Introduction
Human colon cancer is one of the major causes of
morbidity and mortality worldwide. Its tumorigenic mechanism is a
multi-step process related to the genetic instability associated
with genetic alterations in the invariably less well-oxygenated
tumor state (1–6). The unstable hypoxic microenvironment
induces the expression of hypoxia inducible factor-1 (HIF-1), a key
transcriptional regulator which plays a central role in the
regulation of biological processes, including glucose metabolism,
cell proliferation, angiogenesis, migration, and survival (7–13).
HIF-1α activity is dependent on the localization of HIF-1α protein
in the nucleus (14). Upregulation
of the HIF pathway has been shown in aggressive phenotypes of
colorectal cancer (CRC) (15–18).
Endothelial cell-specific molecule-1 (ESM-1), also
known as endocan, is a 50-kDa secretory proteoglycan, which was
originally cloned from a human endothelial cell cDNA library by
Lassalle and collaborators (19).
The structure of ESM-1 is composed of a mature polypeptide of 165
amino acids and a single dermatan sulfate chain covalently linked
to the serine residue at position 137 (19–21).
ESM-1 expression in endothelial and epithelial cells is upregulated
by tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and vascular
epidermal growth factor (VEGF), downregulated by IL-4 and
interferon (IFN)-γ (19,22–24),
and secreted by vascular endothelial cells, epithelial cells lining
distal tubules, bronchi and lung submucosal glands (19,22,25).
In addition to ESM-1 expression in normal human tissue,
differential expression of ESM-1 has been reported in the vascular
endothelium of renal carcinoma (26), breast carcinoma (27,28),
glioblastoma (29), non-small cell
lung cancer (24), and liver cancer
(30). In colon cancer, we reported
that ESM-1 expression was increased in the tissue and serum samples
of CRC patients, and it can be used as a potential serum marker for
early detection of CRC (31). ESM-1
has previously been described to be upregulated by VEGF in
vitro and in vivo (20).
VEGF is a major target gene of HIF-1α regulatory genes (32). In addition, Maurage et al
showed that ESM-1 expression was increased under hypoxic condition
(1% O2) in a glioblastoma cell line (29). Although there are several reports
that ESM-1 overexpression was closely related to the process of
angiogenesis in the endothelial cells from tumor tissues (26,33–35),
whether ESM-1 modulation is directly regulated by HIF-1α or whether
the overexpression of these two proteins in the tissue samples of
CRC patients significantly correlate with each other has not yet
been elucidated.
In this study, we investigated the overexpression of
ESM-1 and HIF-1α in the tissue samples of 143 CRC patients using
RT-PCR and immunohistochemistry. Next, the clinicopathological
significance of ESM-1 immunoreactivity and its correlation with
poor prognosis of CRC was analyzed, and we confirmed the functional
inter-correlation of ESM-1 with HIF-1α in colon cancer cells and
tissues.
Materials and methods
Cell lines and transfection
The human colon cancer cell line, HT29, was
purchased from the American Type Culture Collection (ATCC;
Rockville, MD, USA). The cells were cultured in Dulbecco’s modified
Eagle’s medium (DMEM; Gibco-BRL, Invitrogen, Carlsbad, CA, USA)
supplemented with 2 mM glutamine, 1% penicillin/streptomycin, and
10% fetal bovine serum (FBS; Hyclon, Logan, UT, USA), and kept at
37°C in a humidified incubator, which was maintained with 5%
CO2. siRNA directed against human HIF-1α (5′-CUGAU
GACCAGCAACUUGATT-3′ and 5′-UCAAGUUGCUGGUC AUCAGTT-3′) (siGENOME
SMARTpool, catalog no. M-013858–00) was purchased from Bioneer
(Daejeon, Korea) with its control non-targeting siRNA (catalog no.
1068432), and treated according to the manufacturer’s instructions.
As an example, 12 pmol of HIF-1α siRNA was premixed with 15 μl of
Lipofectamine RNAiMAX reagent (Invitrogen) following the
manufacturer’s instructions, incubated for 20 min at room
temperature (RT), and used to treat colon cancer cells plated on a
60-mm dish with 40% confluency. The plasmid-containing wild type
HIF-1α-coding region was transfected into the HT29 cell lines using
Lipofectamine Plus reagent (Invitrogen), according to the
manufacturer’s instructions.
