Introduction
CD44, a hyaluronan (HA) receptor, serves as an
adhesion molecule in cell-substrate and cell-cell interactions,
lymphocyte recruitment to inflammatory sites and tumor metastasis
(1–4). CD44 isoforms are generated from a
single gene by messenger RNA alternative splicing of at least 12
exons (5). The size of the CD44
molecule ranges from the standard 85–95 kDa form (CD44s) to larger
variant isoforms (CD44v) of 200 kDa or more. The differences in
size are partly due to post-translational modifications, as all
isoforms of CD44 are highly glycosylated (6). The functional characterization of the
different isoforms of this family remains limited.
Invasion is a critical step for metastasis. Tumor
cell invasion involves cell adhesion to the extracellular matrix,
degradation of extracellular matrix components, tumor cell motility
and cell detachment (7). CD44 is
expressed in many types of invasive tumor cells (2,4). It
has been shown in animal models that the injection of reagents that
interfere with the binding of CD44 to its ligand inhibits local
tumor growth and metastatic spread (8,9).
Soluble CD44 can be shed from cell surfaces through a proteolytic
process by metalloproteinase (10,11).
This cleavage is followed by γ-secretase-dependent release of CD44
intracellular domain (ICD) (12).
This proteolytic cleavage of CD44 is involved in tumor invasion and
metastasis (10). During tumor
metastasis, cells detach from the primary tumor, penetrate the
basement membrane into the connective tissue, and invade adjacent
structures, including lymph and blood vessels. The tumor cells are
subsequently transported to metastatic sites via the lymph and/or
blood. However, the mechanisms by which CD44 promotes tumor
metastasis are poorly understood.
Matrix metalloproteinases (MMPs) are a group of
proteases that are involved in extracellular matrix degradation;
the functioning of these proteases requires the presence of zinc.
Since MMPs degrade extracellular matrix, they participate in tumor
cell invasion and migration (13).
The MMP family is currently divided into two categories:
soluble-type MMPs and membrane-type MMPs (MT-MMPs) (14,15).
One of these proteins, MT1-MMP, is often-expressed in invasive
cancer cells and in endothelial cells during angiogenesis (16,17)
and its substrates include type I, II and III collagen, laminin-1,
-5, vitronectin, fibronectin and aggrecan (18). MT1-MMPs also activate other proMMPs,
including proMMP-2 and proMMP-13 (19,20).
The expression of MT1-MMP on the cell surface may trigger various
activation cascades. Research has shown that the extracellular
domain of CD44 undergoes cleavage on the surface of cancer cells
and this cleavage process plays a decisive role during tumor cell
migration (21). This process of
inducing CD44 de-adhesion through metalloproteinase interaction
appears to be significantly involved in cell movement.
In this report, we have identified a new function
for HA, its involvement in the induction of MT1-MMP expression in
breast cancer cells and the subsequent mediation of cellular
migration during the invasion process. Based on these new findings,
we suggest that the activation signals resulting from HA
stimulation may be involved in tumor cell metastasis. We propose
that one function of HA oligosaccharides in tumor cells may be to
induce MT1-MMP expression, which, at least for a number of tumor
cell types, may be a critical step in the formation of metastatic
colonies.
Materials and methods
Cell culture
The human breast carcinoma cell lines, MDA-MB-435s
and MDA-MB-231, were obtained from the Food Industry Research and
Development Institute (Hsinchu, Taiwan). Cells were grown in
Leibovitz’s L-15 medium supplemented with 15% fetal bovine serum
(FBS; HyClone, Logan, UT, USA), 10 μg/ml of insulin, 100 U/ml of
penicillin, and 100 μg/ml of streptomycin at 37°C in a humidified
atmosphere of 5% CO2.
Antibodies
Mouse monoclonal antibody against human MT1-MMP and
rabbit monoclonal antibody (Ab) against CD44 were purchased from
R&D Systems (Minneapolis, MN, USA). Mouse monoclonal Ab against
CD44 and horseradish peroxidase-conjugated goat anti-mouse
secondary Ab were both purchased from NeoMarkers (Fremont, CA,
USA). Rabbit polyclonal Ab against MT1-MMP and rhodamine-conjugated
goat anti-rabbit secondary Ab was purchased from Chemicon
International, Inc. (San Diego, CA, USA). FITC-conjugated bovine
anti-mouse secondary Ab was purchased from Santa Cruz Biotechnology
(Santa Cruz, CA, USA).
Flow cytometry
MDA-MB-435s cells were cultured in a serum-free
medium (Leibovitz’s L-15 medium) overnight and then HA was added
(0.05 mg/ml; 0.5 mg/ml) for 36 h. Next, the cells were trypsinized
and suspended in Leibovitz’s L-15 medium at a concentration of
5×106 cells/ml, and then a 1-ml sample was incubated for
45 min at 4°C with 150 μl of various non-labeled mouse anti-human
antibodies followed by fluorescein isothiocyanate (FITC)-conjugated
anti-mouse immunoglobulin G (IgG) antibodies (10 mg/ml) for 1 h at
room temperature. Finally, the cells were washed twice with
phosphate-buffered saline (PBS; pH 7.4), centrifuged, and fixed in
1.5 ml of 4% paraformaldehyde. Control samples were incubated with
PBS instead of primary antibody. A FACScan machine
(Becton-Dickinson, Franklin Lakes, NJ, USA) was used to analyze
antibody binding.
