Introduction
In 2008, genome-wide mutational analysis with 22
glioblastomas (GBM) performed by Parson et al (1) found recurrent point mutations
affecting the isocitrate dehydrogenase 1 (IDH1) and
IDH2 genes. This novel point mutation was thus placed in the
spotlight of brain cancer biology (2–29). The
mutation in IDH1 consistently occurred in exon 4 at codon
132, where a CGT→CAT transition of a single amino acid from
arginine to histidine (R132H) occurred (1,16), and
less frequently in IDH2, at the corresponding amino acid
R172 (28). IDH1 and
IDH2 mutations have been found with high frequency in lower
grade astrocytic and oligodendroglial neoplasms compared with in
GBM (1,2,18,23,28).
In addition, although a low incidence of IDH1 mutation was
found in primary GBM, it is more frequently mutated in secondary
GBM (2,28). The accumulating research regarding
this IDH1 mutation has generated many insights (30–33);
one is the prognostic usefulness of IDH1 mutation, as
mutated tumors have a better prognosis. The other is that
IDH1 mutation has already become an essential diagnostic
marker for brain tumors (1,22,28,34).
Also, in a multivariate analysis, IDH1 mutation was
confirmed as an independent prognostic factor in patients with
gliomas (18,23). Among the notable genetic profiles in
gliomas, 1p 19q co-deleted genotype and MGMT methylation
were tightly associated with IDH1 mutation, but IDH1
mutation was mutually exclusive with EGFR gene amplification
and loss of chromosome 10 (23).
Substantial research effort into IDH1 mutation has
concentrated on its mechanistic role. Wild-type IDH1
catalyzed the oxidative carboxylation of isocitrate (ICT) to
α-ketoglutarate (α-KG), yielding reduced nicotinamide adenine
dinucleotide phosphate (NADPH) (23). However, mutated IDH1 in a
tumor inhibited IDH1-mediated conversion of ICT to α-KG and
induced hypoxia-inducible factor 1α (HIF-1α) (29). Moreover, mutated IDH1
acquired the ability to catalyze α-KG to R(−)-2-hydroxyglutarate
[R(−)-2HG] (6,20). Identification of IDH1
mutation in gliomas not only improved physicians’ ability to
predict disease progression but also prompted researchers to
re-evaluate the disease entity. The study of IDH1 mutations
will change the treatment options and drug regimens used in gliomas
in the near future. Although the need for such research is
increasing, no information is available regarding IDH1/IDH2
mutations in Korean brain tumor patients. Therefore, to determine
the prevalence and prognostic impact of IDH1/IDH2 mutations
in the Korean population, we investigated a series of 134 glioma
patients. Additionally, we compared IDH1 mutations with
other genomic profiles commonly associated with gliomas.
Materials and methods
Case selection
Tumor tissue was from human brain tumor specimens
diagnosed in the Department of Neuropathology at the Seoul National
University Hospital from 1999 to 2011. This study included 41
oligodendrogliomas (LO), 47 anaplastic oligodendrogliomas (AO), and
46 primary GBM. This study was approved by the Institutional Review
Board of Seoul National University Hospital (H-1201-037-394).
DNA extraction and PCR amplification for
IDH1 sequencing
Tumor areas were manually microdissected from 6-μm
unstained histological sections. DNA was isolated from tumor tissue
using the DNeasy Blood and Tissue kit (Qiagen, Valencia, CA)
according to the manufacturer’s instructions.
Template DNA (1 μl) was added to 100 μl of PCR
reaction solution [10 μl 10X MG™Taq-HF buffer, 10 μl 2 mM
MG™dNTP mixture, 5 μl 10 pmol primer (2X), 1 μl MGTaq-HF
polymerase, distilled water]. IDH1 forward primer (5′-ACC AAA TGG
CAC CAT ACG A-3′) and reverse primer (5′-GCA AAA TCA CAT TAT TGC
CAA C-3′) generated a 130-bp PCR product; IDH2 forward primer
(5′-GCT GCA GTG GGA CCA CTA TT-3′) and reverse primer (5′-TGT GGC
CTT GTA CTG CAG AG-3′) generated a 293-bp PCR product (Table I). PCR amplification was performed
using AmpliTaq Gold PCR Master Mix (Applied Biosystems, Inc.,
Foster City, CA). The reaction mixture was subjected to an initial
denaturation at 95°C for 10 min, followed by 35 cycles of
amplification consisting of denaturation at 95°C for 30 sec,
annealing at 55°C for 30 sec, and extension at 72°C for 60 sec.