Antibodies and western blotting
Cells were washed with phosphate-buffered saline
(PBS) and lysed with cell lysis buffer [20 mM Tris-HCl, pH 7.5, 150
mM NaCl, 1 mM EGTA, 1 mM EDTA, 1% NP-40, 2.5 mM sodium
pyrophosphate, 1 mM Na3VO4, 1 mM NaF, and
Complete Protease Inhibitor Cocktail (Roche, Indianapolis, IN,
USA)] on ice for 30 min. SDS-PAGE was used to resolve 30–50 μg of
the lysate by using 10 or 12% gels and transferred to PVDF
membranes (Millipore, Billerica, MA, USA). The membranes were
incubated with primary antibodies followed by incubation with
peroxidase-conjugated anti-rabbit or anti-mouse IgG antibodies
(Calbiochem, EMD Chemicals Inc., San Diego, CA, USA) and ECL
reagent (Amersham Biosciences Inc., Piscataway, NJ, USA) for band
visualization. To verify equal loading and adequate transfer, the
membranes were probed with anti-γ-tubulin antibody (Santa Cruz
Biotechnology, Inc., (Santa Cruz, CA, USA). The primary antibodies
were anti-ESM-1 (Abnova, Taipei, Taiwan) and anti-HIF-1α (Novus
Biologicals, Littleton, CO, USA).
RT-PCR analysis
A 2-step RT–PCR reaction was performed using reverse
transcriptase with oligo-dT primer, and Taq polymerase
(Takara, Shiga, Japan), with specific primer pairs. Total RNA was
isolated by a standard protocol (36), and cDNA was synthesized using the
AccuScript High Fidelity First Strand cDNA synthesis kit
(Stratagene, La Jolla, CA, USA) following the manufacturer’s
instructions. One microliter of the synthesized cDNA was used per
20 μl of PCR reaction mixture, which comprised 0.2 U ExTaq DNA
polymerase, 1X buffer, and 1 mM dNTP mix (Takara), with specific
primer pairs, and amplified as follows: 1 cycle of 94°C for 5 min;
then 30 cycles of 94°C for 45 sec, 56°C for 45 sec and 72°C for 1
min; followed by a final extension of 7 min at 72°C, using the
GeneAmp PCR System 2700 (Applied Biosystems, Foster City, CA, USA).
PCR primers were designed using the Primer3 program purchased from
Bioneer. ESM-1 gene-specific primers used for PCR were
5′-GCCCTTCCTTGGTAGG TAGC-3′ (sense) and 5′-TGTTTCCTATGCCCCAGAAC-3′
(antisense). The PCR products were separated on a 1.5% agarose gel,
stained with ethidium bromide, visualized by Gel Doc 2000 UV
transilluminator (Bio-Rad Laboratories, Hercules, CA, USA), and
analyzed using Quantity One software (Bio-Rad Laboratories). Each
sample was tested more than 2 times, and the representative data
are shown. The primers used for ESM-1 real-time RT-PCR were
5′-AAGGC TGCTGATGTAGTTC-3′ (sense), 5′-GCTATTTATGGAAGT
GTATGTGTTT-3′ (antisense), gapdh; 5′-AGTCAGCCGCAT CTTCTT-3′
(sense), 5′-GCCCAATACGACCAAATCC-3′ (antisense). Optimized PCR was
carried out as follows: 1 cycle of 95°C for 10 sec; 40 cycles of
95°C for 5 sec, 60°C for 30 sec, and 95°C for 15 sec; and a final
extension at 60°C for 15 sec. The relative levels of gene
expression were normalized to GAPDH expression.
Patient samples and
immunohistochemistry
Human colorectal carcinoma samples were obtained
from patients who underwent routine surgery for colorectal cancer
at the Department of Surgery, Eulji University Hospital, between
January 2000 and June 2005. Our study protocol (Protocol No. 10–24)
was approved by our Institutional Review Board, Eulji University
Hospital. For immunohistochemical study, 143 colorectal carcinoma
tissue samples and paired normal mucosal tissue samples taken from
a site distant from the tumor lesion were fixed in 10%
neutralized-buffered formalin solution for 24 h and embedded in
paraffin wax. Serial sections (4-μm) were cut and mounted on
charged glass slides (Superfrost Plus; Fisher Scientific,
Rochester, NY, USA). IHC conditions for ESM-1 and HIF-1α were
optimized and evaluated by 2 independent pathologists. In brief,
tissue sections were microwaved twice for 10 min in citrate buffer
(pH 6.0) for antigen retrieval. The sections were then treated with
3% hydrogen peroxide in methanol to quench the endogenous
peroxidase activity, followed by incubation with 1% BSA. Mouse
monoclonal antibodies against ESM-1 (Abnova, Taipei, Taiwan) and
HIF-1α (Novus Biologicals) were used at dilutions of 1:200 and
1:50, respectively. The tissue sections were incubated with
antibody overnight at 4°C in a wet chamber. The sections were
stained using a standard EnVision-HRP kit (Dako, Glostrup, Denmark)
and developed with diaminobenzidine as a substrate. An irrelevant
mouse IgG of the same isotype or antibody dilution solution served
as a negative control.
