Introduction
Lung cancer is the leading cause of cancer-related
mortality world-wide, and was responsible for 1.38 million deaths
in 2008 (1). According to the WHO
estimation, China will become one of the countries that have a
relatively high incidence of lung cancer in the 21st century
(2). Lung adenocarcinoma (AD),
accounted for ~40% of all lung cancers, is currently one of the
most common histological types and its incidence has gradually
increased in recent years in many countries (3). The conventional chemotherapy and
radiotherapy of lung cancer have limited the success in controlling
lung cancer, necessitating the development of new treatment
strategy. Various approaches for lung cancer treatment including
induction of differentiation and apoptosis have been attempted
(4).
The thyroid transcription factor 1 (TTF-1/Nkx-2.1)
is expressed normally and selectively in thyroid follicular and
parafollicular C cells, type II pneumocytes and the non-ciliated
bronchiolar epithelium (Clara cells) (5,6).
During lung development, TTF-1 plays a critical role in lung
morphogenesis and respiratory epithelial cell differentiation and
regulates the expression of lung-specific genes such as surfactant
proteins A (SP-A), B and C and Clara cell secretory protein
(7). In adult lung, TTF-1
expression is suppressed in the epithelium of proximal airways, but
persists in the epithelia of lung parenchyma, leading to a
proximal-to-distal gradient in TTF-1 expression. This gradient has
been shown to be disturbed in pulmonary hypoplasia (8). TTF-1 could be a useful
immunohistochemical marker to distinguish lung AD from squamous
cell carcinoma (SCC) or large cell carcinomas (LCC). Among the
human NSCLC, TTF-1 is expressed in 60–90% of AD, in 0–27% of SCC
and in 0–25% of LCC (9–11). There have been a few conflicting
reports on the prognostic value of TTF-1 overexpression in lung
cancer patients. Berghmans et al(12) reviewed published data from 1999–2005
that examined the relation between the TTF-1 expression and patient
survival in lung cancer. Of the 10 eligible studies, 5 found that
TTF-1 expression had no significant impact of survival, 4 reported
that increased TTF-1 expression was associated with better
survival, while 1 study reported that TTF-1 expression was
associated with poorer survival. The result indicated that
TTF-1-positivity could be a favorable prognostic marker. Few
studies referred to possible significance of TTF-1 expression in
lung carcinogenesis, despite its crucial role in lung development
and maintenance of pulmonary function. Significant inverse
correlation has been found between TTF-1 expression and
proliferative activity evaluated by Ki-67 protein (13).
The aim of the present study was to detect the
expression of TTF-1 in human NSCLC cell lines and to evaluate the
association of overexpressed TTF-1 with Ki-67 and apoptosis in A549
cell line. We were interesting in A549 cells because the cells have
high tumorigenicity and low expression of TTF-1 (data not shown).
The TTF-1 expression was found at a very low frequency in NSCLC
cell lines, including adenocarcinoma cell lines. Overexpressed
TTF-1 could downregulate the expression of Ki-67 and induce the
apoptosis of A549 cell line. We also investigated
immunohistochemically the expressions of TTF-1 and Ki-67 in Xuanwei
lung adenocarcinoma, located in southwestern Chinese province of
Yunnan. The patients with strong immunohistochemical expression of
TTF-1 were statistically associated with well-differentiated
phenotype (P=0.006) and inversely correlated with Ki-67 expression
(P=0.016).
Materials and methods
NSCLC cell lines and cell culture
A549 cells obtained from the American Type Culture
Collection, were cultured in RPMI-1640 medium supplemented with 10%
fetal bovine serum. Cell cultures were maintained in a humidified
atmosphere of 5% CO2 at 37°C.
TTF-1 mRNA quantitated by qRT-PCR
mRNAs from the 10 NSCLC cell lines (AD: A549, MGH24,
MGH8 and H1264; SCC: H157, H520 and MGH7; LCC: H460 and RVH6849;
adenosquamous carcinoma: H125) were extracted using Qiagen RNeasy
Mini kit according to the manufacturer's protocol and reverse
transcribed into cDNAs (Invitrogen, Carlsbad, CA). Primers for qPCR
were designed using Primer Express v1.5 (Applied Biosystems, Foster
City, CA). qPCR was performed using the SYBR® Green PCR
Master Mix (Applied Biosystems). A Mx3000PR QPCR system
(Stratagene, Cedar Creek, TX). The expression level of TTF-1 was
normalized to that expression of TBP. The primer sequences were F:
5′-GAGGGAGG AGCAGCCCC-3′, R: 5′-CCACTTTCTTGTAGCTTTCCT CCA-3′ for
TTF-1; and F: 5′-TTTGCTGCGGTAATCATG AGG-3′, R:
5′-ATTTTCTTGCTGCCAGTCTGG-3′ for TBP. Ten nanogram cDNA was used in
each QPCR reaction and A549 was used as a calibrator. Relative mRNA
was calculated using the formula, 2-ΔΔCt(14). The TTF-1 relative mRNA levels were
higher or lower than two times of the average of A549 mRNA, which
were defined as TTF-1 overexpression or downregulation,
respectively.
Construction of the TTF-1 expression
plasmid
Commercial TTF-1 purchased from ATCC (Manassas, VA,
USA; Catalog no. 1056387) was extracted from the DH10B of
Escherichia coli using Qiagen Plasmid Midi kit (Qiagen Inc.,
Valencia, CA, USA). The amplified TTF-1 was inserted into the
pENTRCMV-ON plasmid at HpaI and NotI sites. The
recombinant plasmid pENTRCMV-ON-TTF-1 was confirmed by sequencing
(3730, Applied Biosystems).