 | Table IAmplification and sequencing
primers. |
Table I
Amplification and sequencing
primers.
| Name | Sequence |
|---|
| IDH1-F | 5′-ACC AAA TGG CAC
CAT ACG A-3′ |
| IDH1-R | 5′-GCA AAA TCA CAT
TAT TGC CAA C-3′ |
| IDH2-F | 5′-GCT GCA GTG GGA
CCA CTA TT-3′ |
| IDH2-R | 5′-TGT GGC CTT GTA
CTG CAG AG-3′ |
Direct sequencing
Purified PCR products were sequenced using two IDH1
primers, as described in Table I.
Sequencing was performed using a BigDye terminator cycle sequencing
kit v.3.1. (Applied Biosystems). Sequencing products were resolved
on an Applied Biosystems model 3730XL automated DNA sequencing
system (Applied BioSystems).
Immunohistochemistry
The primary antibodies used in the
immunohistochemical study of formalin-fixed, paraffin-embedded
sections are summarized in Table
II, based on our previous reports (35,36).
 | Table IIAntibodies used in this study. |
Table II
Antibodies used in this study.
| Name | Manufacturer | Antigen
retrieval | Dilution |
|---|
| P53 | Dako, Glostrup,
Denmark | EDTA,
microwave | 1:800 |
| PTEN | Dako, Glostrup,
Denmark | EDTA,
microwave | 1:100 |
Fluorescence in situ hybridization
(FISH)
Analysis of chromosome 1p, 19q deletion, and
EGFR gene status was conducted by FISH using Vysis probes,
as per our previous report (35,36).
O6-methylguanine-DNA methyltransferase
(MGMT) methylation-specific polymerase chain reaction (MSP)
analysis
Analysis of methylation of the MGMT promoter
was performed using the methylation-specific polymerase chain
reaction (MSP) technique, as described previously (37).
Statistical analyses
Fisher’s exact test was used to examine associations
between IDH1 mutations and genetic alterations. The t-test
was used to assess the relationship of IDH mutations with
the absence or presence of genetic alterations with age. Overall
survival of patients with LO, AO was estimated by the Kaplan-Meier
method and compared using a log-rank test. All statistical analyses
were performed with SPSS version 18 (SPSS Inc., Chicago, IL,
USA).
Results
IDH1 mutation frequencies in various
brain tumors
The patients ranged in age from 3 to 71 years (mean
41.3 years). The male-to-female ratio was 1.5:1. We analyzed DNA
from 134 formalin-fixed, paraffin-embedded tissue samples from
archival surgical specimens. We found 72 (53.7%) mutations in codon
132 of IDH1 (Fig. 1). All were
G395A (Arg132His). IDH1 mutation frequencies differed
according to histologic subtype, affecting 30 (73.2%) of 41 LO, 39
(82.9%) of 47 AO and three (6.5%) of 46 primary GBM cases at
IDH1 codon 132. These results are summarized in Table III.
 | Table IIIIDH1 mutation frequencies in
134 brain tumors. |
Table III
IDH1 mutation frequencies in
134 brain tumors.
| IDH1 | |
|---|
|
| |
|---|
| Mutant | Wild | Total number | Percent (%) |
|---|
| LO | 30 | 11 | 41 | 73.2 |
| AO | 39 | 8 | 47 | 82.9 |
| Primary GBM | 3 | 43 | 46 | 6.5 |
| Total | 72 | 62 | 134 | 53.7 |
Oligodendroglioma (LO)
The 41 LO patients ranged in age from 23 to 69 years
(mean 41.1 years). They were operated on from 1999 to 2009. The
follow-up duration was 9.9 to 130.5 months. Tumor recurrence
occurred in 14 cases, and one case was lost to follow-up. Of the 14
patients with recurrence, 12 had an IDH1 mutation. Of the 26
patients without recurrence, 18 had an IDH1 mutation. Of the
38 patients whose samples were subjected to p53 immunostaining, 8
revealed simultaneous p53 expression and IDH1 mutation.
