Introduction
The accumulation of unfolded proteins can be induced
by agents such as tunicamycin (TM), a naturally occurring
antibiotic that induces ER stress by inhibiting the first step in
the biosynthesis of N-linked oligosaccharides in cells (1,2).
Inhibition of N-linked glycosylation by TM results in cell death in
various cell types (1,3,4). ER
stress triggers a unique pathway of apoptosis that is mediated via
activation of ER-resident caspases (5,6).
CD44, a highly glycosylated cell surface adhesion
molecule involved in cell-matrix interactions, was originally
identified as a receptor for hyaluronate and later found to have
affinity for various molecules (7).
CD44 has many variant forms, which are generated by alternative
splicing of at least 10 exons named v1-v10 (8). Normal cells usually express standard
CD44 (CD44s), which lacks the entire group of variant exons
(9,10). Soluble CD44v6 is considered as a
useful marker of tumor prognosis (11). For example, the malignancy grade of
colon cancer is related to the expression of CD44v6 (12). Specifically, tumor progression
requires crosstalk between neighboring tumors, wherein the tumor
matrix plays an essential role and CD44v6 coordinates tumor
matrix-triggered motility and apoptosis resistance (13). Many tumor cells resistant to
apoptosis express CD44v6, which co-localizes and interacts with
Fas, thereby inhibiting Fas-mediated apoptosis (13,14).
Thus, CD44v6 ectodomain shedding may have important effects on the
apoptosis of tumor cells.
Matrix metalloproteinases (MMPs) are extracellular
zinc-dependent endopeptidases involved in the degradation and
remodeling of the extracellular matrix (ECM) in physiological and
pathological processes in vertebrates (15). Since all MMPs are synthesized as
inactive proenzymes, their ability to act as proteases is dependent
on the presence of appropriate activation mechanisms (16). In addition to their role in ECM
turnover, MMPs also target other proteinases, latent growth
factors, cell surface receptors, cytokines, cell adhesion molecules
and the release of apoptotic Fas ligands (17). Based on structural relationships and
substrate specificities, MMPs have been divided into 4 subfamilies:
collagenases, stromelysins, gelatinases and membrane-tethered MMPs
(MT-MMPs). Further, the ADAM (a disintegrin and metalloproteinase)
family of metzincin proteinases is closely related to MMPs
(18).
ADAM10 has a wide variety of substrates and can
remodel ECM components in addition to membrane proteins (19). ADAM10 has been implicated in the
shedding of CD44v6 ectodomain from the cell surface (20–22).
Release of CD44v6 ectodomain from the cell surface results in
dynamic regulation of the interaction between CD44 and the ECM.
CD44v6 ectodomain cleavage event also initiates the CD44-mediated
intracellular signaling pathway (22). The proteolytic activity of MMP-9
(gelatinase B) has been implicated in various physiological and
pathological conditions. Specifically, the extracellular domain of
CD44 binds MMP-9, which cleaves CD44s as well as CD44v6 (9,23).
Further, the MMP-13 (collagenase-3) cascade results in the
activation of pro-MMP-9 to MMP-9 (24,25),
and the inhibition of MMP-13 prevents MMP-9 activation (25).
Considering the importance of CD44v6 to apoptosis
resistance, we determined the expression profiles of MMP-9, MMP-13
and ADAM10, which are responsible for CD44v6 ectodomain shedding
during TM-induced apoptosis of Caki-2 cells.
Materials and methods
Reagents
Caki-2 human renal carcinoma cells were obtained
from the American Type Culture Collection (ATCC; Rockville, MD).
Tunicamycin (TM) and MTT were purchased from Sigma (St. Louis, MO).
GI254023X (ADAM10-specific inhibitor) was obtained from
GlaxoSmithKline (Stevenage, UK). MMP-9/-13 inhibitor, GM6001 and
anti-MMP-9 were purchased Calbiochem (La Jolla, CA). Monoclonal
anti-CD44v6 and polyclonal anti-ADAM10 and MMP-13 antibodies were
purchased from Chemicon International, Inc. (Temecula, CA).
Monoclonal anti-β-actin, polyclonal anti-PARP, anti-GADD153,
anti-KDEL and anti-procaspase-3 were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA).
Cell culture
Cells were plated onto 6-well culture plates and
maintained at 37˚C in 5% CO2 and 95% air. The culture
medium consisted of Dulbecco’s modified Eagle’s medium (DMEM; Gibco
Invitrogen, Carlsbad, CA), 10% (v/v) fetal bovine serum (FBS) and
1% (v/v) penicillin-streptomycin. Cells were plated at a density of
0.6×106 cells/well, incubated for 24 h, and then treated
with TM (0.01, 0.1 and 5 μg/ml) for 24 h.
Preparation of conditioned medium
After serum cultures for 24 h, cells were washed
twice with serum-free DMEM. Then, cells were treated with or
without TM (0.01, 0.1 and 5 μg/ml) in serum-free DMEM for 24 h,
after which serum-free conditioned medium was collected. To examine
the levels of MMP-9, MMP-13, ADAM10 and cleaved CD44v6 ectodomain,
proteins were precipitated from equal-volume aliquots of
supernatant using 10% ice-cold trichloroacetic acid (TCA). The
precipitates were washed twice with 100% acetone, air-dried,
dissolved in RIPA buffer and stored at -20˚C until use.
Western blot analysis
Equal volumes of conditioned media or equal amounts
of protein lysate (30 μg) in RIPA buffer were separated by 7.5 or
10% SDS-PAGE under reducing conditions. After transfer,
nitrocellulose membranes were incubated for 1 h with primary
antibodies against anti-CD44v6, ADAM10, MMP-13, PARP, caspase-3,
CHOP, GRP78 and β-actin. The results were visualized using
horseradish peroxidase (HRP)-conjugated second antibodies, along
with an enhanced chemiluminescence kit.
