Introduction
Thyroid carcinoma is the most common endocrine
malignancy in developed countries, accounting for ~1% of total
human cancers, and is more frequent in women than in men. Thyroid
cancer was recognized as the sixth most frequently diagnosed cancer
in women (1).
The majority (95%) of thyroid carcinomas originate
from follicular epithelial cells. They are classified as well
differentiated, poorly differentiated or undifferentiated
(anaplastic) neoplasms. The well differentiated thyroid tumors
comprise follicular thyroid cancer (FTC) and papillary thyroid
cancer (PTC). These cancers are highly curable, however cause death
in over 10% of cases (2).
PTC is the most common thyroid malignancy,
accounting for >80 % of cases and it does not have a benign
counterpart. In contrast, follicular neoplasms comprise both benign
and malignant (FTC) lesions. FTC, accounting for ~10% of thyroid
carcinomas, morphologically overlaps benign follicular neoplasm.
These two types of thyroid tumors do not differ appreciably.
Currently, diagnostic criteria for malignancy are based on
histological assessment of invasive features including full
penetration of the tumor capsule and/or invasion of blood vessels
in or beyond the capsule (3).
Follicular lesions can not be accurately diagnosed by fine needle
aspiration cytology as the crucial diagnostic features are missing
in this method (3). Screening for
molecular markers characteristic for thyroid tumors seems to be a
promising way to improve traditional morphological cytopathology
(reviewed in ref. 4).
The HtrA family of proteins consists of
evolutionarily conserved ATP-independent serine proteases,
homologues of the HtrA protein from the bacterium Escherichia
coli. The common feature of HtrA proteins is the presence of
trypsin-like proteolytic domain and at least one PDZ domain at the
C-terminus. Members of the family have been identified in most
organisms, from bacteria to humans. The defense against cellular
stresses inducing aberrations in protein structure is believed to
be a general function of the HtrAs (reviewed in refs. 5 and 6).
At least four human HtrA proteases have been
identified. They are involved in maintenance of mitochondrial
homeostasis, apoptosis and cell signaling, and disturbances in
their functions may contribute to the development of several
diseases including cancer, arthritis and neurodegenerative
disorders (reviewed in refs. 6 and
7).
HtrA1 was originally identified as a gene
downregulated in SV40-transformed fibroblasts (8). Several lines of evidence indicate that
HtrA1 functions as a tumor suppressor, promoting cell death.
Downregulation of HtrA1 expression contributes to cancer
cell survival and is correlated with tumor progression and
metastasis (9–11). HtrA1 expression was found to
be downregulated in many types of cancer cell lines (10), primary tumors such as ovarian
(10–12) and endometrial cancers (13,14),
and also in metastatic foci of melanoma, lung and prostate cancers
compared to primary tumors (9,15).
Additionally, in primary ovarian cancer a progressive decrease of
HtrA1 expression correlated with an increasing degree of
malignancy has been observed (12).
In endometrial tumors HtrA1 expression was decreased with
increasing grades of endometrial cancer (13). In glioma and mesothelioma loss of
HtrA1 expression was correlated with poor prognosis
(16,17). Moreover, it has been demonstrated
that diminished level of HtrA1 protein in ovarian and gastric
tumors is associated with poor clinical response to platinum-based
chemotherapy (11).
Molecular mechanism of HtrA1 function in cancer
development is unclear. It was demonstrated that HtrA1 triggers
death of cancer cells by induction of apoptosis (10,11).
It was also shown that HtrA1 functions as an inhibitor of TGF-β
proteins (18,19) which are regulators of cell growth
and differentiation and whose involvement in cancer development has
been proven. While at the very early stages of oncogenesis TGF-β
proteins function as tumor suppressors, at the advanced stages they
stimulate tumor progression and metastasis (20).
The HtrA2/Omi protein is a mitochondrial serine
protease which under physiological conditions functions as a
quality control protease and facilitates cell survival. However, in
stressful conditions HtrA2 switches from a protector to a
proapoptotic protein. Upon apoptotic stimuli HtrA2 is released into
cytosol where it promotes apoptosis in a caspase-dependent manner
by degradation of the Inhibitor of Apoptosis Proteins (IAPs) as
well as in a caspase-independent manner via its proteolytic
activity (21–26). HtrA2 degrades proteins other than
IAPs, exhibiting antiapoptotic properties (e.g., Hax-1 and
ped/pea15) (27,28). Moreover, Liu et al (29) showed that the protease is implicated
in anoikis, cell death induced by disruption of cell attachment or
contact with extracellular matrix. Resistance to anoikis is a
common feature of cancer cells, contributing to metastasis
(30). To date, several reports
have been published arguing the involvement of HtrA2 in cancer
development, with its expression variable, depending on cancer type
(reviewed in ref. 7).
The HtrA3 protein was initially discovered as a
pregnancy-related serine protease (PRSP) implicated in the
development of embryo and placentation (31–33).
So far, two isoforms of HtrA3, a long, 50 kDa variant (HtrA3-L) and
a short, 40 kDa variant (HtrA3-S), produced by alternative mRNA
splicing, have been identified (34). Both HtrA3 isoforms are serine
proteases, but HtrA3-S lacks the PDZ domain at its C-terminal end.
Since the PDZ domains are involved in substrate binding the HtrA3
isoforms may recognize different substrates and thus have slightly
different functions. High homology of HtrA3 with HtrA1 (58%), an
identical domain organization and similar expression pattern in
human tissues suggest similar activity and physiological functions
(34). Indeed, HtrA3 acts as an
inhibitor of TGF-β signaling pathway (35). Moreover, evidence exists showing
involvement of HtrA3 in modulation of chemotherapy-induced
cytotoxicity (36,37). Recently, it has been proposed that
HtrA proteins could be novel targets in cancer therapy (reviewed in
refs. 6 and 7).
HtrA proteins are involved in oncogenesis, are
prospective therapeutic targets and finding new molecular markers
in thyroid cancer is necessary. To date, there is no information
available concerning involvement of HtrA proteins in thyroid
cancer. These facts prompted us to evaluate levels of human HtrA1,
HtrA2 and HtrA3 (long and short variants) proteins in thyroid
normal and tumor (benign and malignant) tissues. Since HtrA1 and
HtrA3 are believed to function in TGF-β signaling, we included
evaluation of the TGF-β1 protein level. The relative protein levels
were estimated by immunoblotting technique and correlations between
the protein levels and thyroid tumor type, histopatological type of
cancer and patient gender were analyzed.