Sixteen of 20 patients whose samples were subjected to PTEN
immunostaining expressed PTEN and the IDH1 mutation. The 1p
19q co-deletion was found in 32 of 38 patients by FISH. Of the 32
patients with the 1p 19q co-deletion, 22 had the IDH1
mutation. None of our LO revealed EGFR gene amplification or high
polysomy. In LO, we analyzed the relationship between the
IDH1 mutation and factors, such as age, sex, recurrence and
1p 19q co-deletion. There was no statistically significant
correlation between IDH1 mutation status and other factors
except p53. In patients with both IDH1 mutation and
MGMT methylation, p53 immunoexpression was a significant
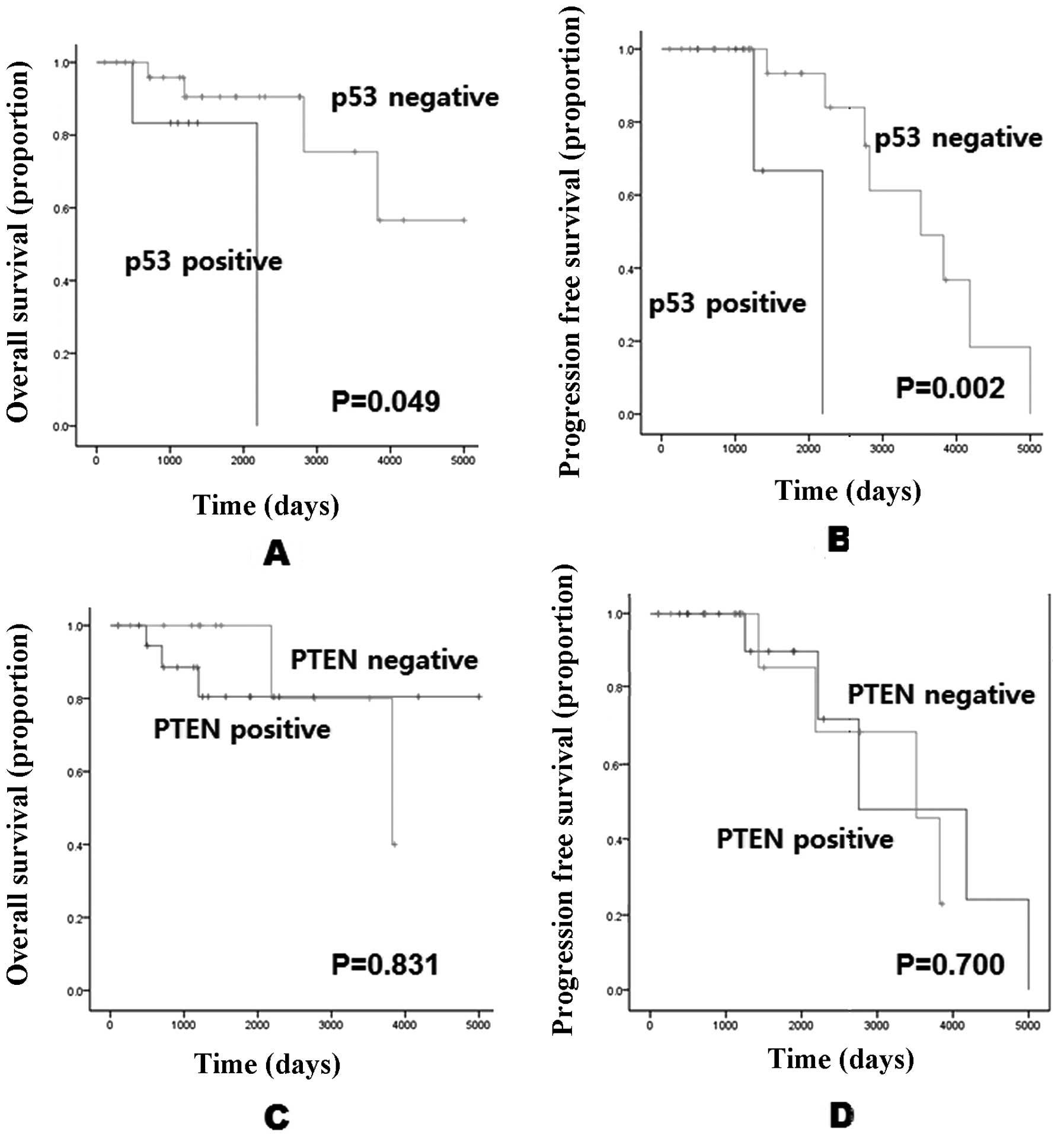
negative prognostic factor (p=0.049) (Fig. 3).