Western blot analysis
For immunoblotting on polyvinylidene difluoride
(PVDF) membranes (Amersham; Piscataway, NJ, USA), cells per
treatment group were pooled, rinsed briefly with PBS, then lysed on
ice for 10 min in 1 ml of PBS containing 1% sodium dodecyl sulfate,
0.5 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 10 μg/ml
aprotinin, 5 μg/ml pepstatin, 10 μg/ml soybean trypsin inhibitor,
and 0.5 mM dithiothreitol. Following centrifugation for 20 min at
1,300 g and 4°C, the supernatant was removed and centrifuged at
6,000 g for 1 h at 4°C. The final supernatant was used as the
cytosolic fraction and the pellet as the membrane fraction. Equal
amounts (50 μg of protein) of the membrane fractions or cytosolic
fractions were run on 10% polyacrylamide gels and transferred to
PVDF membranes in a transfer buffer [4 parts 25 mM Tris/200 mM
buffer (pH 8.0) and 1 part methanol]. The membranes were then
blocked for 1 h at room temperature with 50 mM Tris HCl, 150 mM
NaCl, 0.05% Tween-20 [Tris buffered-saline with Tween-20 (TBST), pH
7.0], containing 5% non-fat dry milk, and then incubated overnight
at 4°C with the primary Ab. The membranes were then washed with
TBST and exposed to the horseradish peroxidase-conjugated secondary
Ab for 1 h at room temperature. The bound Ab was detected by the
enhanced chemiluminescence method (Perkin-Elmer Life Sciences,
Waltham, MA, USA).
RNA extraction and real-time polymerase
chain reaction analysis
Total RNA was extracted from both untreated
(control) and treated cells using RNeasy purification reagent
(Qiagen; Valencia, CA, USA) and then a sample (1 μg) was reverse
transcribed with M-MLV reverse transcriptase for 30 min at 42°C in
the presence of oligo-dT primer. PCR was performed using specific
primers designed from the published sequence of each cDNA as
follows; the oligonucleotides (sense 5′-GTGATGGATGGATA CCCAATGC-3′
and antisense 5′-GAACGCTGGCAGTAAA GCAGTC-3′) corresponding to human
MT1-MMP were used for specific amplification of a 786 bp fragment
of MT1-MMP mRNA. The initial temperature for the RT-PCR was 95°C
for 5 min, followed by 35 cycles of denaturation at 95°C for 30
sec, annealing at 55°C for 30 sec, and elongation at 72°C for 30
sec, with an additional 7-min incubation at 72°C after completion
of the last cycle. To exclude the possibility of contaminating the
genomic DNA, the PCRs were also run without reverse transcriptase.
The amplified cDNA was separated by electrophoresis through a 2%
agarose gel, stained, and photographed under ultraviolet light.
cDNA was used for PCR with specific primers in the presence of
SYBR-Green I (LightCycler®-FastStart DNA Master SYBR
Green I; Roche, Basel, Switzerland). The sequences of the primers
were: MT1-MMP forward, CGCTA CGCCATCCAGGGTCTCAAA, reverse,
CGCTCATCAT CGGGCAGCACAAAA; GAPDH forward, CACCATCTT CCAGGAGCGAG,
reverse, TCACGCCACAGTTTCCCGGA (Mission Biotech, Taiwan). A
LightCycler® 480 (Roche Diagnostics, Indianapolis, IN,
USA) was used for real-time PCR.
Immunofluorescence stain and confocal
laser scanning microscopic analysis
Breast cancer cells (1×105) were cultured
on glass coverslips, serum-starved for 3 h, and then treated with
HA for various times. Following incubation, the cells were fixed in
4% paraformaldehyde for 15 min, washed with PBS, and pre-incubated
in blocking solution (5% non-fat milk in PBS) for 15 min. After
being washed with PBS, the cells were incubated with diluted
anti-MT1-MMP monoclonal (m) Ab (1:500) or anti-CD44 mAb (1:500) in
PBS for 60 min at room temperature. After washing with PBS, the
cells were incubated with diluted FITC-conjugated secondary
antibody or rhodamine-conjugated secondary antibody for 60 min at
room temperature. The samples were then washed with PBS and mounted
in a mounting medium (Vector; North Hollywood, CA, USA) and
visualized using a confocal microscope (Leica; Wetzlar, Germany)
with a 100/1.30 oil immersion objective and an appropriate filter.
There was negligible immunofluorescence in the controls without
primary antibodies.
Migration assay
The migration assay used was a transwell, the upper
chamber of which consisted of cell culture inserts coated with 50
μl of Matrigel at 37°C in an incubator overnight. The wells were
washed 3 times with PBS. Sub-confluent breast cancer cell lines
were trypsinized and re-suspended in a cell culture medium
containing 5% FBS. Then, 1×105 cells were added to the
upper chamber and incubated overnight at 37°C in a humidified 5%
CO2 environment. Subsequently, cells were starved for 12
h, and MT1-MMP specific Abs or MMP inhibitors were added to the
cells for 1 h before treating the cells with HA for 60 h. Cells
that had invaded through the matrix and become adherent to the
undersurface of the filter were quantified using Hoechst stain and
observed with a fluorescence microscope.
Statistical analysis
The results are expressed as the mean ± standard
deviation (SD) of at least 3 experiments and comparisons were
analyzed by one-way ANOVA. A P-value <0.05 was considered to
indicate a statistically significant difference.
