Introduction
Acute promyelocytic leukemia (APL) is characterized
by a balanced reciprocal translocation between chromosome 15 and
17, resulting in the fusion of the promyelocytic leukemia (PML) and
retinoic acid receptor genes (1–3).
All-trans retinoic acid (ATRA) has become the first-line
treatment for patients with APL due to its high clinical complete
remission rate (4,5). For patients with relapsed or
refractory APL who are resistant to the conventional treatment
protocols, a new arsenic-based therapy has been established.
Previous clinical studies demonstrated that 90% of relapsed
patients achieved remission by treatment with arsenic trioxide
(ATO) (6,7).
NB4, the first APL cell line with t(15;17), was
established from marrow samples obtained from a patient with APL
receiving retinoic acid (8). NB4
has been widely used to investigate the cellular and molecular
mechanisms of ATRA and ATO (9–14).
Cell biology studies using NB4 and APL blasts obtained from
patients have revealed that ATO exerts dose-dependent dual effects
on APL cells. Apoptosis is induced when cells are treated with
1.0–2.0 μM ATO, while partial differentiation is observed when low
concentrations (0.1–0.5 μM) are used (13,14).
Aquaporin-9 (AQP9), a transmembrane transporter belonging to
aquaporin superfamily, has been recognized as a major pathway of
arsenic uptake, and its expression is associated with ATO
sensitivity in either leukemia cells (12) or human-derived normal cells
(15). Although several
investigators reported that ATRA induced AQP9 expression (12), ATO incorporation has not been
investigated well when these two agents are concomitantly
administered.
Myeloid growth factors such as granulocyte
colony-stimulating factor (G-CSF) promote proliferation, survival
and differentiation of leukemic cells (9,16,17).
Although it remains controversial whether G-CSF alone induces
differentiation in APL blasts, differentiation induction in APL
cells by ATRA could be enhanced by G-CSF in vitro and in
vivo (9,11,17).
Furthermore, a previous report has demonstrated that the
differentiation-inducing activities of ATRA and ATO were abrogated
by specific G-CSF neutralization, suggesting a requirement for
G-CSF for differentiation of APL cells (9).
Although clinical analyses revealed the
effectiveness of combination treatment with ATO and ATRA (18,19),
its efficacy and mechanisms of the combination administration are
not yet well understood. In this study, we examined the effect of
ATO, ATRA and G-CSF alone or in combination on HT93A cells. While
most of in vitro experiments on APL have been conducted
using NB4 cells, another t(15;17)-positive APL cell line, HT93A,
was used for this study. HT93A was obtained from peripheral blood
of an APL patient without receiving ATRA or ATO (20–23).
Cellular responses to ATRA or ATO treatment in HT93A cells may be
different from those in NB4 cells since diverse cell types with
distinct characteristics exist in individual patient with different
clinical background, although both possess t(15;17).
Therefore, we investigated the alterations in
morphology, cell surface markers, apoptosis and AQP9 expression in
HT93A cells treated with ATRA or ATO in the presence or absence of
G-CSF. We also attempted to investigate the contribution of G-CSF
to the differentiation induction by ATRA or ATO. Our data suggest
the enhanced differentiation induction of combination treatment
with ATRA+G-CSF or ATO+G-CSF in APL.
Materials and methods
Reagents
ATO was purchased from Sigma (St. Louis, MO, USA)
and dissolved in 1 M sodium hydroxide solution, diluted with
phosphate-buffered saline (PBS), sterilized by filtration (0.22-μm
filter) and used as the stock solution. Recombinant human G-CSF
(Filgrastim) was obtained from Kyowa Hakko Kirin Co, Ltd. (Tokyo,
Japan) and dissolved in PBS to prepare the stock solution and
stored at 4°C until use. ATRA was purchased from Sigma and
dissolved in ethanol to obtain a final concentration of 2 mM and
stored at −20°C in the dark. The vehicle reagent, ethanol (final
concentration <0.05%), did not affect cell viability and
differentiation. Phycoerythrin (PE)-conjugated mouse anti-human
CD11b IgG2α and CD34 IgG1 as well as
fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD15
IgM were used for the assessment of differentiation induction and
were obtained from Beckton-Dickinson (San Jose, CA, USA).
FITC-conjugated mouse anti-human CD11c IgG1 was obtained
from eBioscience, Inc. (San Diego, CA, USA). Anti-rat AQP9 antibody
(rabbit) was purchased from Alpha Diagnostic International, Inc.
(San Antonio, TX, USA). The Apoptosis Detection kit I containing
annexin V-FITC, propidium iodide (PI) and 10X annexin V binding
buffer was purchased from Beckton-Dickinson.
Cell culture and treatment
HT93A, a human APL cell line established from
peripheral blood of a patient with APL (16), was provided from Dr Kenji Kishi
(Shibata Hospital, Shibata, Japan) and Dr Yuko Sato (National
Center for Global Health and Welfare, Japan). HT93A was maintained
in RPMI-1640 medium (Gibco-BRL, Grand Island, NY, USA) supplemented
with 10% heat-inactivated fetal bovine serum (FBS) (Gibco-BRL), 100
U/ml penicillin and 100 μg/ml streptomycin (Gibco-BRL) at 37°C in a
humidified atmosphere (5% CO2 in air). Cells were seeded
at a density of 1×105 cells/ml and treated with ATO,
ATRA and G-CSF, alone or in combination. Cell viability was
assessed by the trypan blue exclusion assay.
Determination of apoptosis
Apoptosis was analyzed using the Apoptosis Detection
kit I according to the manufacturer’s instructions. In brief, cells
were washed twice with cold PBS and then resuspended in 1X annexin
V binding buffer. After annexin V-FITC/PI staining for 15 min at
room temperature in the dark, the cells were analyzed using flow
cytometry (Cyto ACE-150; Jasco, Tokyo, Japan) within 1 h. The
percentage of apoptotic cells was determined by annexin V/PI
staining. The sum total of early apoptotic cells, annexin V (+) PI
(−), and late apoptotic cells, annexin V (+) PI (+), was regarded
as the total number of apoptotic cells. Cells were considered
viable if they did not show annexin V/PI staining.
