Introduction
The 5-year survival for patients with squamous cell
carcinoma of the head and neck is only 30%, mainly due to the
frequent presence of metastasis at diagnosis (1). The pattern of regional cervical
metastasis in this disease is remarkably consistent. A better
understanding of this unique metastatic process is necessary to
enable the development of therapies designed to prevent tumor
dissemination.
Chemokines and their respective receptor that
regulate chemotaxis and the transendothelial migration of
leukocytes during immune and inflammatory reactions were recently
observed to play an important role in the metastasis of various
types of cancer (2,3). Chemokine receptors have been found to
be expressed in these invasive cells in a distinct and non-random
pattern. In particular, metastatic SCCHN cells have been shown to
express chemokine receptor 7 (CCR7), which may enable their access
to the lymphatic system and facilitate spread to regional lymph
nodes (4–7). However, the signaling mechanisms
mediated by CCR7 and induced by CCL19 have yet to be elucidated in
SCCHN cells.
Proline-rich kinase-2 (Pyk2) is a non-receptor
protein tyrosine kinase structurally related to focal adhesion
kinase. Pyk2 has a restricted tissue expression primarily in
neuronal and hematopoietic tissues. The tyrosine 402 (Y402) at Pyk2
serves as the primary autophosphorylation site that is essential
for Pyk2 activity and function (8).
Pyk2 is rapidly activated by G-protein coupled receptor agonists,
growth factors, cytokines and stress signals, thus regulating
various functions such as cell adhesion, migration, proliferation
and survival (9,10). Pyk2 is also involved in regulating
the migration and invasion of human glioma, prostate and breast
cancer cells (11–13). It has previously been demonstrated
that the activaton of Pyk2 by CCR7 was involved in dendritic cells
(DC) (14). Therefore, we
hypothesized that Pyk2 might be involved in CCR7 mediated signals
in SCCHN cells. Our results showed that CCL19 induced the
activation of Pyk2 and the activation of cofilin which altered
actin cytoskeletal rearrangement, and this signal pathway was
crucial for the chemotaxis and migration of SCCHN cells.
Materials and methods
Cell line and human tumor samples
The metastatic SCCHN cell line PCI-37B, which
strongly expresses CCR7, was a kind gift from the University of
Pittsburgh. Cells were cultured in DMEM medium (Invitrogen,
Carlsbad, CA, USA), which contained 10% (v/v) heat-inactivated
fetal bovine serum (Gibco-BRL Corp., Grand Island, NY, USA), 100
U/ml penicillin and 100 U/ml streptomycin.
Sixty specimens of SCCHN tumors with the adjacent
metastatic (or normal) lymph nodes and 10 specimens of normal human
oral mucosal tissue were obtained from the Head and Neck Tumor
Center, School of Stomatology, China Medical University. All the
specimens were obtained with the consent of the patients before
surgery and in accordance with Health Insurance Portability. The
classification of SCCHN, including primary tumors (T), regional
lymph nodes (N), distant metastasis (M) and stage grouping, was
determined according to the rules of the International Union
Against Cancer (UICC) for Head and Neck Cancer (tumor node
metastasis, TNM classification, 1997).
Reagents and antibodies
The CCR7 chemokine ligand (CCL19, MIP-3β) and the
anti-hCCR7 mAb were purchased from R&D Systems (Minneapolis,
MN, USA). The Pyk2 inhibitor (Tyrphostin A9) was purchased from
Calbiochem (San Diego, CA, USA). Anti-Pyk2, anti-phospho-Pyk2(402)
and anti-p-cofflin were bought from Santa Cruz Biotechnology (Santa
Cruz, CA, USA). TRITC-labelled phalloidin was from Sigma (St.
Louis, MO, USA).
Chemotaxis assay
Chemotaxis in response to chemokine was determined
by measuring the number of cells migrating through a polycarbonate
filter (8-μm pore size) in 24-well transwell chambers in triplicate
in DMEM with 0.5% (w/v) BSA (Invitrogen). The cell suspensions
(2×105 cell/200 μl), were placed in the top chamber of
the filter. Aliquots of the chemokine were added to the wells.
After 24 h, cells in each lower well were counted under a light
microscope in at least five different fields (original
magnification, ×200). The mean ± SD was recorded for each
condition, and index calculated based on the control, random
migration.
Matrigel invasion assay
Cell invasion was quantified in vitro using
Matrigel-coated semipermeable, modified inserts with a pore size of
8 μm. The analysis of invasion assay was performed as described in
the chemotaxis assay incubated with CCL19 for 36 h.