FACS analysis
Cells (~1×106) were suspended in 100 μl
of PBS, and 200 μl of 95% ethanol were added while vortexing. Then,
the cells were incubated at 4˚C for 1 h, washed with PBS, and
resuspended in 250 μl of 1.12% sodium citrate buffer (pH 8.4)
together with 12.5 μg of RNase. Incubation was continued at 37˚C
for 30 min. The cellular DNA was then stained by applying 250 μl of
propidium iodide (50 μg/ml) for 30 min at room temperature. The
stained cells were analyzed by fluorescent activated cell sorting
(FACS) on a FACScan flow cytometer to determine relative DNA
content based on red fluorescence.
MTT assay
Cell viability was determined by MTT assay. Caki-2
cells in 96-well plates were incubated with TM (5 μg/ml) in the
presence of several MMP inhibitors for 24 h. Then, 10 μl of stock
MTT solution (5 mg/ml in PBS) were added to each well, after which
cells were incubated for another 4 h at 37˚C. The supernatants were
then aspirated carefully, and 100 μl of DMSO were added to each
well. The plates were shaken for an additional 5 min on a plate
shaker, and the absorbance values were read using a Microplate
Reader (Bio-Rad, Hercules, CA) at 570 nm.
Zymography
To profile secreted gelatinases, gelatin gel
zymography was performed as previously described (15). SDS-containing 8% polyacrylamide gels
were co-polymerized with gelatin as substrates for the
identification of MMP-9 in culture medium. Conditioned medium was
loaded onto the gel without boiling and electrophoresed under
non-reducing conditions. The gels were then shaken for 1 h in a
2.5% solution of Triton X-100 at room temperature to remove SDS and
were incubated at 37˚C in reaction buffer (50 mM Tris-HCl, pH 7.5;
10 mM CaCl2) overnight to allow proteinases to react
with the substrate. Bands containing gelatin-degrading MMP-9 appear
as clear bands on a dark blue stained background after staining
with Coomassie brilliant blue R-250 (Bio-Rad). Both proenzyme and
proteolytically activated forms of MMPs were visualized by
zymography.
Statistical analysis
Three or more separate experiments were performed.
Statistical analysis was performed by paired Student’s t-test or
ANOVA.
Results
TM-induced ER stress and PARP cleavage
and apoptosis of Caki-2 cells
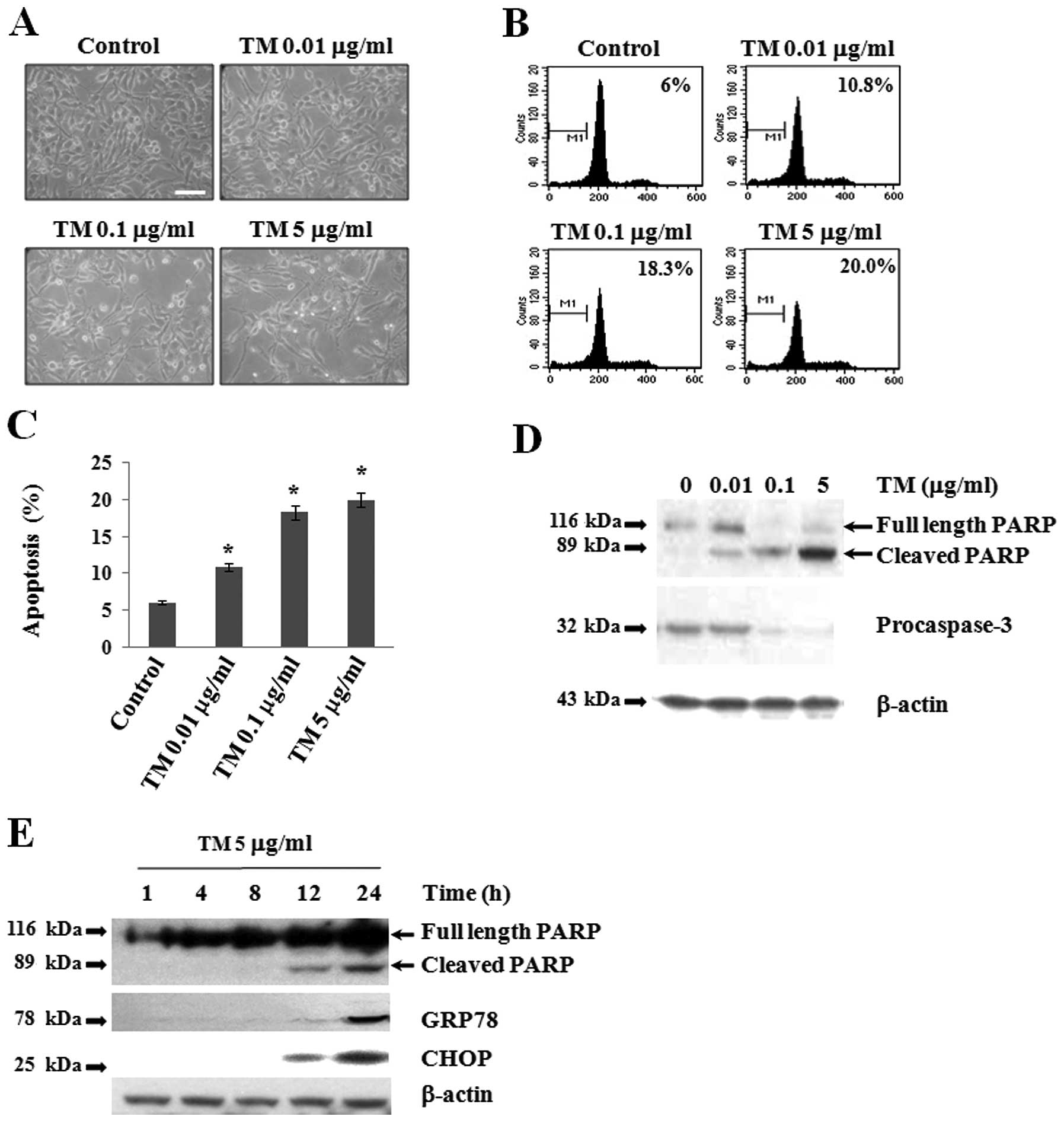
To investigate the effect of TM on the apoptosis of
Caki-2 cells, cells were treated with TM (0.01, 0.1 and 5 μg/ml) in
serum-free medium for 24 h. Three established criteria were used to
assess apoptosis in our system. Firstly, we examined changes in
cell morphologies after TM treatment and observed apoptotic
characteristics, such as cell shrinkage and detachment of cells