Assessment of immunostaining and
statistical analysis
Each slide was evaluated for ESM-1 and HIF-1α
immunoreactivity by using a semi-quantitative scoring system for
both the intensity of the stain and the percentage of positive
neoplastic cells. In the colorectal and mucosal cells, ESM-1
immunoreactivity corresponded to the cytoplasm and HIF-1α to the
nuclei. The intensity of membrane staining was coded as follows: 0,
weaker than that in the adjacent normal-appearing mucosal
epithelium; 1, similar to that in the adjacent mucosal epithelium;
and 2, stronger than that in the adjacent mucosal epithelium. The
percentage of cells displaying a stronger staining intensity than
that in the adjacent mucosal epithelium was scored as either 1
(0–24% tumor cells stained), 2 (25–49% tumor cells stained), 3
(50–74% tumor cells stained), or 4 (75–100% tumor cells stained).
For the purpose of statistical analysis, the median of this series
(25% of malignant cells showing a stronger intensity than adjacent
colonic epithelium) was used as a cut-off value to distinguish
tumors with low (<25%) or high (>25%) levels of ESM-1 and
HIF-1α expression. The relationship between the results of the
immunohistochemical study and the clinicopathological parameters
was determined using the SPSS software package (version 14.0; SPSS
Inc., Chicago, IL, USA). The correlation between staining index
scores and other categorical factors was analyzed using the
Pearson’s Chi-square test of independence.
Prognostic parameter for recurrence-free
survival and overall survival
Recurrence-free survival was defined as the time
from the date of surgery to the first date of recurrence of cancer,
or death from any cause. Overall survival was defined as the time
from the date of surgery to the date of last follow-up or death
from any cause. The median follow-up period for all patients was
54.2 months (inter-quartile range, 23.2–80.7). Survival and median
survival curves were estimated using the Kaplan-Meier method. The
log-rank test was used to evaluate the statistical significance of
differences in survival distribution. Multivariate analysis was
carried out using the Cox proportional hazards regression analysis.
Results were considered statistically significant if P-values were
<0.05.
Results
ESM-1 is differentially expressed in
human CRC tissues
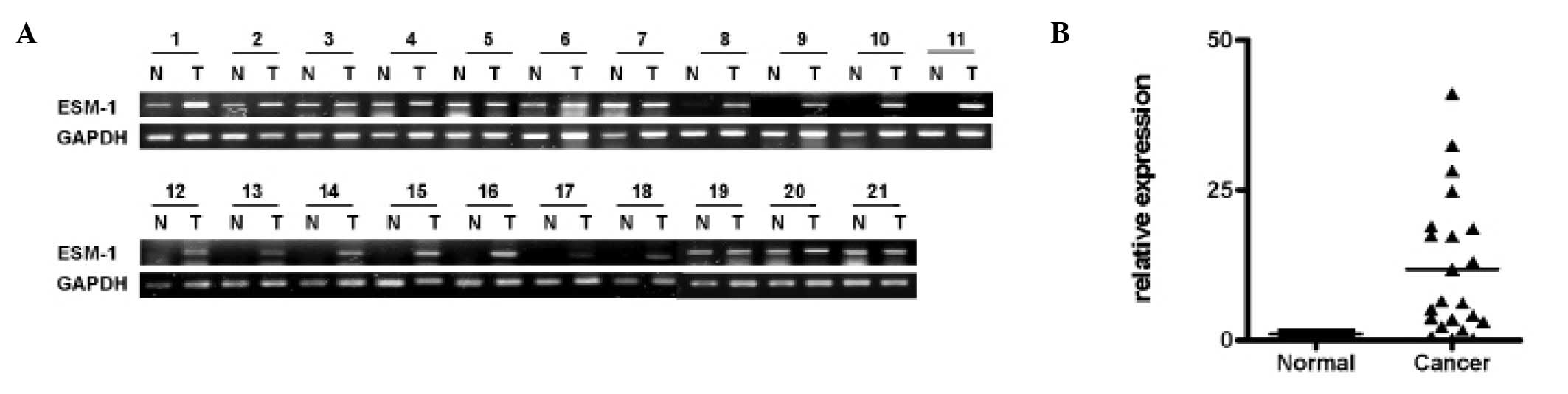
To compare the ESM-1 expression levels in the colon
cancer tissues, we examined the mRNA level of ESM-1 by performing
RT-PCR or real-time RT-PCR analysis on pairs of tissue containing
normal and tumor tissue samples from the same donor. GAPDH was used
as a reference gene to correct for the variations for mRNA in
individual samples. As shown in Fig. 1A
and B, 21 cases of colon cancer tissues, randomly selected from
clinically diagnosed patients, showed a significant increase in
ESM-1 mRNA expression compared to the normal tissue from the same
patients.
