Introduction
Malignant mesothelioma (MM) from the serosal
membranes of the body cavities, is a particularly aggressive cancer
which is characterised by rapid progression, late metastases, and
poor prognosis (1). Although
surgery, radiotherapy, chemotherapy, and/or their combinations have
been used as therapeutic modalities, median patient survival is
8–18 months (2). MM cells exhibit
resistance to many chemotherapeutic agents, including doxorubicin
and cisplatin, which are nevertheless widely used to treat MM
(3). A recent report of a phase III
study showed that the combination of pemetrexed and cisplatin is
more effective than cisplatin alone with differences in response
rate of 41.3 versus 16.3% (4).
However, most of the patients relapsed within a year after starting
the treatment. Therefore, new therapeutic approaches are urgently
needed for MM patients. In addition to conventional chemotherapy,
there have been many advances in targeted therapies for several
cancers, such as epidermal growth factor receptor (5). The Src family of kinases (SFK), which
is a family of intracellular non-receptor tyrosine kinases, is one
candidate molecule that could hold promise in the treatment of
cancer patients, including MM (6).
SFK constitutes a family of 11 non-receptor tyrosine
kinases; Src, Fyn, Yes, Blk, Yrk, Frk, Fgr, Hck, Lck, Lyn and Rgr
that share similar structural and biochemical properties (7). Of the members, c-Src, Fyn, and Yes are
widely expressed in tissues and appear to play an important role in
the regulation of cell adhesion, cell growth, and differentiation
(8). The activated forms of SFK,
particularly c-Src, are capable of transforming many different cell
types (9), and the activation or
overexpression of human SFK has been observed in a range of human
cancers (10). A member of SFK, Yes
is the cellular counterpart of the viral v-Yes protein encoded by
the Yamaguchi avian sarcoma virus (11). Amongst SFK, Yes exhibits the highest
homology with 70% identity outside the N-terminus with c-Src. In
v-Yes a C-terminal truncation, as in v-Src, allows the kinase to be
constitutively active and highly oncogenic due to the removal of
the negative regulatory Tyr. Such an activating mechanism has not
been reported in human cancer, however, Yes is found frequently
activated in colorectal cancer (CRC). Nonetheless, Yes activation
in CRC correlates more closely with poor prognosis than does c-Src
activation (12,13). It was clearly demonstrated that Yes
regulates specific oncogenic signaling pathways important for CRC
progression that is not shared with c-Src (13). In our preliminary experiment, we
observed that some MM cells showed overexpression of Yes compared
to c-Src. Based on this observation, we hypothesized that Yes also
played an important role in the appearance of malignancy in MM. In
this context, the present study was undertaken to confirm this
hypothesis.
Materials and methods
Reagents
All culture reagents were purchased from Invitrogen
(Carlsbad, CA, USA). VBL was obtained from Wako Pure Chemicals
(Osaka, Japan). Non-specific (NS) small interfering RNA (siRNA), HP
validated siRNAs for c-Src (cat no. SI02664151), Yes (cat no.
SI00302218), and Fyn (cat no. SI00605451) and HiPerfect
transfection reagent were obtained from Qiagen Japan (Tokyo,
Japan). PCR primers were also purchased from Qiagen. Other
chemicals were purchased from Sigma (St. Louis, MO, USA), unless
otherwise noted. All antibodies were purchased from Cell Signaling
Technology (Danvers, MA, USA).
Cell culture
Human non-malignant transformed mesothelial cell
(Met5A) and MM cells (H28, H2052, H2452 and MSTO-211H) obtained
from ATCC (Manassas, VA, USA), were routinely maintained in
RPMI-1620 medium supplemented with 10% fetal bovine serum and
penicillin-streptomycin at 37˚C in an atmosphere of 5%
CO2.
Cell growth analysis
The cells were cultured on microtiter plates
(3×104 cells/well) and treated with siRNA treatment as
described in Transfection of short interfering RNA (siRNA).
Cell viability was then determined using the Cell Proliferation
Assay kit with WST-1 reagent (Sigma), according to the
manufacturer’s instructions.