from the plate in a dose-dependent manner (Fig. 1A). Secondly, apoptosis of Caki-2
cells was confirmed using flow cytometric analysis to detect
hypo-diploid cell populations with and without TM treatment for 24
h. TM treatment markedly increased the accumulation of sub-G1 phase
cells and induced apoptosis in a dose-dependent manner (Fig. 1B and C). Caspase-3 is one of the key
executioners of apoptosis. Further, PARP-1 is known to function
during DNA repair and to bind DNA strand breaks during apoptosis
(26). Thirdly, to determine
whether or not caspase-3 activation and PARP cleavage are involved
in TM-induced apoptosis, cells were treated with TM (0.01, 0.1 and
5 μg/ml) for 24 h (Fig. 1D). In
western blot analysis with anti-pro-caspase-3 antibody, high levels
of pro-caspase-3 (32 kDa) were detected in cell lysates of the
control culture, whereas TM treatment downregulated the expression
of pro-caspase-3 in a dose-dependent manner. Interestingly,
pro-caspase-3 protein was not detected at all upon TM treatment at
5 μg/ml, which suggests that procaspase-3 was presumably cleaved
into active caspase-3 (27).
Furthermore, we found that the cleavage of PARP increased upon TM
treatment in a dose-dependent manner, accompanied by concomitant
downregulation of procaspase-3 expression. Further, PARP cleavage
was barely detectable in the control culture, which indicates that
caspase-3 was involved in TM-induced apoptosis. The ER stress
response is involved in the activation of ATF6 and subsequent
induction of GRP78 and GADD153 (CHOP), a key component in ER
stress-mediated apoptosis (28).
Using western blot analysis, we examined whether or not both GRP78
and CHOP are induced during TM-induced apoptosis. For this, cells
were treated with 5 μg/ml of TM in serum-free medium for 1, 4, 8,
12 and 24 h (Fig. 1E). The protein
levels of GRP78 and CHOP were detected after 12 h of TM treatment,
and higher levels of both proteins were clearly detected at 24 h.
Simultaneously, we clearly observed PARP cleavage at 12 and 24 h.
These results indicate that TM-induced ER stress stimulated
apoptosis of Caki-2 cells.
TM-induced CD44v6 ectodomain shedding and
secretion of ADAM10
Cultured cells interact with a matrix via adhesion
molecules for proper function, but TM treatment induced cell
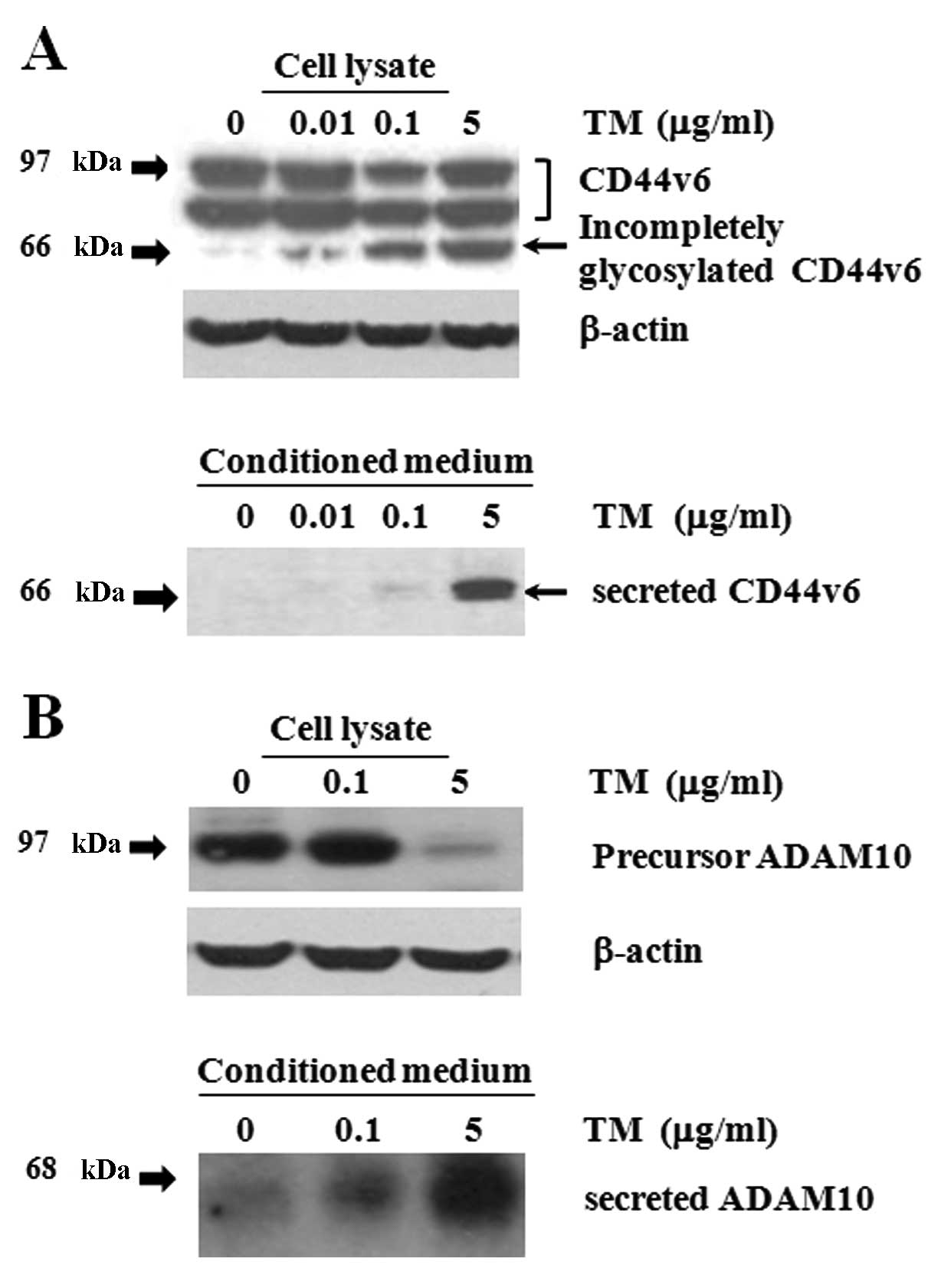
detachment from plates in addition to apoptosis (Fig. 1). To measure the levels of CD44v6 as
well as its cleaved form, equal amounts of lysate proteins were
immunoblotted with a monoclonal anti-CD44v6 antibody. The core
protein of CD44s is 37 kDa with an apparent molecular mass of ~85
kDa due to glycosylation (22).