Materials and methods
Patients and specimens
Specimens were collected from 40 patients undergoing
surgical treatment for thyroid pathology at the Medical University
of Gdansk. Both tumor and unchanged thyroid tissues (excised from
thyroid in areas free of macroscopically visible abnormalities)
were obtained from each patient. The collecting of tissues was
supervised by a pathologist. Tissues were immediately frozen in
liquid nitrogen and stored at −70°C. Tissue characteristics are
presented in Table I.
 | Table ICharacteristics of specimens. |
Table I
Characteristics of specimens.
| Tissue type | No. of cases |
|---|
| Benign/Normal | 20/20 |
| Malignant |
| Follicular
carcinoma/Normal | 12/12 |
| Papillary
carcinoma/Normal | 8/8 |
Chemicals
Immobilon PSQ Transfer Membrane (0.2 μm pore size)
for immunoblotting was purchased from Millipore (Millipore Corp.,
Bedford, USA). Other chemicals were purchased from Sigma or Fluka
(Poznan, Poland) and were of the highest purity.
Preparation of tissue extracts
Protein extracts were prepared as described
previously by Narkiewicz et al (12,14).
Briefly, ~30 mg of thyroid tissue was homogenized with 0.2 ml of
cold 10 mM Tris pH 7.4 buffer containing 150 mM KCl and centrifuged
(15000 × g, 30 min) at 4°C. Supernatant was collected, stored at
−70°C and subjected to protein analysis.
Protein assay and electrophoresis
Proteins were quantified by Amido Black staining
(38). SDS-PAGE electrophoresis was
carried out according to Laemmli (39).
Immunoblotting (western blot
analysis)
The immunoblotting was performed essentially as
described by Pound (40). Samples
of tissue lysates containing equal amounts of total protein were
resolved by SDS-PAGE and electrotransferred onto Immobilon
membrane. Samples of thyroid lesions and unchanged thyroid tissues
were resolved on the same gel together with a reference sample. The
same sample was used as the reference in all assays for a given
protein (HtrA or TGF-β1). The chosen reference sample was the
normal tissue in which the level of the tested protein was close to
average. Proteins bound to the membrane surface were stained by a
standard procedure with the Ponceau S dye. This step was introduced
into the procedure in order to confirm proper sample loading,
protein resolution by SDS-PAGE and electrotransfer, and also to
check if protein amounts in the samples were comparable.
Polyclonal rabbit immunoglobulins against a given
HtrA protein or monoclonal mouse anti-TGF-β1 immunoglobulins were
used as primary antibodies. Polyclonal antibodies against the rat
HtrA1 and human HtrA2 recombinant proteins were raised as described
previously (41). Polyclonal rabbit
anti- HtrA3 and monoclonal mouse anti-TGF-β1 antibodies were
purchased from Abcam (USA). As the secondary antibodies, goat
anti-rabbit or anti-mouse HRP-conjugated IgG were used (Sigma). To
visualize HtrA and TGF-β1 proteins chemiluminescence detection was
performed using Lumi-Light Western Blotting Substrate from Roche
(Warsaw, Poland). The relative levels of the HtrA or TGF-β1
proteins were calculated as a ratio of the tested protein band
intensity to the intensity of a corresponding protein (HtrA or
TGF-β1) in the reference sample resolved on the same gel. The blots
were also probed with mouse monoclonal antibody against GAPDH
(Abcam) used as a loading control.
Statistical analysis
Protein band intensity was assessed by densitometric
analysis using 1Dscan EX 3.0 software (Scanalytics, Inc., USA) and
band intensity ratios were calculated. Each assay was repeated 3
times and the differences did not exceed 10%. Statistica 7.1 PL was
used for statistical analysis. The Student’s t-test was used to
compare the groups. Analysis of variance was performed using ANOVA
tests and post-hoc NIR Fisher’s test. Statistical significance was
assumed at P<0.05.
Results
The levels of HtrA2, HtrA3-S and HtrA3-L
are increased in thyroid cancer
We assayed the relative levels of HtrA1, HtrA2 and
of the two isoforms of HtrA3 proteins in thyroid malignant (n=20)
and benign tumor tissues (n=20) as well as in control
macroscopically unchanged thyroid tissues. The control tissues were
grouped according to their source, e.g., the thyroids with benign
or malignant lesions. To measure relative levels of HtrA proteins,
lysates of the tissues containing equal amounts of total protein
were resolved electrophoretically and subjected to western blot
analysis.