Transfection of recombinant plasmid of
pENTRCMV-ON-TTF-1
A549 cells were seeded (2×105 cells/well)
in 6-well tissue culture plates. After 24 h of incubation, they
reached 90–95% confluence and were transfected with recombinant
plasmid (pENTR-CMV-ON-TTF-1) or blank plasmid (pENTR-CMV) in
serum-free medium using Lipofectamine™ 2000 (Invitrogen) and were
treated with the transfection reagent alone as control group. Each
plasmid or blank plasmid (4 μg) and 10 μl of Lipofectamine 2000
were diluted in serum-free medium (250 μl), left at room
temperature for 5 min, mixed immediately, and incubated for 20 min
at room temperature. The mixture was then added to wash A549 cells,
and after 5 h of incubation, the medium was replaced with full
medium for 24 h.
Immunohistochemistry of TTF-1 and Ki-67
in A549 cells
Briefly, A549 cells of transfected TTF-1 or blank
plasmid were washed in PBS, trypsinized, then mounted on to
polylysine-coated slides and fixed with ice-cold acetone for 20 min
at room temperature to dry. The cells were incubated with primary
mouse monoclonal antibody, purchased from Maixin Bio, Ltd. (Fuzhou,
China), against human TTF-1 or Ki-67, which both were prediluted
for 60 min at room temperature. The cells were stained using a
commercial kit and visualized using freshly prepared solutions of
diaminobenzidine DAB (Maixin Bio, Ltd.). The transfected A549 cells
were counted at a ×400 magnification in 5 fields and slides were
independently assessed by two researchers. For Ki-67, cells with
nucleolar staining were considered as positive. The number of cells
with positive staining in high-power fields was divided by the
total number of A549 cells in those fields and the result was
expressed as a percentage.
Flow cytometric determination of the
apoptotic rate
A flow cytometric analysis was performed by Coulter
Epics XL flow cytometer (Beckman Coulter, Miami, FL, USA). Briefly,
the cells which were treated TTF-1, blank plasmid and transfection
reagent alone were centrifuged, washed in phosphate-buffered saline
(PBS), and treated with 10 ng/ml RNase and 0.1% Triton X-100 for 15
min. Subsequently, cells were stained with propidium iodide (5
mg/ml) for 30 min. The sample was read on a Coulter Epics XL flow
cytometer and the percentages of cells in the apoptotic sub-G1 and
the G1, S and G2/M phases were calculated using WinCycle software
(Phoenix Flow Systems, San Diego, CA).
Patients and tissue samples
Primary tumor specimens from 62 Xuanwei lung
carcinomas were obtained by surgical resection between 2008 and
2010 at the several hospitals affiliated to Kunming Medical
College. All hematoxylin and eosin stained slides of the tissue
samples were reviewed, and the pathological diagnoses of the
histologic grades and types were confirmed by pathologists.
Histologic typing was performed according to the World Health
Organization diagnostic criteria for lung carcinomas (1999) and all
cases were adenocarcinomas. The pathologic stages were assessed
according to the TNM classification of AJCC staging system (1997).
The hospital records of all 62 patients were reviewed to obtain the
clinicopathological variables such as gender, age, smoking history,
grade and stage.
Immunohistochemistry for TTF-1 and Ki-67
in Xuanwei lung adenocarcinomas
Formalin-fixed paraffin-embedded tissue samples were
investigated. After rehydration, deparaffinized 4-μm sections were
pretreated by microwave epitope retrieval (750 W for 15 min in
citrate buffer 10 mmol, pH 6.0) antibodies. Prior to the
application of the primary antibody, endogenous peroxidase activity
was inhibited with 5% hydrogen peroxide and a biotin with bovine
albumin blocking step was performed. Tissue sections (4 μm) of all
tumors were incubated with monoclonal antibodies directed against
TTF-1 (1:100 dilution)and Ki-67 (1:100 dilution) (all from Maixin
Bio, Ltd.). The primary antibody was detected using a secondary
biotinylated antibody and a streptavidin-peroxidase conjugate
according to the manufacturer's instructions. Hematoxylin was used
as the nuclear counterstain. Immunostaining was evaluated by a
semi-quantitative method according to the percentage of positive
tumor cells, (0; 1+, <25%; 2++, 26–50%; 3+++, >50%]. To
exclude equivocal reactions, at least 1% of positive cells were
required for a diagnostically relevant positive reaction. Only
nuclear labelling with anti-TTF-1 was considered to be a positive
reaction. Cases were considered positive for Ki-67 expression, when
>10% of tumor cells were reactive, because the median value of
Ki-67 proliferative fraction was about 10% in all cases.
Statistical analysis
All analyses were conducted using by SPSS (version
17.0) for Windows software. One-Way ANOVA test was used to compare
the rate of apoptosis of control, blank plasmid and TTF-1 group,
and to compare the Ki-67 expression between TTF-1 group and blank
plasmid group. P<0.05 were considered statistically
significant.
Spearman rank-correlation test and Wilcoxon rank-sum
test were used to examine the association between TTF-1 positive
status and clinicopathological features. The association between
TTF-1 and Ki-67 positive status was examined by Spearman
rank-correlation test. P<0.05 were considered statistically
significant.