Extensive post-translational glycosylation of different CD44
variants produces proteins with molecular masses ranging from 80 to
200 kDa (29). Using western blot
analysis, high levels of uncleaved CD44v6 (97 and 90 KDa) were
strongly detected in control cell lysates cultured for 24 h
(Fig. 2A). Interestingly, three
major bands (97, 90 and 66 kDa) corresponding to CD44v6 were
detected in TM-treated cultures; secreted CD44v6 (66 kDa; arrow)
was only detected in TM-treated cells in a dose-dependent manner,
presumably due to the inhibition of N-linked glycosylation.
Furthermore, to detect shedding of CD44v6 ectodomain, conditioned
medium from cells cultured for 24 h was subjected to western blot
analysis (Fig. 2A). As expected,
high levels of the secreted form of CD44v6 (66 kDa) were detected
in cells treated with TM (5 μg/ml). In contrast, secretion of
CD44v6 was not detected at all in control cultures. Figs. 1 and 2 together indicate that cleavage of CD44v6
ectodomain is regulated during TM-induced apoptosis of Caki-2
cells.
ADAM10 is expressed as an inactive pro-form (97
kDa), which is later processed to a shorter, active form (68 kDa)
(22). We next examined the
presence of active and secreted forms of ADAM10 in TM-treated
cultures (Fig. 2B). Using western
blot analysis, only the single 97-kDa protein was detected in cell
lysates of control and TM (0.1 μg/ml)-treated cultures at 24 h. In
contrast, the 97-kDa protein was barely detectable in lysates of TM
(5 μg/ml)-treated cells. Although ADAM10 is a membrane-anchored
glycoprotein, it is present in the pericellular ECM of the
developing corneal stroma, and the secreted form is detected in
cultured cells (21). Further, we
found high levels of the 68-kDa band present in the collected
conditioned medium in TM (5 μg/ml)-treated cells, showing that the
active form of ADAM10 was secreted. These data suggest that CD44v6
ectodomain cleavage was mediated at least in part by active ADAM10
during TM-induced apoptosis.
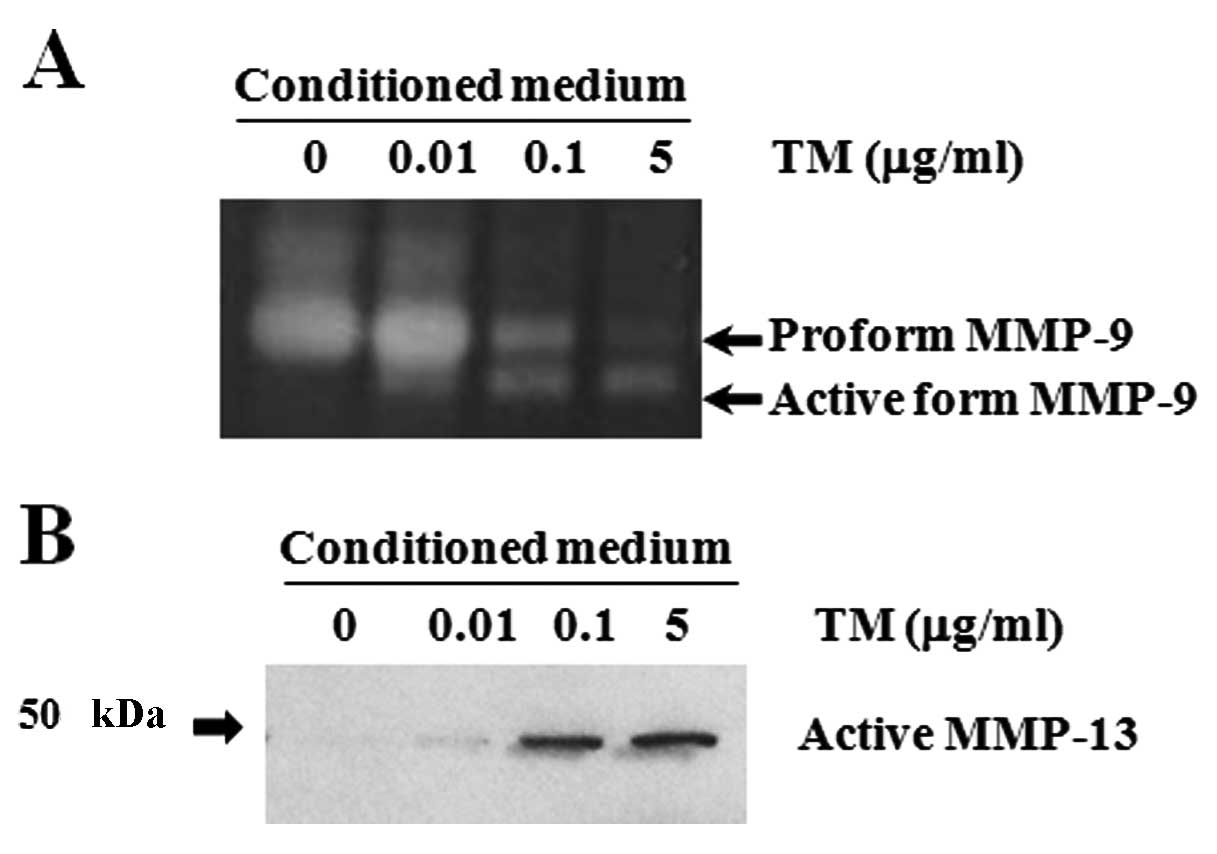
TM-stimulated activation of MMP-9 and
expression of active MMP-13
CD44 serves as a docking molecule to retain MMP-9
activity at the cell surface (30),
and it leads to activation of pro-MMP-9 (31) and MMP-9 during secretion (32). MMP-9-induced CD44 cleavage can be
inhibited by an MMP-9 inhibitor (33). To determine whether or not a causal
relationship exists between MMP-9 activity and CD44v6 cleavage,
gelatin zymography was performed to detect MMP-9 protein (Fig. 3A). In control culture, the pro-form
of MMP-9 (92 kDa) was detected at high levels in conditioned
medium, whereas the active form (82 kDa) was not detected at all.