Differentiation analysis
Differentiation induction was confirmed by
morphology and expression of surface markers. For morphological
assessment, cytospin preparations of treated cells stained with
Wright-Giemsa were evaluated by light microscopy. Myeloid
maturation with cell surface markers was analyzed by flow cytometry
(Cyto ACE-150; Jasco) using antibodies for CD11b, CD11c, CD15 and
CD34 as previously described with minor modifications (16). In brief, ~1×106 cells
were washed with PBS containing 2.5% FBS and 0.5% NaN3
(PBSF) and stained with PE-conjugated mouse anti-human CD11b
IgG2α and CD34 IgG1, FITC-conjugated mouse
anti-human CD15 IgM and FITC-conjugated mouse anti-human CD11c
IgG1 for 30 min at 4°C in the dark. Cells were then
washed 3 times with PBSF and analyzed by flow cytometry with a
minimum acquisition of 10,000 events. Non-binding mouse IgG-PE,
IgG-FITC or IgM-FITC isotype antibodies (Beckton-Dickinson) were
used as controls.
Analysis of AQP9 expression
Cells were harvested and washed with PBSF, followed
by staining with rabbit anti-rat AQP9 antibody at a concentration
of 10 μg/ml for 30 min at 4°C in the dark. After washing 3 times
with PBSF, the cells were stained with FITC-conjugated goat
anti-rabbit antibody and analyzed by flow cytometry. Immunostaining
specificity was controlled by omission of primary or secondary
antibodies.
Analysis of intracellular arsenic
accumulation (As[intra])
Cells were harvested and counted to give accurate
viable cell numbers, which were used to normalize As[intra]. After
washing 3 times with PBS, the cells were pelleted by centrifugation
and stored at −20°C until analysis. After transfer to 15-ml
polypropylene centrifuge tubes, cell pellets were mixed with
HNO3 (0.1 ml) at room temperature for 10 min, and
incubated at 80°C on a hot plate for 90 min. The cell suspension
was diluted with Milli-Q water to 3 ml and analyzed using
inductively coupled plasma mass spectrometry (ICP-MS)
(ELAN® DRC-e; PerkinElmer SCIEX, Ontario, Canada) for
total arsenic determination, as described previously (24).
Statistical analysis
Experiments were independently repeated and results
are shown as mean ± standard deviation (SD) of 3 assays. A
two-tailed, paired Student’s t-test or Mann-Whitney U test was used
and a P-value <0.05 was considered to be significant.
Results
Growth inhibition and apoptosis by ATO in
HT93A cells
First, we investigated whether HT93A cells showed
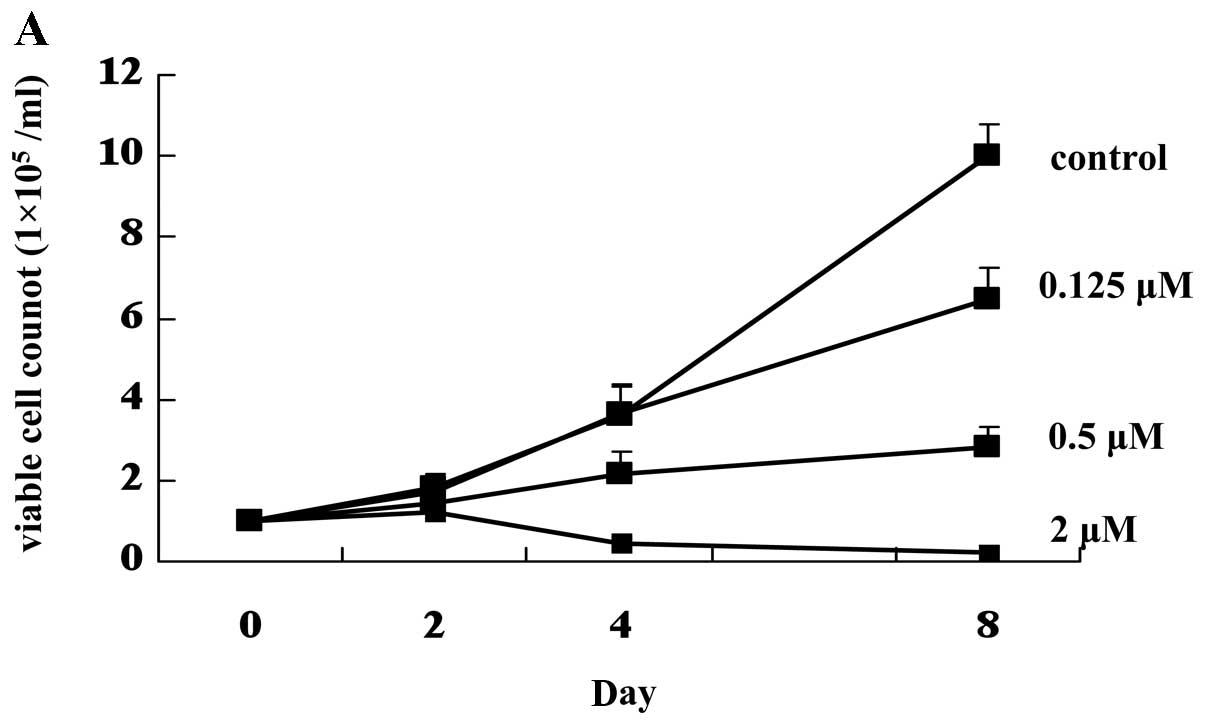
sensitivity to ATO treatment. ATO inhibited growth of HT93A cells
in a dose-dependent manner (Fig.
1A). After treatment with ATO for 4 days, apoptosis induction
was determined by Apoptosis Detection kit I. Similar to previous
findings that ATO induced apoptosis at concentrations ranging from
1 to 2 μM in vivo and in vitro (6,13,14),
the same range of concentrations of ATO significantly induced
apoptosis in HT93A cells in a dose-dependent manner (Fig. 1B). The addition of 1 μM ATRA and/or
50 ng/ml G-CSF to 2 μM ATO did not affect apoptosis compared to ATO
treatment alone. Total apoptotic cell ratio was 38.2% in ATO+ATRA
and 34.6% in ATO+ATRA+G-CSF treated cells, respectively. The ratio
was not different between ATO alone, ATO+ATRA and ATO+ATRA+G-CSF
groups (data not shown).
