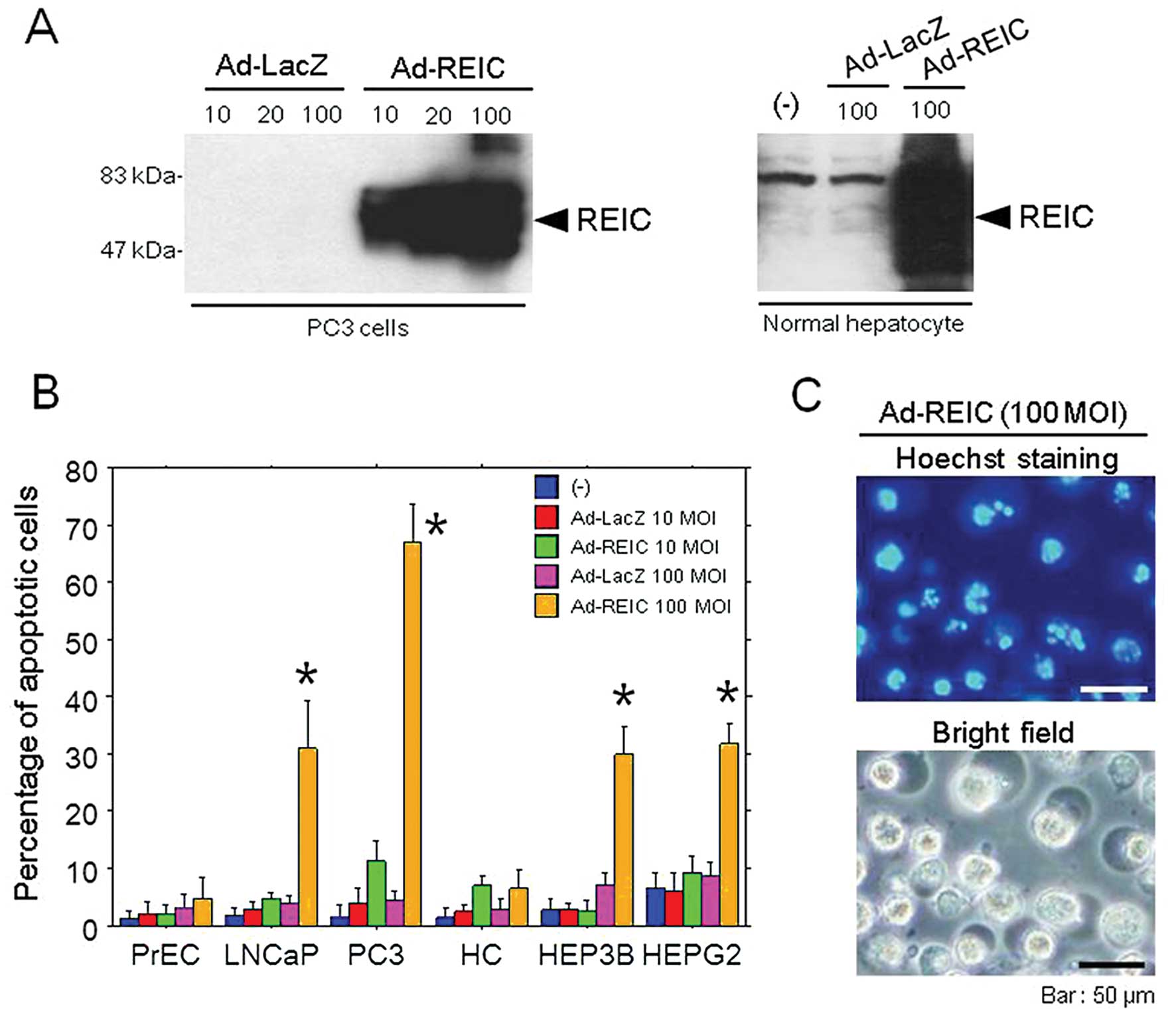
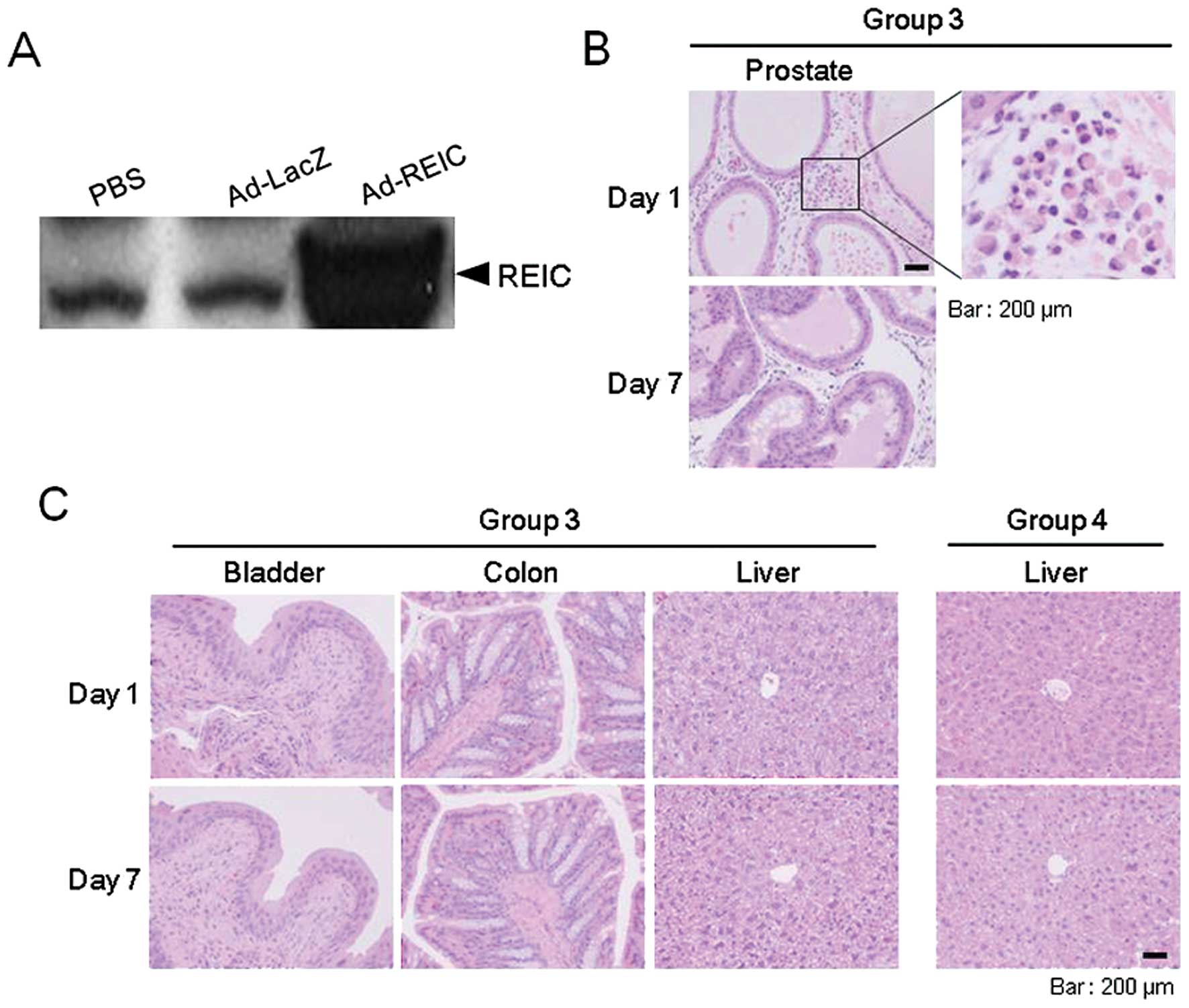
|
1
|
Tsuji T, Miyazaki M, Sakaguchi M, Inoue Y
and Namba M: A REIC gene shows downregulation in human immortalized
cells and human tumor-derived cell lines. Biochem Biophys Res
Commun. 268:20–24. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hsieh SY, Hsieh PS, Chiu CT and Chen WY:
Dickkopf-3/REIC functions as a suppressor gene of tumor growth.
Oncogene. 23:9183–9189. 2004.PubMed/NCBI
|
|
3
|
Abarzua F, Sakaguchi M, Takaishi M, Nasu
Y, Kurose K, Ebara S, Miyazaki M, Namba M, Kumon H and Huh NH:
Adenovirus-mediated overexpression of REIC/Dkk-3 selectively
induces apoptosis in human prostate cancer cells through activation
of c-Jun-NH2-kinase. Cancer Res. 65:9617–9622. 2005. View Article : Google Scholar
|
|
4
|
Kashiwakura Y, Ochiai K, Watanabe M,
Abarzua F, Sakaguchi M, Takaoka M, Tanimoto R, Nasu Y, Huh NH and
Kumon H: Downregulation of inhibition of differentiation-1 via
activation of activating transcription factor 3 and Smad regulates
REIC/Dickkopf-3-induced apoptosis. Cancer Res. 68:8333–8341. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang K, Watanabe M, Kashiwakura Y, Li SA,
Edamura K, Huang P, Yamaguchi K, Nasu Y, Kobayashi Y, Sakaguchi M,
et al: Expression pattern of REIC/Dkk-3 in various cell types and
the implications of the soluble form in prostatic acinar
development. Int J Oncol. 37:1495–1501. 2010.PubMed/NCBI
|
|
6
|
Mao B, Wu W, Davidson G, Marhold J, Li M,
Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, et al: Kremen
proteins are Dickkopf receptors that regulate Wnt/beta-catenin
signaling. Nature. 417:664–667. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abarzua F, Sakaguchi M, Tanimoto R,
Sonegawa H, Li DW, Edamura K, Kobayashi T, Watanabe M, Kashiwakura
Y, Kaku H, et al: Heat shock proteins play a crucial role in
tumor-specific apoptosis by REIC/Dkk-3. Int J Mol Med. 20:37–43.