In contrast, both the pro- and active forms of MMP-9 were detected
upon 0.1 μg/ml of TM treatment. Interestingly, pro-MMP-9 activity
dramatically declined and became undetectable, whereas the active
form of MMP-9 was observed upon 5 μg/ml of TM treatment. These data
indicate that MMP-9 underwent proteolysis consistent with its
activation during apoptosis in response to TM treatment. Given the
evidence that MMP-13 is able to activate pro-MMP-9 to MMP-9
(25), we examined the level of
active MMP-13 protein during TM-induced apoptosis of Caki-2 cells
using conditioned medium by western blot analysis (Fig. 3B). High levels of proteolytically
active MMP-13 protein (48 kDa) were strongly detected in TM (0.1
and 5 μg/ml)-treated cells at 24 h. In contrast, no MMP-13 protein
was detected in control cells, suggesting a possible role for
MMP-13 in MMP-9 activation.
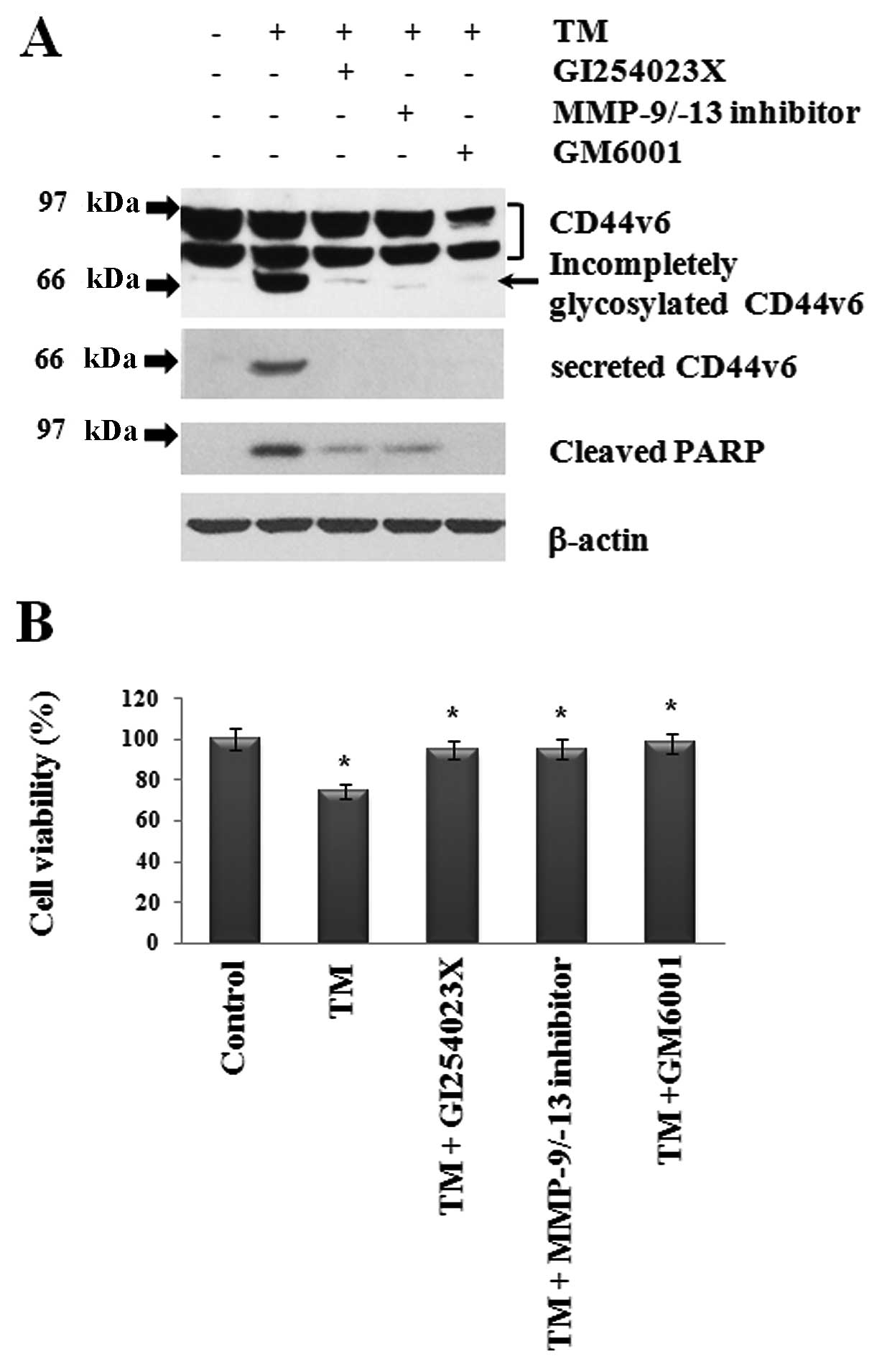
Inhibition of TM-induced PARP cleavage
and apoptosis by treatment with MMP inhibitors
TM-induced PARP cleavage and MMP activation occurred
in a dose-dependent manner during apoptosis (Figs. 1–3).