Results
Effects of HA on MT1-MMP expression on
MDA-MB-435s cells
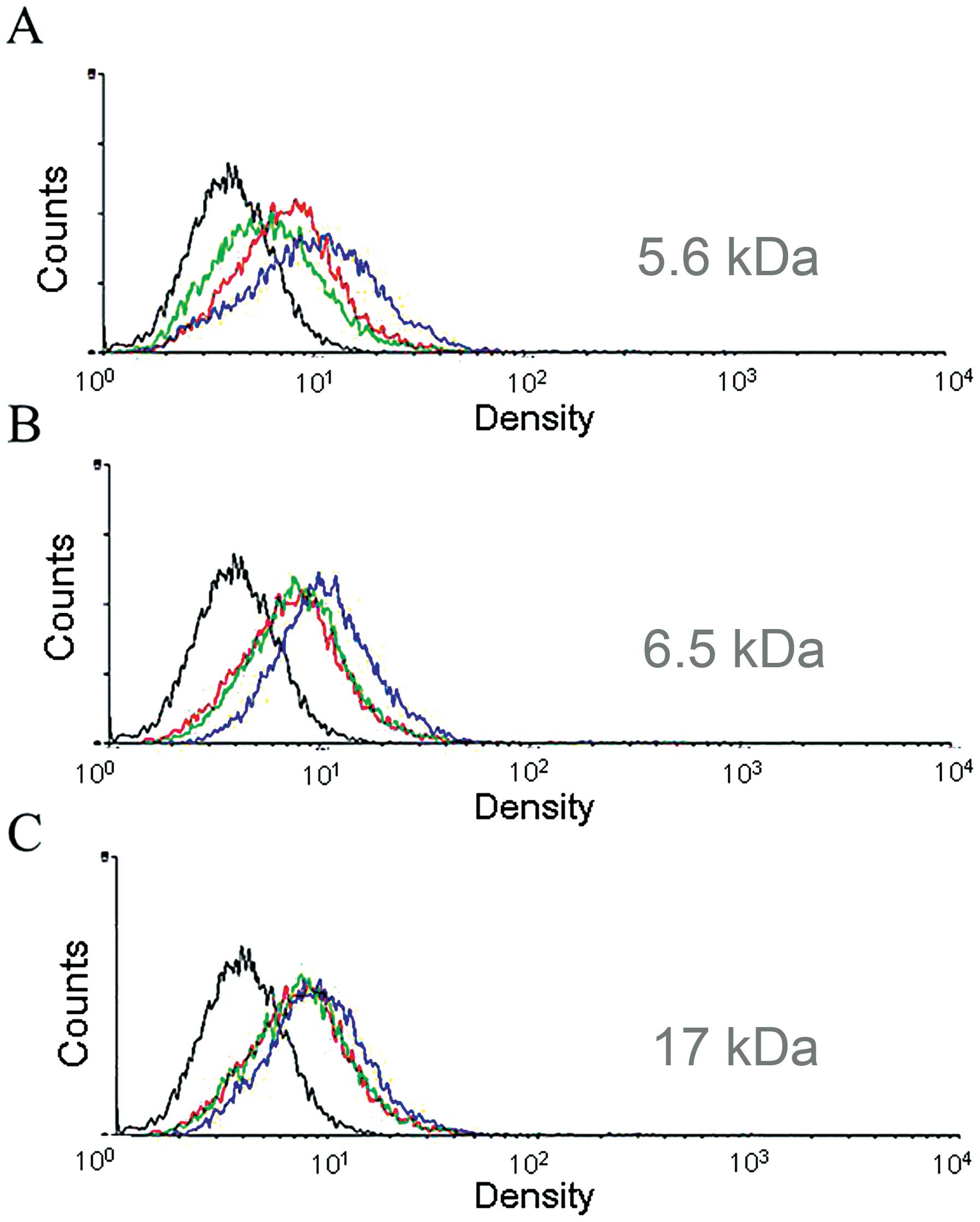
In order to observe the effect of HA on MT1-MMP
expression following stimulation, MDA-MB-435s cells were treated
for different times with 17, 5.6 and 6.5 kDa HA (0.05 mg/ml or 0.5
mg/ml) (Fig. 1). HA 5.6 kDa and 6.5
kDa (0.5 mg/ml) stimulation after 36 h revealed an increased
expression of MT1-MMP on the cell membrane (Fig. 1A and B). This result showed that HA
oligosaccharide (5.6 and 6.5 kDa) stimulation of MDA-MB-435s cells
induced a significant amount of MT1-MMP expression on the cell
membrane. Thus, 6.5 kDa HA was used in the following
experiments.
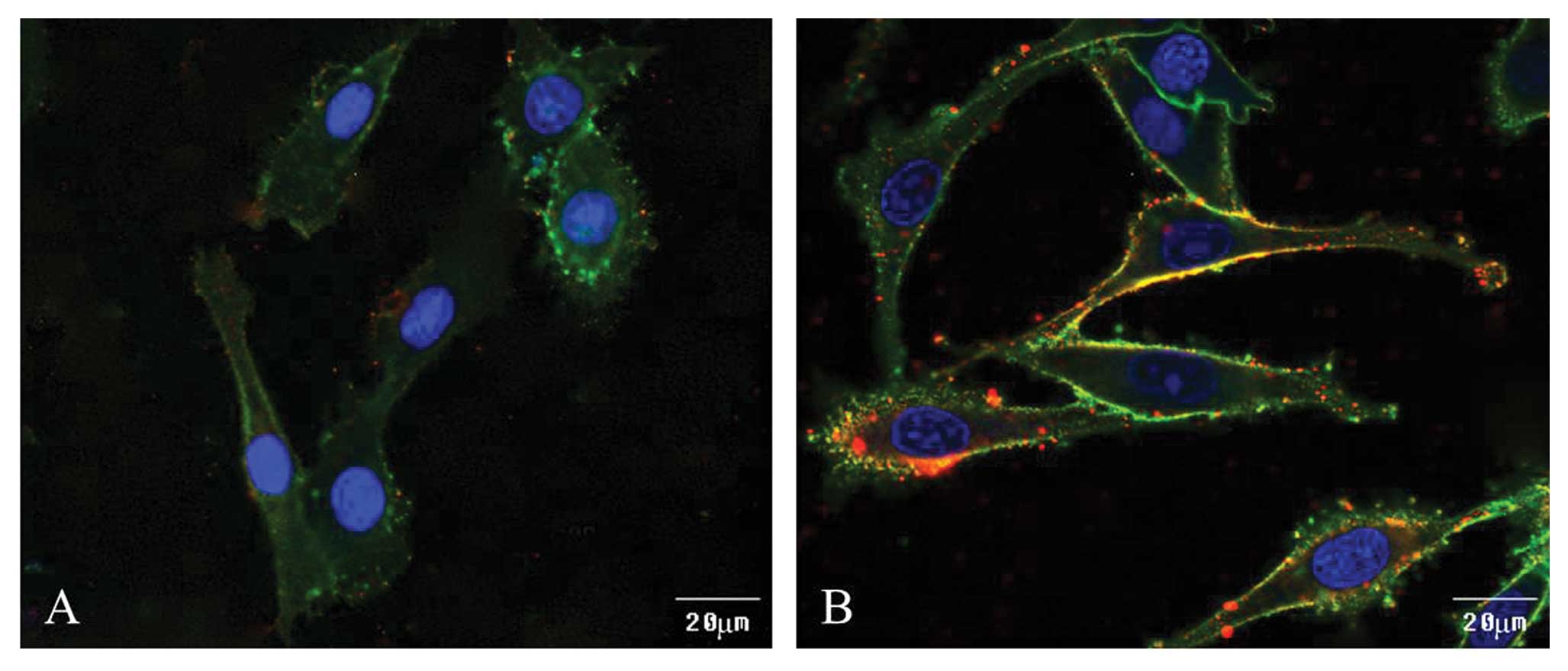
Immunofluorescence observation of CD44
and MT1-MMP expression following HA stimulation
We further investigated the link between CD44 and
HA-induced MT1-MMP expression. After 36 h of HA stimulation,
MDA-MB-435s cells were fixed, blocked, treated with mouse anti-CD44
mAb, rabbit anti-MT1-MMP polyclonal Ab, FITC-conjugated bovine
anti-mouse Ab, and rhodamine-conjugated goat anti-rabbit Ab. The
cells were then mounted and placed under a confocal microscope for
observation. The results show that under normal conditions in
MDA-MB-435s cells, the CD44 receptor was localized on the cell
membrane and small amounts of MT1-MMP were localized in the cytosol
(Fig. 2A). After HA stimulation for
36 h, the expression of MT1-MMP increased on the cell membrane and
colocalized with CD44 (Fig.