Western blotting
Briefly, 70–80% confluent cells were serum-starved
for 24 h. Following treatment, cells were lysed in M-PER reagent
(Pierce) containing 1 mM PMSF and phosphatase inhibitors and
centrifuged at 4°C, 12,000 rpm for 30 min. The supernatant protein
was normalized and 80 μg of protein was size-fractionated and
immunoblotted with the indicated mAbs, we determined Pyk2,
p-Pyk2(402) and p-cofilin protein expression.
Actin polymerization assay
PCI-37B cells pretreated with/without CCR7 mAb and
Pyk2 inhibitor Tyrphostin A9 were fixed, permeabilized and stained
with TRITC-labeled phalloidin. Following labeling, the samples were
washed three times for 10 min each in PBS to remove the
unincorporated label. F-actin distribution following CCL19
stimulation was evaluated by confocal laser scanning microscope
(CLSM, Leica SP2, Germany).
Immunohistochemical analysis
Immunohistochemical staining used conventional
horseradish peroxidase immunohistochemical staining methods. In
brief, 5-μm sections of the specimens were deparaffinized and
hydrated with 0.6% H2O2 in methanol to
inhibit endogenous peroxidase, performed antigen retrieval and
incubated with normal blocking serum for 10 min. The sections were
then incubated with primary antibodies (1:100): rabbit anti-Pyk2
polyclonal antibody overnight at 4°C. Immunodetection was performed
using peroxidase labeled secondary antibody (R&D Systems) and
diaminobenzidine for visualization. All the sections were
counterstained with hematoxylin (Sigma). Negative controls included
omission of the primary antibody. The cell morphology was analyzed
by microscopy (Nikon Eclipse 80i, Tokyo, Japan) at ×100–400
magnification. According to the percentage of positive tumor cells,
all these cells were scored as negative (−), <10% or no
staining; weak positive (+), 11–50%; positive (++), 51–75%; or
strongly positive (+++), >75%.
Statistical analysis
Numerical data were expressed as the mean ± standard
deviation (SD). Statistical differences between two groups were
evaluated using Student’s t-test. Correlation between Pyk2