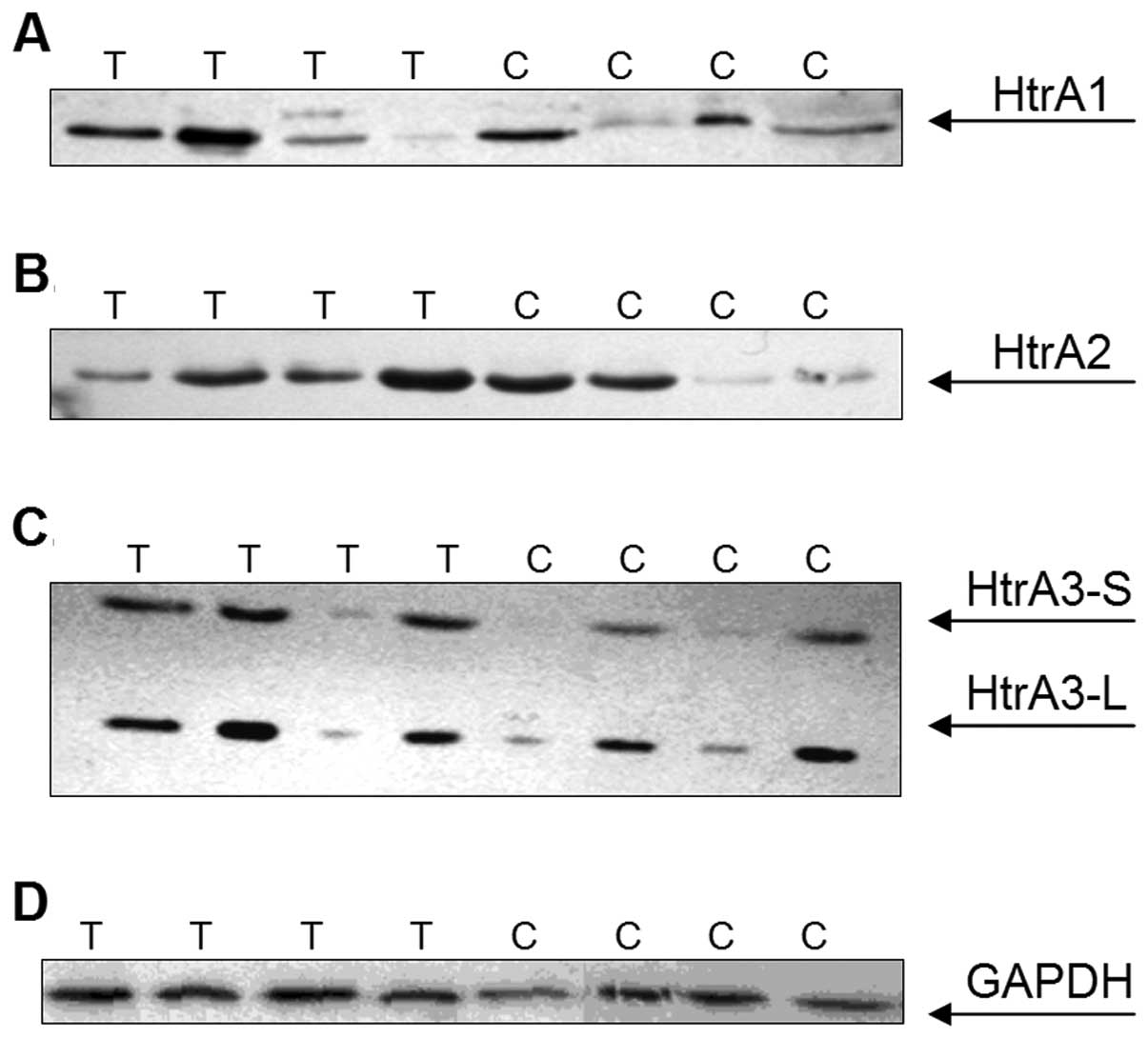
As predicted, polyclonal antibodies against HtrA1
and HtrA2 detected bands of 50 and 40 kDa, respectively,
corresponding to the mature forms of these HtrA proteins (Fig. 1A and B). Immunoblotting with
anti-HtrA3 antibodies revealed two bands of around 50 and 40 kDa,
representing a long (HtrA3-L) and a short (HtrA3-S) mature isoform
of the protein (Fig. 1C).
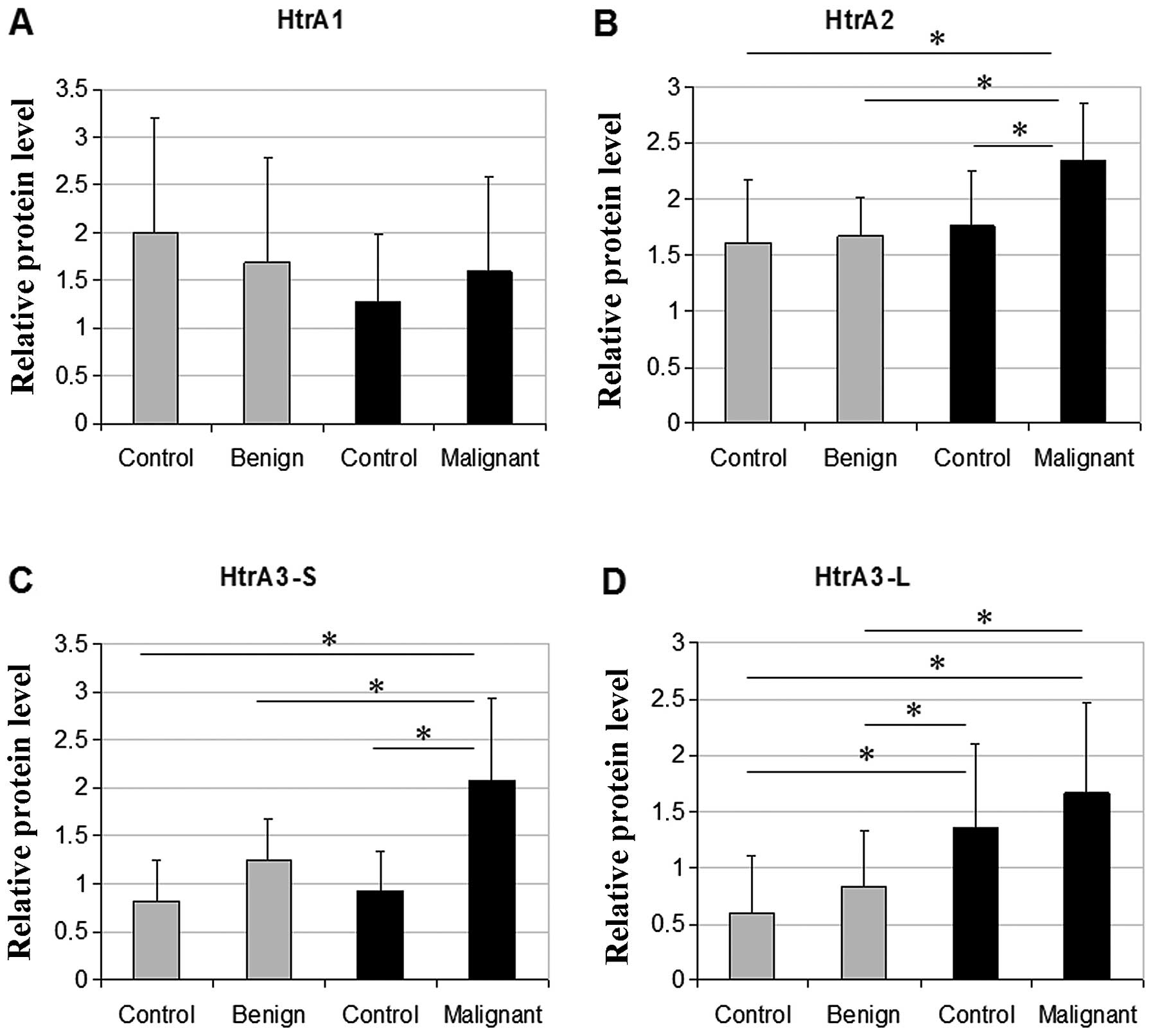
The levels of HtrA2 and HtrA3-S were significantly
higher in a group of thyroid malignant tumors compared to control
tissues and benign thyroid lesions (Fig. 2B and C), with more dramatic increase
found for HtrA3-S. The HtrA3-S protein level in malignant tumor
tissues was 2.3-fold higher compared to benign tumor tissues and
~1.6-fold higher compared to each of the control groups (one from
patients with benign lesions and one from patients with malignant
tumors). The HtrA3-L protein level was significantly increased in
malignant tumor tissues compared to benign tumor tissues, and also
to control tissues from patients with benign tumors. Interestingly,
the HtrA3-L expression was higher in control tissues from
patients with thyroid cancer compared to control tissues from
patients with benign lesions (Fig.