Results
TTF-1 is lowly expressed in most NSCLC
cell lines
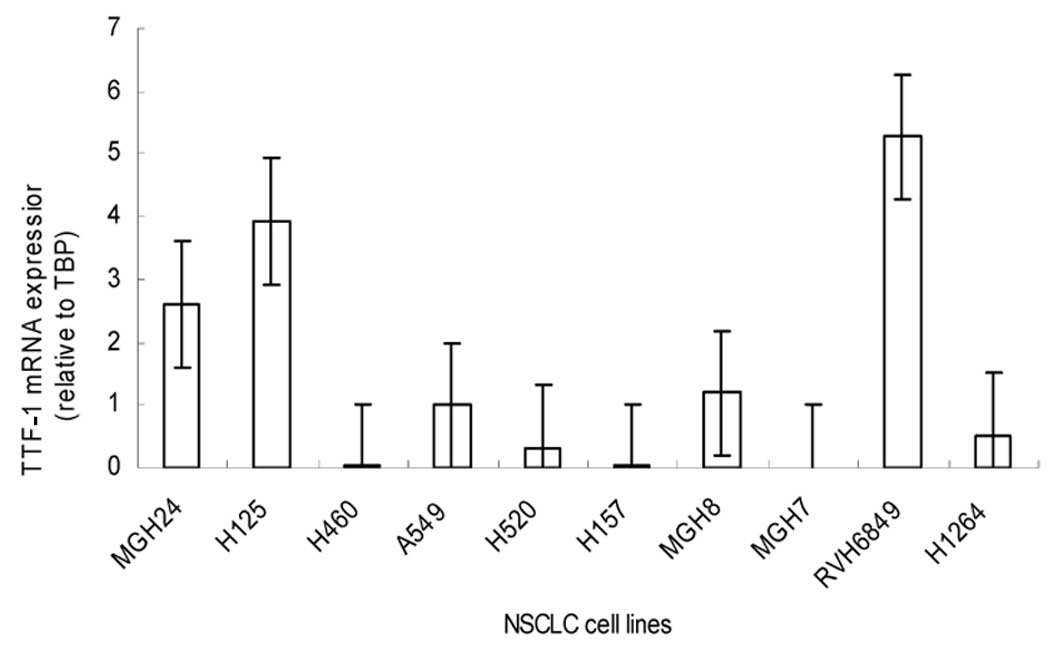
The TTF-1 mRNA transcript levels in 10 NSCLC cell
lines showed that the median TTF-1 mRNA expression levels relative
to the average of A549 cells were 2.6 for MGH24, 3.9 for H125, 0.02
for H460, 0.3 for H520, 0.02 for H157, 1.1 for MGH8, 0.00 for MGH7,
5.2 for RVH6849 and 0.5 for H1264. Only the mRNAs level of MGH24,
H125 and RVH6849 showed overexpression while other 7 NSCLC cell
lines lowered or lacked the expression of TTF-1 mRNA, which was
consistent with the previous reports on TTF-1 expression in lung
cancer cell lines (15) (Fig. 1). We therefore used the A549 to
study the role of TTF-1 in lung cancer cells.
Construction of TTF-1 expression
vector
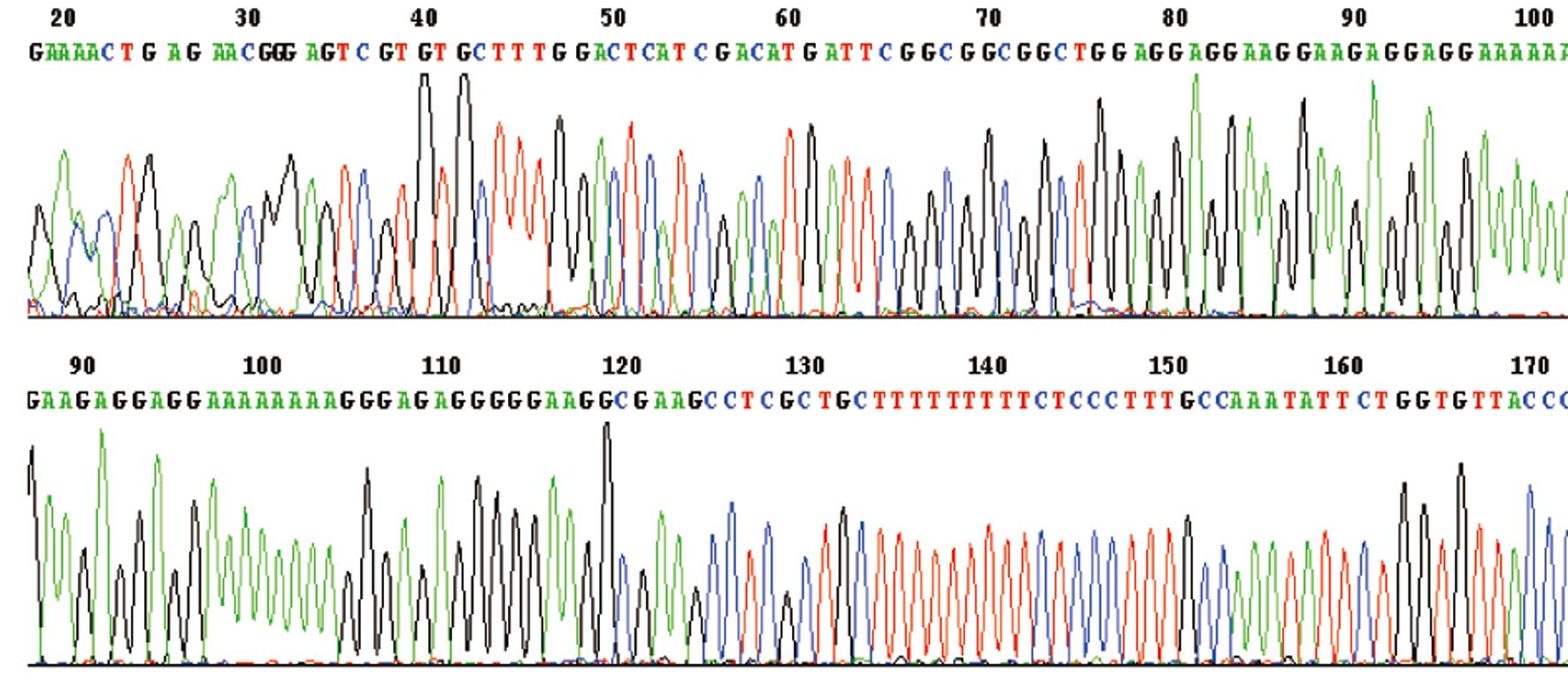
Fig. 2 shows that
the correct TTF-1 expression vector was obtained. The obtained
sequence of the TTF-1 gene was identified by sequence.