Cell cycle and apoptosis analysis
After the siRNA treatment the cells were harvested
by trypsinization, washed with PBS, re-suspended in 70% ethanol in
PBS, and kept at 4˚C for ≤30 min. Before analysis, cells were
washed again with PBS and resuspended and incubated for 30 min in
PBS containing 0.05 mg/ml propidium iodide, 1 mM EDTA, 0.1% Triton
X-100, and 1 mg/ml RNase A. The suspension was then passed through
a nylon mesh filter, and the ratio of each fraction in cell cycle
was analyzed on a Becton-Dickinson FACScan (Franklin Lakes, NJ,
USA), and the ratio of subG1 population was estimated to confirm
the induction of apoptosis.
Transfection of short interfering RNA
(siRNA)
Each molecule was downregulated by short interfering
RNAs (siRNAs) targeting each molecule. For transfection, the cells
were seeded in each plate and transfected with HiPerfect
transfection reagent according to the manufacturer’s instructions.
Then the cells were treated with the siRNA for 48 h, and
subsequently, knockdown of each by siRNA was confirmed by
RT-real-time PCR. As a negative control, NSsiRNA was used. Also,
after the siRNA treatment for 48 h, WST-1 and immunoblot analysis
were performed.
Gene expression analysis
Total RNA was isolated by using SV Total RNA
Isolation System (Promega, Madison, WI, USA) and cDNA was
synthesized as previously described (14). Real-time PCR was performed by using
an ABI PRISM 7000 Sequence Detection System (Applied Biosystems
Japan Ltd. Tokyo, Japan) and SYBR Premix Ex Taq™ (Takara Bio Inc.,
Shiga, Japan) according to the manufacturer’s instructions. The
primers used were from Qiagen, and each product number was as
follows: ribosomal protein CL32 (PRL32), QT01668198; c-Src,
QT00039326; Yes, QT00037940; Fyn, QT00054005.
Immunoblot analysis
Immunoblot analysis was performed as previously
described (14). Briefly, cell
lysate was prepared in Cell Lysis/Extraction Reagent (Sigma)
including phosphatase inhibitor cocktail 1, phosphatase inhibitor
cocktail 2, and protease inhibitor cocktail, and 10 μg total
protein extract from each sample was loaded onto a 10%
SDS-polyacrylamide gel. After electrophoresis, proteins were
transferred to nitrocellulose membranes. The blots were incubated
with each antibody. Each immunoreactive band was detected using the
ECL system (Amersham) and a cooled CCD camera-linked Cool Saver
System (Atto, Osaka Japan). Molecular sizing was done using Rainbow
MW marker (Amersham). Protein concentrations were determined using
DC Protein Assay System (Bio-Rad, Hercules, CA, USA). Also,
membrane/cytoplasm separations were done using Subcellular Protein
Fractionation kit according to the manufacturer’s instructions
(Pierce, ThermoScientific, Tokyo, Japan).
Statistical analysis
Data were analyzed by one-way ANOVA followed by
Student’s t-test or Dunnett’s multiple-range test. P<0.05 was
considered significant.
