lu W, Ton S, et al: Arsenic
trioxide-mediated growth inhibition in gallbladder carcinoma cells
via down-regulation of Cyclin D1 transcription mediated by Sp1
transcription factor. Biochem Biophys Res Commun. 360:684–689.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin LM, Li BX, Xiao JB, Lin DH and Yang
BF: Synergistic effect of all-trans-retinoic acid and arsenic
trioxide on growth inhibition and apoptosis in human hepatoma,
breast cancer, and lung cancer cells in vitro. World J
Gastroenterol. 11:5633–5637. 2005.PubMed/NCBI
|
|
13
|
Friesen C, Lubatschofski A, Kotzerke J,
Buchmann I, Reske SN and Debatin KM: Beta-irradiation used for
systemic radioimmunotherapy induces apoptosis and activates
apoptosis pathways in leukaemia cells. Eur J Nucl Med Mol Imaging.
30:1251–1261. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xia S, Yu S, Fu Q, et al: Inhibiting
PI3K/Akt pathway increases DNA damage of cervical carcinoma HeLa
cells by drug radiosensitization. J Huazhong Univ Sci Technolog Med
Sci. 30:360–364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Giammarile F, Mognetti T and Resche I:
Bone pain palliation with strontium-89 in cancer patients with bone
metastases. Q J Nucl Med. 45:78–83. 2001.PubMed/NCBI
|
|
16
|
Falkmer U, Jarhult J, Wersall P and
Cavallin-Stahl E: A systematic overview of radiation therapy
effects in skeletal metastases. Acta Oncol. 42:620–633. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gkialas I, Iordanidou L, Galanakis I and
Giannopoulos S: The use of radioisotopes for palliation of
metastatic bone pain. J BUON. 13:177–183. 2008.PubMed/NCBI
|
|
18
|
Finlay IG, Mason MD and Shelley M:
Radioisotopes for the palliation of metastatic bone cancer: a
systematic review. Lancet Oncol. 6:392–400. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gunawardana DH, Lichtenstein M, Better N
and Rosenthal M: Results of strontium-89 therapy in patients with
prostate cancer resistant to chemotherapy. Clin Nucl Med. 29:81–85.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rasch-Isla Munoz A and Catano Catano JG:
Usefulness of bone-specific alkaline phosphatase for bone
metastases detection in prostate cancer. Arch Esp Urol. 57:693–698.
2004.(In Spanish).
|
|
21
|
Wetzler M, Brady MT, Tracy E, et al:
Arsenic trioxide affects signal transducer and activator of
transcription proteins through alteration of protein tyrosine
kinase phosphorylation. Clin Cancer Res. 12:6817–6825. 2006.
View Article : Google Scholar
|
|
22
|
Kang SH, Song JH, Kang HK, et al: Arsenic
trioxide-induced apoptosis is independent of stress-responsive
signaling pathways but sensitive to inhibition of inducible nitric
oxide synthase in HepG2 cells. Exp Mol Med. 35:83–90. 2003.
View Article : Google Scholar
|
|
23
|
Xiao YF, Liu SX, Wu DD, Chen X and Ren LF:
Inhibitory effect of arsenic trioxide on angiogenesis and
expression of vascular endothelial growth factor in gastric cancer.
World J Gastroenterol. 12:5780–5786. 2006.PubMed/NCBI
|
|
24
|
Shen ZY, Zhang Y, Chen JY, et al:
Intratumoral injection of arsenic to enhance antitumor efficacy in
human esophageal carcinoma cell xenografts. Oncol Rep. 11:155–159.
2004.PubMed/NCBI
|
|
25
|
Shao QS, Ye ZY, Ling ZQ and Ke JJ: Cell
cycle arrest and apoptotic cell death in cultured human gastric
carcinoma cells mediated by arsenic trioxide. World J
Gastroenterol. 11:3451–3456. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ning S and Knox SJ: Optimization of
combination therapy of arsenic trioxide and fractionated
radiotherapy for malignant glioma. Int J Radiat Oncol Biol Phys.