2B).
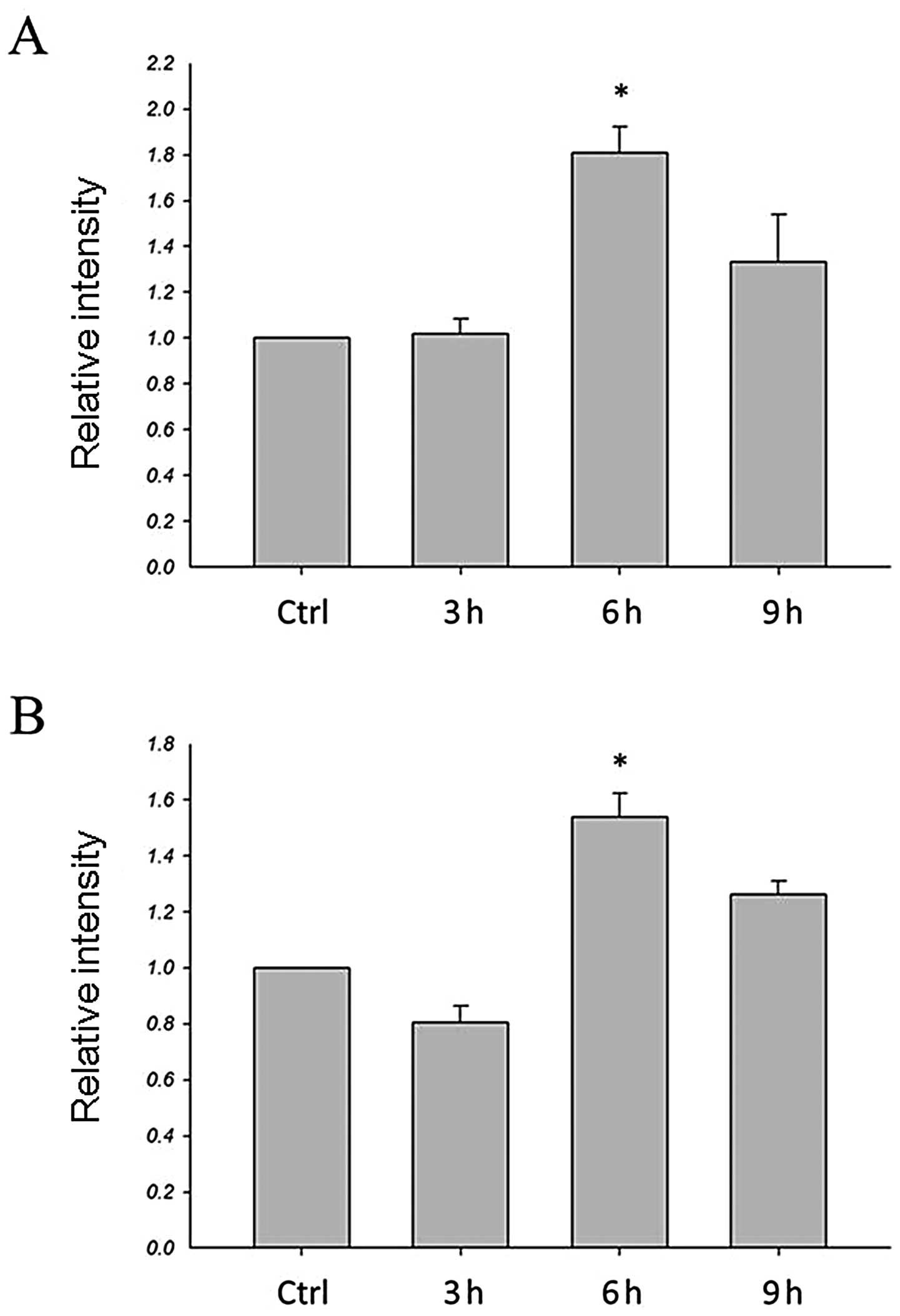
Gene expression levels in HA treated
breast cancer cells
To determine whether HA has an effect on MT1-MMP
expression, the expression levels of the MT1-MMP gene were examined
by real-time PCR. As shown in Fig.
3, real-time PCR was used to quantify the gene expression
levels of HA-treated cells compared to the control. For both
MDA-MB-435s and MDA-MB-231 cells, mRNA levels for the MT1-MMP were
significantly increased in HA-treated cells at 6 h (Fig. 3). Thus, HA is capable of inducing
breast cancer cells which express the MT1-MMP gene.