Overexpression of TTF-1 in A549 cell
line
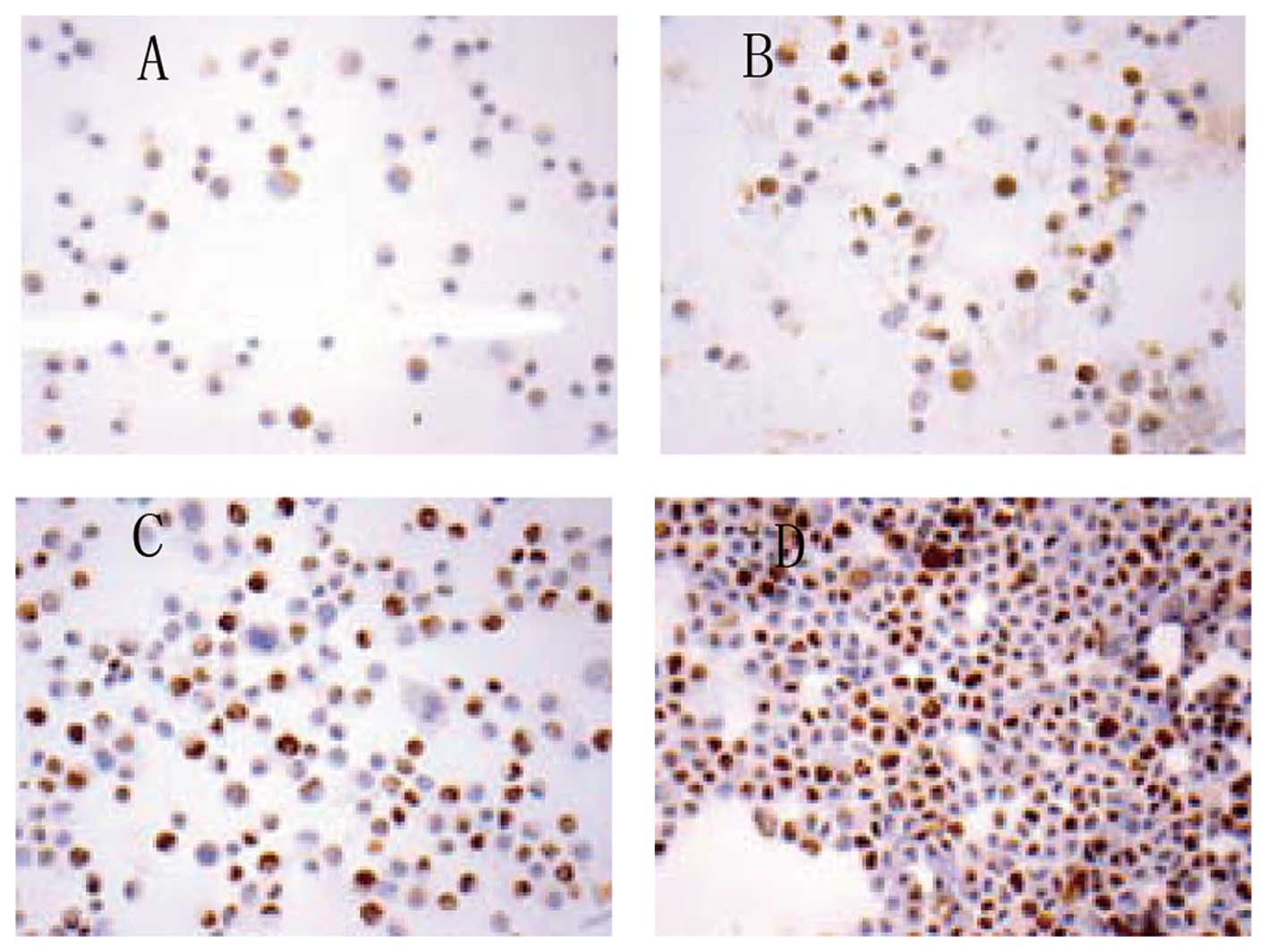
As expected, TTF-1 staining was found in cytoplasm
only in untransfected TTF-1 A549 cells (Fig. 3A), consistent with previous report
(15). In contrast, TTF-1 staining
was seen in both the cytoplasm and nucleus when A549 cells were
transfected with TTF-1 (Fig. 3B),
consistent with that observed in tissue sections of primary lung
adenocarcinoma.
TTF-1 expression suppresses proliferation
of A549 cells by regulating Ki-67 expression in vitro
Overexpression of TTF-1 decreased the proliferation
of A549 cells. The percentage of Ki-67-positive cells was 42.9±3.1%
in TTF-1 group (Fig. 3C) vs.