Differentiation of HT93A cells by ATO,
ATRA and G-CSF
To clarify the expression profiling of
differentiation markers of HT93A cells, the expression of 23
surface markers was comprehensively investigated after 1 μM of ATRA
for 8 days. CD11b, CD11c and CD15, associated with myeloid
maturation, were significantly upregulated by ATRA treatment. On
the contrary, the expression of CD34, associated with progenitor
cells, was significantly decreased. Therefore, we used these
markers for further differentiation experiments (Table I).
 | Table IProfile of surface antigen expression
in HT93A cells before and after ATRA treatment. |
Table I
Profile of surface antigen expression
in HT93A cells before and after ATRA treatment.
| Antigens | Clones | Positivity of
control (%) | Positivity after
ATRA treatment (%) | Differentiation
marker expression |
|---|
| CD2 | MT910 | 0.4 | 0.7 | |
| CD3 | SK7 | 0.8 | 1.0 | |
| CD4 | SK3 | 0.1 | 0.1 | |
| CD5 | 53-7.3 | 0.3 | 1.1 | |
| CD7 | M-T701 | 0.6 | 0.9 | |
| CD8 | SK-1 | 0.4 | 0.8 | |
| CD10 | HI10a | 0.2 | 0.1 | |
| CD11b | D12 | 0.3 | 13.6 | Upregulated |
| CD11c | S-HCL-3 | 0.4 | 16.9 | Upregulated |
| CD13 | L138 | 0.3 | 3.2 | |
| CD14 | MϕP9 | 0.1 | 6.7 | |
| CD15 | MMA | 19.4 | 44.5 | Upregulated |
| CD16 | NKP15 | 0.4 | 0.4 | |
| CD19 | 4G7 | 0.5 | 0.8 | |
| CD20 | L27 | 0.2 | 0.1 | |
| CD33 | WM53 | 99.0 | 99.5 | |
| CD34 | 8G12 | 66.2 | 30.9 | Downregulated |
| CD41 | HIP8 | 0.6 | 7.6 | |
| CD56 | MY31 | 99.5 | 99.8 | |
| CD117 | YB5.B8 | 0.4 | 0 | |
| KORSA | KOR-SA3544 | 0.2 | 0.5 | |
| HLA-DR | L243 | 0.4 | 4.6 | |
| MPO | MPO-7 | 2.4 | 2.3 | |
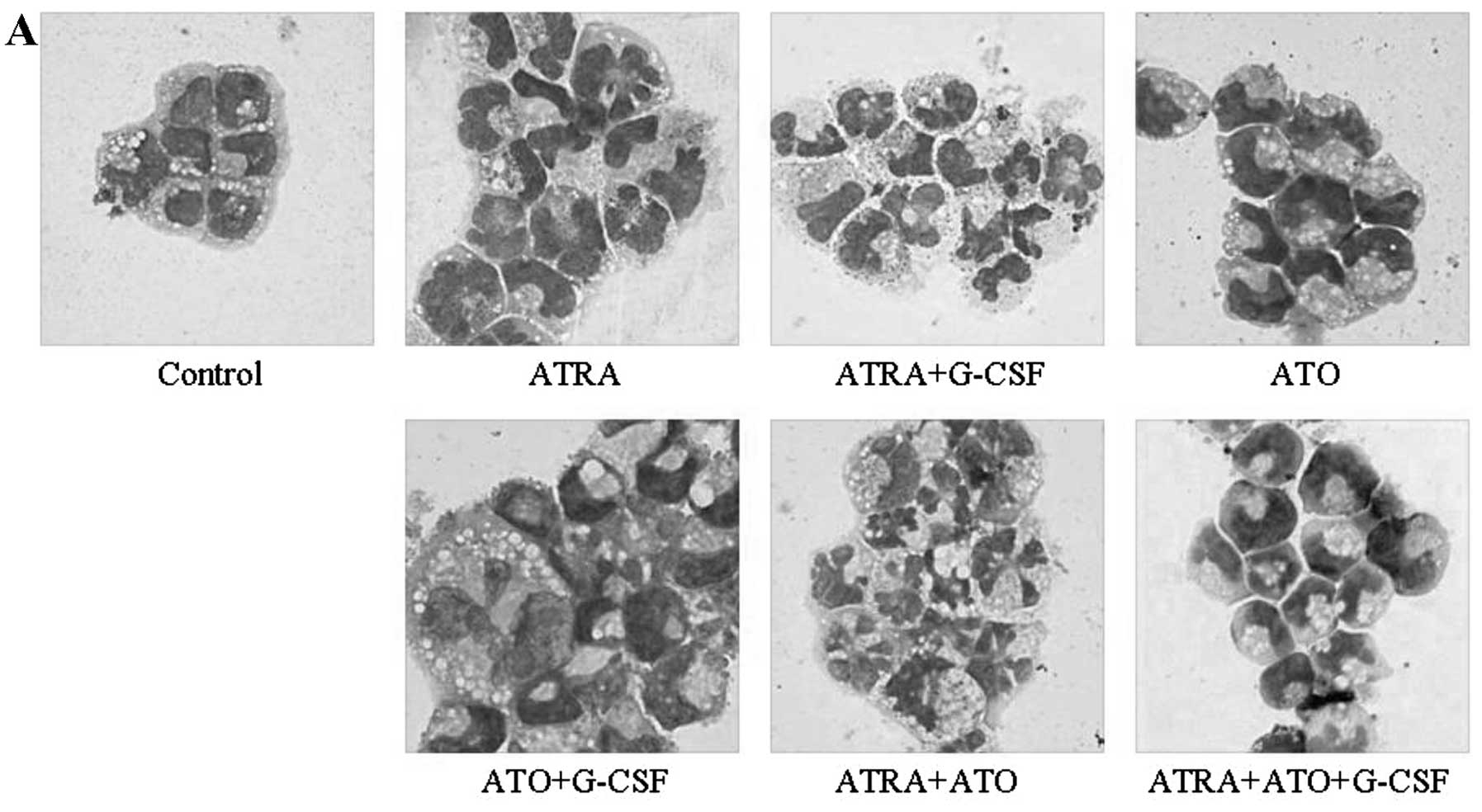
After treatment with ATRA (1 μM), ATO (0.125 μM) and
G-CSF (50 ng/ml), alone or in combination, morphological changes in
HT93A cells were examined (Fig.
2A). HT93A cells treated with 1 μM ATRA for 8 days underwent
remarkable differentiation-associated changes with condensation and
lobulation of nuclei. The differentiation-inducing activities of
these reagents were assessed by examining alterations in CD11b,
CD11c, CD15 and CD34 expression (Fig.