Anaplastic oligodendroglioma (AO)
The 47 AO patients ranged in age from 26 to 69 years
(mean 44.9 years). Of the 12 patients with recurrence, 10 had the
IDH1 mutation. Of the 35 patients without recurrence, 29 had
the IDH1 mutation. Of the 42 patients whose samples were
subjected to p53 immunostaining, 6 revealed simultaneous p53
expression and IDH1 mutation. Twenty of 37 patients whose
samples were subjected to PTEN immunostaining expressed PTEN and
the IDH1 mutation. The 1p 19q co-deletion was found in 40 of
47 patients by FISH. Of the 40 patients with the 1p 19q
co-deletion, 33 had the IDH1 mutation. Seven cases without
the 1p 19q co-deletion had the IDH1 mutation. EGFR
FISH was performed in 47 cases. Forty-five of these revealed no
amplification of the EGFR gene, and 37 of these had the
IDH1 mutation. Only 2 cases of AO showed EGFR
FISH-positive (2 high polysomy). These two EGFR
FISH-positive cases had the IDH1 mutation. Thus, we could
not see the mutual exclusion between IDH1 mutation and EGFR
positivity. Forty-one of 43 cases subjected to MGMT MSP
revealed methylation of the MGMT promoter, and 35 of these
had the IDH1 mutation. In AO, there was no statistically
significant correlation between the IDH1 mutation and other
factors, such as sex, recurrence, 1p 19q co-deletion, EGFR
FISH. In the patients with both IDH1 mutation and MGMT methylation,
p53 immunoexpression was a significant negative prognostic factor
as in LO (p=0.002) (Fig. 3).
Glioblastoma (GBM)
The 46 GBM patients ranged in age from 3 to 71 years
(mean 39.6 years). Among the 41 patients without recurrence, two
had the IDH1 mutation, and 39 did not. Of the 5 patients
with recurrence, one had the IDH1 mutation. The 1p 19q
co-deletion was not found in any of the 46 patients by FISH. Three
of 46 cases without the 1p 19q co-deletion had the IDH1
mutation. EGFR FISH was positive in 7 (15.2%) out of 46
performed. One of 7 cases with EGFR gene amplification and 2
of 39 patients without EGFR gene amplification had the IDH1
mutation. Therefore, EGFR gene amplification and IDH1 mutation were
not mutually exclusive (p=0.398), but it needs to be studied in
more cases. Fourteen (30.4%) cases of 46 GBM performed MGMT
MSP revealed methylation of the MGMT promoter. Two of 14
cases with MGMT methylation and one of 32 cases without methylation
of the MGMT promoter had the IDH1 mutation. We
analyzed the relationship between IDH1 mutation status and
other factors, such as sex, recurrence, 1p 19q co-deletion,
EGFR FISH and MGMT MSP and found no statistically
significant correlation.
Survival analysis
We analyzed the prognostic impact of IDH1
mutation in LO, AO, and GBM. The follow-up duration was 9.93–130.5
months, 3.4–166.6 months and 9 days to 79.8 months in LO, AO and
GBM. The median survival was 68.4, 54.2 and 19.7 months in LO, AO
and GBM, respectively. The overall survival rate was 82.9, 78.7 and
0% in LO, AO and GBM, respectively. In GBM, 1-, 2- and 3-year
survival rates were 60.9, 28.4 and 13.0%, respectively. In LO,
overall survival was higher in IDH1-mutated LO, compared
with non-mutated LO (p=0.03; Fig.
2A). Also, in AO, overall survival was higher in IDH1
mutated AO compared with non-mutated AO (p=0.013; Fig. 2B). In contrast to LO and AO, overall
survival was higher in IDH1 non-mutated GBM, compared with
mutated GBM (p=0.587; Fig. 2C), but
this result was not statistically significant, because IDH1
mutated GBM was only 3 cases. Also, progression-free survival was
not statistically different between IDH1 mutated and
non-mutated tumors in the LO (p=0.708; Fig. 2D), AO (p=0.938; Fig. 2E), and GBM (p=0.173; Fig. 2F) groups. We analyzed survival in
patients with both IDH1-mutated and MGMT methylated LO and
AO according to p53 and PTEN expression. The overall survival and
progression-free survival of patients with p53-negative tumors were
significantly longer than in those with p53-positive tumors
(p=0.049 and 0.002; Fig. 3A and B).