Hypoxic stress induces ESM-1 expression
via HIF-1α in CRC cells
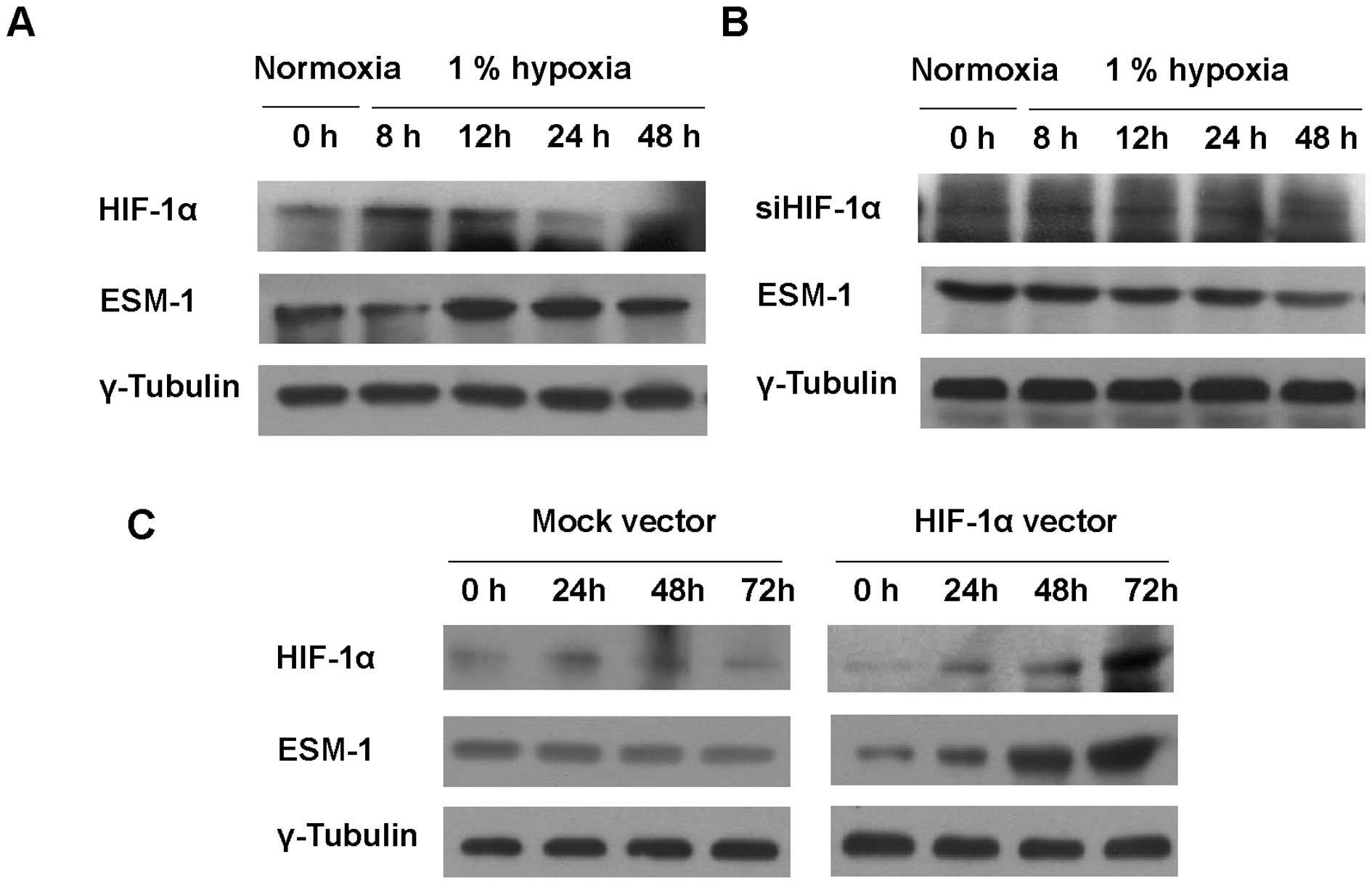
To investigate the effect of hypoxic stress on ESM-1
expression in colon cancer cells, HT29 cells were exposed to 1%
hypoxia. Under a hypoxic condition, the protein level of HIF1-α was
maximized at 8 and 12 h and was gradually decreased to reach basal
level; ESM-1 showed the same trend (Fig. 2A). To test whether ESM-1 induction
is correlated with HIF-1α, siRNA targeting HIF-1α was transfected
into HT29 cells and the cells were then incubated for indicated
times under a hypoxic condition. siRNA targeting HIF-1α attenuated
the induction of ESM-1 under a hypoxic condition (Fig. 2B). When HIF-1α was overexpressed in
order to confirm whether ESM-1 expression is led by HIF-1α, the
expression level of ESM-1 was found to be consistent with that of
HIF-1α (Fig. 2C). Taken together,
transcription factor HIF-1α, which is induced under hypoxic
conditions, induced ESM-1 expression.