2B). Alterations in the expression of cell surface markers
including a significant increase in CD11b, CD11c and CD15 and a
significant decrease in CD34 concurred with the morphological
changes. Furthermore, the number of cells containing
multi-lobulated nuclei was further increased when the cells were
treated with the combination of ATRA and G-CSF compared to when
treated with ATRA alone. The increase in differentiation-inducing
activities due to combination treatment was confirmed by a
significant increase in CD11b and CD11c expression and a
significant decrease in CD34 expression.
Similar to a previous report in which a low dose of
ATO (0.1–0.5 μM) induced differentiation of NB4 cells (13), 0.125 μM ATO also induced
differentiation in HT93A cells as confirmed by the appearance of
jelly bean-shaped nuclei in almost all cells, accompanied by a
significant increase in CD11c and CD15 expression. When cells were
treated with the combination of ATO and G-CSF, G-CSF augmented
differentiation by ATO, as confirmed by the observation of
multi-lobulated nuclei and increased CD11b expression. No
differentiation induction was observed in HT93A cells treated with
G-CSF alone. Furthermore, ATO augmented morphological changes by
ATRA as indicated by the appearance of an increasing number of
cells containing multi-lobulated nuclei, whereas the expression
surface markers did not change as compared to that observed when
with ATRA alone.
CD11b expression in ATRA+ATO+G-CSF-treated cells was
higher than that in ATRA+ATO-treated cells. However, CD11b and
CD11c expression was significantly lower than that in
ATRA+G-CSF-treated cells (P<0.01). Furthermore, fewer
ATRA+ATO+G-CSF-treated cells showed multi-lobulated nuclei than
ATRA+G-CSF-treated cells. Therefore, the combination of ATRA and
G-CSF showed maximum differentiation, which was inhibited by ATO
addition.
Effects of ATRA alone or in combination
with G-CSF on cell viability
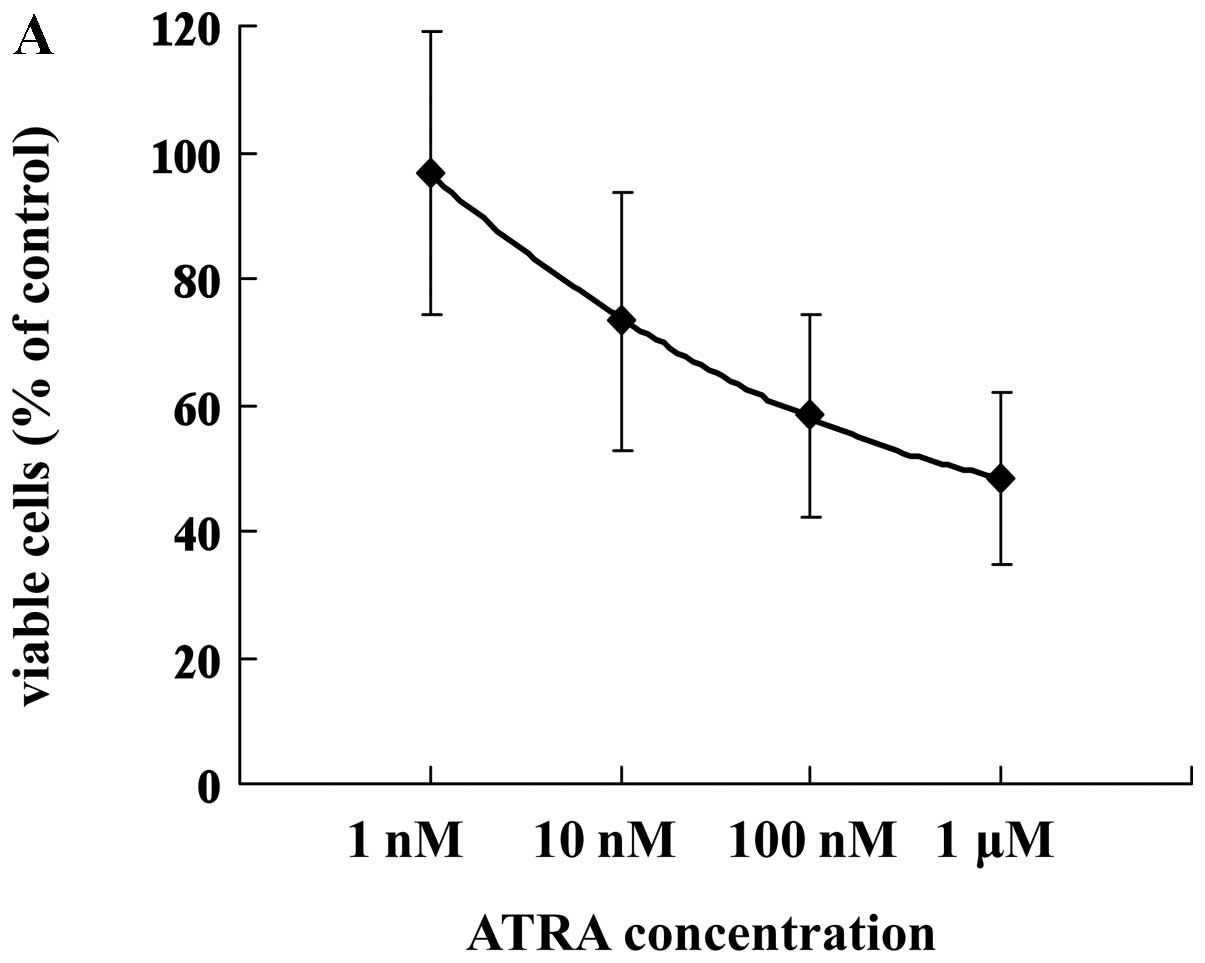
Treatment with ATRA for 7 days inhibited growth in a
dose-dependent manner, as observed by the trypan blue dye exclusion
assay (Fig. 3A). Because G-CSF
stimulates growth of leukemia cells, the effect of G-CSF on cell
growth in HT93A cells treated with or without ATRA was
investigated. The addition of 50 ng/ml G-CSF to ATRA significantly
increased the number of viable cells (Fig. 3B).