expression and SCCHN clinical stage was analyzed by χ2
test. Values of P<0.05 were considered to indicate statistically
significant differences. All statistical analyses were performed
with the software SPSS 13.0.
Results
Pyk2 is overexpressed in squamous cell
carcinoma of the head and neck
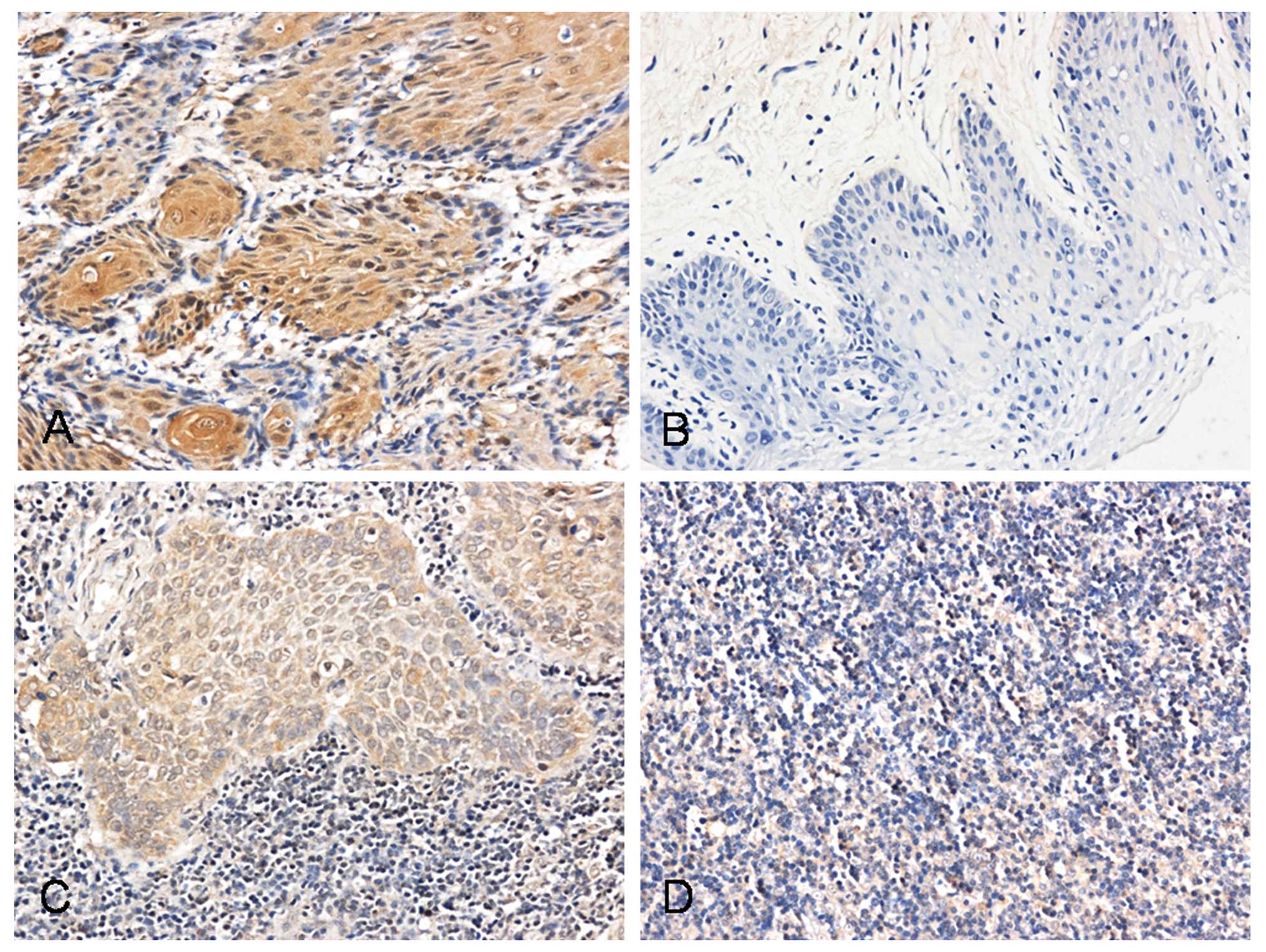
Using immunohistochemistry, we investigated the
expression of Pyk2 in SCCHN tumor tissues, metastatic lymph nodes,
normal lymph nodes and oral mucosal tissues. Pyk2 was found in the
cell membrane and cytoplasm in tumor cells and lymph node
metastatic cells. The number of stained cells was low or absent in
normal lymph nodes and oral mucosal tissues (Fig. 1 and Table I). The expression of Pyk2 was
significantly correlated with cervical lymph node metastasis and
SCCHN clinical stage (P<0.05)
 | Table ICorrelation between Pyk2 expression
and clinicopathological factors of SCCHN. |
Table I
Correlation between Pyk2 expression
and clinicopathological factors of SCCHN.
| Clinicopathological
characteristics | No. of cases | + to +++ | − | Statistical analysis
(χ2 test) |
|---|
| Age (years) |
| ≥60 | 36 | 20 | 16 | 0.049 |
| <60 | 24 | 15 | 9 | |
| Tumor size |
| T1, T2 | 49 | 28 | 21 | 0.156 |
| T3, T4 | 11 | 7 | 4 | |
| Clinical stage |
| I, II | 28 | 11 | 17 | 7.837a |
| III, IV | 32 | 24 | 8 | |
| Nodal metastasis |
| No | 30 | 11 | 19 | 11.589a |
| Yes | 30 | 24 | 6 | |
Correlation of the migration ability and
the activities of CCR7 and Pyk2 in the metastatic SCCHN cell
line
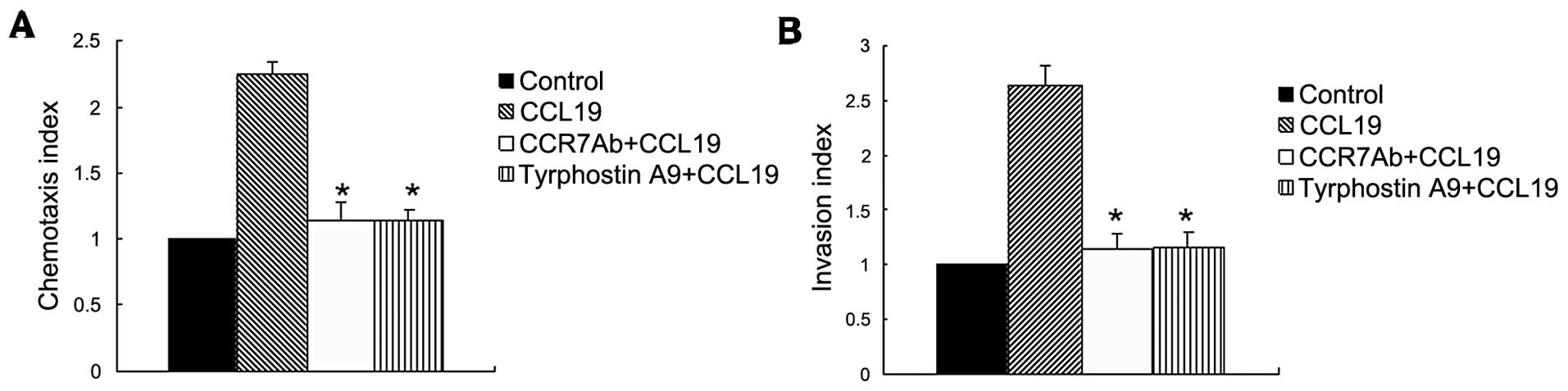
To investigate the molecular mechanism that
regulates the chemotaxis and migration ability of SCCHN, we
utilized the metastatic tumor cell line (PCI-37B) which expresses
CCR7, then analysed their capability to migrate in vitro in
response to the respective chemokine ligand CCL19. This experiment
showed that CCL19 enhanced chemotaxis of SCCHN significantly as
compared with background control levels established with media
alone. The Pyk2 inhibitior and anti-CCR7 mAb significantly blocked
CCL19-induced cell chemotaxis, as shown in Fig. 2A.