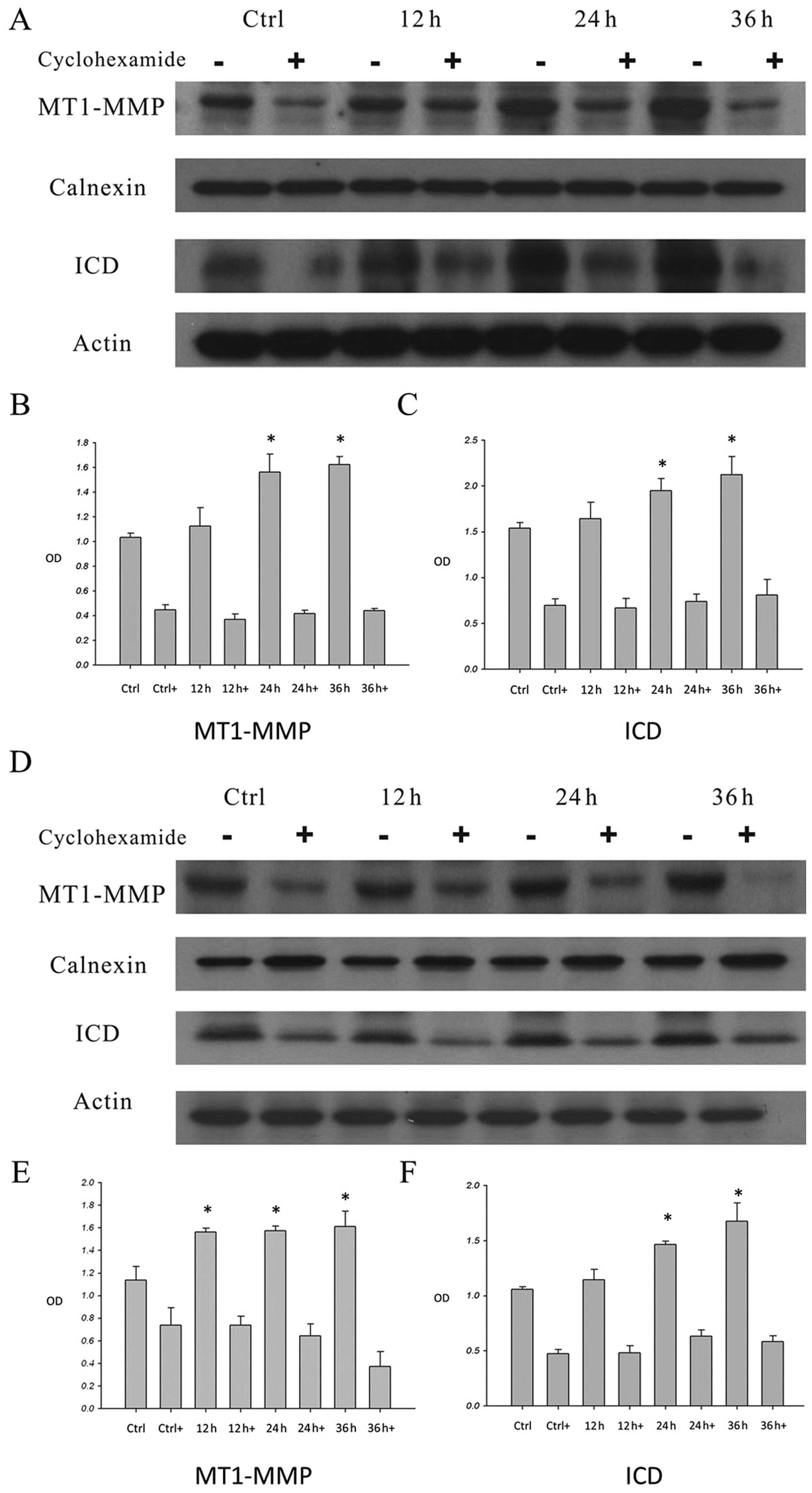
The effects of cycloheximide on
HA-induced MT1-MMP expression and CD44 cleavage on the cell
membrane
Western blot analysis revealed that when the
MDA-MB-435s (Fig. 4A–C) and
MDA-MB-231 cells (Fig. 4D–F) were
incubated with HA, MT1-MMP expression was increased. When the cells
were pre-treated with cycloheximide and then stimulated with HA for
12, 24 and 36 h, the expression of MT1-MMP was decreased (Fig. 4A, B, D and E). Moreover, after HA
treatment for 12, 24 and 36 h, the CD44 ICD was increased after 12
h of stimulation (Fig. 4A, C, D and
F). When cells were pre-treated with cycloheximide, the ICDs
were reduced (Fig. 4A, C, D and F).
These results further confirmed that HA induced the expression of
MT1-MMP through protein synthesis. Moreover, HA-induced MT1-MMP was
involved in the process of CD44 cleavage.