Upregulation of AQP9 expression in HT93A
cells by ATRA and ATO
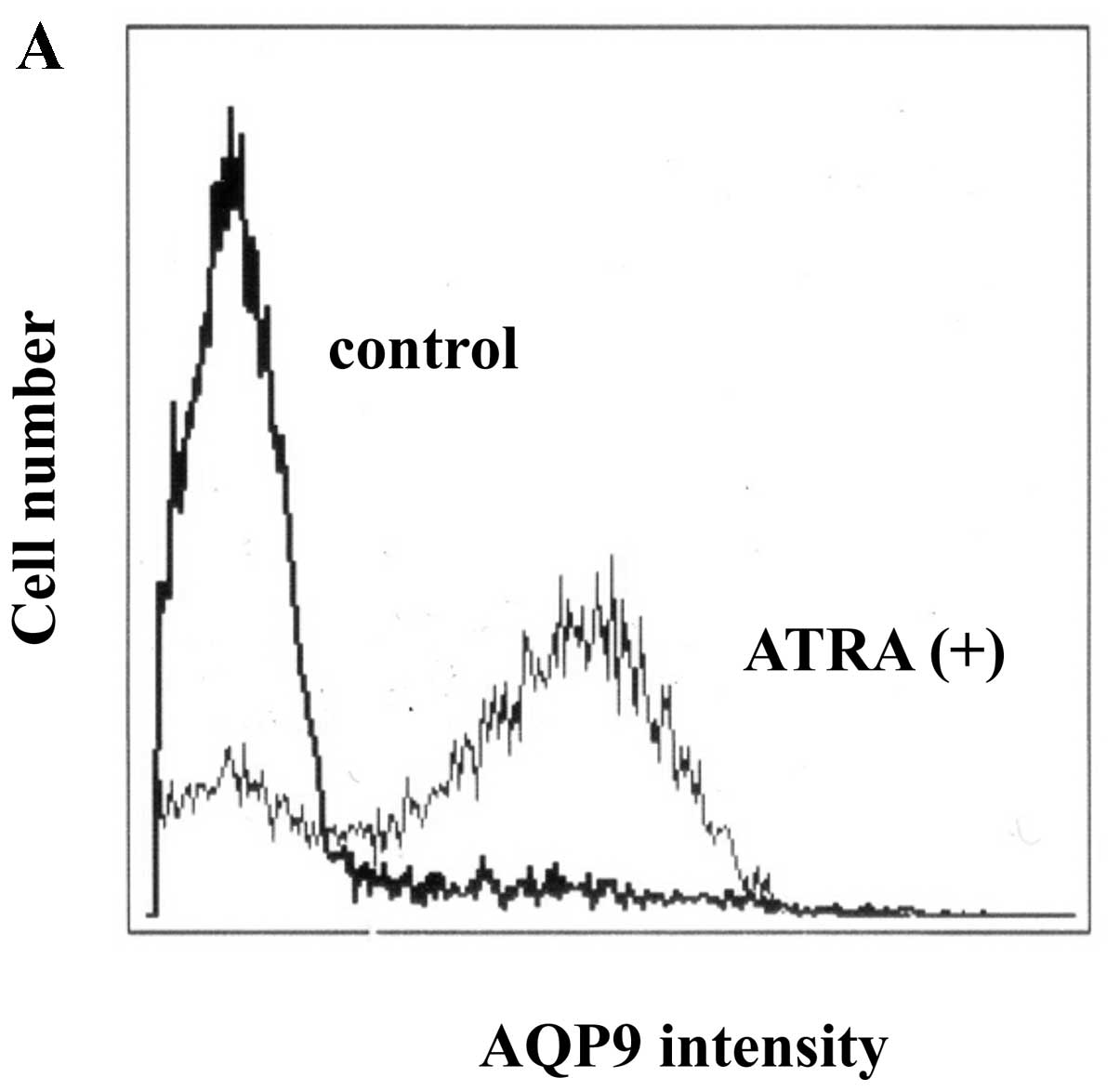
AQP9 expression in ATRA-treated HT93A cells was
investigated using flow cytometry. As shown in Fig. 4A, after treatment with ATRA (1 μM)
for 7 days, ATRA significantly upregulated AQP9 in HT93A cells. ATO
also induced AQP9 expression, although expression was lower than
that observed with ATRA. G-CSF addition did not enhance AQP9
expression in either ATRA- or ATO-treated cells (Fig. 4B). Moreover, G-CSF alone did not
affect AQP9 expression (data not shown), indicating no apparent
effect of G-CSF on AQP9 expression. Time-dependent upregulation of
AQP9 expression was observed when cells were treated with 1 μM ATRA
for 4 and 7 days (Fig. 4C).
Furthermore, dose-dependent upregulation of AQP9 was observed when
HT93A cells were treated with various concentrations of ATRA (1
nM-1 μM) for 7 days (Fig. 4D),
accompanied by ATRA-induced differentiation (Fig. 4E).