Results
Expression patterns of SFK in MM cell
lines
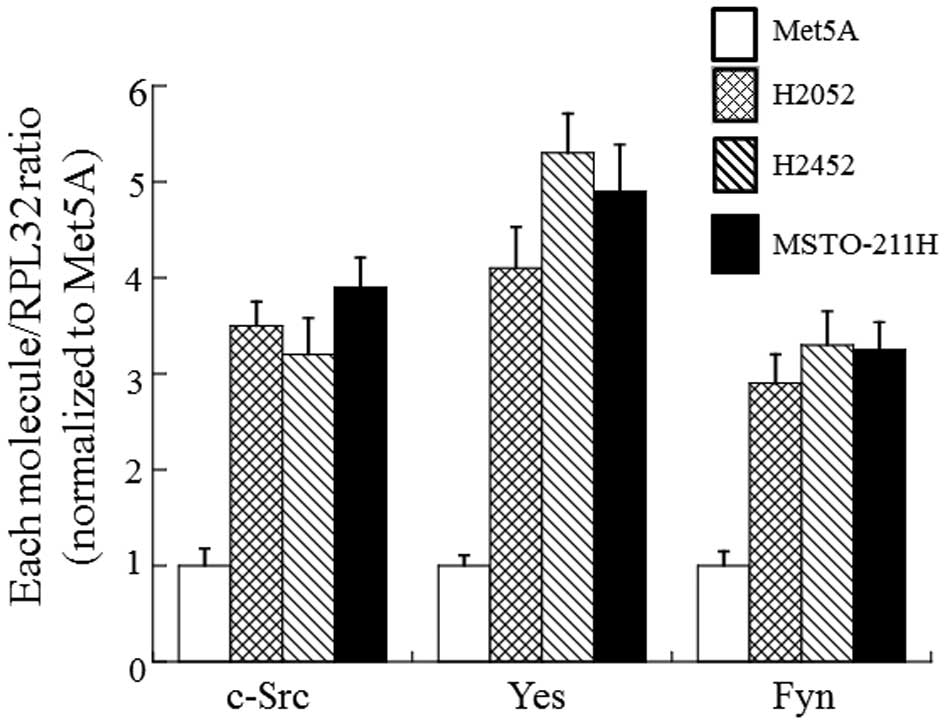
Similarly to other tumors, SFK was commonly
activated in MM cells and primary MM specimens (15). In order to determine which molecule
of SFK is expressed in MM cells, we compared expression patterns of
SFK in non-tumorigenic mesothelial cells (Met5A) and three
different types of MM cells (H2052, H2452 and MSTO-211H). As shown
in Fig. 1, at least three different
members of SFK, c-Src, Yes and Fyn were expressed in all cell lines
tested. Compared to Met5A cells, the three MM cells showed
significantly higher expression levels in the three members of SFK.
Of these members of SFK, the level of Yes was the highest. Besides
c-Src, Yes, and Fyn, another SFK member, Lyn, was also detected,
but the level was lower than other molecules (data not shown).
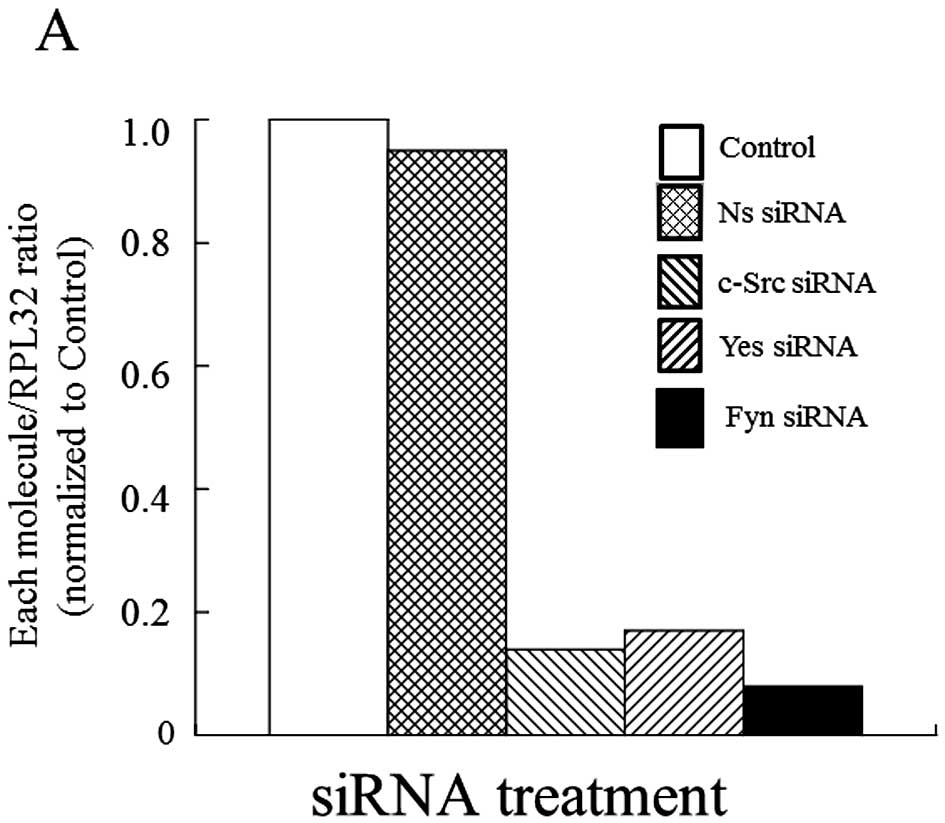
Contribution of Yes to cell growth in MM
cells
Recent reports showed that SFK members played
different roles in the appearance of malignant phenotypes on tumor
cells (9,10), so we estimated which molecule of the
three SFKs examined could contribute to cell growth in MM cells. As
shown in Fig. 2, only knock down of
Yes by siRNA significantly reduced cell growth (-42%) in H2452
cells under almost the same silencing condition of the SFK members.