2D).
We did not find any statistically significant
differences in the HtrA1 expression between the thyroid
tumors and the control tissues (Fig.
2A). No statistical correlation between the HtrA protein
relative levels and patient gender was found (data not shown).
Follicular thyroid carcinoma and
papillary thyroid carcinoma differ in expression of HtrA1 and
HtrA3-S
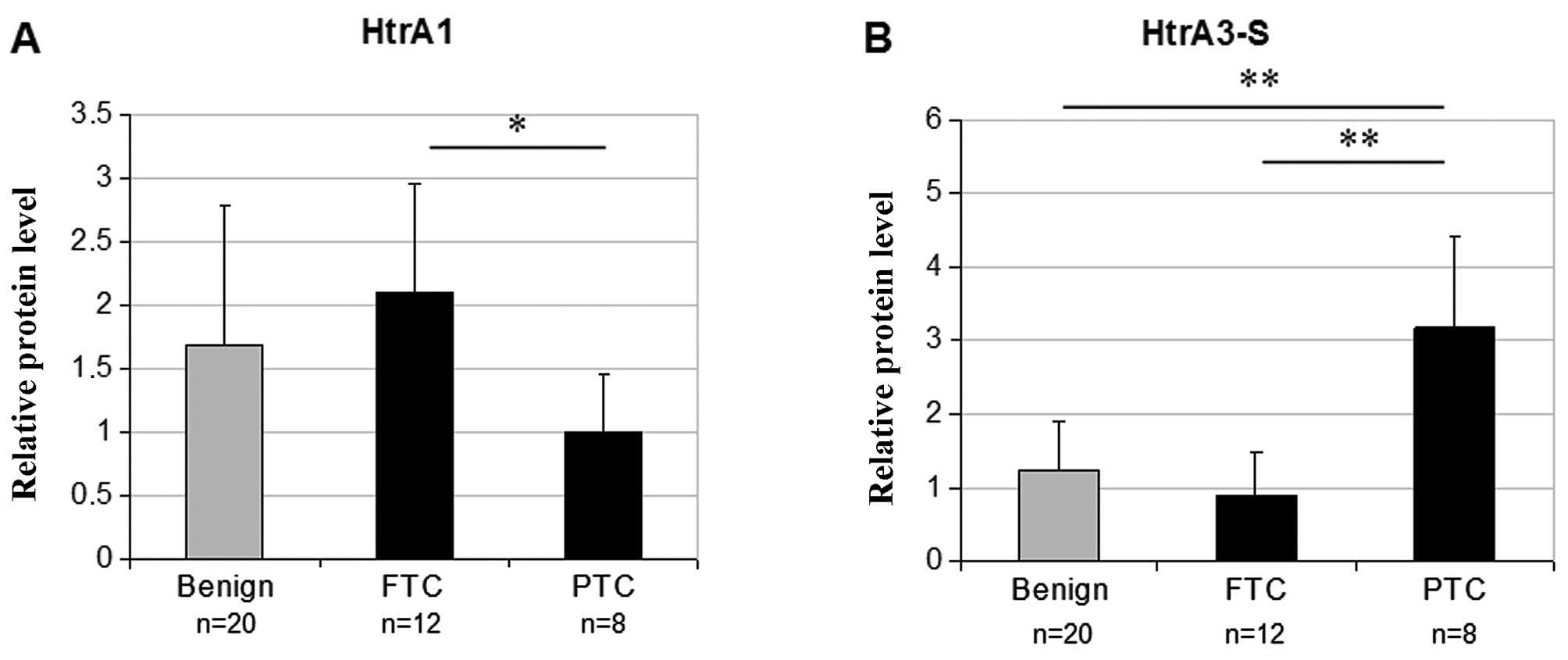
To evaluate the relationship between the HtrA gene
expression and histological type of thyroid cancer, we divided
patients with thyroid carcinoma (n=20) according to
histopathological analysis of the tumor tissues into two groups:
patients with follicular thyroid carcinoma (FTC) (n=12) and
patients with papillary thyroid carcinoma (PTC) (n=8), and then
compared the relative HtrA protein levels in these groups. As shown
in Table II, the HtrA3-S protein
level was 3.6-fold higher in PTC compared to the FTC (P<0.001)
while the relative HtrA1 protein level was ~2-fold higher in FTC
compared to PTC (P=0.045). We also found that the relative protein
level of HtrA3-S in PTC but not in FTC was significantly higher
compared to benign tumor tissues (Fig.
3). There were no significant differences in HtrA2 and HtrA3-L
levels between FTC and PTC (Table
II). Thus, these two types of thyroid carcinoma differ in
expression of HtrA3-S and HtrA1.
 | Table IIRelative levels of HtrA proteins and
TGF-β1 in different histological types of thyroid carcinoma. |
Table II
Relative levels of HtrA proteins and
TGF-β1 in different histological types of thyroid carcinoma.