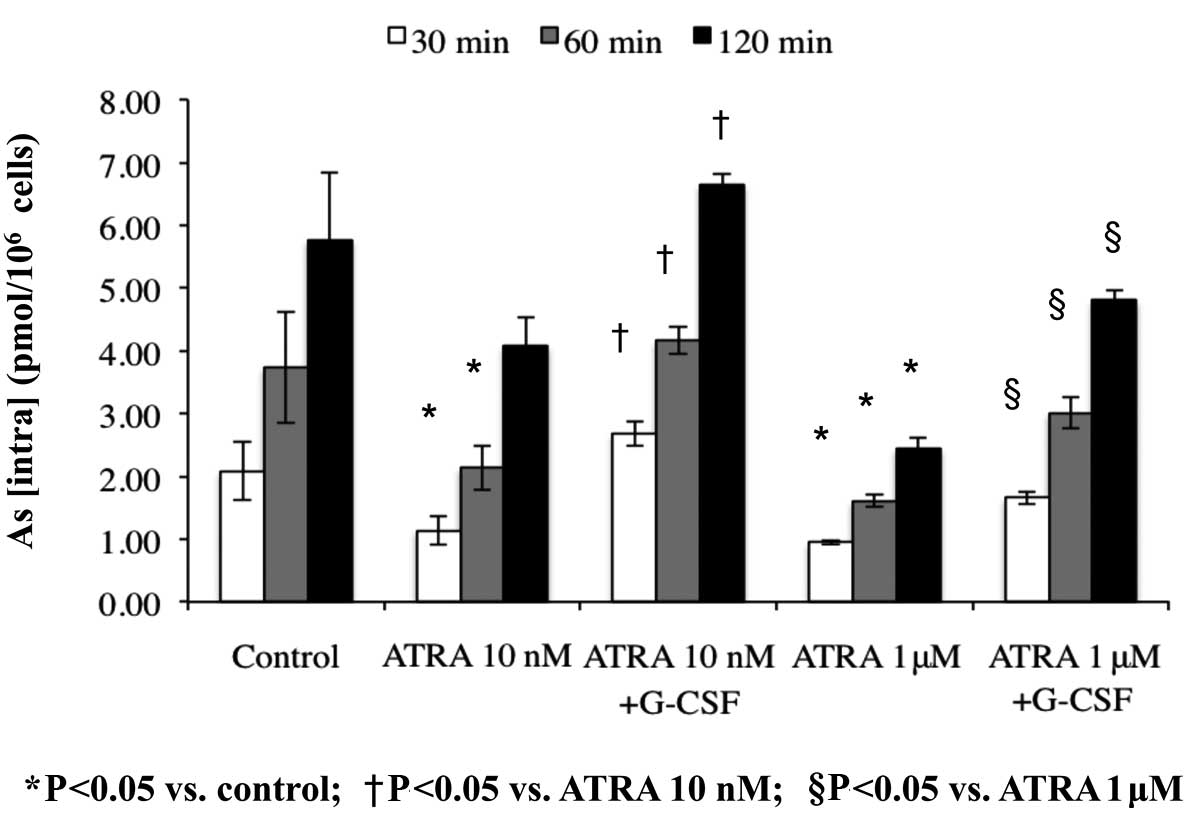
As[intra] in HT93A cells treated with
ATRA alone or in combination with G-CSF
Arsenic uptake was measured to examine whether
increased AQP9 expression contributes to ATO uptake. After
treatment with 10 nM or 1 μM ATRA in the presence or absence of 50
ng/ml G-CSF for 7 days and exposure to 0.5 μM ATO for 30, 60 and
120 min, As[intra] was determined using ICP-MS. Compared to the
control group exposed with ATO alone, As[intra] significantly
decreased to 42.3% and 70.7% due to treatment with either 10 nM or
1 μM ATRA for 120 min, respectively. However, it should be noted
that G-CSF addition recovered As[intra] to control levels (Fig. 5). G-CSF alone did not affect arsenic
uptake (data not shown).
Discussion
In this study, we demonstrated for the first time
that ATO induced growth inhibition and apoptosis in HT93A cells.
ATO (≥1 μM) significantly induced apoptosis in HT93A cells, which
is similar to that observed in NB4 cells (14). Lower concentration of ATO induced
differentiation of HT93A cells. On the basis of our previous study,
in which the blood level of ATO in APL patients was investigated
(24,25), plasma concentration of ATO in either
bone marrow or peripheral blood kept the effective level of
differentiation induction for HT93A cells. Next, we demonstrated
that both ATRA and ATO induced differentiation in HT93A cells. The
differentiation induced by ATO was lesser than that induced by
ATRA, as previously reported (13,14).
We observed the induction of the surface antigen CD15, but not
CD11b, after treatment with ATO in HT93A cells. CD15 is an
arsenic-induced sensitive differentiation marker as shown in
clinical studies (26). It is
noteworthy that CD34 expression, well-known to be present in
hematopoietic progenitor cells and absent in NB4 cells (8,18),
significantly decreased after treatment with ATRA in HT93A cells.
These results suggest that CD34, CD11b and CD15 could be used as a
parameter to evaluate the level of differentiation in clinical
therapy as shown in our previous clinical observation (25). There are only a few APL cell lines
and most in vitro experiments are performed with NB4 cells.
Because HT93A cells induced differentiation and apoptosis with ATO,
the cell line is a useful experimental target for arsenic treatment
as well as NB4 cells.
In agreement with previous reports (20), we demonstrated that G-CSF augmented
differentiation induced by ATO in HT93A cells. ATO increases mRNA
and protein levels of p21/WAF1 (27) which inhibits cell cycle in the S
phase. In concurrent use with G-CSF and ATO, it is possible that
G-CSF recruits quiescent leukemic cells to the S phase, rendering
them more sensitive to ATO. Similar increase of CD11b and CD11c
expression was observed when G-CSF was added to ATRA. Among several
combinations, maximum differentiation was observed with the
combination of ATRA and G-CSF. It has been shown that G-CSF
restores ATRA sensitivity in APL cells both in vitro and
in vivo, possibly through G-CSF receptors (9,28–31).
These previous findings and our results suggest that therapeutic
strategies based on ATRA or ATO in combination with G-CSF may
improve the clinical efficacy of differentiation therapy against
APL.
A meta-analysis of recent clinical trials with the
combination of ATRA and ATO treatment of APL indicates favorable
outcomes compared to ATO alone (19). However, it is still unclear whether
this combination is better than ATRA alone. Dai et al
(32) reported that complete
remission rates in ATRA- and ATRA+ATO-treated patients with APL
were similar (>90%). Furthermore, in our experiment, ATO
addition did not enhance ATRA-induced differentiation except for
morphological changes, probably because ATRA functioned well.
AQP9 is responsible for transporting small uncharged
molecules and plays a crucial role in arsenic uptake (12,33).
Previous studies demonstrated that pretreatment of a myeloid
leukemia cell line HL-60 with ATRA or vitamin D upregulates AQP9
expression, leading to a significant increase in arsenic uptake and
ATO-induced cytotoxicity in the presence of ATO (12,34).
However, a synergistic effect of ATRA and ATO was not observed in
differentiation and apoptosis in HT93A cells. We demonstrated for
the first time that As[intra] decreased after treatment with ATRA,
although AQP9 expression increased. Jing et al (35) reported that the pretreatment with
ATRA decreased ATO-induced apoptosis in ATRA-sensitive NB4 cells,
but not in ATRA-resistant cells. Possibly because HT93A cells were
ATRA-sensitive, a synergistic effect between ATRA and ATO was not
shown.
It is worth indicating that G-CSF addition
significantly recovered As[intra], whereas AQP9 expression remained
the same as that when treated with ATRA alone. It has been reported
that growth factors including G-CSF have multiple properties that
may affect the fate of both normal and neoplastic cells; these
properties include promotion of proliferation, survival and
differentiation (9,16,17).