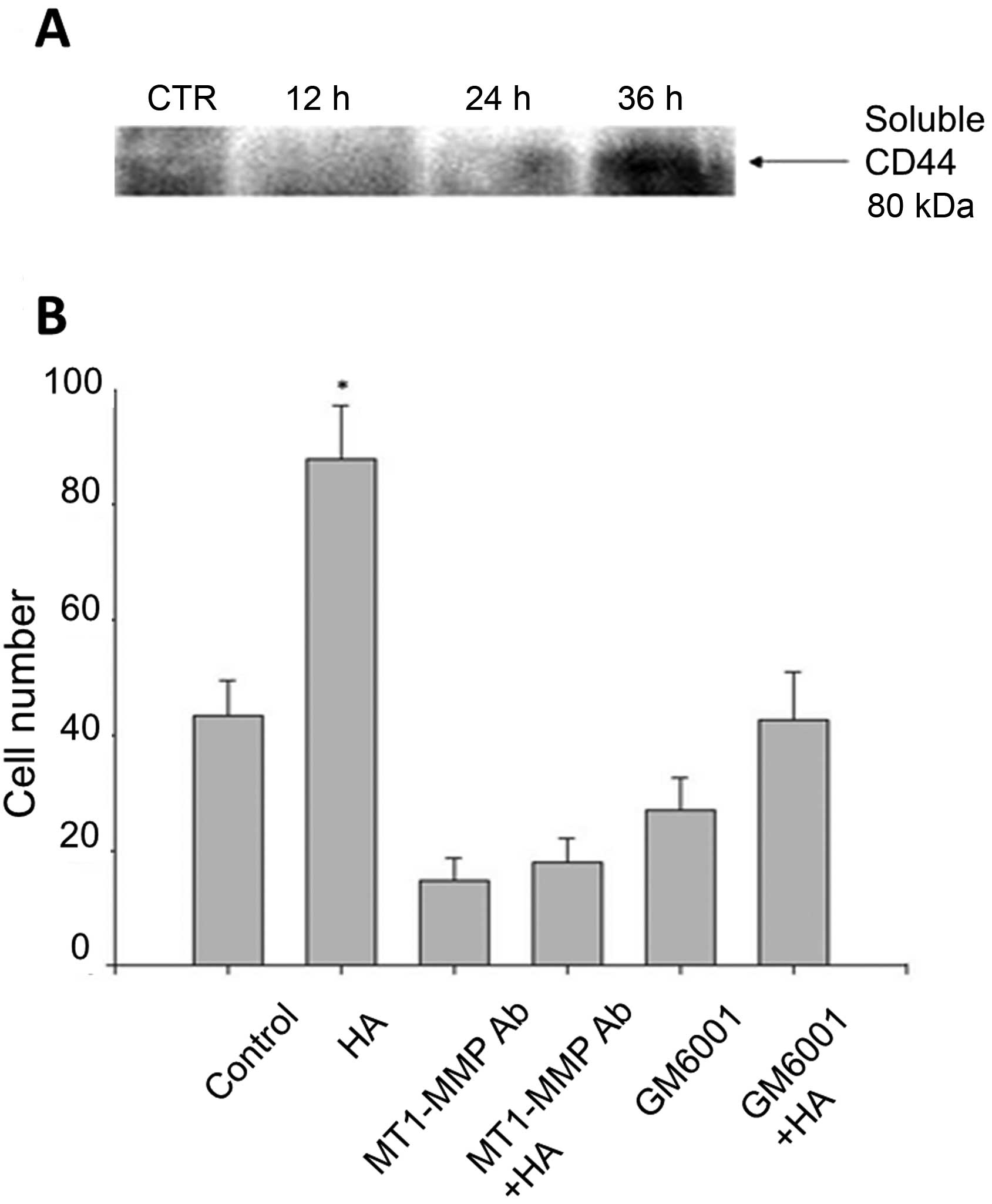
Invasiveness of breast cancer cells
determined by migration assay following HA stimulation
Penetration of the cells through the Matrigel has
been suggested to be similar to the invasive activities that occur
during the metastatic process in vivo. A transwell coated
with Matrigel was adopted to study the invasive behavior of the
breast cancer cells. MDA-MB-435s cells were pre-treated with
MT1-MMP specific antibody or MMP inhibitor for 1 h, then stimulated
with or without HA for 60 h, each individual well was followed to
count the cells that had migrated through the membrane. MDA-MB-435S
cells pre-treated with MT1-MMP specific antibody or MMP inhibitor
reduced cell migration (Fig.
5).
Discussion
Matrix metalloproteinases (MMPs) comprise a family
of zinc-dependent edopeptidases that can cleave virtually any
component of the extracellular matrix (18) and play an important role in cancer
cell invasion and metastasis (18).
During metastasis, cancer cells break down the extracellular matrix
to clear the path for movement, and then enter the blood vessels or
the lymphatics (18). Membrane
type-MMPs are a small group in the MMP family, consisting of six
known members. Membrane type-MMPs are located on cell surfaces and
degrade protein (22). Membrane
type-MMPs are found in a number of different cancer cells and they
are capable of breaking down extracellular matrix molecules, such
as collagen type I and III, fibronectin, laminin-1 and -5 and
aggrecan (15,23).
Hyaluronan (HA) generally exists as a high molecular
mass polymer (in excess of 1,000 kDa) as a component of the
extracellular matrix under physiologic conditions (24). Lower molecular mass HA has been
detected in association with certain pathologic conditions, such as
inflammation (25) and tumors
(26–28). The low molecular mass HA fragments
induce a variety of biological events, such as cell proliferation
(28) and angiogenesis (29). High levels of angiogenic HA
fragments have been detected in several types of human tumor, such
as bladder (28), prostate
(30) and mesothelioma cancer
(26). High levels of hyaluronidase
activity are also found in prostate and bladder cancers (30,31),
which generate HA fragments. It is possible that the autoregulatory
degradation of HA in tumor tissues may enhance tumor invasion and
metastasis. HA has been found to enhance tumor cell adhesion and
migration (32) and to activate the
Ras-mitogen-activated protein kinase and phosphoinositide 3-kinase
pathways (33). These reports
suggested that HA and/or its degradation products may be involved
in the CD44 signaling that enhances tumor motility.
Although high and low molecular weight HA can induce
different effects in the same cells, in most studies the size of HA
was not monitored. We found that by adding 6.5 kDa and 5.6 kDa HA
oligosaccharide to stimulate MDA-MB-435s cells, MT1-MMP expression
was increased dramatically after 36 h of treatment, but not the 17
kDa HA oligosaccharide. Therefore, in further study, we used a 6.5
kDa HA oligosaccharide. We also used real-time PCR to observe
MT1-MMP mRNA expression and found that MT1-MMP mRNA may be detected
after 1 h of HA stimulation and is maintained until 6 h of
stimulation in MDA-MB-435s and MDA-MB-231 cells. To confirm that HA
stimulation of MT1-MMP is conducted through protein synthesis, we
pre-treated breast cancer cells with cycloheximide. The results of
western blot analysis and flow cytometry both showed that
HA-induced MT1-MMP expression was blocked by pre-treatment with
cycloheximide. From the above experiments, we suggest that HA
stimulation of MT1-MMP is conducted through protein synthesis.