| Protein | FTC (n=12) | PTC (n=8) | P-value |
|---|
| HtrA1 | 2.10 | 1.01 | 0.045 |
| HtrA2 | 2.56 | 2.47 | 0.882 |
| HtrA3-S | 0.88 | 3.17 | <0.001 |
| HtrA3-L | 1.41 | 2.26 | 0.331 |
| TGF-β1 | 0.76 | 0.67 | 0.544 |
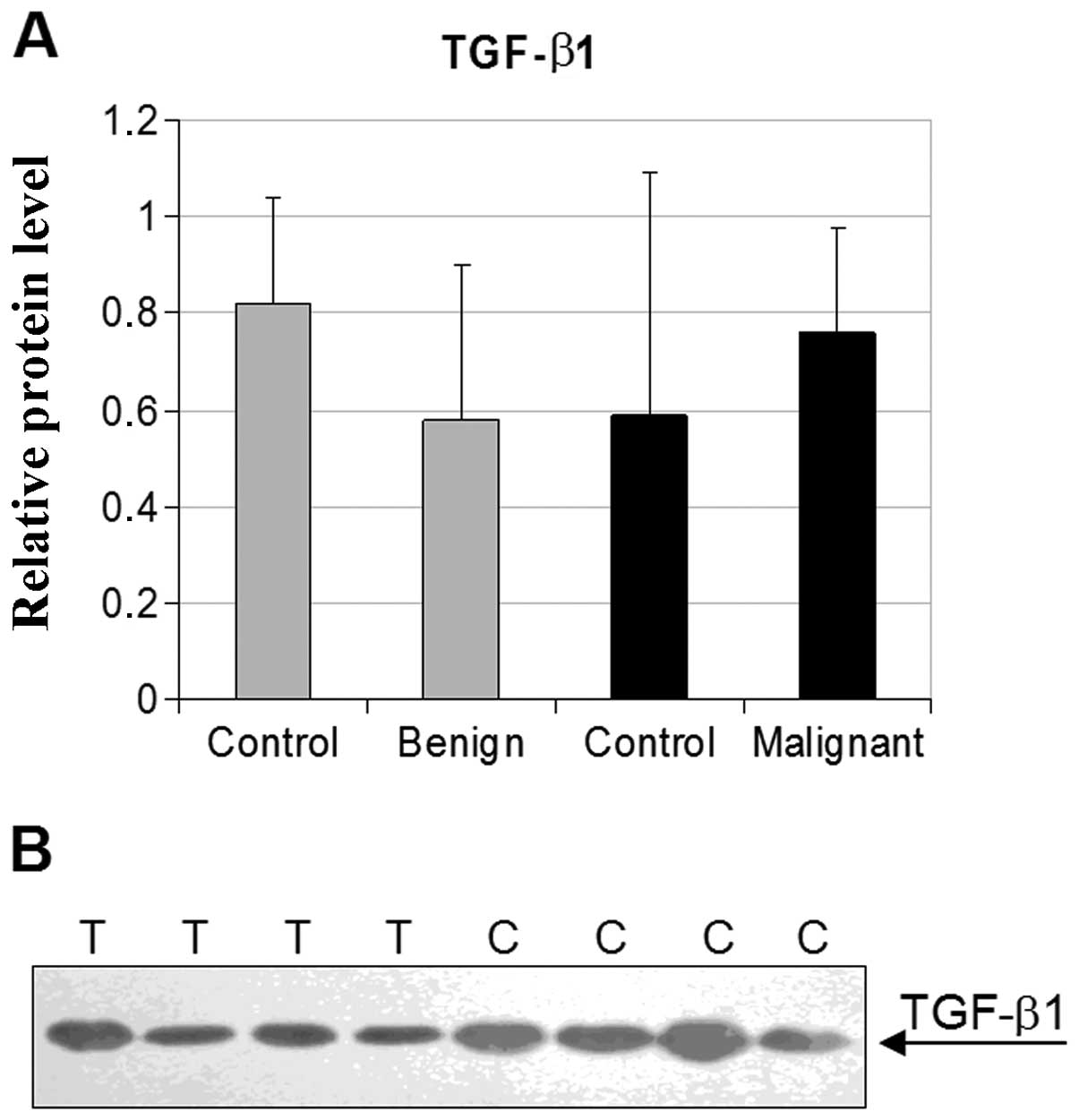
The TGF-β1 expression in thyroid benign
and malignant tumor tissues
As shown in Fig. 4
no significant differences between the TGF-β1 relative protein
levels in healthy controls and thyroid benign or malignant tumor
tissues were found. We did not find any statistically significant
correlation between the TGF-β1 expression and histological
type of thyroid cancer (Table II)
or patient gender (data not shown).
Discussion
In the present study we evaluated the expression of
the genes encoding members of HtrA serine proteases family, human
HtrA1, HtrA2 and HtrA3, and of the
transforming growth factor β1 (TGF-β1) in thyroid normal and
tumor tissues. The relative HtrA and TGF-β1 protein levels were
evaluated by western blot technique and correlated with
histological type of cancer and patient gender. Moreover, for the
first time, the levels of both isoforms of HtrA3, HtrA3-S (short)
and HtrA3-L (long) in tumor tissues were analyzed.
We found an increase in the HtrA2 level in thyroid
malignant tumors compared to normal thyroid tissues and to benign
thyroid lesions (Fig. 2). This
indicates involvement of HtrA2 in the development of thyroid cancer
and suggests that the elevated HtrA2 level may be correlated with
thyroid cancer malignancy. These results are in agreement with
several other reports showing HtrA2 involvement in oncogenesis.
HtrA2 was found to be overexpressed in prostate carcinoma
while normal prostate tissue and benign prostatic hyperplasia
showed none or weak expression (42); it was highly expressed in advanced
gastric adenocarcinomas (43), in
lung cancers (44,45) and in advanced tumors induced in
Syrian hamster kidneys by prolonged estrogenization (41). On the other hand, the HtrA2 protein
levels were reduced in endometrial (13,14),
ovarian (12) and breast cancers
(46,47). The mechanism of HtrA2 function in
oncogenesis is not known, however, its involvement in promoting
apoptosis and anoikis makes an obvious link with cancer cells which
evade both types of cell death. The increased HtrA2 levels observed
in this study might reflect a cellular defense response to
oncogenesis aimed at triggering cell death.
However, the question arises why overexpressed HtrA2
with its proapoptotic function does not protect from tumor
development. One possibility is that HtrA2 level might not be
sufficient to trigger cell death. Trencia et al (27) demonstrated that overproduction of
antiapoptotic ped/pea15 prevents apoptosis triggered by HtrA2. Thus
induction of apoptosis mediated by HtrA2 might be dependent on the
relative levels of the active protease and antiapoptotic proteins,
such as ped/pea15 or IAPs. On the other hand, HtrA2 might be
sequestered in mitochondria and not released into the
cytoplasm.
It was demonstrated that activating point mutations
of ras are frequent in differentiated thyroid cancers and
regarded as crucial oncogenic events contributing to the transition
from follicular adenoma to follicular carcinoma (reviewed in ref.