In addition to chemotactic ability, we evaluated the
invasive capacity mediated by CCR7 in the metastatic SCCHN cell
line. In vitro invasion through Matrigel was assessed after
exposure of these cells to the CCR7 ligand, CCL19, in the presence
or absence of Tyrphostin A9 or pretreatment with a CCR7-specific
blocking mAb. Fig. 2B shows that
treatment of cells with tyrphostin A9 significantly abolished the
CCL19-induced invasive capacity (P<0.01), indicating the
invasive capacity mediated by CCR7 required Pyk2 activity.
Role of CCR7 in regulating the Pyk2
activity in metastatic SCCHN
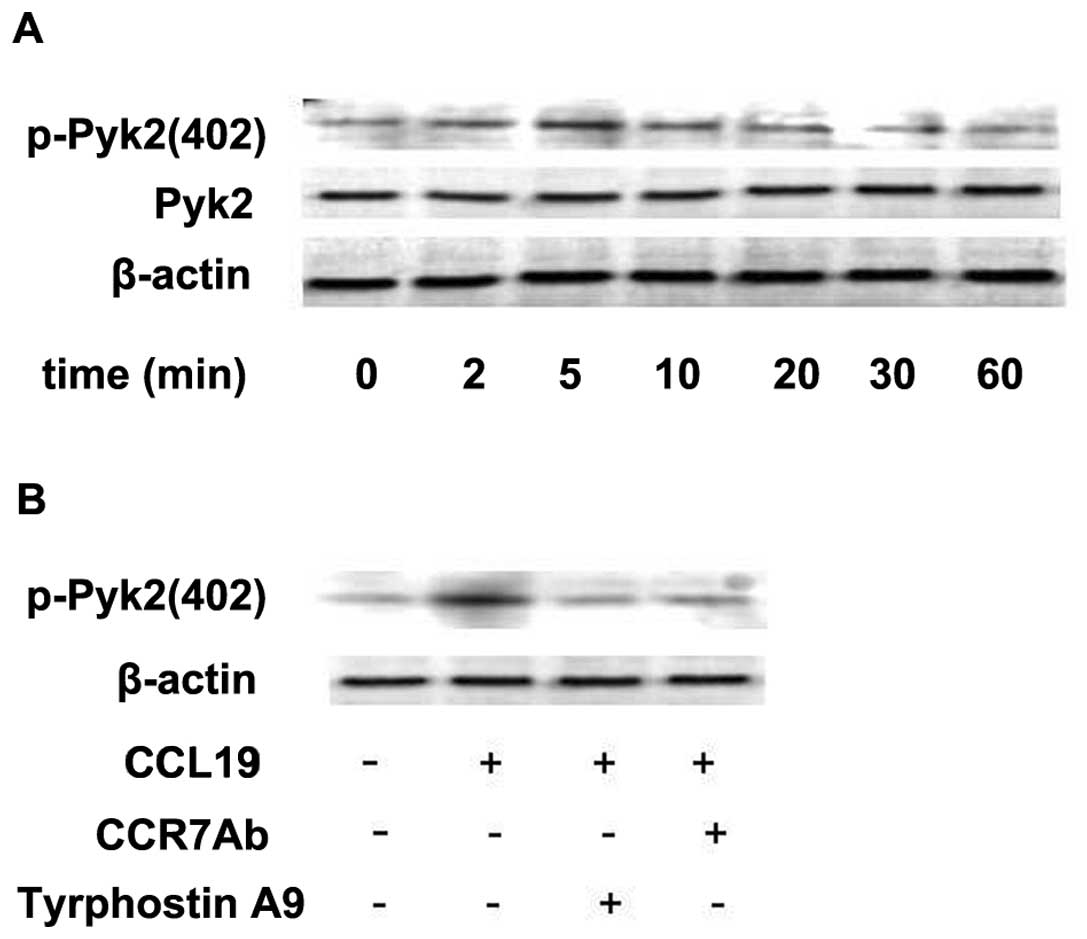
Since tyrosine kinase Pyk2 has been shown to be
involved in the signaling from chemokine receptors (11), we examined whether stimulation of
metastatic SCCHN cells with CCL19 modulates the activity of this
kinase. The metastatic SCCHN cell line (PCI-37B) was stimulated
with chemokines for different time periods. We found that CCL19
induced a transient increase in the activity of Pyk2 (Fig. 3A). Autophosphorylation of Pyk2
increased as early as 2 min after the addition of chemokine to the
cells, reached a maximum after 5–10 min and returned to basal
levels after 60 min (Fig. 3A). To