Association of ESM-1 and HIF-1α
expression levels with the clinicopathological characteristics
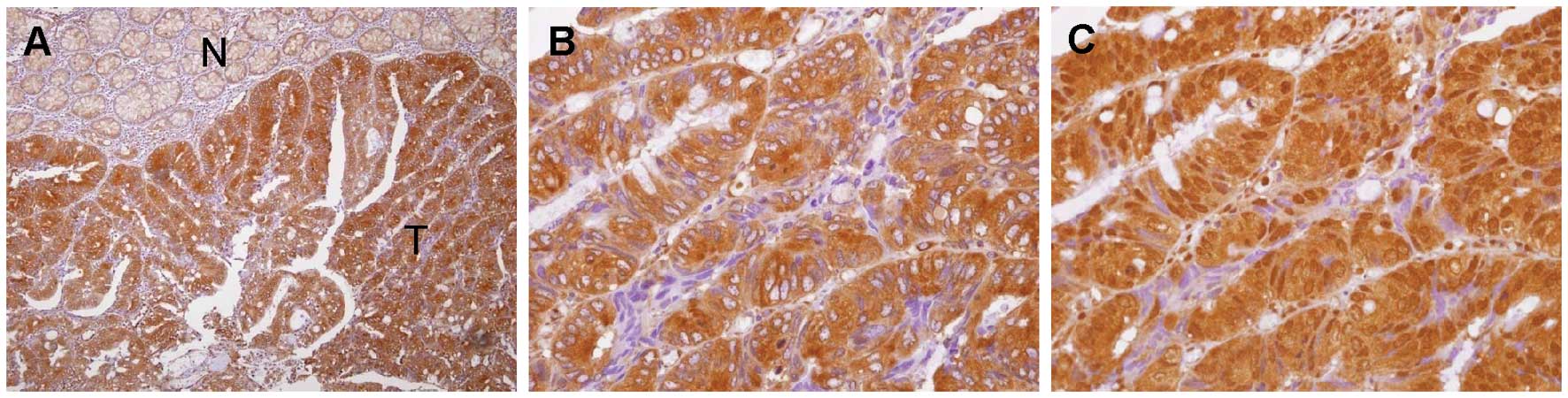
ESM-1 and HIF-1α levels were evaluated by
immunohistochemical analysis. The elevated expression of ESM-1 and
HIF-1α was detectable in 76 (53.1%) and 66 (46.1%) CRC cases,
respectively. ESM-1 immunoreactivity was found primarily in the
cytosol, but expression of ESM-1 in the cell membrane was
occasionally noted in some malignant cells. HIF-1α was noted
predominantly in the nucleus. Fig.
3 shows representative expression patterns of ESM-1 (Fig. 3A and B) and HIF-1α (Fig. 3C) in CRC tissues. Clinical and
pathological characteristics of the 143 CRC patients who underwent
surgical resection are summarized in Table I. The median age at the time of
surgical resection was 60.3 years. A high level of ESM-1 expression
was observed in 76 (53.1%) of the 143 patients. When we tested for
an association between the level of ESM-1 expression and the
clinicopathological factors potentially predictive of prognosis,
tumor size, depth of invasion, nodal status, distant metastasis,
and Dukes’ stage, these clinicopathological variables showed a
statistically significant association with ESM-1 expression status.
We also tested for an association between HIF-1α status and these
variables. A significant correlation between high nuclear HIF-1α
and Dukes’ stage (P=0.005) was noted (data not shown). A
significant correlation between high nuclear HIF-1α and ESM-1
(P<0.001) was also noted (Table
II).
 | Table IClinicopathological variables and the
expression status of ESM-1. |
Table I
Clinicopathological variables and the
expression status of ESM-1.