48). Activated oncogenic Ras is a
strong inhibitor of anoikis. Recent studies by Liu et
al (29) showed that Ras
inhibits anoikis by preventing the release of mitochondrial
HtrA2 protease into the cytoplasm. Thus, it is possible that
sequestration of HtrA2 in mitochondria may repress cell death and
facilitate metastasis contributing to cancer promotion.
In western blotting experiments using anti-HtrA3
antibody we detected two bands corresponding to both, the 50
(HtrA3-L) and 40 kDa (HtrA3-S) isoforms of HtrA3 in thyroid tumor
and control tissues, and the short isoform was predominant in both
tissue types. Both isoforms of HtrA3 transcripts were found
in a range of human tissues with long (heart, skeletal muscle) or
short form (placenta, kidney) being predominant in some of them. In
some of tissues only long (lung, small intestine) or short (brain)
form was detected (34).
Former studies concerning the HtrA3
expression in diverse tumors were based on analysis of the HtrA3-S
protein level only, since the HtrA3-L level was usually too low to
be quantified (12,14). In the present study we found that
both HtrA3-S and HtrA3-L expression levels were
elevated in malignant tumors when compared to benign lesions.
Furthermore, HtrA3-S but not HtrA3-L level was increased in cancers
when compared to control tissues. The observed differences in
expression of the HtrA3 isoforms in thyroid tumors indicate
the implication of both HtrA3 isoforms in the development of
thyroid cancer and also suggest that HtrA3-S and HtrA3-L could play
different roles in thyroid carcinogenesis. Contrary to our results,
showing a significant increase in HtrA3 level, previous reports
revealed a significant decrease of HtrA3 expression in ovarian
(12), endometrial (13,14)
and lung cancers (37). However,
the molecular mechanisms of HtrA3 function in cancer development
remains unclear. Recent studies demonstrated that HtrA3 is
localized in mitochondria and upon cytotoxic stress induced by
etoposide or cisplatin treatment is released into the cytosol where
it promotes apoptosis via its proteolytic activity (36,49).
Thus, overexpression of the HtrA3 gene in thyroid tumors
might be a response to stress created by oncogenesis, aimed at
enhancing apoptosis but not sufficient to activate death of tumor
cells due to a prevalence of antiapoptotic signals.
Interestingly, we have also found a significant
increase in expression of HtrA3-L in control tissues from
patients with thyroid cancer compared to control tissues from
patients with benign lesions which suggests that the elevated level
of the long form of HtrA3 might predispose to the development of
malignant form of thyroid tumor.
In the present study we have observed a correlation
between HtrA1 and HtrA3-S protein levels and histological type of
thyroid cancer. We found that the HtrA1 level was increased in
follicular thyroid carcinoma (FTC) compared to papillary thyroid
carcinoma (PTC), while the HtrA3-S level was much higher in PTC
compared to FTC (Table II). This
observation suggests that the HtrA1 and HtrA3 proteins may play
different roles in the development of PTC and FTC. It is worth to
note that not only was the HtrA3-S level in PTC significantly
higher compared to FTC but also compared to benign lesions
(Fig. 4). Since there were no
significant differences in HtrA3-L levels between PTC and FTC (data
not shown), these findings taken together support the hypothesis of
diverse functions of HtrA3 isoforms in thyroid cancer development.
Both, the long and the short form of HtrA3 are serine proteases,
but HtrA3-S lacks the PDZ domain at the C-terminal end. The PDZ
domains are known as regulatory elements and specificity
determinants of the HtrA proteins (reviewed in refs. 5 and 6).
It was demonstrated that HtrA3 requires its proteolytic activity to
trigger apoptosis and PDZ-deleted HtrA3 variant was slightly less
prone to trigger platinum-induced apoptosis of lung cancer cells
than its full-length counterpart (36). Taking into account these facts, the
HtrA3 isoforms may recognize different substrates and play slightly
distinct roles. However, to date no physiological substrates have
been identified and further studies are required to clarify the
precise roles of HtrA3 isoforms in carcinogenesis.
Currently, fine-needle aspiration (FNA) is the most
widely used preoperative test for initial evaluation of thyroid
nodule and diagnostic criteria for malignancy are based on the
histological assessment of thyroid specimens (3). Several studies focused on molecular
markers that could improve the diagnostic accuracy of cytological
analysis after FNA (4). It is
tempting to speculate that assaying levels of HtrA2, HtrA3-S and
HtrA3-L might serve as an adjuvant facilitating the differentiation
between the benign and malignant lesions. However, more thyroid
cancer samples need to be analyzed. We evaluated expression level
of TGF-β1 in control and tumor thyroid tissues. However, no
significant differences between the TGF-β1 relative protein levels
in healthy controls and thyroid benign or malignant tumor tissues
have been found. Also, we did not find any correlation between the
TGF-β1 expression and histological type of thyroid cancer or
patient gender. It has been shown that the TGF-β1 protein level was
elevated in endometrial cancer (14). Moreover, a significant negative
correlation between the levels of TGF-β1 and HtrA1 and HtrA3
suggested the involvement of HtrA proteins in TGF-β1 protein
regulation (14). However, such
correlation has not been found in ovarian cancer (12). These findings together with our
results suggest that the regulation of TGF-β1 pathway by HtrA
proteins may be tissue-specific and could play different role in
the development of distinct tumors.
To our knowledge this is the first study
demonstrating upregulation of HtrA2 and HtrA3 proteins levels in
thyroid tumors and presenting differences in expression of the long
and the short isoforms of HtrA3 in cancer.
Our results indicate involvement of HtrA2 and HtrA3
proteins in thyroid cancerogenesis and suggest different roles of
HtrA3-S and HtrA3-L in the cancer development. Our findings open
the way for further investigation of the role of the HtrA proteins
in thyroid pathogenesis and also suggest the possibility to
consider HtrA proteins as molecular markers in diagnostics of
thyroid cancer.
References
|
1
|
Fassnacht M, Kreissl MC, Weismann D and
Allolio B: New targets and therapeutic approaches for endocrine
malignancies. Pharmacol Ther. 123:117–141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Urken ML: Management of
well-differentiated thyroid cancer in 2010: perspectives of a head
and neck surgical oncologist. Endocr Pract. 16:903–912. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kapur U and Wojcik EM: Follicular neoplasm
of the thyroid-vanishing cytologic diagnosis? Diagn Cytopathol.