Also, we observed the same effect on cell growth in H2052 and
MSTO-211H cells (data not shown). These results suggest that Yes
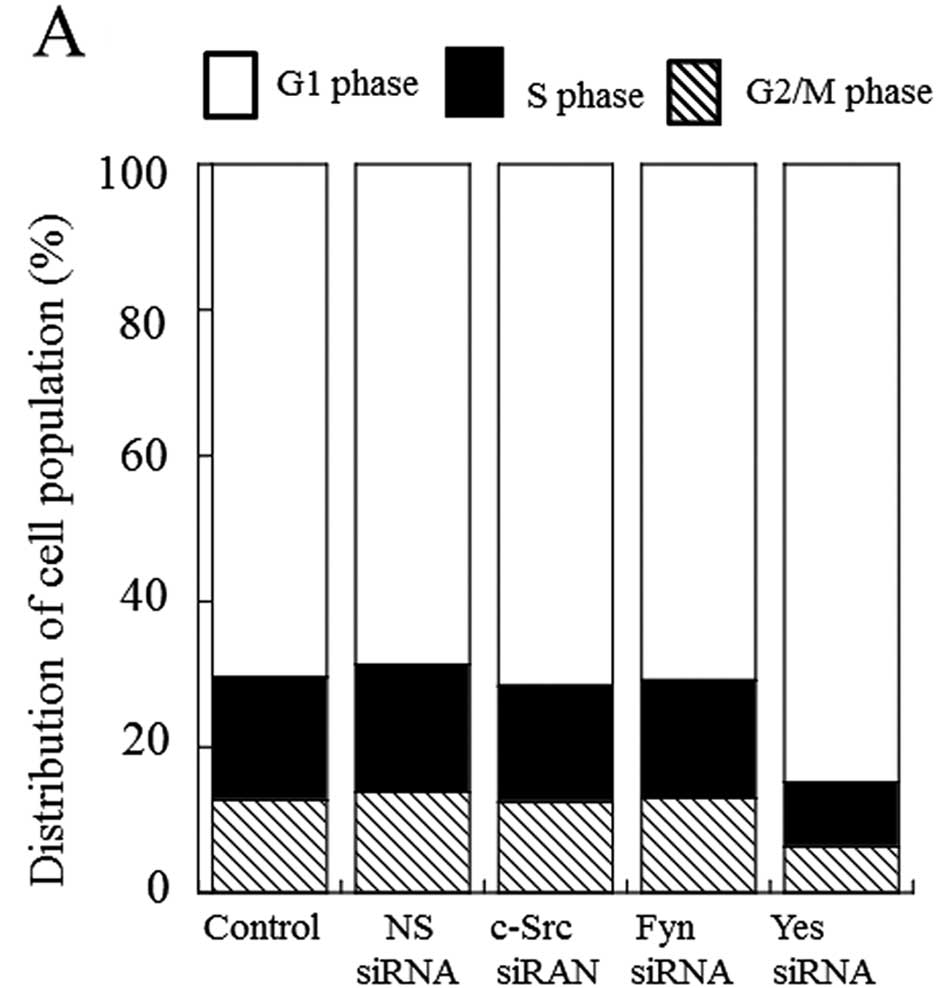
plays an important role in cell growth control of MM cells. With
respect to cell cycle regulation under knockdown of Yes, G1 arrest
was induced in H2452 cells (Fig.