CD44 is expressed in many types of metastatic tumor
cells (2,4,34). The
soluble form CD44 can be shed from cell surfaces through a
proteolytic process (35). CD44
cleavage contributes to the regulation of CD44-HA interactions
required for the migration process, and consequently promotes
CD44-mediated cancer cell migration. This proteolytic cleavage of
CD44 is involved in tumor invasion and metastasis (12). MT1-MMP appears to possess CD44
shedding capabilities and promotes cell migration (35). Moreover, MT1-MMP and CD44 were
colocalized at the migration front, which is important in cell
migration (35). CD44H has been
found to link MT1-MMP to the cytoskeleton and regulate its
localization (36). This
interaction is critical for the shedding of CD44H and the cell
migration (36). MT1-MMP appears to
possess CD44 shedding capabilities and promotes cell migration as
demonstrated by the colocalization of MT1-MMP and CD44, which is
important for cell migration (11).
Accumulating evidence suggests that MT1-MMP plays a pivotal role in
tumor cell migration and invasion (36–38).
In this study, we found that CD44 is colocalized with HA-induced
MT1-MMP on the cell membrane. After 24 h of HA stimulation, there
was a marked increase in the ICD of CD44.
These results suggest that 6.5 kDa HA
oligosaccharide stimulation of human breast cancer cells induces
the expression of MT1-MMP, and that MT1-MMP and CD44 were
colocalized on the cell surface which further causes CD44 cleavage.
CD44 cleavage has been demonstrated to play a critical role in
CD44-mediated tumor cell migration by providing off-setting changes
in adhesive interactions between CD44 and extra cellular matrix
(9,39). In order to analyze the correlation
between HA stimulation of MT1-MMP expression, CD44 cleavage and
breast cancer cell mobility, we used Matrigel-coated transwell to
observe tumor cell migration. After 60 h of HA stimulation, there
was a clear increase in the invasive capabilities of the stimulated
cells, and this migration may be inhibited by specific anti-MT1-MMP
Ab or MMP inhibitors.
MMP-1, -2, -9 and MT1-MMP are involved in cancer
invasion (40). Membrane type 1-MMP
degrades the extracellular matrix, activating other MMPs (18), and acts as a shedding enzyme
involving CD44 cleavage and promotes the cell movement (10,35).
Through tissue staining it has been found that invasive breast
cancer cells have higher levels of CD44 than normal tissue
(2,41). In the present study, we demonstrated
that 6.5 kDa HA oligosaccharide stimulation of the human breast
cancer cell lines MDA-MB-435s and MDA-MB-231 was able to induce the
expression of MT1-MMP through protein synthesis. Our data also
suggest that HA-induced MT1-MMP and CD44 are colocalized on the
cell surface and that CD44 showed signs of cleavage. A significant
increase in the invasive capabilities of HA-stimulated breast
cancer cells was observed using the migration assay. These
observations indicate that HA induced MT1-MMP expression, which
then caused CD44 cleavage and thus enhanced the invasive abilities
of the cancer cells. We believe that the results of this study
yield significant insight into breast cancer invasion and
metastasis. In conclusion, we found that HA oligosaccharide-induced
MT1-MMP expression in breast cancer cells may be an element of the
sequence of molecular events preceding metastasis.
Acknowledgements
This study was supported by a research grant from
the National Science Council, Taiwan (NSC 95-2320-B-010-036-MY2,
NSC 97-2320-B-010-019-MY3) and a grant from the Ministry of
Education, Aim for the Top University Plan to H.S.W.
References
|
1
|
Lesley L, Hyman R and Kincade PW: CD44 and
its interaction with extracellular matrix. Adv Immunol. 54:271–335.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Naor D, Sionov RV and Ish-Shalom D: CD44:
structure, function, and association with the malignant process.
Adv Cancer Res. 71:241–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shimizu Y, Van Seventer GA, Siraganian R,
et al: Dual role of the CD44 molecule in T cell adhesion and
activation. J Immunol. 143:2457–2463. 1989.PubMed/NCBI
|
|
4
|
Gunthert U, Hofmann M, Rudy W, et al: A
new variant of glycoprotein CD44 confers metastatic potential to
rat carcinoma cells. Cell. 65:13–24. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Screaton GR, Bell MV, Jackson DG, et al:
Genomic structure of DNA encoding the lymphocyte homing receptor
CD44 reveals at least 12 alternatively spliced exons. Proc Natl
Acad Sci USA. 89:12160–12164. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Underhill CB: CD44: the hyaluronan
receptor. J Cell Sci. 103:293–298. 1992.
|
|
7
|
Liotta LA: Tumor invasion and metastases -
role of the extracellular matrix: Rhoads Memorial Award Lecture.
Cancer Res. 46:1–7. 1986.PubMed/NCBI
|
|
8
|
Guo UJ, Ma J, Wang J, et al: Inhibition of
human melanoma growth and metastasis in vivo by anti-CD44
monoclonal antibody. Cancer Res. 54:1561–1565. 1994.PubMed/NCBI
|
|
9
|
Zahalka MA, Okon E, Gosslar U, et al:
Lymph node (but not spleen) invasion by murine lymphoma is both
CD44- and hyaluronate-dependent. J Immunol. 154:5345–5355.
1995.PubMed/NCBI
|
|
10
|
Okamoto I, Kawano Y, Tsuiki H, et al: CD44
cleavage induced by a membrane-associated metalloprotease plays a
critical role in tumor cell migration. Oncogene. 18:1435–1446.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhong J, Cornelsen Gencay MM, Bubendorf L,
et al: ERK1/2 and p38 MAP kinase control MMP-2, MT1-MMP, and TIMP
action and affect cell migration: a comparison between mesothelioma
and mesothelial cells. J Cell Physiol. 207:540–552. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okamoto I, Kawano Y, Murakami D, et al:
Proteolytic release of CD44 intracellular domain and its role in
the CD44 signaling pathway. J Cell Biol. 155:755–762. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mignatti P and Rifkin DB: Biology and
biochemistry of proteinases in tumor invasion. Physiol Rev.