In fact, G-CSF transiently initiates and promotes the growth of
responding acute myeloid leukemia cells but long-term proliferation
is not sustained (16). G-CSF
increased the number of viable HT93A cells. We speculate that ATRA
decreased cell viability and, consequently, low cell metabolism
resulted in reduced arsenic uptake by ATRA-sensitive cells. G-CSF
may enhance arsenic uptake through increased cell viability.
In summary, we report for the first time the effects
of ATO on HT93A cells, showing that ATO induced differentiation at
low concentrations and apoptosis at high concentrations. We also
report that G-CSF not only promoted differentiation-inducing
activities of both ATRA and ATO but also made APL cells vulnerable
to increased arsenic uptake, although G-CSF alone showed no
influence on these cellular responses. The combination of ATRA and
G-CSF showed maximum differentiation and ATO addition was not
beneficial. Thus, our observations provide new insight into
combination therapy with ATRA, ATO and G-CSF for the treatment of
APL.
Acknowledgements
We thank Dr Kenji Kishi and Dr Yuko Sato for
providing the HT93A cell line. We also thank Eiko Ishizuka for
technical assistance. This work was supported in part by grants
from Japan China Medical Association to B.Y.
References
|
1
|
Goddard AD, Borrow J, Freemont PS and
Solomon E: Characterization of a zinc finger gene disrupted by the
t(15;17) in acute promyelocytic leukemia. Science. 254:1371–1374.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tong JH, Dong S, Geng JP, Huang W, Wang
ZY, Sun GL, Chen SJ, Chen Z, Larsen CJ and Berger R: Molecular
rearrangements of the MYL gene in acute promyelocytic leukemia
(APL, M3) define a breakpoint cluster region as well as some
molecular variants. Oncogene. 7:311–316. 1992.PubMed/NCBI
|
|
3
|
de Thé H, Chomienne C, Lanotte M, Degos L
and Dejean A: The t(15;17) translocation of acute promyelocytic
leukaemia fuses the retinoic acid receptor alpha gene to a novel
transcribed locus. Nature. 347:558–561. 1990.PubMed/NCBI
|
|
4
|
Melnick A and Licht JD: Deconstructing a
disease: RARα, its fusion partners, and their roles in the
pathogenesis of acute promyelocytic leukemia. Blood. 93:3167–3215.
1999.
|
|
5
|
Burnett AK, Grimwade D, Solomon E,
Wheatley K and Goldstone AH: Presenting white blood cell count and
kinetics of molecular remission predict prognosis in acute
promyelocytic leukemia treated with all-trans retinoic acid:
result of the Randomized MRC Trial. Blood. 93:4131–4143.
1999.PubMed/NCBI
|
|
6
|
Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM,
Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, et al: Use of arsenic
trioxide (As2O3) in the treatment of acute
promyelocytic leukemia (APL): II. Clinical efficacy and
pharmacokinetics in relapsed patients. Blood. 89:3354–3360.
1997.PubMed/NCBI
|
|
7
|
Soignet SL, Maslak P, Wang ZG, Jhanwar S,
Calleja E, Dardashti LJ, Corso D, DeBlasio A, Gabrilove J,
Scheinberg DA, et al: Complete remission after treatment of acute
promyelocytic leukemia with arsenic trioxide. N Engl J Med.
339:1341–1348. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lanotte M, Martin-Thouvenin V, Najman S,
Balerini P, Valensi F and Berger R: NB4, a maturation inducible
cell line with t(15;17) marker isolated from a human acute
promyelocytic leukemia (M3). Blood. 77:1080–1086. 1991.PubMed/NCBI
|
|
9
|
Matsui W, Smith BD, Vala M, Beal N, Huff
CA, Diehl LF and Jones RJ: Requirement for myeloid growth factors
in the differentiation of acute promyelocytic leukaemia. Br J
Haematol. 128:853–862. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Caprodossi S, Pedinotti M, Amantini C,
Santoni G, Minucci S, Pelicci PG and Fanelli M: Differentiation
response of acute promyelocytic leukemia cells and PML/RARα
leukemogenic activity studies by real-time RT-PCR. Mol Biotechnol.
30:231–238. 2005.
|
|
11
|
Cunha De Santis G, Tamarozzi MB, Sousa RB,
Moreno SE, Secco D, Garcia AB, Lima AS, Faccioli LH, Falcão RP,
Cunha FQ and Rego EM: Adhesion molecules and Differentiation
Syndrome: phenotypic and functional analysis of the effect of ATRA,
As2O3, phenylbutyrate, and G-CSF in acute
promyelocytic leukemia. Haematologica. 92:1615–1622.
2007.PubMed/NCBI
|
|
12
|
Leung J, Pang A, Yuen WH, Kwong YL and Tse
EW: Relationship of expression of aquaglyceroporin 9 with arsenic
uptake and sensitivity in leukemia cells. Blood. 109:740–746. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J,
Cai X, Han ZG, Ni JH, Shi GY, Jia PM, et al: Use of arsenic
trioxide (As2O3) in the treatment of acute
promyelocytic leukemia (APL): I. As2O3 exerts
dose-dependent dual effects on APL cells. Blood. 89:3345–3353.
1997.PubMed/NCBI
|
|
14
|
Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ,
Si GY, Jin XL, Tang W, Li XS, Xong SM, et al: In vitro studies on
cellular and molecular mechanisms of arsenic trioxide
(As2O3) in the treatment of acute
promyelocytic leukemia: As2O3 induces
NB4 cell apoptosis with downregulation of Bcl-2
expression and modulation of PML-RARα/PML proteins. Blood.
88:1052–1061. 1996.PubMed/NCBI
|
|
15
|
Yoshino Y, Yuan B, Kaise T, Takeichi M,
Tanaka S, Hirano T, Kroetz DL and Toyoda H: Contribution of
aquaporin 9 and multidrug resistance-associated protein 2 to
differential sensitivity to arsenite between primary cultured
chorion and amnion cells prepared from human fetal membranes.