| | ESM-1 expression
level | |
|---|
| |
| |
|---|
| | Negative/low | High | |
|---|
| |
|
| |
|---|
|
Characteristics | Total | n (%) | n (%) | P-value |
|---|
| Age (years) |
| <50 | 26 | 12 (17.9) | 14 (18.4) | 0.937 |
| ≥50 | 117 | 55 (82.1) | 62 (81.6) | |
| Gender |
| Female | 68 | 35 (52.2) | 33 (43.4) | 0.292 |
| Male | 75 | 32 (47.8) | 43 (56.6) | |
| Site |
| Right/transverse
colon | 34 | 14 (20.9) | 20 (26.3) | 0.447 |
| Left colon and
rectum | 109 | 53 (79.1) | 56 (73.7) | |
| Size (cm in
diameter) | | | | 0.030 |
| <5 | 61 | 35 (52.2) | 26 (34.2) | |
| ≥5 | 82 | 32 (47.8) | 50 (65.8) | |
|
Differentiation |
| Well | 34 | 20 (29.9) | 14 (18.4) | 0.266 |
| Moderately | 82 | 36 (53.7) | 46 (60.5) | |
| Poorly | 27 | 11 (16.4) | 16 (21.1) | |
| Depth of
invasion |
| T1 | 5 | 5 (7.5) | 0 (0.0) | <0.001 |
| T2 | 21 | 16 (23.9) | 5 (6.6) | |
| T3 | 105 | 43 (64.2) | 62 (81.6) | |
| T4 | 12 | 3 (4.5) | 9 (11.8) | |
| Nodal status |
| N0 | 67 | 44 (65.7) | 23 (30.3) | <0.001 |
| N1 | 23 | 10 (14.9) | 13 (17.1) | |
| N2 | 53 | 13 (19.4) | 40 (52.6) | |
| Distant
metastasis |
| M0 | 121 | 63 (94.0) | 58 (76.3) | 0.003 |
| M1 | 22 | 4 (6.0) | 18 (23.7) | |
| Dukes’ stage |
| A | 8 | 7 (10.4) | 1 (1.3) | <0.001 |
| B | 58 | 36 (53.7) | 22 (28.9) | |
| C | 56 | 20 (29.9) | 36 (47.4) | |
| D | 21 | 4 (6.0) | 17 (22.4) | |
 | Table IICorrelation between ESM-1 and HIF-1α
expression status. |
Table II
Correlation between ESM-1 and HIF-1α
expression status.
| | HIF-1α
expression |
|---|
| |
|
|---|
| Frequency | Total | Low/negative, n
(%) | High, n (%) |
|---|
| ESM-1
expression |
| Low, n (%) | 67 | 51 (76.1) | 16 (23.9) |
| High, n (%) | 76 | 26 (34.2) | 50 (65.8) |
A high level of ESM-1 expression is an
independent prognostic factor for disease recurrence and a worse
survival outcome
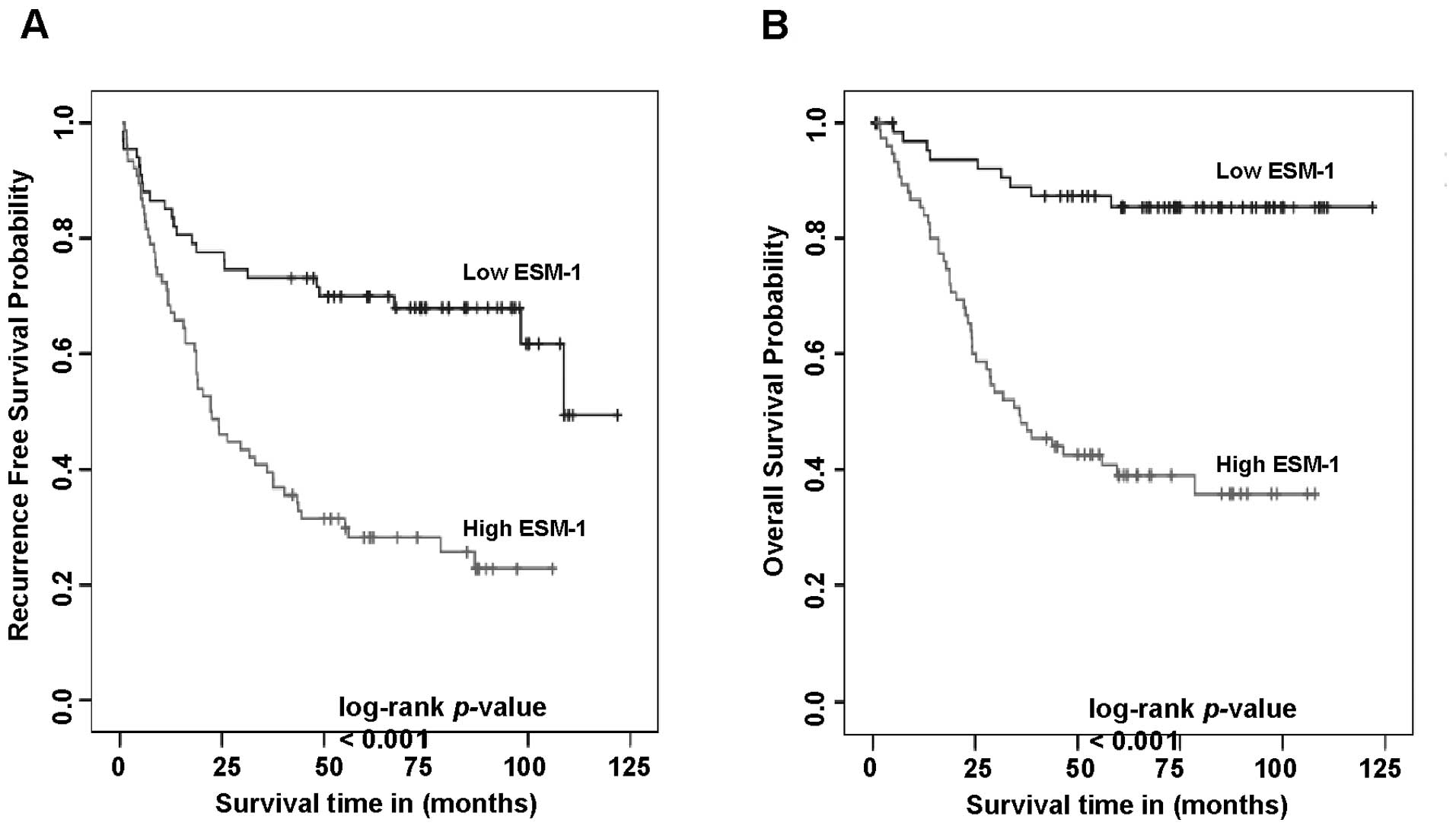
We first carried out univariate analyses to examine
whether the expression status of ESM-1 correlates with
recurrence-free survival. A total of 40 patients (28.0%) presented
with recurrence during the follow-up period. At the end of the
follow-up, 81 (56.6%) patients were alive, and 62 had died. The
analysis showed that a high level of ESM-1 expression was
negatively associated with recurrence-free survival (P<0.001),
as shown in Fig. 4A. A high level
of ESM-1 expression also correlated significantly with negative
overall survival (P<0.001). Cumulative overall survival curves
of patients were significantly split by ESM-1 expression status
(Fig. 4B). The mean overall
survival times for patients with high and low levels of ESM-1
expression, and all patients were 53.4, 100.4, and 78.1 months,
respectively. We carried out multivariate analyses to assess the
predictive value of ESM-1 expression status for recurrence-free
survival and overall survival by adjusting for other potentially
prognostic factors, including age, gender, tumor site, tumor size,