50.9±3.3% in the blank plasmid group (Fig. 3D) and the difference was significant
(P<0.05) (Table I).
 | Table IOverexpression of TTF-1 decreased the
proliferation of A549 cells by regulating Ki-67. |
Table I
Overexpression of TTF-1 decreased the
proliferation of A549 cells by regulating Ki-67.
| Ki-67-positive cells
(%) |
|---|
|
|
|---|
| Cell | TTF-1 | Blank plasmid |
|---|
| A549 | 42.9±3.1 | 50.9±3.3a |
TTF-1 overexpression enhances apoptosis
of A549 cells in vitro
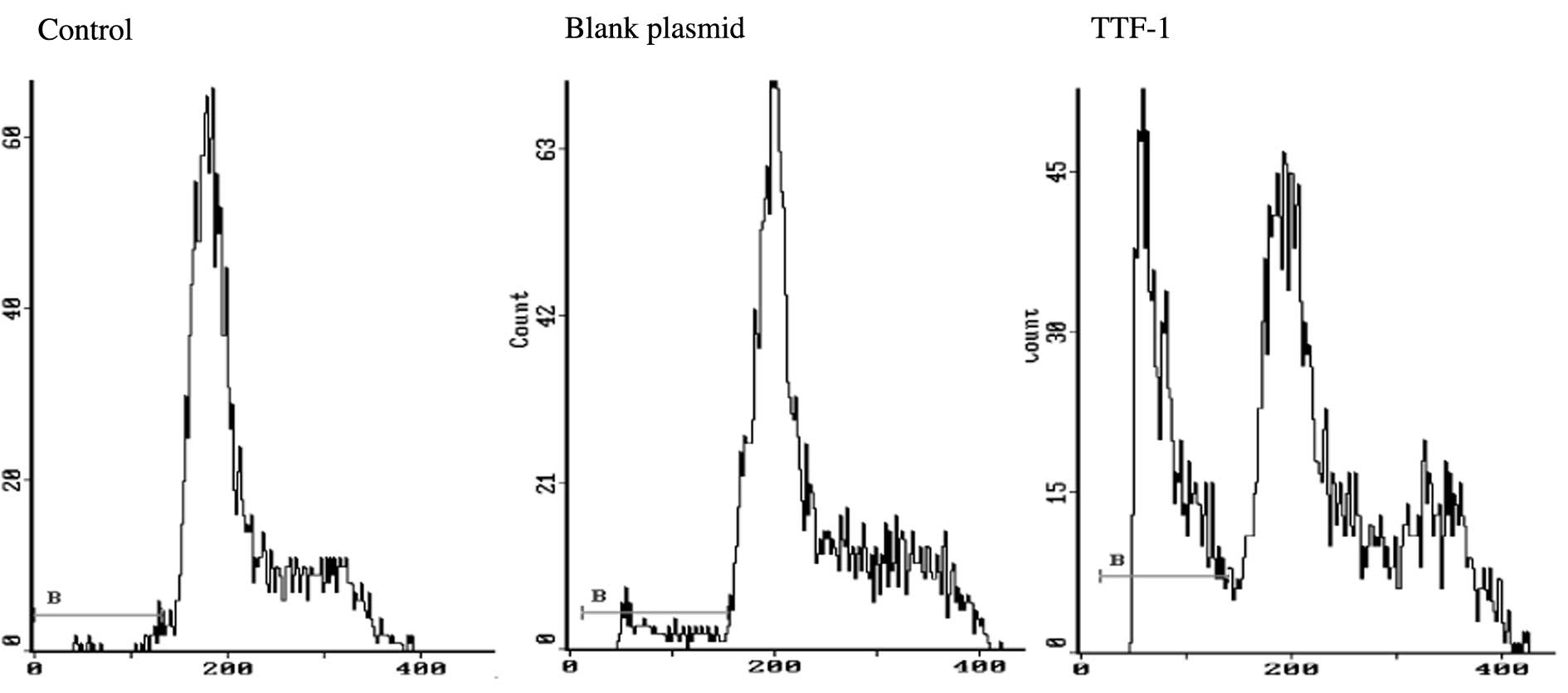
Fig. 4 and Table II show the apoptotic results in
A549 cell, blank plasmid and TTF-1 groups. The apoptotic rates were
1.622±0.286, 2.522±0.703 and 24.122±3.198 in control, blank plasmid
and TTF-1 groups, respectively. The difference was significant
(P<0.05).
 | Table IIApoptotic rate of TTF-1 transient
transfection compared with control groups in vitro. |
Table II
Apoptotic rate of TTF-1 transient
transfection compared with control groups in vitro.