Toxicol Appl Pharmacol. 257:198–208. 2011. View Article : Google Scholar
|
|
16
|
Pébusque MJ, Lafage M, Lopez M and Mannoni
P: Preferential response of acute myeloid leukemias with
translocation involving chromosome 17 to human recombinant
granulocyte colony-stimulating factor. Blood. 72:257–265. 1998.
|
|
17
|
Souza LM, Boone TC, Gabrilove J, Lai PH,
Zsebo KM, Murdock DC, Chazin VR, Bruszewski J, Lu H, Chen KK, et
al: Recombinant human granulocyte colony-stimulating factor:
effects on normal and leukemic myeloid cells. Science. 232:61–65.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu J, Liu YF, Wu CF, Xu F, Shen ZX, Zhu
YM, Li JM, Tang W, Zhao WL, Wu W, et al: Long-term efficacy and
safety of all-trans retinoic acid/arsenic trioxide-based
therapy in newly diagnosed acute promyelocytic leukemia. Proc Natl
Acad Sci USA. 106:3342–3347. 2009.PubMed/NCBI
|
|
19
|
Wang H, Chen XY, Wang BS, Rong ZX, Qi H
and Chen HZ: The efficacy and safety of arsenic trioxide with or
without all-trans retinoic acid for the treatment of acute
promyelocytic leukemia: a meta-analysis. Leuk Res. 35:1170–1177.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kishi K, Toba K, Azegami T, Tsukada N,
Uesugi Y, Masuko M, Niwano H, Hashimoto S, Sakaue M, Furukawa T, et
al: Hematopoietic cytokine-dependent differentiation to eosinophils
and neutrophils in a newly established acute promyelocytic leukemia
cell line with t(15;17). Exp Hematol. 26:135–142. 1998.PubMed/NCBI
|
|
21
|
Iijima Y, Ito T, Oikawa T, Eguchi M,
Eguchi-Ishimae M, Kamada N, Kishi K, Asano S, Sakaki Y and Sato Y:
A new ETV6/TEL partner gene, ARG (ABL-related gene or ABL2),
identified in an AML-M3 cell line with a t(1;12)(q25;p13)
translocation. Blood. 95:2126–2131. 2000.PubMed/NCBI
|
|
22
|
Makishima M, Umesono K, Shudo K, Naoe T,
Kishi K and Honma Y: Induction of differentiation in acute
promyelocytic leukemia cells by 9-cis retinoic acid
alpha-tocopherol ester (9-cis tretinoin tocoferil). Blood.
91:4715–4726. 1998.PubMed/NCBI
|
|
23
|
Uesugi Y, Fuse I, Toba K, Kishi K,
Furukawa T, Koike T and Aizawa Y: Involvement of SHP-1, a
phosphotyrosine phosphatase, during myeloid cell differentiation in
acute promyelocytic leukemia cell lines. Eur J Haematol.
62:239–245. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoshino Y, Yuan B, Miyashita SI, Iriyama
N, Horikoshi A, Shikino O, Toyoda H and Kaise T: Speciation of
arsenic trioxide metabolites in blood cells and plasma of a patient
with acute promyelocytic leukemia. Anal Bioanal Chem. 393:689–697.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iriyama N, Yoshino Y, Yuan B, Horikoshi A,
Hirabayashi Y, Hatta Y, Toyoda H and Takeuchi J: Speciation of
arsenic trioxide metabolites in peripheral blood and bone marrow
from an acute promyelocytic leukemia patient. J Hematol Oncol.
5:1–11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shao W, Fanelli M, Ferrara FF, Riccioni R,
Rosenauer A, Davison K, Lamph WW, Waxman S, Pelicci PG, Lo Coco F,
et al: Arsenic trioxide as an inducer of apoptosis and loss of
PML/RARα protein in acute promyelocytic leukemia cells. J Natl
Cancer Inst. 90:124–133. 1998.PubMed/NCBI
|
|
27
|
Wang X, Gao P, Long M, Lin F, Wei JX, Ren
JH, Yan L, He T, Han Y and Zhang HZ: Essential role of cell cycle
regulatory genes p21 and p27 expression in inhibition of breast
cancer cells by arsenic trioxide. Med Oncol. 28:1225–1254. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Higuchi T, Kizaki M and Omine M: Induction
of differentiation of retinoic acid-resistant acute promyelocytic
leukemia cells by the combination of all-trans retinoic acid
and granulocyte colony-stimulating factor. Leuk Res. 28:525–532.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jansen JH, de Ridder MC, Geertsma WM,
Erpelinck CA, van Lom K, Smit EM, Slater R, vd Reijden BA, de Greef
GE, Sonneveld P and Löwenberg B: Complete remission of t(11;17)
positive acute promyelocytic leukemia induced by all-trans
retinoic acid and granulocyte colony-stimulating factor. Blood.
94:39–45. 1999.PubMed/NCBI
|
|
30
|
Tsurumi H, Tojo A, Takahashi T, Moriwaki
H, Asano S and Muto Y: The combined effects of all-trans
retinoic acid and granulocyte colony-stimulating factor as a
differentiation induction therapy for acute promyelocytic leukemia.
Intern Med. 32:648–650. 1993.
|
|
31
|
Gianní M, Terao M, Zanotta S, Barbui T,
Rambaldi A and Garattini E: Retinoic acid and granulocyte
colony-stimulating factor synergistically induce leukocyte alkaline
phosphatase in acute promyelocytic leukemia cells. Blood.
83:1909–1921. 1994.
|
|
32
|
Dai CW, Zhang GS, Shen JK, Zheng WL, Pei
MF, Xu YX, Cao YX, Yi Y, Yang JJ, Peng HL, et al: Use of
all-trans retinoic acid in combination with arsenic trioxide
for remission induction in patients with newly diagnosed acute
promyelocytic leukemia and for consolidation/maintenance in CR
patients. Acta Haematol. 121:1–8. 2009.
|
|
33
|
Shinkai Y, Sumi D, Toyama T, Kaji T and
Kumagai Y: Role of aquaporin 9 in cellular accumulation of arsenic
and its cytotoxicity in primary mouse hepatocytes. Toxicol Appl
Pharmacol. 237:232–236. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bhattacharjee H, Carbrey J, Rosen BP and
Mukhopadhyay R: Drug uptake and pharmacological modulation of drug
sensitivity in leukemia by AQP9. Biochem Biophys Res Commun.
322:836–841. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jing Y, Wang L, Xia L, Chen GQ, Chen Z,
Miller WH and Waxman S: Combined effect of all-trans
retinoic acid and arsenic trioxide in acute promyelocytic leukemia
cells in vitro and in vivo. Blood. 97:264–269. 2001.PubMed/NCBI
|