2007.PubMed/NCBI
|
|
8
|
Edamura K, Nasu Y, Takaishi M, Kobayashi
T, Abarzua F, Sakaguchi M, Kashiwakura Y, Ebara S, Saika T,
Watanabe M, et al: Adenovirus-mediated REIC/Dkk-3 gene transfer
inhibits tumor growth and metastasis in an orthotopic prostate
cancer model. Cancer Gene Ther. 14:765–772. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watanabe M, Kashiwakura Y, Huang P, Ochiai
K, Futami J, Li SA, Takaoka M, Nasu Y, Sakaguchi M, Huh NH and
Kumon H: Immunological aspects of REIC/Dkk-3 in monocyte
differentiation and tumor regression. Int J Oncol. 34:657–663.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanimoto R, Abarzua F, Sakaguchi M,
Takaishi M, Nasu Y, Kumon H and Huh NH: REIC/Dkk-3 as a potential
gene therapeutic agent against human testicular cancer. Int J Mol
Med. 19:363–368. 2007.PubMed/NCBI
|
|
11
|
Kawasaki K, Watanabe M, Sakaguchi M,
Ogasawara Y, Ochiai K, Nasu Y, Doihara H, Kashiwakura Y, Huh NH,
Kumon H and Date H: REIC/Dkk-3 overexpression downregulates
P-glycoprotein in multidrug-resistant MCF7/ADR cells and induces
apoptosis in breast cancer. Cancer Gene Ther. 16:65–72. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin Y, Murata H, Sakaguchi M, Kataoka K,
Watanabe M, Nasu Y, Kumon H and Huh NH: Partial sensitization of
human bladder cancer cells to a gene-therapeutic adenovirus
carrying REIC/Dkk-3 by downregulation of BRPK/PINK1. Oncol Rep.
27:695–699. 2012.PubMed/NCBI
|
|
13
|
Freytag SO, Khil M, Stricker H, Peabody J,
Menon M, DePeralta-Venturina M, Nafziger D, Pegg J, Paielli D,
Brown S, et al: Phase I study of replication-competent
adenovirus-mediated double suicide gene therapy for the treatment
of locally recurrent prostate cancer. Cancer Res. 62:4968–4976.
2002.PubMed/NCBI
|
|
14
|
Kubo H, Gardner TA, Wada Y, Koeneman KS,
Gotoh A, Yang L, Kao C, Lim SD, Amin MB, Yang H, et al: Phase I
dose escalation clinical trial of adenovirus vector carrying
osteocalcin promoter-driven herpes simplex virus thymidine kinase
in localized and metastatic hormone-refractory prostate cancer. Hum
Gene Ther. 14:227–241. 2003. View Article : Google Scholar
|
|
15
|
van der Linden RR, Haagmans BL,
Mongiat-Artus P, van Doornum GJ, Kraaij R, Kadmon D,
Aguilar-Cordova E, Osterhaus AD, van der Kwast TH and Bangma CH:
Virus specific immune responses after human neoadjuvant
adenovirus-mediated suicide gene therapy for prostate cancer. Eur
Urol. 48:153–161. 2005.PubMed/NCBI
|
|
16
|
Su C, Cao H, Tan S, Huang Y, Jia X, Jiang
L, Wang K, Chen Y, Long J, Liu X, et al: Toxicology profiles of a
novel p53-armed replication-competent oncolytic adenovirus in
rodents, felids, and nonhuman primates. Toxicol Sci. 106:242–250.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Belloc F, Dumain P, Boisseau MR,
Jalloustre C, Reiffers J, Bernard P and Lacombe F: A flow
cytometric method using Hoechst 33342 and propidium iodide for
simultaneous cell cycle analysis and apoptosis determination in
unfixed cells. Cytometry. 17:59–65. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maciorowski Z, Delic J, Padoy E,
Klijanienko J, Dubray B, Cosset JM, Dumont J, Magdelénat H and
Vielh P: Comparative analysis of apoptosis measured by Hoechst and
flow cytometry in non-Hodgkin’s lymphomas. Cytometry. 32:44–50.
1998.PubMed/NCBI
|
|
19
|
Muruve DA, Barnes MJ, Stillman IE and
Libermann TA: Adenoviral gene therapy leads to rapid induction of
multiple chemokines and acute neutrophil-dependent hepatic injury
in vivo. Hum Gene Ther. 10:965–976. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sakaguchi M, Kataoka K, Abarzua F,
Tanimoto R, Watanabe M, Murata H, Than SS, Kurose K, Kashiwakura Y,
Ochiai K, et al: Overexpression of REIC/Dkk-3 in normal fibroblasts
suppresses tumor growth via induction of interleukin-7. J Biol
Chem. 284:14236–14244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ochiai K, Watanabe M, Ueki H, Huang P,
Fujii Y, Nasu Y, Noguchi H, Hirata T, Sakaguchi M, Huh NH,
Kashiwakura Y, Kaku H and Kumon H: Tumor suppressor REIC/Dkk-3
interacts with the dynein light chain, Tctex-1. Biochem Biophys Res
Commun. 412:391–395. 2011. View Article : Google Scholar : PubMed/NCBI
|