cell differentiation and tumor stage. The results corroborated a
worse survival outcome in patients with a high level of ESM-1
expression. In a multivariate Cox regression analysis, the
independent prognostic factors significantly associated with
overall survival were ESM-1 (P=0.001) and tumor stage (P=0.039).
The relative risk (RR) of death was more than 3 times higher in
patients with high ESM-1 levels (RR, 3.062; 95% CI, 1.630–5.751)
than those with low ESM-1 levels. A high level of ESM-1 expression
was also predictive of increased disease recurrence with a P-value
of <0.001. The RR of disease recurrence for patients with high
ESM-1 level was 2.65 (95% CI, 1.543–4.550). The results from Cox
proportional hazards analysis are summarized in Table III.
 | Table IIIMultivariate Cox proportional hazards
analysis for recurrence-free and overall survival. |
Table III
Multivariate Cox proportional hazards
analysis for recurrence-free and overall survival.
| | Recurrence-free
survival | Overall
survival |
|---|
| |
|
|
|---|
| n | RR (95% CI) | P-value | Median (95%
CI) | P-value |
|---|
| ESM-1 level |
| Low/negative | 77 | 1.000 | 0.010 | 1.000 | 0.001 |
| High | 76 | 2.109
(1.196–3.716) | | 3.531
(1.632–7.644) | |
| Age (years) |
| <50 | 26 | 1.000. | 0.900 | 1.000 | 0.123 |
| ≥50 | 117 | 0.962
(0.531–1.744) | | 1.862
(0.845–4.104) | |
| Gender |
| Female | 68 | 1.000 | 0.131 | 1.000 | 0.387 |
| Male | 75 | 1.432
(0.899–2.282) | | 1.278
(0.733–2.228) | |
| Site |
| Right colon | 34 | 1.000 | 0.011 | 1.000 | 0.096 |
| Left colon | 109 | 2.197
(1.195–4.041) | | 1.804
(0.901–3.612) | |
| Size (cm,
diameter) |
| <5 | 61 | 1.000 | 0.344 | 1.000. | 0.104 |
| ≥5 | 82 | 1.27
(0.774–2.085) | | 1.626
(0.905–2.922) | |
|
Differentiation |
| Well | 34 | 1.000 | 0.236 | 1.000 | 0.009 |
| Moderately | 81 | 0.771
(0.411–1.446) | | 0.894
(0.404–1.979) | |
| Poorly | 28 | 1.299
(0.589–2.864) | | 2.758
(1.100–6.915) | |
| Dukes’ stage |
| A and B | 66 | 1.000 | 0.020 | 1.000 | 0.039 |
| C and D | 77 | 1.942
(0.981–3.027) | | 2.046
(0.846–3.242) | |
Discussion
Intra-tumoral hypoxia is a major event that occurs
in most solid tumors (37,38), and HIF is a key transcription factor
activating survival machinery in cancer cells under intra-tumoral
hypoxic conditions (39). To date,
there has been one report suggesting the modulation of ESM-1
expression by hypoxia in human glioblastoma cells in addition to
TNF-α, fibroblast growth factor (FGF)-2 and VEGF (29). Although we could anticipate the
functional correlation of HIF-1α with ESM-1, there was no in
vitro or in vivo evidence to suggest whether there is a
significant direct correlation between ESM-1 and HIF-1α. Therefore,
in this study, we screened the tissues of 143 CRC patients, and
showed that overexpression of ESM-1 in CRC was closely related to
the restricted overexpression of HIF-1α in the nuclei of CRC cells.