define the role of CCR7 mAb in regulating Pyk2 activity we examined
the activity of this kinase in the cells pretreated with/without
CCR7 mAb and Pyk2 inhibitor Tyrphostin A9. As shown in Fig. 3B, CCL19 induced Pyk2 activation to a
level 2- to 3-fold above the baseline, which was determined by
incubation with media alone. Phosphorylation of this molecule was
blocked selectively by the Pyk2 inhibitor (Tyrphostin A9) and CCR7
mAb. These experiments were repeated at least three times with
similar results.
CCR7 inhibits
phosphorylation/inactivation of cofilin that is mediated by
Pyk2
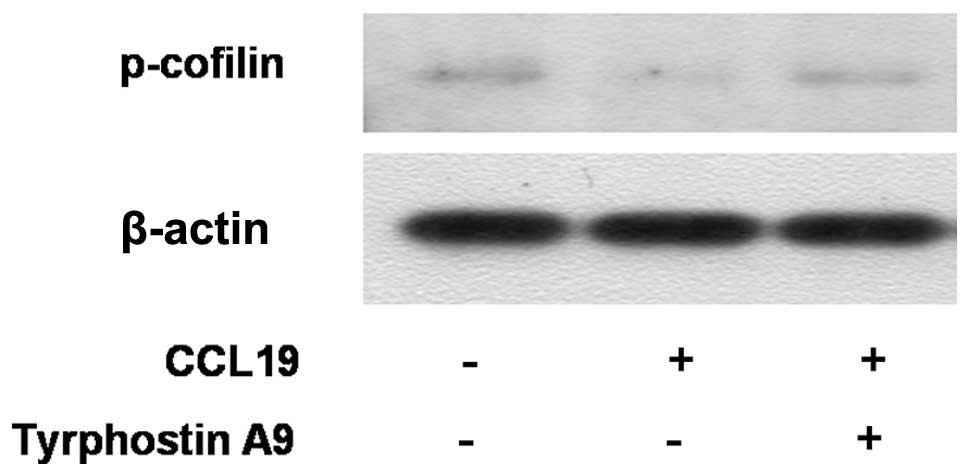
Cofilin mediates lamellipodium extension and
polarized cell migration by stimulating actin filament dynamics at
the leading edge of migrating cells. Cofilin is inactivated by
phosphorylation at Ser-3. We analyzed whether cofilin is downstream
of Pyk2. The western blot experiment showed that CCL19 inhibited
phosphorylation/inactivation of cofilin in SCCHN cells. Markedly,
Tyrphostin A9 blunted the decrease in phosphorylation of cofilin
(Fig. 4), indicating that cofilin
is downstream of Pyk2. In summary, the result indicates that CCR7
inhibits phosphorylation/inactivation of cofilin that is mediated
by Pyk2.