3A). The silencing of Yes induced by siRNA significantly
increased SubG1 population in H2452 cells by ~45% (Fig. 3B). H2052 and MSTO-211H cells showed
similar results (data not shown). Overall, it seems that the
knockdown of Yes-mediated cell growth control mainly depends on G1
arrest in the cell cycle.
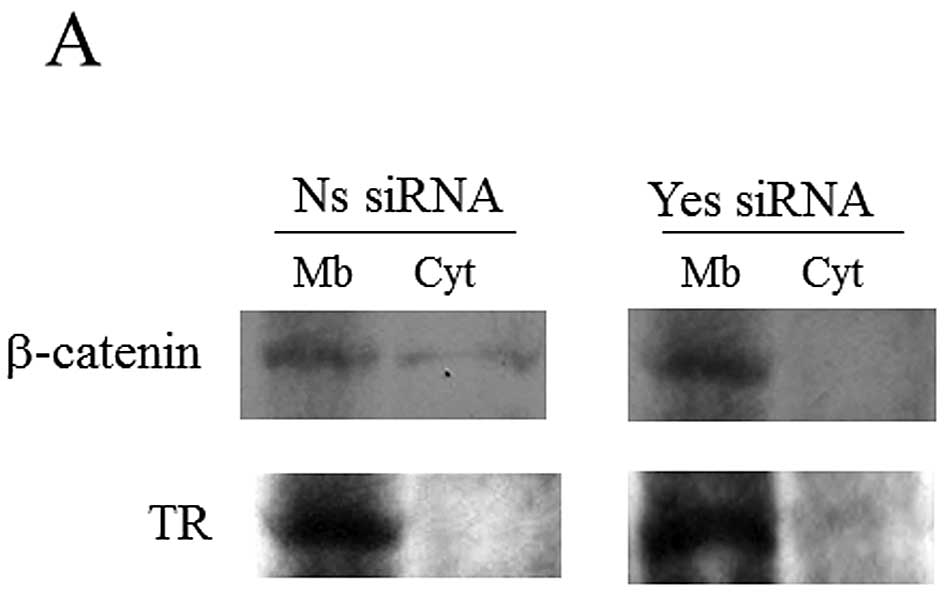
Effect of Yes knockdown on β-catenin
localization and signaling
In a colon carcinoma cell study (16), Yes knockdown induced β-catenin
accumulation in membrane and induced the inactivation of β-catenin
signaling. Thus, we next determined whether Yes silencing could
affect β-catenin localization and signaling in H2452 cells. As
shown in Fig. 4A, biochemical
analysis of β-catenin cytosolic and membrane fractions showed that
Yes knockdown restored the localization of the catenin to the
membrane fraction. The knockdown of Yes reduced the level of cyclin
D, which is necessary for transition of G1 to S phase in cell cycle
and a target molecule of β-catenin signaling (Fig. 4B) (17). Furthermore, a reduction in EphB3 (a
target molecule of β-catenin signaling) mRNA level was observed
upon Yes depletion (data not shown). These results suggest that Yes
knockdown affects β-catenin localization and signaling in H2452
cells. Also, we confirmed similar results in H2052 and MSTO-211H
cells (data not shown). Finally, in order to confirm this effect of
Yes depletion, we estimated if the knockdown of Yes could influence
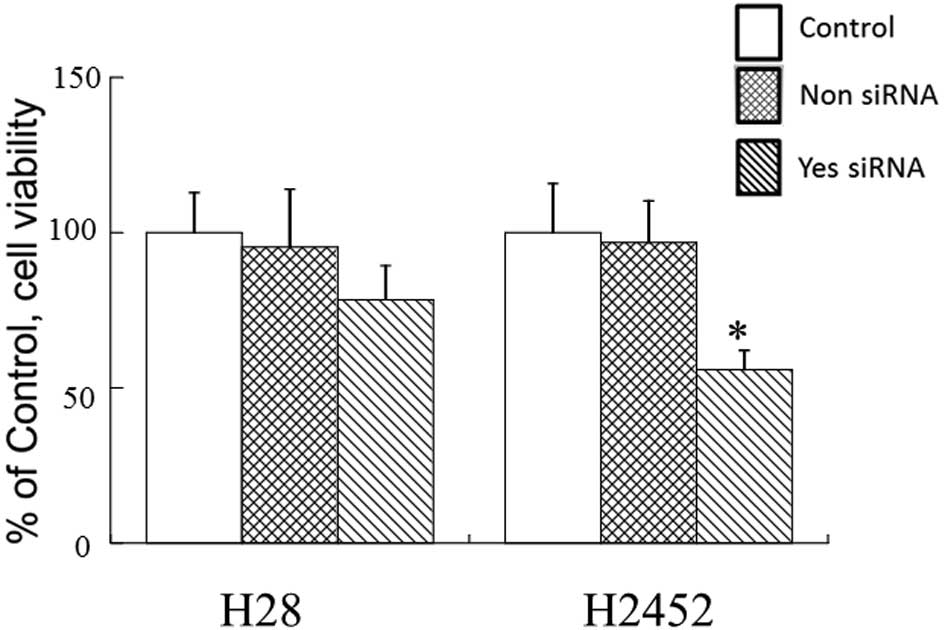
cell growth in H28 cells which are deficient in β-catenin (18). As a result, Yes silencing had less
effect on cell growth in H28 cells compared to H2452 cells which
expressed β-catenin (Fig. 5). We
confirmed that the knockdown level was almost the same between the
two cell types (data not shown). These observations completely
support the above speculation.
Discussion
MM is an aggressive malignancy, the incidence of
which is expected to increase due to its association with asbestos
exposure. A number of chemotherapeutic agents have been used,
either alone or in combination, to treat MM with the latter
multi-agent regimen generally having the highest response rates
(19). Nonetheless, despite the
current therapies, the prognosis for many MM patients is very poor.
Several signal molecules related to growth and survival are
constitutively activated in MM cells (20) and simultaneous suppression of
multi-target molecules is required for an effective therapeutic