| | Apoptotic rate
(%) |
|---|
| |
|
|---|
| Cell | N | Control | Blank plasmid | TTF-1 |
|---|
| A549 | 9 | 1.622±0.286 | 2.522±0.703 | 24.122±3.198a |
Clinicopathological data of Xuanwei lung
adenocarcinomas and correlation with TTF-1
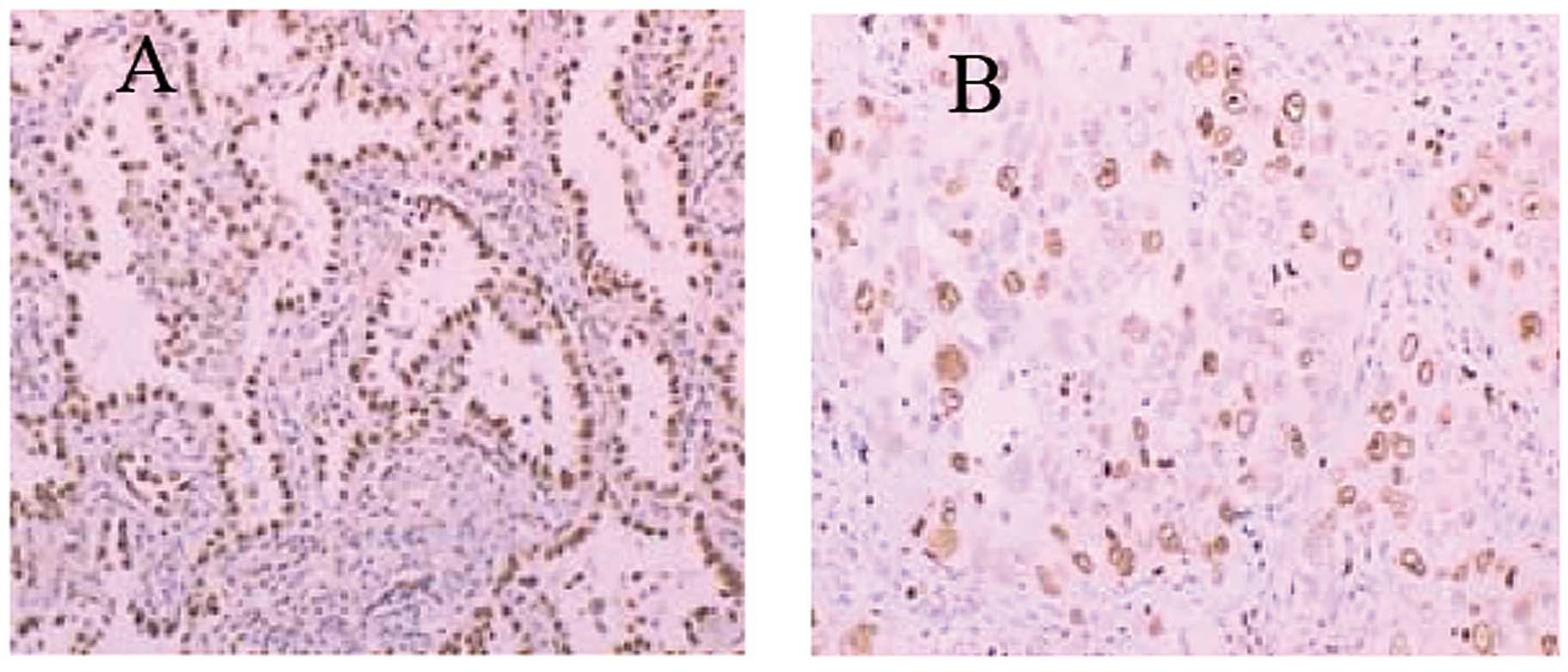
Patient profiles are shown in Table III and Fig. 5A. Sixty-two Xuanwei lung
adenocarcinomas, based on the WHO classification (1999), consisted
of 23 men and 39 women, with median ages of 52.2 and 50.1 years,
respectively. According to the AJCC staging system, 62 patients
were classified as stage I–III (43 in stage I–II and 19 in stage
III). The patients were graded as 29 cases of grade 1 (well
differentiated), 33 of grade 2 (moderately and poorly
differentiated).
 | Table IIICorrelations between TTF-1 expression
and clinicopathological features in Xuanwei lung
adenocarcinomas. |
Table III
Correlations between TTF-1 expression
and clinicopathological features in Xuanwei lung
adenocarcinomas.
| | TTF-1
immunoreactivity | |
|---|
| |
| |
|---|
| Clinicopathological
features | No. of cases | − | + | ++ | +++ | P-value |
|---|
| Gender |
| Male | 23 | 2 | 4 | 8 | 9 | 0.829 |
| Female | 39 | 2 | 9 | 11 | 17 | |
| Age (years) |
| <60 | 54 | 1 | 12 | 17 | 24 | 0.073 |
| ≥60 | 8 | 3 | 1 | 2 | 2 | |
| Smoking
history |
| Smokers | 22 | 2 | 4 | 5 | 11 | 0.579 |
| Non-smokers | 40 | 2 | 9 | 14 | 15 | |
|
Differentiation |
| Well | 29 | 0 | 3 | 10 | 16 | 0.006 |
|
Moderately/poorly | 33 | 4 | 10 | 9 | 10 | |
| Stage |
| Early stage (I and
II) | 43 | 2 | 9 | 12 | 20 | 0.307 |
| Locally advanced
stage (III) | 19 | 2 | 4 | 7 | 6 | |
Clinicopathological variables such as gender, age,
smoking history and stage were not significantly associated with
TTF-1 expression, although TTF-1 expression tend to be higher in
<60 years group. However, TTF-1 was expressed significantly
higher in well differentiation than in moderately/poorly
differentiation groups (P<0.05).
Association of TTF-1 expression with
Ki-67 in Xuanwei lung adenocarcinomas
The statistical analysis for the relationships
between TTF-1 and Ki-67 is shown in Table IV and Fig. 5B. Significant inverse correlation
was found between TTF-1 expression and proliferative activity
evaluated by Ki-67 protein (P<0.05).
 | Table IVCorrelation between TTF-1 expression
and Ki-67 proliferative activity in Xuanwei lung
adenocarcinomas. |
Table IV
Correlation between TTF-1 expression
and Ki-67 proliferative activity in Xuanwei lung
adenocarcinomas.
| | TTF-1
expression | |
|---|
| |
| |
|---|
| Ki-67
immunoreactivity | No. of cases | - | + | ++ | +++ | P-value |
|---|
| Positive | 23 | 3 | 7 | 7 | 6 | 0.016 |
| Negative | 39 | 1 | 6 | 12 | 20 | |
Discussion
In the present study, we demonstrated i) TTF-1 mRNA
expression in human lung cancer cell lines had very low frequency,
ii) overexpressed TTF-1 increased apoptosis and repressed the
expression of Ki-67 in A549 cell line and iii) strong
immunohistochemical expression of TTF-1 was statistically
associated with well-differentiated phenotype and inverse
correlation with Ki-67 expression in Xuanwei lung
adenocarcinomas.