73:161–195. 1993.PubMed/NCBI
|
|
14
|
Nagase H and Woessner JF Jr: Matrix
metalloproteinases. J Biol Chem. 274:21491–21494. 1999. View Article : Google Scholar
|
|
15
|
Seiki M: Membrane-type matrix
metalloproteinases. APMIS. 107:137–143. 1999. View Article : Google Scholar
|
|
16
|
Hiraoka N, Allen E, Apel IJ, et al: Matrix
metalloproteinases regulate neovascularization by acting as
pericellular fibrinolysins. Cell. 95:365–377. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Galvez BG, Matias-Roman S, Albar JP, et
al: Membrane type 1-matrix metalloproteinase is activated during
migration of human endothelial cells and modulates endothelial
motility and matrix remodeling. J Biol Chem. 276:37491–37500. 2001.
View Article : Google Scholar
|
|
18
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sato H, Takino T, Okada Y, et al: A matrix
metalloproteinase expressed on the surface of invasive tumour
cells. Nature. 370:61–65. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Knauper V, Will H, Lopez-Otin C, et al:
Cellular mechanisms for human procollagenase-3 (MMP-13) activation.
Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to
generate active enzyme. J Biol Chem. 271:17124–17131. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kuo YC, Su CH, Liu CY, et al: Transforming
growth factor-beta induces CD44 cleavage that promotes migration of
MDA-MB-435s cells through the up-regulation of membrane type
1-matrix metalloproteinase. Int J Cancer. 124:2568–2576. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Seiki M: The cell surface: the stage for
matrix metalloproteinase regulation of migration. Curr Opin Cell
Biol. 14:624–632. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Okada Y: Tumor cell-matrix interaction:
pericellular matrix degradation and metastasis. Verh Dtsch Ges
Pathol. 84:33–42. 2000.PubMed/NCBI
|
|
24
|
Laurent TC and Graser JR: Hyaluronan.
FASEB J. 6:2397–2404. 1992.PubMed/NCBI
|
|
25
|
Balazs EA, Watson D, Duff IF, et al:
Hyaluronic acid in synovial fluid. I Molecular parameters of
hyaluronic acid in normal and arthritis human fluids. Arthritis
Rheum. 10:357–376. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dahl IM and Laurent TC: Concentration of
hyaluronan in the serum of untreated cancer patients with special
reference to patients with mesothelioma. Cancer. 62:326–330. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumar S, West DC, Ponting JM, et al: Sera
of children with renal tumours contain low-molecular-mass
hyaluronic acid. Int J Cancer. 44:445–448. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lokeshwar VB, Obek C, Soloway MS, et al:
Tumor-associated hyaluronic acid: a new sensitive and specific
urine marker for bladder cancer. Cancer Res. 57:773–777.
1997.PubMed/NCBI
|
|
29
|
West DC, Hampson IN, Arnold F, et al: The
effect of hyaluronate and its oligosaccharides on endothelial cell
proliferation and monolayer integrity. Science. 228:1324–1326.
1985.
|
|
30
|
Lokeshwar VB, Rubinowicz D, Schroeder GL,
et al: Stromal and epithelial expression of tumor markers
hyaluronic acid and HYAL1 hyaluronidase in prostate cancer. J Biol
Chem. 276:11922–11932. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pham JT, Blick NL and Lokeshwar VB:
Tumor-derived hyaluronidase: a diagnostic urine marker for
high-grade bladder cancer. Cancer Res. 57:778–783. 1997.PubMed/NCBI
|
|
32
|
Itano N, Atsumi F, Sawai T, et al:
Abnormal accumulation of hyaluronan matrix diminishes contact
inhibition of cell growth and promotes cell migration. Proc Natl
Acad Sci USA. 99:3609–3614. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sohara Y, Ishiguro N, Machida K, et al:
Hyaluronan activates cell motility of v-Src-transformed cells via
Ras-mitogen-activated protein kinase and phosphoinositide
3-kinase-Akt in a tumor-specific manner. Mol Biol Cell.
12:1850–1868. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sneath RJ and Mangham DC: The normal
structure and function of CD44 and its role in neoplasia. Mol
Pathol. 51:191–200. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kajita M, Itoh Y, Chiba T, et al:
Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes
cell migration. J Cell Biol. 153:893–904. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mori H, Tomari T, Koshikawa N, et al: CD44
directs membrane-type 1 matrix metalloproteinase to lamellipodia by
associating with its hemopexin-like domain. EMBO J. 21:3949–3959.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ueda J, Kajita M, Suenaga N, et al:
Sequence-specific silencing of MT1-MMP expression suppresses tumor
cell migration and invasion: importance of MT1-MMP as a therapeutic
target for invasive tumors. Oncogene. 22:8716–8722. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Annabi B, Bouzeghrane M, Moumdjian R, et
al: Probing the infiltrating character of brain tumors: inhibition
of RhoA/ROK-mediated CD44 cell surface shedding from glioma cells
by the green tea catechin EGCg. J Neurochem. 94:906–916. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Goebeler M, Kaufmann D, Brocker EB, et al:
Migration of highly aggressive melanoma cells on hyaluronic acid is
associated with functional changes, increased turnover and shedding
of CD44 receptors. J Cell Sci. 109:1957–1964. 1996.PubMed/NCBI
|
|
40
|
Hotary K, Allen E, Punturieri A, et al:
Regulation of cell invasion and morphogenesis in a
three-dimensional type I collagen matrix by membrane-type matrix
metalloproteinases 1, 2, and 3. J Cell Biol. 149:1309–1323. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Iida N and Bourguignon LY: New CD44 splice
variants associated with human breast cancers. J Cell Physiol.
162:127–133. 1995. View Article : Google Scholar : PubMed/NCBI
|