However, PTEN expression did not affect the patients’ survival.
Discussion
Recently, IDH1 mutation has been recognized
as a strong prognostic factor in brain tumors. In low-grade
astrocytic and oligodendroglial tumors (WHO grades II and III), the
impact on prognosis was magnified several times. However, no Korean
studies evaluating IDH1 mutation frequencies in brain tumors
had previously been conducted, so we attempted to discover the
IDH1 mutation frequency and prognostic impact in a Korean
patient population. The methods for detection of IDH1
mutation have changed. Recently, a monoclonal antibody that detects
the R132H IDH1 mutation was developed. This antibody has
been applied to everyday pathologic practice (5). Immunohistochemical research with this
monoclonal antibody is easy, and inexpensive, but has some
limitations. The best way to identify the IDH1 mutation is
by direct sequencing; thus, we performed direct sequencing instead
of immunohistochemistry for IDH1. According to our data, WHO
grades II and III astrocytic and oligodendroglial tumors exhibit
high mutation frequencies. Although the case numbers were
insufficient to reflect tumor frequencies, our IDH1 mutation
results were similar to other published results. In accordance with
other studies, we found that IDH1 mutation was a strong
prognostic factor in oligodendroglioma and anaplastic
oligodendroglioma. Detection of the IDH1 mutation was
extremely helpful in brain tumors. Using the same approach with the
1p 19q co-deletion, IDH 1 mutation was an excellent prognostic
marker. The usefulness of the IDH1 mutation in brain tumors
has been reported previously. In addition to its predictive
utility, IDH1 also has possibilities as a diagnostic marker.
IDH1 is a powerful differential diagnostic marker for round
and clear-cell brain tumors mimicking oligodendroglioma, including
dysembryoplastic neuroepithelial tumor (DNT), extraventricular
neurocytoma (EVN), and clear-cell ependymoma, because the
IDH1 mutation is not present in these or other glioneuronal
tumors, such as central neurocytoma and gangliogliomas. Therefore,
we performed additional sequencing to detect the IDH1
mutation in 5 cases of PGNT and 11 of EVN. The IDH1 mutation
was not detected in these tumor entities, however, the number of
cases was small. Thus, we suggest the IDH1 mutation as a
reasonable adjunctive analysis in daily practice, especially for
diagnosis of brain tumors with clear-cell morphology.
We analyzed the relationship between IDH1
mutation and other aspects of the genetic profile, including 1p 19q
co-deletion, EGFR amplification, and MGMT methylation in LO, AO,
and GBM. In LO and AO, the IDH1 mutation was more frequent
in tumors with the 1p and 19q co-deletion than those without 1p 19q
co-deletion. Also, in AO, the IDH1 mutation was more
frequent in MGMT-methylated tumors. Although none of these findings
was statistically significant, these data are similar to those in
other reports. The statistical significance of these associations
between genetic profile and the IDH1 mutation should be
verified in a larger series. In contrast to the report by Hartmann
et al (13), the mean age of
the two groups in this study was not significantly different. These
results are summarized in Table
IV.
 | Table IVThe relationship of IDH1
mutations to genetic alterations according to age. |
Table IV
The relationship of IDH1
mutations to genetic alterations according to age.
| Entity | IDH1 | Number | Mean age | SD | P-value |
|---|
| LO | Mutant | 30 | 41.67 | 10.1 | 0.578 |
| Wild | 11 | 39.55 | 12.34 | |
| AO | Mutant | 39 | 45.77 | 10.22 | 0.856 |
| Wild | 8 | 45.5 | 11 | |
| GB | Mutant | 3 | 48.67 | 10.26 | 0.456 |
| Wild | 43 | 39.37 | 21.04 | |
In conclusion, we identified IDH1 mutation
frequencies in various brain tumors; IDH1 mutation was a
strong prognostic factor in gliomas. Besides its role as a
prognostic marker, the IDH1 mutation may be a useful
diagnostic marker. We also confirmed the lack of a difference in
mean age between the IDH1 wild-type and mutant groups. Among
patients with both IDH1 mutation and MGMT methylation, p53
immunoexpression was a significant negative prognostic factor in
both LO and AO, but PTEN loss did not affect these patients’
survival. However, we found no association between IDH1
mutation and other genetic profiles commonly associated with
gliomas. These results remain to be proven in a larger study.