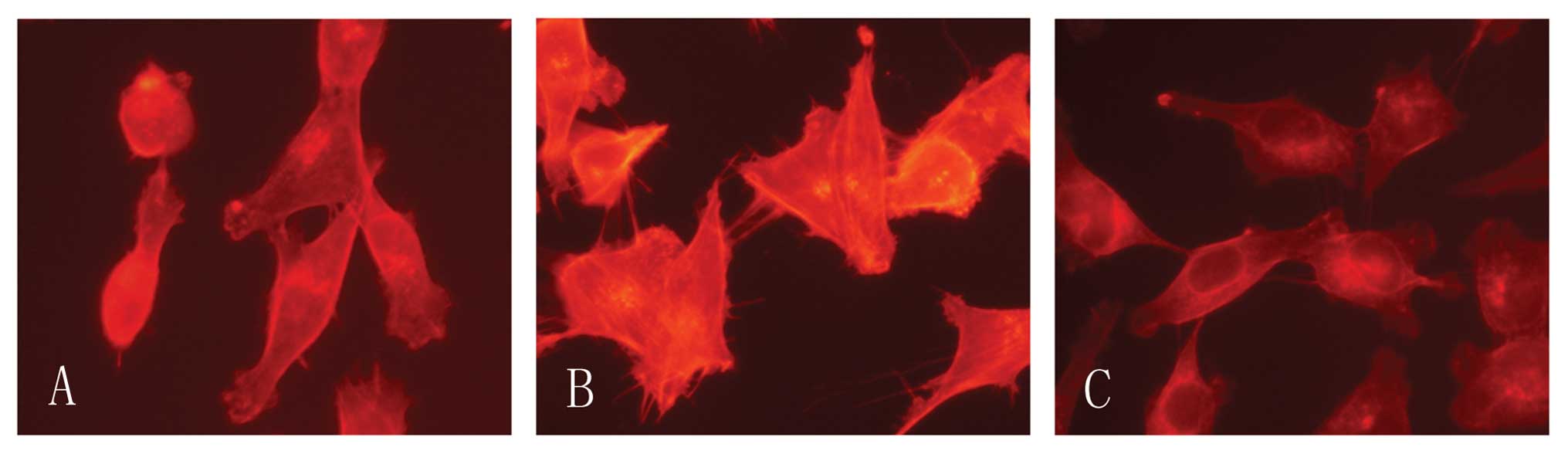
CCR7 induces F-acin rearrangement
Cell motility involves regulation of the actin
cytoskeleton and the actin-severing protein cofilin regulates actin
organization. We found that CCR7 activation lead F-actin
polymerization and pseudopodia formation. In untreated cells we
observed a scattered distribution of F-actin (Fig. 5). In the cells treated with CCL19
F-actin arrays and pseudopodia were formatted, while these effects
were blocked by Tyrphostin A9. We therefore consider that the actin
cytoskeletal rearrangement induced by CCL19 requires Pyk2.
Discussion
The chemotaxis and migration ability of tumor cells
plays a critical role for successful metastasis (14). CCR7 has been shown to be involved in
the metastasis of SCCHN (4), and
previous studies have presented a picture of the function of this
gene (15–18). However, the exact role of CCR7 in
tumor progression and its underlying mechanism remain unclear. Pyk2
is also involved in regulating the migration and invasion of a
variety of different tumor types. ErbB-2 via Pyk2 upregulates the
adhesive ability of androgen receptor-positive human prostate
cancer cells (19). Pyk2 has been
found to be crucial to cell motility and SOCS3 can regulate Pyk2
pro-migratory function in lung cancer (20). In breast cancer cells, Pyk2
participates in CXCR4-induced chemotactic and chemoinvasive
signaling pathways (21). However,
there is no report on the role of Pyk2 in SCCHN. Our experiments
showed that Pyk2 is highly expressed in SCCHN and metastatic lymph
node cells. The expression of Pyk2 was significantly correlated
with cervical lymph node metastasis and SCCHN clinical stage.
Hence, we considered that Pyk2 is probably crucial for the
development and progress of SCCHN.
Tyrosine kinase Pyk2 has been involved in the
signaling from chemokine receptors in different cells. In breast
cancer cells, CXCL12 induces the phosphorylation at residues 402
and 579/580 of Pyk2, Pyk2 inhibitors significantly blocks
CXCL12-induced chemotaxis and chemoinvasion and so the role of Pyk2
is indicated in CXCL12-induced breast cancer cell migration and
invasion (22). In T cells, Pyk2
becomes activated in response to chemokine receptors and is
important for cell spreading and migration (23). Some results indicate that Pyk2 acts
as a receptor-proximal link between integrin and chemokine receptor
signaling, and the Pyk2/Rac pathway plays a pivotal role in the
control of NK cell transendothelial migration (24). We examined the role of Pyk2 in
CCR7-induced metastasis of SCCHN cells. We found that CCL19 (CCR7
ligand) induced the activation of Pyk2 in metastasic SCCHN cells
and the actvation was blocked by CCR7 mAb. Then, we used the Pyk2
inhibitor, Tyrphostin A9, to analyze the role of Pyk2 in the
chemotaxis and migration of SCCHN cells. The results revealed the
predominant role of CCR7 in upregulating the Pyk2 activity which
was important for the chemotaxis and migration ability of SCCHN
cells. Further experiments are required to clarify if other
molecules are involved in the CCR7/Pyk2 pathway.