In the present study, we demonstrated very low
frequency of TTF-1 mRNA expression in human lung cancer cell lines.
TTF-1 expression was found in 30% (3/10) of NSCLC cell lines and in
25% (1/4) of AD lines. Fujita et al reported similar result
(15). They evaluated the
expressions of mRNA and protein of TTF-1 in 9 human NSCLC cell
lines by RT-PCR and immunohistochemistry, respectively. For the
mRNA expression, the positive rate was 44% (4/9) for all NSCLC and
50% (2/4) for AD. For TTF-1 protein expression, intranuclear
staining was found in only 33% (2/6) in AD. Therefore, it appears
that the TTF-1 mRNA expression in lung AD cell lines is generally
very low, suggesting gene silencing at the transcription level. It
has also been reported that the TTF-1 expression is closely related
to lung differentiation. Since cell lines usually have an increased
growth rate and lack essential antigen expressions, most
established cell lines might lose their ability to express
TTF-1.
Few studies refer to possible significance of TTF-1
expression in carcinogenesis and results have been conflicting. In
the current study, we found that overexpressed TTF-1 downregulated
the expression of Ki-67, a marker of cell proliferation, and
induced apoptosis of lung cancer cell line A549.
In thyroid tumor, there appears to be a progressive
decrease of TTF-1 nuclear staining from follicular adenoma to
well-differentiated carcinoma, then to anaplastic carcinoma,
consistent with the progressive dedifferentiation and increasing
malignancy of thyroid tumors (16)
The loss of TTF-1 and Pax8 (thyroid-specific transcription factor)
correlated with the aggressiveness of thyroid carcinoma and their
overexpression induced differentiation of anaplastic thyroid
carcinoma (16–18). TTF-1 seems able to induce
differentiation and decrease proliferation of tumor cells in
thyroid tumors.
Transcription factors involved in apoptosis have
been previously reported such as the p53 gene. Several studies have
shown that transfection with wild-type p53 alone or a combination
of wild-type p53 with exposure to amifostine, a cytoprotective
agent can directly promote cells into apoptosis and/or growth
arrest when p53 was overexpressed (19,20).
To our knowledge, direct induction of apoptosis by TTF-1
overexpression has not been reported previously. Fukazawa et
al(21) showed that the
proapoptotic gene Bcl-2-associated X protein (Bax) inserted into
the TTS (TTF-1 gene under the control of hTERT promoter and hSPA1
promoter) system (TTS/Bax) induced selectively apoptosis of
pulmonary adenocarcinoma and the role of TTF-1 was only a
tissue-specific gene to target pulmonary adenocarcinoma. Our result
showed that the apoptotic rate was obviously >2 times higher in
TTF-1 group than in empty vector groups in A549 cell line. Further
studies are necessary to demonstrate which apoptosis pathways are
affected by TTF-1 overexpression. Weir et al(22) have identified 31 recurrent focal
events, including 24 amplifications and 7 homozygous deletions in
primary lung adenocarcinomas. Among the 24 amplicon regions, the
chromosome 14q13.3 (TTF-1 gene) was the most common focal amplicon.
TTF-1 knockdown led to a decrease in colony formation in lung
adenocarcinoma lines. The discrepant results remain largely
unexplained and deserve further studies.
In human NSCLC, TTF-1 protein is predominantly
expressed in 60–90% of AD (9–11). In
the present study, TTF-1 was expressed in 58 (93%) of 62 of AD and
was higher than the previous studies. Recently, Yatabe et
al(23) reported that
TTF-1-positive adenocarcinoma differed from TTF-1-negative
adenocarcinoma.
The prevalence of female and non-smoker in
TTF-1-positive tumor was observed in their study. In our study, the
female cases are more than male cases and about 91% of the males
were tobacco smokers, whereas only one female smoked. We have a
small number of patients in the present study and large number
patients are needed in further research. In our study, the patients
with strong immunohistochemical expression of TTF-1 were
statistically associated with well-differentiated phenotype. The
results support previous findings that TTF-1 controlled
differentiation of tumor and limited metastatic potential in a
mouse model. Winslow et al(24) demonstrated that TTF-1-negativity was
pathognomonic of high-grade poorly differentiated tumors and TTF-1
expression was low/absent in almost all lymph node and distant
macrometastases in their mouse model. In addition, our results
showed that patients with strong immunohistochemical expression of
TTF-1 were inversely correlated with Ki-67 expression in Xuanwei
lung adenocarcinomas, consistent with previous results. Pelosi
et al and Myong (10,13)
reported that TTF-1-positive expression group was inversely
correlated with Ki-67 and was correlated with better survival
compared to TTF-1-negative patients.
In conclusion, we have demonstrated a suppressive
effect of TTF-1 in NSCLC cell line. Overexpressed TTF-1
downregulates Ki-67 and induces apoptosis. Strong TTF-1
immunohistochemical expression is statistically associated with
well-differentiated phenotype and inverse correlation with Ki-67 in
Xuanwei lung adenocarcinomas. Thus TTF-1 might have antitumor
effects.