Therefore, we further examined whether or not inhibition of MMP-9,
MMP-13 and ADAM10 in TM-treated cells decreases both CD44v6
ectodomain and PARP cleavage while increasing cell viability
(Fig. 4). As expected, TM-induced
cleavage of CD44v6 and PARP at 24 h was greatly reduced by
treatment with MMP-9/-13 inhibitor, ADAM10-specific inhibitor
(GI254023X) and GM6001, which is a broad spectrum MMP inhibitor
(Fig. 4A). Surprisingly, incomplete
glycosylation of CD44v6 induced by TM was also abrogated by
treatment with all MMP inhibitors. Furthermore, we performed MTT
assay to check cell viability (Fig.
4B). As expected, TM-induced apoptosis was reduced by all MMP
inhibitors. These data indicate that TM-induced apoptosis was
mediated in part by MMP-9, MMP-13 and ADAM10 functions.
Discussion
In the present study, we examined the connection
between MMP expression/activity and apoptosis, and we found that
MMP-9, MMP-13 and ADAM10 are intimately involved in TM-induced
cleavage of CD44v6 ectodomain and apoptosis of Caki-2 cells. We
further provide direct evidence that TM induces MMP-9 activation
and expression of active MMP-13 and ADAM10, and that inhibition of
ADAM10 or MMP-9/MMP-13 activity increases Caki-2 cell
viability.
Induction of cleavage of CD44v6
ectodomain during TM-induced cell apoptosis
CD44 functionally interacts with growth factors
deposited in the ECM (7) and growth
factor receptors, which trigger activation of signal transduction
cascades (13,34), thereby regulating cell
proliferation. On the cytoplasmic side, the interaction between
CD44 and NF2 proteins can be pivotal in determining whether cells
proliferate or undergo apoptosis (35). Normal cells expressing CD44s are
susceptible to cleavage of PARP and apoptosis (36). In contrast, CD44v6 coordinates
activation of anti-apoptotic molecules and mediates apoptosis
resistance via the PI3K/Akt and ras-raf-MAPK pathways (13). In addition, cells expressing high
levels of CD44v6 are resistant to PARP cleavage as well as
Fas-mediated apoptosis depending on the cell type (14). Therefore, we can assume that high
levels of CD44v6 are constitutively expressed in Caki-2 cells for
the purpose of apoptosis resistance (Fig. 2). Treatment with TM either slightly
reduced or did not affect cell surface CD44 expression, but it has
been shown to inhibit hyaloranate binding by CD44v and destroy
clustering of CD44v6 in carcinoma cells (37,38).
Likewise, high levels of uncleaved CD44v6 (97 and 90 kDa) were
detected similarly in both control and TM-treated cultures
(Fig. 2A). On the other hand, when
cells were treated with 5 μg/ml of TM, presumably a de-glycosylated
CD44v6 (66-KDa) band was strongly detected in cell lysates.
However, a CD44v6 (66-kDa) band was not detected at all in control
cultures. Soluble CD44 and CD44v6 have been identified in cultured
supernatants in human prostate tumor cell lines (39). In this study, high levels of soluble
CD44v6 ectodomain (66 kDa) were detected only in TM (5
μg/ml)-treated conditioned medium (Fig.
2A), suggesting that this band represents the cleaved form of
whole CD44v6 (97 or 90 kDa) and not the incompletely glycosylated
form of CD44v6 (66 kDa) detected in cell lysates. Soluble CD44 can
compete with cancer cell membrane CD44 for matrix-binding sites and
exert anticancer effects, including decreased tumorigenicity and
increased apoptosis (40). Although
we demonstrated changes in CD44v6 glycosylation upon treatment with
TM, we do not yet know whether or not de-glycosylation of other
molecules on the cell surface influences CD44v6 function.
Collectively, our present data indicate that incomplete
glycosylation of CD44v6 as well as ectodomain shedding by TM may
play roles in the apoptosis of Caki-2 cells.
Sequential proteolytic cleavages in the ectodomain
and intramembranous domain of CD44 play critical roles in various
disease pathologies (41). ADAM10
serves as the constitutive functional sheddase of CD44 in several
melanoma cell lines, and soluble CD44 can abolish cell
proliferation (20). In addition,
silencing of ADAM10, but not MMP-14 (MT1-MMP), stimulates cell
proliferation in a CD44-dependent manner (20). Note that the secreted form of active
ADAM10 (68 kDa) was highly abundant upon 5 μg/ml of TM treatment
during cleavage of CD44v6 ectodomain and PARP at 24 h (Fig. 2B). Furthermore, inhibition of ADAM10
activity by GI254023X treatment reduced TM-induced PARP cleavage
and increased the viability of Caki-2 cells (Fig. 4). Taken together, our study suggests
that the secreted form of active ADAM10 may be involved in the
cleavage of CD44v6 ectodomain, which modulates apoptosis of Caki-2
cells.