Acknowledgements
This study was supported by Basic Research Program
through the National Research Foundation of Korea (NRF) funded by
the Ministry of Education, Science and Technology
(2011-0003666).
Abbreviations:
|
EGFR
|
epidermal growth factor receptor
|
|
FISH
|
fluorescence in situ
hybridization
|
|
MGMT-MSP
|
O6-methylguanine-DNA methyltransferase
methylation-specific polymerase chain reaction
|
References
|
1
|
Parsons DW, Jones S, Zhang X, et al: An
integrated genomic analysis of human glioblastoma multiforme.
Science. 321:1807–1812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Balss J, Meyer J, Mueller W, Korshunov A,
Hartmann C and von Deimling A: Analysis of the IDH1 codon 132
mutation in brain tumors. Acta Neuropathol. 116:597–602. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bleeker FE, Atai NA, Lamba S, et al: The
prognostic IDH1 (R132) mutation is associated with reduced NADP
(+)-dependent IDH activity in glioblastoma. Acta Neuropathol.
119:487–494. 2010.
|
|
4
|
Bujko M, Kober P, Matyja E, et al:
Prognostic value of IDH1 mutations identified with PCR-RFLP assay
in glioblastoma patients. Mol Diagn Ther. 14:163–169. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Capper D, Weissert S, Balss J, et al:
Characterization of R132H mutation-specific IDH1 antibody binding
in brain tumors. Brain Pathol. 20:245–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dang L, White DW, Gross S, et al:
Cancer-associated IDH1 mutations produce 2-hydroxyglutarate.
Nature. 462:739–744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
De Carli E, Wang X and Puget S: IDH1 and
IDH2 mutations in gliomas. N Engl J Med. 360:2248author reply 2249.
2009.
|
|
8
|
Dubbink HJ, Taal W, van Marion R, et al:
IDH1 mutations in low-grade astrocytomas predict survival but not
response to temozolomide. Neurology. 73:1792–1795. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ducray F, Marie Y and Sanson M: IDH1 and
IDH2 mutations in gliomas. N Engl J Med. 360:248author reply 2249.
2009.
|
|
10
|
Ferroli P, Acerbi F and Finocchiaro G:
From standard treatment to personalized medicine: role of IDH1
mutations in low-grade glioma evolution and treatment. World
Neurosurg. 73:234–236. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Frezza C, Tennant DA and Gottlieb E: IDH1
mutations in gliomas: when an enzyme loses its grip. Cancer Cell.
17:7–9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gravendeel LA, Kloosterhof NK, Bralten LB,
et al: Segregation of non-p. R132H mutations in IDH1 in distinct
molecular subtypes of glioma. Hum Mutat. 31:E1186–E1199. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hartmann C, Meyer J, Balss J, et al: Type
and frequency of IDH1 and IDH2 mutations are related to astrocytic
and oligodendroglial differentiation and age: a study of 1,010
diffuse gliomas. Acta Neuropathol. 118:469–474. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hayden JT, Fruhwald MC, Hasselblatt M,
Ellison DW, Bailey S and Clifford SC: Frequent IDH1 mutations in
supratentorial primitive neuroectodermal tumors (sPNET) of adults
but not children. Cell Cycle. 8:1806–1807. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Horbinski C, Kelly L, Nikiforov YE, Durso
MB and Nikiforova MN: Detection of IDH1 and IDH2 mutations by
fluorescence melting curve analysis as a diagnostic tool for brain
biopsies. TJ Mol Diagn. 12:487–492. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Horbinski C, Kofler J, Kelly LM, Murdoch
GH and Nikiforova MN: Diagnostic use of IDH1/2 mutation analysis in
routine clinical testing of formalin-fixed, paraffin-embedded
glioma tissues. J Neuropathol Exp Neurol. 68:1319–1325. 2009.
View Article : Google Scholar
|
|
17
|
Houillier C, Wang X, Kaloshi G, et al:
IDH1 or IDH2 mutations predict longer survival and response to
temozolomide in low-grade gliomas. Neurology. 75:1560–1566. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ichimura K, Pearson DM, Kocialkowski S, et
al: IDH1 mutations are present in the majority of common adult
gliomas but rare in primary glioblastomas. Neurooncology.