agent against MM. In a recent study, it was found that SFK is a
promising molecular target to perform an effective treatment in MM
(21). However, at present, which
member of SFK is absolutely required for effective MM treatment is
unresolved. The aim of the present study was to address this
issue.
It has been demonstrated that, of members in SFK,
c-Src, Yes and Fyn were constantly activated in MM, through
phospho-protein proteomic screen analysis (22). Actually, we observed that
overexpression of three subtypes of SFK occurred in two
histologically different types of MM cells compared to
non-tumorigenic mesothelial cells. In a previous study, it has been
reported that the contribution of some SFK members to oncogenic
activity in each tissue is redundant (16). In order to clearly address this
issue in MM, we utilized siRNA knockout technology. As a result,
only Yes silencing was found to be associated with suppression of
cell growth in MM cells, indicating that Yes is a central mediator
of cell growth in MM cells.
In other studies, inhibition of SFK activation by a
specific inhibitor suppresses cell growth of most of the examined
MM cell lines, mainly due to G1 arrest in cell cycle (15). Reinforcing this, we have obtained
similar results in our study (23).
Similarly, our present study showed that the silencing of Yes
contributed to G1 arrest in the cell cycle. These results suggest
that, of SFK members, Yes is the main molecule to drive cell cycle
progression in MM cells. With respect to a mechanism on
Yes-mediated cell growth in MM cells, we can speculate that Yes
stimulates cell growth via the activation of β-catenin signaling
(14). In that study it was clearly
demonstrated that the localization of β-catenin is changed from
cytoplasm and nucleus to cell membrane by the knockdown of Yes in
colon carcinoma cells and that the alteration of the localization
is closely associated with loss of several malignant phenotypes
such as invasion in the carcinoma cells. It is well known that
β-catenin localized in the nucleus acts as a transactivator
targeting for genes stimulating cell growth, that is, nuclear
β-catenin forms a complex with the transcription factor TCF and
induces the expression of downstream target genes including c-myc
and cyclin D1, together with other transcriptional co-factors, such
as CREB binding protein (CBP) (24). Of the target genes, cyclin D1 is a
positive regulator of the cell cycle and promotes G1 to S phase
transition in cell cycle (17).