Induction of active MMP-13 and activation
of MMP-9 during TM-induced apoptosis
Pro-MMP-9 is activated by osteoarthritic
chondrocytes via the MMP-13 cascade (24). In addition, MMP-13 can initiate bone
resorption and activate pro-MMP-9 in vitro, and MMP-13
inhibitor prevents MMP-9 activation (25). Pro-MMP-9 was converted into active
MMP-9 by TM treatment in a dose-dependent manner (Fig. 3A). Importantly, the temporal
expression pattern of active MMP-13 protein was correlated with
MMP-9 activation (Fig. 3B).
Inhibition of caspase suppresses induction of MMP-9 expression
(42). Further, as PARP-1 is
involved in the transcriptional activation of MMP-9, induction of
apoptosis through caspase activation and PARP-1 cleavage results in
re-repression of MMP-9 promoter activity (42). In our study, inhibition of both
MMP-9 and MMP-13 reduced TM-induced PARP cleavage, but increased
viability of Caki-2 cells (Fig. 4).
Taken together (Figs. 3 and
4), the active forms of both MMP-9
and MMP-13 exhibit similar temporal expression patterns, which may
be correlated with TM-induced apoptosis.
CD44 retains MMP-9 activity at the cell surface
(30), and surface expression of
CD44 along with activation of MMP-9 on the cell surface are
interdependent (9). In addition,
soluble CD44 mediates secretion of MMP-9 (32). The CD44v6-matrix interaction
stimulates MMP-9 production and promotes MMP-9 binding to CD44
(43). MMP-9 acts as a processing
enzyme for CD44 cleavage, which is inhibited by both
transcriptional knockdown of MMP-9 and MMP-9 specific inhibitor
(33). Collectively, our findings
of TM-mediated active MMP-13 protein induction and MMP-9 activation
in combination with caspase-3 activation and PARP cleavage
(Figs. 1, 3, and 4)
suggest that TM may participate in the regulation of CD44v6
cleavage via MMP-9 and MMP-13 during the apoptosis of Caki-2
cells.
In summary, these findings collectively support our
hypothe- sis that apoptosis of Caki-2 cells in vitro is
mediated by the temporal regulation of multiple active MMPs (e.g.,
MMP-9, MMP-13 and ADAM10). We further show that TM-induced CD44v6
ectodomain cleavage and apoptosis occurs through an MMP-dependent
mechanism. Further studies will be necessary to understand the
precise molecular mechanism of these effects.
Acknowledgements
This study was supported by the Basic Science
Research Program of the National Research Foundation of Korea
(NRF), which is funded by the Ministry of Education, Science and
Technology (2011-0004933). This work was also supported by a Korean
Research Foundation Grant (KRF-2006-521-C00148 and
KRF-2009-0075108).
Abbreviations:
|
TM
|
tunicamycin
|
|
MMPs
|
matrix metalloproteinases
|
|
ADAM
|
a disintegrin and
metalloproteinase
|
References
|
1
|
Dolai S, Pal S, Yadav RK and Adak S:
Endoplasmic reticulum stress-induced apoptosis in Leishmania
through Ca2+-dependent and caspase-independent
mechanism. J Biol Chem. 286:13638–13646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Elbein AD: Inhibitors of the biosynthesis
and processing of N-linked oligosaccharide chains. Annu Rev
Biochem. 56:497–534. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Delom F, Fessart D and Chevet E:
Regulation of calnexin sub-cellular localization modulates
endoplasmic reticulum stress-induced apoptosis in MCF-7 cells.
Apoptosis. 12:293–305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dricu A, Carlberg M, Wang M and Larsson O:
Inhibition of N-linked glycosylation using tunicamycin causes cell
death in malignant cells: role of down-regulation of the
insulin-like growth factor 1 receptor in induction of apoptosis.
Cancer Res. 57:543–548. 1997.PubMed/NCBI
|
|
5
|
Hitomi J, Katayama T, Taniguchi M, Honda
A, Imaizumi K and Tohyama M: Apoptosis induced by endoplasmic
reticulum stress depends on activation of caspase-3 via caspase-12.
Neurosci Lett. 357:127–130. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakagawa T, Zhu H, Morishima N, et al:
Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and
cytotoxicity by amyloid-β. Nature. 403:98–103. 2000.PubMed/NCBI
|
|
7
|
Yu Q and Stamenkovic I: Localization of
matrix metalloproteinase 9 to the cell surface provides a mechanism
for CD44-mediated tumor invasion. Genes Dev. 13:35–48. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arch R, Wirth K, Hofmann M, et al:
Participation in normal immune responses of a metastasis-inducing
splice variant of CD44. Science. 257:682–685. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Desai B, Ma T, Zhu J and Chellaiah MA:
Characterization of the expression of variant and standard CD44 in
prostate cancer cells: identification of the possible molecular
mech+anism of CD44/MMP9 complex formation on the cell surface. J
Cell Biochem. 108:272–284. 2009.PubMed/NCBI
|
|
10
|
Naor D, Sionov RV and Ish-Shalom D: CD44:
structure, function, and association with the malignant process.
Adv Cancer Res. 71:241–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamane N, Tsujitani S, Makino M, Maeta M
and Kaibara N: Soluble CD44 variant 6 as a prognostic indicator in
patients with colorectal cancer. Oncology. 56:232–238. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wielenga VJ, Heider KH, Offerhaus GJ, et
al: Expression of CD44 variant proteins in human colorectal cancer
is related to tumor progression. Cancer Res. 53:4754–4756.
1993.PubMed/NCBI
|
|
13
|
Jung T, Gross W and Zoller M: CD44v6
coordinates tumor matrix-triggered motility and apoptosis
resistance. J Biol Chem. 286:15862–15874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mielgo A, van Driel M, Bloem A, Landmann L
and Gunthert U: A novel antiapoptotic mechanism based on
interference of Fas signaling by CD44 variant isoforms. Cell Death
Differ. 13:465–477. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fini ME and Girard MT: The pattern of
metalloproteinase expression by corneal fibroblasts is altered by
passage in cell culture. J Cell Sci. 97:373–383. 1990.PubMed/NCBI
|
|
16
|
Girard MT, Matsubara M, Kublin C, Tessier
MJ, Cintron C and Fini ME: Stromal fibroblasts synthesize
collagenase and stromelysin during long-term tissue remodeling. J
Cell Sci. 104:1001–1011. 1993.PubMed/NCBI
|
|
17
|
Sternlicht MD and Werb Z: How matrix
metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol.