35:525–528. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Freitas BC and Cerutti JM: Genetic markers
differentiating follicular thyroid carcinoma from benign lesions.
Mol Cell Endocrinol. 321:77–85. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clausen T, Southan C and Ehrmann M: The
HtrA family of proteases implications for protein composition and
cell fate. Mol Cell. 10:443–455. 2002.PubMed/NCBI
|
|
6
|
Zurawa-Janicka D, Skorko-Glonek J and
Lipińska B: HtrA proteins as targets in therapy of cancer and other
diseases. Expert Opin Ther Targets. 14:665–679. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chien J, Campioni M, Shridhar V and Baldi
A: HtrA serine proteases as potential therapeutic targets in
cancer. Curr Cancer Drug Targets. 9:451–468. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zumbrumm J and Treub B: Primary structure
of a putative serine protease specific for IGF-binding proteins.
FEBS Lett. 398:187–192. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baldi A, De Luca A, Morini M, Battista T,
Felsani A, Baldi F, Catricala C, Amantea A, Noonan DM, Albini A, et
al: The HtrA1 serine protease is down-regulated during human
melanoma progression and represses growth of metastatic melanoma
cells. Oncogene. 21:6684–6688. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chien J, Staub J, Hu SI, Erickson-Johnson
MR, Couch FJ, Smith DI, Crowl RM, Kaufmann SH and Shridhar V: A
candidate tumor suppressor HtrA1 is down-regulated in ovarian
cancer. Oncogene. 23:1636–1644. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chien J, Aletti G, Baldi A, Catalano V,
Muretto P, Keeney GL, Kalli KR, Staub J, Ehrmann M, Cliby WA, et
al: Serine protease HtrA1 modulates chemotherapy-induced
cytotoxicity. J Clin Invest. 116:1994–2004. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Narkiewicz J, Klasa-Mazurkiewicz D,
Zurawa-Janicka D, Skorko-Glonek J, Emerich J and Lipińska B:
Changes in expression of human HtrA1, HtrA2 and
HtrA3 genes in ovarian cancer. Clin Biochem. 41:561–569.
2008.
|
|
13
|
Bowden MA, Di Nezza-Cossens LA, Jobling T,
Salamonsen LA and Nie G: Serine proteases HtrA1 and HtrA3 are
down-regulated with increasing grades of human endometrial cancer.
Gynecol Oncol. 103:253–260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Narkiewicz J, Lapińska-Szumczyk S,
Zurawa-Janicka D, Skorko-Glonek J, Emerich J and Lipińska B:
Expression of human HtrA1, HtrA2, HtrA3 and
TGF-β1 genes in primary endometrial cancer. Oncol Rep.
21:1529–1537. 2009.
|
|
15
|
Esposito V, Campioni M, De Luca A,
Spugnini EP, Baldi F, Cassandro R, Mancini A, Vincenzi B, Groeger
A, Caputi M and Baldi A: Analysis of HtrA1 serine protease
expression in human lung cancer. Anticancer Res. 26:3455–3459.
2006.PubMed/NCBI
|
|
16
|
Kotliarow Y, Steed ME, Christopher N,
Walling J, Su Q, Center A, Heiss J, Rosenblum M, Mikkelsen T,
Zenklusen JC and Fine HA: High-resolution global genomic survey of
178 gliomas reveals novel regions of copy number alterations and
allelic imbalances. Cancer Res. 66:9428–9436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baldi A, Mottolese M, Vincenzi B, Campioni
M, Mellone P, Di Marino M, di Crescenzo VG, Visca P, Menegozzo S,
Spugnini EP, et al: The serine protease HtrA1 is a novel prognostic
factor for human mesothelioma. Pharmacogenomisc. 9:1069–1077. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oka C, Tsujimoto R, Kajikawa M,
Koshiba-Takeuchi K, Ina J, Yano M, Tsuchiya A, Ueta Y, Soam A,
Kanda H, et al: HtrA1 serine protease inhibits signaling mediated
by Tgfβ family proteins. Development. 131:1041–1053.
2004.PubMed/NCBI
|
|
19
|
Launay S, Maubert E, Lebeurrier A,
Tennstaedt A, Campioni M, Docagne F, Gabriel C, Dauphinot L, Potier
MC, Ehrmann M, et al: HtrA1-dependent proteolysis of TGF-β controls
both neuronal maturation and development survival. Cell Death
Differ. 15:1408–1416. 2008.PubMed/NCBI
|
|
20
|
Inman GJ: Switching TGFβ from a tumor
suppressor to a tumor promoter. Curr Opin Genet Dev. 21:93–99.
2011.