Amplification of the gene encoding cyclin D1 and overexpression of
cyclin D1 protein have frequently been found in several types of
human malignant neoplasms (25). In
this study, we observed that the silencing of Yes caused G1 arrest
in the cell cycle, possibly due to the reduction of cyclin D level.
Since we also observed that Yes silencing induced a reduction in
EphB3 (a target molecule of β-catenin signaling) mRNA level, the
decrease of cyclin D level might partly depend on the inactivation
of β-catenin signaling by Yes siRNA treatment. This speculation can
be completely supported by the present data in which Yes knockdown
has less effect on cell growth in H28 cells, being deficient of
β-catenin signaling, than on H2452 cells in which β-catenin
signaling is present.
The reason why Yes has a specific effect on cell
growth in MM cells is still unclear at present. As a possible
mechanism, it has been proposed that specific subcellular
localization of SFK family members leads to phosphorylation of
specific substrates and subsequent outcome of specific cellular
events. Actually, a recent report has shown that the difference of
localization among SFK family members regulates SFK signaling
specificity leading to, for example, mitogenesis or neoplastic
transformation (26). Also the
possibility of interaction between substrates and the unique SH3 or
SH2 domains of these SFK may give rise to an additional mechanism
for selective signaling. Similarly it was demonstrated in a
previous study with colon cancer that one mechanism by which Yes
regulates its oncogenic activity is by modulation of β-catenin
subcellular localization counteracting its nuclear transcriptional
activity, where this cellular process was regulated by tyrosine
phosphorylation (16). In order to
further clarify the specific transforming activities of Yes,
additional signaling pathways regulated by Yes should be
elucidated. Finally, this determination may lead to establishment
of a new effective treatment for MM.
Acknowledgements
This study was supported by a research grant for
Health Sciences Focusing on Drug Innovation from the Japan Health
Sciences Foundation (KHC1023).
Abbreviations:
|
CRC
|
colorectal cancer
|
|
MM
|
malignant mesothelioma
|
|
RT-real-time PCR
|
reverse transcription-real-time
polymerase chain reaction
|
|
siRNAs
|
short interfering RNAs
|
|
SFK
|
the Src family of kinases
|
References
|
1
|
Carbone M, Kratzke RA and Testa JR: The
pathogenesis of mesothelioma. Semin Oncol. 29:2–17. 2002.
View Article : Google Scholar
|
|
2
|
Nowak AK, Lake RA, Kindler HL and Robinson
BW: New approaches for mesothelioma: biologics, vaccines, gene
therapy, and other novel agents. Semin Oncol. 29:82–96. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tomek S, Emri S, Krejcy K and Manegold C:
Chemotherapy for malignant pleural mesothelioma: past results and
recent developments. Br J Cancer. 88:167–174. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vogelzang NJ, Rusthoven JJ, Symanowski J,
et al: Phase III study of pemetrexed in combination with cisplatin
versus cisplatin alone in patients with malignant pleural
mesothelioma. J Clin Oncol. 21:2636–2644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takeuchi K and Ito F: Receptor tyrosine
kinases and targeted cancer therapeutics. Biol Pharm Bull.
34:1774–1780. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Benati D and Baldari CT: SRC family
kinases as potential therapeutic targets for malignancies and
immunological disorders. Curr Med Chem. 15:1154–1165. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sen B and Johnson FM: Regulation of Src
family kinases in human cancers. J Signal Transduct. 2011:1–14.
2011. View Article : Google Scholar
|
|
8
|
Thomas SM and Brugge JS: Cellular
functions regulated by Src family kinases. Annu Rev Cell Dev Biol.