17:463–516. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smith KM, Gaultier A, Cousin H, Alfandari
D, White JM and DeSimone DW: The cysteine-rich domain regulates
ADAM protease function in vivo. J Cell Biol. 159:893–902. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Anderegg U, Eichenberg T, Parthaune T, et
al: ADAM10 is the constitutive functional sheddase of CD44 in human
melanoma cells. J Invest Dermatol. 129:1471–1482. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huh MI, Lee YM, Seo SK, et al: Roles of
MMP/TIMP in regulating matrix swelling and cell migration during
chick corneal development. J Cell Biochem. 101:1222–1237. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nagano O, Murakami D, Hartmann D, et al:
Cell-matrix interaction via CD44 is independently regulated by
different metalloproteinases activated in response to extracellular
Ca(2+) influx and PKC activation. J Cell Biol. 165:893–902.
2004.PubMed/NCBI
|
|
23
|
Pacheco-Rodriguez G, Steagall WK, Crooks
DM, et al: TSC2 loss in lymphangioleiomyomatosis cells correlated
with expression of CD44v6, a molecular determinant of metastasis.
Cancer Res. 67:10573–10581. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dreier R, Grassel S, Fuchs S, Schaumburger
J and Bruckner P: Pro-MMP-9 is a specific macrophage product and is
activated by osteoarthritic chondrocytes via MMP-3 or a
MT1-MMP/MMP-13 cascade. Exp Cell Res. 297:303–312. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hernandez Rios M, Sorsa T, Obregon F, et
al: Proteolytic roles of matrix metalloproteinase (MMP)-13 during
progression of chronic periodontitis: initial evidence for
MMP-13/MMP-9 activation cascade. J Clin Periodontol. 36:1011–1017.
2009.PubMed/NCBI
|
|
26
|
Lazebnik YA, Kaufmann SH, Desnoyers S,
Poirier GG and Earnshaw WC: Cleavage of poly(ADP-ribose) polymerase
by a proteinase with properties like ICE. Nature. 371:346–347.
1994. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ferreira KS, Kreutz C, Macnelly S, et al:
Caspase-3 feeds back on caspase-8, Bid and XIAP in type I Fas
signaling in primary mouse hepatocytes. Apoptosis. 17:503–515.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ponta H, Sleeman J, Dall P, Moll J,
Sherman L and Herrlich P: CD44 isoforms in metastatic cancer.
Invasion Metastasis. 14:82–86. 1994.PubMed/NCBI
|
|
30
|
Spessotto P, Rossi FM, Degan M, et al:
Hyaluronan-CD44 interaction hampers migration of osteoclast-like
cells by down-regulating MMP-9. J Cell Biol. 158:1133–1144. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Samanna V, Ma T, Mak TW, Rogers M and
Chellaiah MA: Actin polymerization modulates CD44 surface
expression, MMP-9 activation, and osteoclast function. J Cell
Physiol. 213:710–720. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Seipel D, Oliveira BC, Resende TL, et al:
Toxoplasma gondii infection positively modulates the macrophages
migratory molecular complex by increasing matrix
metalloproteinases, CD44 and alpha v beta 3 integrin. Vet
Parasitol. 169:312–319. 2010. View Article : Google Scholar
|
|
33
|
Chetty C, Vanamala SK, Gondi CS, Dinh DH,
Gujrati M and Rao JS: MMP-9 induces CD44 cleavage and CD44 mediated
cell migration in glioblastoma xenograft cells. Cell Signal.
24:549–559. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ponta H, Sherman L and Herrlich PA: CD44:
from adhesion molecules to signalling regulators. Nat Rev Mol Cell
Biol. 4:33–45. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Morrison H, Sherman LS, Legg J, et al: The
NF2 tumor suppressor gene product, merlin, mediates contact
inhibition of growth through interactions with CD44. Genes Dev.
15:968–980. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wittig BM, Johansson B, Zoller M,
Schwarzler C and Gunthert U: Abrogation of experimental colitis
correlates with increased apoptosis in mice deficient for CD44
variant exon 7 (CD44v7). J Exp Med. 191:2053–2064. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lesley J, English N, Perschl A, Gregoroff
J and Hyman R: Variant cell lines selected for alterations in the
function of the hyaluronan receptor CD44 show differences in
glycosylation. J Exp Med. 182:431–437. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sleeman J, Rudy W, Hofmann M, Moll J,
Herrlich P and Ponta H: Regulated clustering of variant CD44
proteins increases their hyaluronate binding capacity. J Cell Biol.
135:1139–1150. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stevens JW, Palechek PL, Griebling TL,
Midura RJ, Rokhlin OW and Cohen MB: Expression of CD44 isoforms in
human prostate tumor cell lines. Prostate. 28:153–161. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu Q, Toole BP and Stamenkovic I:
Induction of apoptosis of metastatic mammary carcinoma cells in
vivo by disruption of tumor cell surface CD44 function. J Exp Med.
186:1985–1996. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Isacke CM and Yarwood H: The hyaluronan
receptor, CD44. Int J Biochem Cell Biol. 34:718–721. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kobayashi T: Suppression of matrix
metalloproteinase-9 expression in undifferentiated, non-apoptotic
keratinocytes is abrogated by the cleavage of poly(ADP-ribose)
polymerase-1. Apoptosis. 16:1205–1216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yu Q and Stamenkovic I: Transforming
growth factor-beta facilitates breast carcinoma metastasis by
promoting tumor cell survival. Clin Exp Metastasis. 21:235–242.
2004. View Article : Google Scholar : PubMed/NCBI
|