The actin severing protein cofilin is essential for
directing cell migration and chemotaxis in many cell types and is
also important for tumor cell invasion during metastasis (25,26).
Through its severing activity, cofilin increases the number of free
barbed ends to initiate actin polymerization for actin-based
protrusion. In this regard, we notably observed that stimulation of
CCR7 induced dephosphorylation/activation of cofilin. The
Tyrphostin A9 abolished the activation of cofilin, suggesting that
Pyk2 is upstream of this molecule. Our results indicate that
cofilin may mediate the effects of Pyk2 on cell motility.
A high level of actin polymerization is required for
the formation of pseudopodia, which is needed for
chemokine-mediated cell migration and invasion into surrounding
tissues and efficient metastasis formation (27). In our study, we examined
rhodamine-labeled phalloidin staining by inverted microscopy, and
observed reorganization of the actin cytoskeleton of PCI-37B was
enhanced in response to CCL19, and this function was inhibited by
Tyrphostin A9. This result, consistent with previous studies in
hepatocellular carcinoma, demonstrates that Pyk2 upregulates the
formation of pseudopodia and actin stress fiber polymerization of
HCC cells and promotes cell motility (28).
In conclusion, we found that CCR7 promoted tumor
metastasis by sequential activation of Pyk2 and cofilin followed by
rearrangement of F-actin in SCCHN cells. Our results thus reveal a
novel signal pathway that regulates the chemotaxis and migration
ability of SCCHN cells and provide potential targets for advanced
therapy of squamous cell carcinoma of the head and neck.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 30672331), the National
Science Foundation for Young Scholars of China (no. 81102058) and
the Foundation of Education Bureau of Liaoning Province, China (no.
2009A755).
References
|
1
|
Greenlee RT, Hill-Harmon MB, Murray T and
Thun M: Cancer Statistics, 2001. CA Cancer J Clin. 51:15–36. 2001.
View Article : Google Scholar
|
|
2
|
Müller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001.PubMed/NCBI
|
|
3
|
Ben-Baruch A: Site-specific metastasis
formation: chemokines as regulators of tumor cell adhesion,
motility and invasion. Cell Adh Migr. 3:328–333. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J, Xi L, Hunt JL, Gooding W,
Whiteside TL, Chen Z, Godfrey TE and Ferris RL: Expression pattern
of chemokine receptor 6 (CCR6) and CCR7 in squamous cell carcinoma
of the head and neck identifies a novel metastatic phenotype.
Cancer Res. 64:1861–1866. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao ZJ, Liu FY, Li P, Ding X, Zong ZH and
Sun CF: CCL19-induced chemokine receptor 7 activates the
phosphoinositide-3 kinase-mediated invasive pathway through Cdc42
in metastatic squamous cell carcinoma of the head and neck. Oncol
Rep. 25:729–737. 2011.PubMed/NCBI
|
|
6
|
Liu FY, Zhao ZJ, Li P, Ding X, Zong ZH and
Sun CF: Mammalian target of rapamycin (mTOR) is involved in the
survival of cells mediated by chemokine receptor 7 through PI3K/Akt
in metastatic squamous cell carcinoma of the head and neck. Br J
Oral Maxillofac Surg. 48:291–296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhen-Jin Z, Peng L, Fa-Yu L, Liyan S and
Chang-Fu S: PKCα take part in CCR7/NF-κB autocrine signaling loop
in CCR7-positive squamous cell carcinoma of head and neck. Mol Cell
Biochem. 357:181–187. 2011.
|
|
8
|
Park SY, Li H and Avraham S: RAFTK/Pyk2
regulates EGF-induced PC12 cell spreading and movement. Cell
Signal. 19:289–300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Picascia A, Stanzione R, Chieffi P,
Kisslinger A, Dikic I and Tramontano D: Proline-rich tyrosine
kinase 2 regulates proliferation and differentiation of prostate
cells. Mol Cell Endocrinol. 186:81–87. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuwabara K, Nakaoka T, Sato K, Nishishita
T, Sasaki T and Yamashita N: Differential regulation of cell
migration and proliferation through proline-rich tyrosine kinase 2
in endothelial cells. Endocrinology. 145:3324–3330. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roelle S, Grosse R, Buech T, Chubanov V
and Gudermann T: Essential role of Pyk2 and Src kinase activation
in neuropeptide-induced proliferation of small cell lung cancer
cells. Oncogene. 27:1737–1748. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Amicis F, Lanzino M, Kisslinger A, Calì
G, Chieffi P, Andò S, Mancini FP and Tramontano D: Loss of
proline-rich tyrosine kinase 2 function induces spreading and
motility of epithelial prostate cells. Cell Physiol. 209:74–80.