Acknowledgements
This study was supported by Scientific Research Fund
of Yunnan Provincial Education Department (Grant no. 08CO102) and
Yunnan Department of Natural Science and Kunming Medical University
Co-funding (Grant no. 2011FB227).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Yao H, Zhang Z, Xiao Z, et al:
Identification of metastasis associated proteins in human lung
squamous carcinoma using two-dimensional difference gel
electrophoresis and laser capture microdissection. Lung Cancer.
65:41–48. 2009. View Article : Google Scholar
|
|
3
|
Hanagiri T, Baba T, So T, et al: Time
trends of surgical outcome in patients with non-small cell lung
cancer. J Thorac Oncol. 5:825–829. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim KY, Ahn JH and Cheon HG: Apoptotic
action of peroxisome proliferator-activated receptor-γ activation
in human non-small cell lung cancer is mediated via proline
oxidase-induced reactive oxygen species formation. Mol Pharmacol.
72:674–685. 2007.
|
|
5
|
Lazzaro D, Price M, de Felice M and Di
Lauro R: The transcription factor TTF-1 is expressed at the onset
of thyroid and lung morphogenesis and in restricted regions of
foetal brain. Development. 113:1093–1104. 1991.PubMed/NCBI
|
|
6
|
Ikeda K, Clark JC, Shaw-White JR, Stahlman
MT, Boutell CJ and Whitset JA: Gene structure and expression of
human thyroid transcription factor-1 in respiratory epithelial
cells. J Biol Chem. 270:8108–8114. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maeda Y, Hunter TC, Loudy DE, Davé V,
Schreiber V and Whitsitt JA: PARP-2 interacts with TTF-1 and
regulates expression of surfactant protein-B. J Biol Chem.
28:9600–9606. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou H, Morotti RA, Profitt SA, Langston
C, Wert SE, Whitsett JA and Greco MA: Expression of thyroid
transcription factor-1, surfactant proteins, type I cell-associated
antigen, and Clara cell secretory protein in pulmonary hypoplasia.
Pediatr Dev Pathol. 4:364–371. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zamecnik J and Kodet R: Value of thyroid
transcription factor-1 and surfactant apoprotein A in the
differential diagnosis of pulmonary carcinomas: a study of 109
cases. Virchows Arch. 440:353–361. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pelosi G, Fraggeta F, Pasini F, et al:
Immunoreactivity for thyroid transcription factor-1 in stage I
non-small carcinomas of the lung. Am J Surg Pathol. 25:363–372.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kaufmann O and Dietel M: Thyroid
transcription factor-1 is the superior immunohistochemical marker
for pulmonary adenocarcinomas and large cell carcinomas compared to
surfactant proteins A and B. Histopathology. 36:8–16. 2000.
View Article : Google Scholar
|
|
12
|
Berghmans T, Paesmans M, Mascaux C, et al:
Thyroid transcription factor 1 - a new prognostic factor in lung
cancer: a meta-analysis. Ann Oncol. 17:1673–1676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Myong NH: Thyroid transcription factor-1
(TTF-1) expression in human lung carcinomas: its prognostic
implication and relationship with expressions of p53 and Ki-67
proteins. J Korean Med Sci. 18:494–500. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods.
25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujita J, Ohtsuki Y, Bandoh S, et al:
Expression of thyroid transcription factor-1 in 16 human lung
cancer cell lines. Lung Cancer. 39:31–36. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang P, Zuo H, Nakamura Y, Nakamura M,
Wakasa T and Kakudo K: Immunohistochemical analysis of
thyroid-specific transcription factors in thyroid tumors. Pathol
Int. 56:240–245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van der Kallen CJ, Spierings DC, Thijssen
JH, Blankenstein MA and de Bruin TW: Disrupted co-ordination of
Pax8 and thyroid transcription factor-1 gene expression in a
dedifferentiated rat thyroid tumor cell line derived from FRTL-5. J
Endocrinol. 150:377–382. 1996.PubMed/NCBI
|
|
18
|
Puppin C, D'Elia AV, Pellizzari L, et al:
Thyroid-specific transcription factors control Hex promoter
activity. Nucleic Acids Res. 31:1845–1852. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ndoye A, Merlin JL, Leroux A, et al:
Enhanced gene transfer and cell death following p53 gene transfer
using photochemical internalisation of glucosylated PEI-DNA
complexes. J Gene Med. 6:884–894. 2004. View Article : Google Scholar
|
|
20
|
Pataer A, Fanale MA, Roth JA, Swisher SG
and Hunt KK: Induction of apoptosis in human lung cancer cells
following treatment with amifostine and an adenoviral vector
containing wild-type p53. Cancer Gene Ther. 13:806–814. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fukazawa T, Maeda Y, Durbin ML, et al:
Pulmonary adenocarcinoma-targeted gene therapy by a cancer- and
tissue-specific promoter system. Mol Cancer Ther. 6:244–252. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weir BA, Woo MS, Getz G, et al:
Characterizing the cancer genome in lung adenocarcinoma. Nature.
450:893–898. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yatabe Y, Mitsudomi T and Takahashi T:
TTF-1 expression in pulmonary adenocarcinomas. American J Surg
Pathol. 26:767–773. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Winslow MM, Dayton TL, Verhaak RG, et al:
Suppression of lung adenocarcinoma progression by Nkx2-1. Nature.
473:101–104. 2011. View Article : Google Scholar : PubMed/NCBI
|