13:513–609. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ishizawar R and Parsons SJ: C-Src and
cooperating partners in human cancer. Cancer Cell. 6:209–214. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abram CL and Courteidge: Src family
tyrosine kinases and growth factor signaling. Exp Cell Res.
254:1–13. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roche S and Courtneidge SA: v-Yes as a
transforming factor. Oncogenic Cytoplasmic Tyrosine Kinases. Peters
G and Vousden KH: Oxford University Press; Oxford; pp. 87–120.
1997
|
|
12
|
Pena SV, Melhem MF, Meisler AI and
Cartwright CA: Elevated c-Yes tyrosine kinase activity in
premalignant lesions of the colon. Gastroenterology. 108:117–124.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han NM, Curley SA and Gallick GE:
Differential activation of pp60(c-src) and pp62(c-yes) in human
colorectal carcinoma liver metastases. Clin Cancer Res.
2:1397–1404. 1996.PubMed/NCBI
|
|
14
|
Kashiwagi K, Harada K, Yano Y, et al: A
redox-silent analogue of tocotrienol inhibits hypoxia adaptation of
lung cancer cells. Biochem Biophys Res Commun. 365:875–881. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsao AS, He D, Saigal B, et al: Inhibition
of c-Src expression and activation in malignant pleural
mesothelioma tissues leads to apoptosis, cell cycle arrest, and
decreased migration and invasion. Mol Cancer Ther. 6:1962–1972.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sancier F, Dumont A, Sirvent A, et al:
Specific oncogenic activity of the Src-family tyrosine kinase c-Yes
in colon carcinoma cells. Plos One. 6:1–10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kato-Stankiewicz J, Hakimi I, Zhi G, et
al: Inhibitors of Ras/Raf-1 interaction identified by two hybrid
screening revert Ras-dependent transformation phenotypes in human
cnacer cells. Proc Natl Acad Sci USA. 99:14398–14403. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim YM, Ma H, Oehler VG, et al: The gamma
catenin/CBP complex maintains survivin transcription in β-catenin
deficient/depleted cancer cells. Curr Cancer Drug Targets.
11:213–225. 2011.PubMed/NCBI
|
|
19
|
Vogelzang NJ, Rusthoven JJ, Symanowski J,
et al: Phase III study of pemetrexed in combination with cisplatin
versus cisplatin alone in patients with malignant pleural
mesothelioma. J Clin Oncol. 15:493–500. 2003.PubMed/NCBI
|
|
20
|
Kao SC, Lee K, Armstrong NJ, et al:
Validation of tissue microarray technology in malignant pleural
mesothelioma. Pathology. 43:128–132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Johnson FM, Saigal B, Tran H and Donato
NJ: Abrogation of signal transducer and activator of transcription
3 reactivation after Src kinase inhibition results in synergistic
antitumor effects. Clin Cancer Res. 13:4233–4244. 2007. View Article : Google Scholar
|
|
22
|
Menges CW, Chen Y, Mossman BT, et al: A
phosphotyrosine proteomic screen identifies multiple tyrosine
kinase signaling pathways aberrantly activated in malignant
mesothelioma. Genes Cancer. 1:493–505. 2010. View Article : Google Scholar
|
|
23
|
Kashiwagi K, Virgona N, Harada K, et al: A
redox-silent analogue of tocotrienol acts as a potential cytotoxic
agent against human mesothelioma cells. Life Sci. 84:650–656. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cadigan KM: TCFs and Wnt/β-catenin
signaling: more than one way to throw the switch. Curr Top Dev
Biol. 98:1–34. 2012.
|
|
25
|
Saini SS and Klein MA: Targeting cyclin D1
in non-small cell lung cancer and mesothelioma cells by antisense
oligonucleotides. Anticancer Res. 31:3683–3690. 2011.PubMed/NCBI
|
|
26
|
Oneyama C, Ichino T, Saito K, et al:
Transforming potential of Src family kinases is limited by the
cholesterol-enriched membrane microdomain. Mol Cell Biol.
29:6462–6472. 2009. View Article : Google Scholar : PubMed/NCBI
|