11:341–347. 2009.PubMed/NCBI
|
|
19
|
Kang MR, Kim MS, Oh JE, et al: Mutational
analysis of IDH1 codon 132 in glioblastomas and other common
cancers. Int J Cancer. 125:353–355. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Komotar RJ, Starke RM, Sisti MB and
Connolly ES: IDH1 and IDH2 mutations in gliomas and the associated
induction of hypoxia-inducible factor and production of
2-hydroxyglutarate. Neurosurgery. 66:N20–N21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Larsen CJ: Mutations of IDH1 and 2 genes:
a molecular diagnosis of low-grade gliomas. Bull Cancer.
96:641–642. 2009.(In French).
|
|
22
|
Nobusawa S, Watanabe T, Kleihues P and
Ohgaki H: IDH1 mutations as molecular signature and predictive
factor of secondary glioblastomas. Clin Cancer Res. 15:6002–6007.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sanson M, Marie Y, Paris S, et al:
Isocitrate dehydrogenase 1 codon 132 mutation is an important
prognostic biomarker in gliomas. J Clin Oncol. 27:4150–4154. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sonoda Y, Kumabe T, Nakamura T, et al:
Analysis of IDH1 and IDH2 mutations in Japanese glioma patients.
Cancer Sci. 100:1996–1998. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uno M, Oba-Shinjo SM, Silva R, et al: IDH1
mutations in a Brazilian series of glioblastoma. Clinics (Sao
Paulo). 66:163–165. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Van den Bent MJ, Dubbink HJ, Marie Y, et
al: IDH1 and IDH2 mutations are prognostic but not predictive for
outcome in anaplastic oligodendroglial tumors: a report of the
European Organization for Research and Treatment of Cancer Brain
Tumor Group. Clin Cancer Res. 16:1597–1604. 2010.PubMed/NCBI
|
|
27
|
Watanabe T, Nobusawa S, Kleihues P and
Ohgaki H: IDH1 mutations are early events in the development of
astrocytomas and oligodendrogliomas. Am J Pathol. 174:1149–1153.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yan H, Parsons DW, Jin G, et al: IDH1 and
IDH2 mutations in gliomas. N Engl J Med. 360:765–773. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao S, Lin Y, Xu W, et al: Glioma-derived
mutations in IDH1 dominantly inhibit IDH1 catalytic activity and
induce HIF-1alpha. Science. 324:261–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thol F, Weissinger EM, Krauter J, et al:
IDH1 mutations in patients with myelodysplastic syndromes are
associated with an unfavorable prognosis. Haematologica.
95:1668–1674. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Murugan AK, Bojdani E and Xing M:
Identification and functional characterization of isocitrate
dehydrogenase 1 (IDH1) mutations in thyroid cancer. Biochem Biophys
Res Commun. 393:555–559. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Oki K, Takita J, Hiwatari M, et al: IDH1
and IDH2 mutations are rare in pediatric myeloid malignancies.
Leukemia. 25:382–384. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schnittger S, Haferlach C, Ulke M,
Alpermann T, Kern W and Haferlach T: IDH1 mutations are detected in
6.6% of 1414 AML patients and are associated with intermediate risk
karyotype and unfavorable prognosis in adults younger than 60 years
and unmutated NPM1 status. Blood. 116:5486–5496. 2010.
|
|
34
|
Weller M, Felsberg J, Hartmann C, et al:
Molecular predictors of progression-free and overall survival in
patients with newly diagnosed glioblastoma: a prospective
translational study of the German Glioma Network. J Clin Oncol.
27:5743–5750. 2009. View Article : Google Scholar
|
|
35
|
Kim MA, Lee HS, Lee HE, Jeon YK, Yang HK
and Kim WH: EGFR in gastric carcinomas: prognostic significance of
protein overexpression and high gene copy number. Histopathology.
52:738–746. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jeon YK, Park K, Park CK, Paek SH, Jung HW
and Park SH: Chromosome 1p and 19q status and p53 and p16
expression patterns as prognostic indicators of oligodendroglial
tumors: a clinicopathological study using fluorescence in situ
hybridization. Neuropathology. 27:10–20. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Park CK, Park SH, Lee SH, et al:
Methylation status of the MGMT gene promoter fails to predict the
clinical outcome of glioblastoma patients treated with ACNU plus
cisplatin. Neuropathology. 29:443–449. 2009. View Article : Google Scholar : PubMed/NCBI
|