|
|
21
|
Hegde R, Srinivasula SM, Zhang Z, Wassell
R, Mukattash R, Cilenti L, DoBois G, Lazebnik Y, Zevros AS,
Fernandes-Alnemri T and Alnemri ES: Identification of Omi/HtrA2 as
mitochondrial apoptotic serine protease that disrupts inhibitors of
apoptosis protein-caspase interaction. J Biol Chem. 277:432–438.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Martins LM, Iaccarino I, Tenev T,
Gschmeissner S, Totty NF, Lemoine NR, Savopoulos J, Gray CW, Creasy
CL, Dingwall C and Downward J: The serine protease Omi/HtrA2
regulates apoptosis by binding XIAP through a reaper-like motif. J
Biol Chem. 277:439–444. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Verhagen AM, Silke J, Ekert PG, Pakusch M,
Kaufmann H, Connolly LM, Day CL, Tikoo A, Burke R, Wrobel C, et al:
HtrA2 promotes cell death through its serine protease activity and
its ability to antagonize inhibitor of apoptosis protein. J Biol
Chem. 277:445–454. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Srinivasula SM, Gupta S, Datta P, Zhang Z,
Hegde R, Cheong N, Fernandes-Alnemri T and Alnemri ES: Inhibitor of
apoptosis proteins are substrates for the mitochondrial serine
protease Omi/HtrA2. J Biol Chem. 278:31469–31472. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang QH, Church-Hajduk R, Ren J, Newton ML
and Du Ch: Omi/HtrA2 catalytic cleavage of inhibitor of apoptosis
(IAP) irreversibly inactivates IAPs and facilitates caspase
activity in apoptosis. Genes Dev. 17:1487–1496. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seong YM, Choi JY, Park HJ, Kim KJ, Ahn
SG, Seong GH, Kim IK, Kang S and Rhim H: Autocatalytic processing
of HtrA2/Omi is essential for induction of caspase-dependent cell
death through antagonizing XIAP. J Biol Chem. 279:37588–37596.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Trencia A, Fiory F, Maitan MA, Vito P,
Barbagallo AP, Perfetti A, Miele C, Ungaro P, Oriente F, Cilenti L,
et al: Omi/HtrA2 promotes cell death by binding and degrading the
anti-apoptotic protein ped/pea15. J Biol Chem. 279:46566–46572.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cilenti L, Soundarapandrian MM, Kyriasis
GA, Stratico V, Singh S, Gupta S, Bonventre JV, Alnemri ES and
Zervos AS: Regulation of HAX-1 anti-apoptotic protein by Omi/HtrA2
protease during cell death. J Biol Chem. 279:50295–50301. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu Z, Li H, Derouet M, Berezkin A,
Sasazuki T, Shirasawa S and Rosen K: Oncogenic Ras inhibits anoikis
of intestinal epithelial cells by preventing the release of a
mitochondrial pro-apoptotic protein Omi/HtrA2 into the cytoplasm. J
Biol Chem. 281:14738–14747. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Simpson CD, Anyiwe K and Schimmer AD:
Anoikis resistance and tumor metastasis. Cancer Lett. 272:177–185.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nie GY, Li Y, Minoura H, Batten L, Ooi GT,
Findley JK and Salamonsen LA: A novel serine protease of the
mammalian HtrA family is up-regulated in mouse uterus coinciding
with plancentation. Mol Hum Reprod. 9:279–290. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nie GY, Li Y, Hale K, Okada H,
Manuelpillai U, Wallace EM and Salamonsen LA: Serine protease HtrA3
is closely associated with human plancetal development and is
elevated in pregnancy serum. Biol Reprod. 74:366–374. 2006.
View Article : Google Scholar
|
|
33
|
Nie G, Hale K, Li Y, Manuelpillai U,
Wallace EM and Salamonsen LA: Distinct expression and localization
of serine protease HtrA1 in human endometrium and first-trimester
plancenta. Dev Dyn. 235:3448–3455. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nie GY, Hampton A, Li Y, Findlay JK and
Salamonsen LA: Identification and cloning of two isoforms of human
high-temperature requirement factor A3 (HtrA3), characterization of
its genomic structure and comparison of its tissue distribution
with HtrA1 and HtrA2. Biochem J. 371:39–48. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tocharus J, Tsuchiya A, Kajikawa M, Ueta
Y, Oka C and Kawaichi M: Developmentally regulated expression of
Morse HtrA3 and its role as an inhibitor of TGF-β signaling. Dev
Growth Differ. 46:257–274. 2004.PubMed/NCBI
|
|
36
|
Beleford D, Rattan R, Chien J and Shridhar
V: High-temperature requirement A3 (HtrA3) promotes etoposide- and
cisplatin-induced cytotoxicity in lung cancer cell lines. J Biol
Chem. 285:12011–12027. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Beleford D, Liu Z, Rattan R, Quagliuolo L,
Boccellino M, Baldi A, Maguire J, Staub J, Molina J and Shridhar V:
Methylation induced gene silencing of HtrA3 in smoking-related lung
cancer. Cancer Res. 16:398–409. 2010.PubMed/NCBI
|
|
38
|
Lipinska B, Zylicz M and Georgopoulos C:
The HtrA (DegP) protein, essential for Escherichia coli
survival at elevated temperatures, is an endoprotease. J Bacteriol.
172:1791–1797. 1990.PubMed/NCBI
|
|
39
|
Laemmli UK: Cleavage of structural
proteins during assembly of the head of bacteriophage T4. Nature.
227:680–685. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pound JD: Immunochemical protocols.
Methods in Molecular Biology. 80. Walker JM: Humana Press; Totowa,
NJ: 1998, View Article : Google Scholar
|
|
41
|
Zurawa-Janicka D, Kobiela J, Stefaniak T,
Wozniak A, Narkiewicz J, Limon J, Wozniak M and Lipinska B: Changes
in expression of HtrA1 and HtrA2 serine proteases during
estrogen-induced oxidative stress and nephrocarcinogenesis in male
Syrian hamster. Acta Biochem Polon. 55:9–19. 2008.PubMed/NCBI
|
|
42
|
Hu XY, Xu YM, Chen XC, Ping H, Chen ZH and
Zeng FQ: Immunohistochemical analysis of Omi/HtrA2 expression in
prostate cancer and benign prostatic hyperplasia. Acta Pathol
Microbiol Immunol Scand. 114:893–898. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee SH, Lee JW, Kim HS, Kim SY, Park WS,
Kim SH, Lee JY and Yoo NJ: Immunohistochemical analysis of
Omi/HtrA2 expression in stomach cancer. APMIS. 111:586–590. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bhattacharjee A, Richards WG, Staunton J,
Li C, Monti S, Vasa P, Ladd C, Beheshti J, Bueno R, Gillette M, et
al: Classification of human lung carcinomas by mRNA expression
profiling reveals distinct adenocarcionomas subclasses. Proc Natl
Acad Sci USA. 98:13790–13795. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stearman RS, Dwyer-Nield L, Zerbe L,
Blaine SA, Chan Z, Bunn PA, Johnson GL, Hirsch FR, Merrick DT,
Franklin WA, et al: Analysis of orthologous gene expression between
human pulmonary adenocarcinoma and a carcinogen-induced murine
model. Am J Pathol. 167:1763–1775. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sorlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sorlie T, Tibshirani R, Parker J, Hastie
T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et
al: Repeated observation of breast tumor subclasses in independent
gene expression data sets. Proc Natl Acad Sci USA. 100:8418–8423.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Handkiewecz-Junak D, Czarniecka A and
Jarzab B: Molecular prognostic markers in papillary and follicular
thyroid cancer: current status and future directions. Mol Cell
Endocrinol. 322:8–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chien J, Ota T, Aletti G, Shridhar R,
Boccellino M, Quagliuolo L, Baldi A and Shridhar V: Serine protease
HtrA1 associates with microtubules and inhibits cell migration. Mol
Cell Biol. 29:4177–4187. 2009. View Article : Google Scholar : PubMed/NCBI
|