2006.PubMed/NCBI
|
|
13
|
Behmoaram E, Bijian K, Jie S, Xu Y, Darnel
A, Bismar TA and Alaoui-Jamali MA: Focal adhesion kinase-related
proline-rich tyrosine kinase 2 and focal adhesion kinase are
co-overexpressed in early-stage and invasive ErbB-2-positive breast
cancer and cooperate for breast cancer cell tumorigenesis and
invasiveness. Am J Pathol. 173:1540–1550. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Riol-Blanco L, Sánchez-Sánchez N, Torres
A, Tejedor A, Narumiya S, Corbí AL, Sánchez-Mateos P and
Rodríguez-Fernández JL: The chemokine receptor CCR7 activates in
dendritic cells two signaling modules that independently regulate
chemotaxis and migratory speed. J Immunol. 174:4070–4080. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, Zhang X, Thomas SM, Grandis JR,
Wells A, Chen ZG and Ferris RL: Chemokine receptor 7 activates
phosphoinositide-3 kinase-mediated invasive and prosurvival
pathways in head and neck cancer cells independent of EGFR.
Oncogene. 24:5897–5904. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu FY, Zhao ZJ, Li P, Ding X, Guo N, Yang
LL, Zong ZH and Sun CF: NF-κB participates in chemokine receptor
7-mediated cell survival in metastatic squamous cell carcinoma of
the head and neck. Oncol Rep. 25:383–391. 2011.
|
|
17
|
Li P, Zhao ZJ, Liu FY, Sun LY, Ding X,
Zhang WZ, Shang DH and Sun CF: The chemokine receptor 7 regulates
cell adhesion and migration via β1 integrin in metastatic squamous
cell carcinoma of the head and neck. Oncol Rep. 24:989–995.
2010.
|
|
18
|
Li P, Liu F, Sun L, Zhao Z, Ding X, Shang
D, Xu Z and Sun C: Chemokine receptor 7 promotes cell migration and
adhesion in metastatic squamous cell carcinoma of the head and neck
by activating integrin αvβ3. Int J Mol Med. 27:679–687.
2011.PubMed/NCBI
|
|
19
|
Yuan TC, Lin FF and Veeramani S: ErbB-2
via PYK2 upregulates the adhesive ability of androgen
receptor-positive human prostate cancer cells. Oncogene.
26:7552–7559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang S, Guo D, Jiang L, Zhang Q, Qiu X
and Wang E: SOCS3 inhibiting migration of A549 cells correlates
with PYK2 signaling in vitro. BMC Cancer. 28:1502008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Prasad A, Fernandis AZ, Rao Y and Ganju
RK: Slit protein-mediated inhibition of CXCR4-induced chemotactic
and chemoinvasive signaling pathways in breast cancer cells. J Biol
Chem. 279:9115–924. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fernandis AZ, Prasad A, Band H, Klösel R
and Ganju RK: Regulation of CXCR4-mediated chemotaxis and
chemoinvasion of breast cancer cells. Oncogene. 23:157–167. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ostergaard HL and Lysechko TL: Focal
adhesion kinase-related protein tyrosine kinase Pyk2 in T-cell
activation and function. Immunol Res. 31:267–282. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gismondi A, Jacobelli J, Strippoli R,
Mainiero F, Soriani A, Cifaldi L, Piccoli M, Frati L and Santoni A:
Proline-rich tyrosine kinase 2 and Rac activation by chemokine and
integrin receptors controls NK cell transendothelial migration. J
Immunol. 170:3065–3073. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oser M and Condeelis J: The cofilin
activity cycle in lamellipodia and invadopodia. J Cell Biochem.
108:1252–1262. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Rheenen J, Condeelis J and Glogauer M:
A common cofilin activity cycle in invasive tumor cells and
inflammatory cells. J Cell Sci. 122:305–311. 2009.PubMed/NCBI
|
|
27
|
Pollard TD and Borisy GG: Cellular
motility driven by assembly and disassembly of actin filaments.
Cell. 112:453–465. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun CK, Ng KT, Lim ZX, Cheng Q, Lo CM,
Poon RT, Man K, Wong N and Fan ST: Proline-rich tyrosine kinase 2
(Pyk2) promotes cell motility of hepatocellular carcinoma through
induction of epithelial to mesenchymal transition. PLoS One.
6:e188782011. View Article : Google Scholar : PubMed/NCBI
|