65:493–498. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park WH, Seol JG, Kim ES, et al: Arsenic
trioxide-mediated growth inhibition in MC/CAR myeloma cells via
cell cycle arrest in association with induction of cyclin-dependent
kinase inhibitor, p21, and apoptosis. Cancer Res. 60:3065–3071.
2000.PubMed/NCBI
|
|
28
|
Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K
and Linn S: Molecular mechanisms of mammalian DNA repair and the
DNA damage checkpoints. Annu Rev Biochem. 73:39–85. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Umemura S, Takekoshi S, Suzuki Y, Saitoh
Y, Tokuda Y and Osamura RY: Estrogen receptor-negative and human
epidermal growth factor receptor 2-negative breast cancer tissue
have the highest Ki-67 labeling index and EGFR expression: gene
amplification does not contribute to EGFR expression. Oncol Rep.
14:337–343. 2005.
|
|
30
|
Rupnow BA, Murtha AD, Alarcon RM, Giaccia
AJ and Knox SJ: Direct evidence that apoptosis enhances tumor
responses to fractionated radiotherapy. Cancer Res. 58:1779–1784.
1998.PubMed/NCBI
|
|
31
|
Park JW, Choi YJ, Jang MA, et al: Arsenic
trioxide induces G2/M growth arrest and apoptosis after caspase-3
activation and Bcl-2 phosphorylation in promonocytic U937 cells.
Biochem Biophys Res Commun. 286:726–734. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yih LH, Hsueh SW, Luu WS, Chiu TH and Lee
TC: Arsenite induces prominent mitotic arrest via inhibition of G2
checkpoint activation in CGL-2 cells. Carcinogenesis. 26:53–63.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dai J, Weinberg RS, Waxman S and Jing Y:
Malignant cells can be sensitized to undergo growth inhibition and
apoptosis by arsenic trioxide through modulation of the glutathione
redox system. Blood. 93:268–277. 1999.PubMed/NCBI
|
|
34
|
Middleton G, Cox SW, Korsmeyer S and
Davies AM: Differences in Bcl-2- and Bax-independent function in
regulating apoptosis in sensory neuron populations. Eur J Neurosci.
12:819–827. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li L, Story M and Legerski RJ: Cellular
responses to ionizing radiation damage. Int J Radiat Oncol Biol
Phys. 49:1157–1162. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gupta S, Yel L, Kim D, Kim C, Chiplunkar S
and Gollapudi S: Arsenic trioxide induces apoptosis in peripheral
blood T lymphocyte subsets by inducing oxidative stress: a role of
Bcl-2. Mol Cancer Ther. 2:711–719. 2003.PubMed/NCBI
|
|
37
|
Cory S, Huang DC and Adams JM: The Bcl-2
family: roles in cell survival and oncogenesis. Oncogene.
22:8590–8607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu L, Yang D, Wang S, et al: (−)-Gossypol
enhances response to radiation therapy and results in tumor
regression of human prostate cancer. Mol Cancer Ther. 4:197–205.
2005.
|
|
39
|
Anai S, Goodison S, Shiverick K, Hirao Y,
Brown BD and Rosser CJ: Knock-down of Bcl-2 by antisense
oligodeoxynucleotides induces radiosensitization and inhibition of
angiogenesis in human PC-3 prostate tumor xenografts. Mol Cancer
Ther. 6:101–111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shore GC and Viallet J: Modulating the
Bcl-2 family of apoptosis suppressors for potential therapeutic
benefit in cancer. Hematology Am Soc Hematol Educ Program. 226–230.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chou JJ, Li H, Salvesen GS, Yuan J and
Wagner G: Solution structure of BID, an intracellular amplifier of
apoptotic signaling. Cell. 96:615–624. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kumagai T, Shih LY, Hughes SV, et al:
19-Nor-1,25(OH)2D2 (a novel, noncalcemic vitamin D analogue),
combined with arsenic trioxide, has potent antitumor activity
against myeloid leukemia. Cancer Res. 65:2488–2497. 2005.
View Article : Google Scholar
|