We also provided the first report that HIF-1α stimulated the
induction of ESM-1 in CRC cells.
ESM-1 has been studied in a number of cell lines and
human tumor tissues, and has been shown to influence a variety of
normal and pathological processes. ESM-1 is a key player in tumor
progression as well as in the regulation of inflammatory disorders
(23,40,41),
wherein it is either downregulated or overexpressed. In this study,
we showed that a high level of cytosolic ESM-1 is an independent
and clinically significant prognostic indicator for colon cancer
patients who underwent surgery. However, in contradiction to our
results, Zuo et al (42)
showed that a lower expression of ESM-1 was detected in CRC tissue
compared to that in normal colon and rectal tissue samples, and its
expression was positively correlated with tissue differentiation of
CRC. Although we could not explain the exact reason why our results
differ from those of Zuo et al, we are convinced by our
results based on many efforts to confirm our data, including those
of a previous published report (31). It is noteworthy that ESM-1 was
elevated in the colon cancer cells under hypoxic conditions. Since
previous studies showed that HIF-1α is one of the important
regulators in hypoxia, we explored a possible correlation between
ESM-1 and HIF-1α expression status. We showed that ESM-1 was
upregulated in the human colon cell line HT29 under 1% hypoxic
conditions, inhibited by siRNA of HIF-1α, and overexpressed
following HIF-1α plasmid vector transfection, suggesting that ESM-1
may be regulated by HIF-1α in the hypoxic tumor microenvironment
during tumor development and progression (27). Indeed, of the 67 tumors containing a
high level of nuclear HIF-1α immunoreactivity, 51 displayed a high
level of ESM-1 expression. Of the 76 tumors containing a low level
of nuclear HIF-1α, 25 showed a correspondingly low level of ESM-1.
The likelihood of observing a high level of ESM-1 expression in a
tumor containing a high level of nuclear HIF-1α was significantly
greater than that in a tumor with a low level of nuclear HIF-1α
(P<0.01). Ji et al recently reported that ESM-1 was
secreted in human colon cancer tissue and cells (31). ESM-1 is a novel soluble dermatan
sulfate proteoglycan that is secreted from endothelial cells
(19,22), and its expression is regulated by
tumor cell-derived factors, including vascular endothelial growth
factor, in the unstable hypoxic microenvironment (25). This, together with our findings,
suggests that ESM-1 may be involved in hypoxia-associated
angiogenesis during tumor development and progression.
HIFs are essential mediators in regulating
transcription in tumor cells in response to hypoxia (19,39,43).
HIF-1α is the most well-characterized, and HIF-2α (44) has emerged as a non-redundant player,
but the role of full-length HIF-3α (45,46) is
not yet known. It is well known that HIF-1α but not HIF-1β plays an
important role in the HIF-1 pathway and regulates HIF target genes
(47). HIF-1α is induced by hypoxia
in almost all cell types, and is frequently overexpressed in solid
tumors (11,48). Furthermore, the upregulation of
HIF-1α correlates with cancer progression or aggressiveness in many
human tumors, although the prognostic significance of HIF-1α
induced by hypoxia in colon cancer has only been restrictively
elucidated. Using immunohistochemical screening, we showed that
HIF-1α protein was overexpressed in colon cancer tissues, and that
this expression is significantly correlated with Dukes’ stage
(P=0.005) and poor overall survival (data not shown). These results
were coincident with observations of others that HIF-1α
overexpression is correlated with worse clinical prognosis
(14,49).
The present results demonstrated that HIF-1α
enhances ESM-1 expression in response to hypoxia in human CRC. We
suggest that ESM-1, in addition to serving as a prognostic marker,
may also serve as a therapeutic target by using the HIF pathway in
human CRC. Further studies into the potential of ESM-1 inhibition
as an effective means of enhancing tumor response to treatment
and/or delaying tumor progression are warranted.
Acknowledgements
This study was supported by a grant of the Korea
Healthcare Technology R&D Project, Ministry for Health, Welfare
& Family Affairs, Republic of Korea (A090509) and by the
Next-Generation BioGreen 21 (SSAC, PJ008107), Rural Development
Administration, Republic of Korea.
References
|
1
|
Cannito S, Novo E, Compagnone A, et al:
Redox mechanisms switch on hypoxia-dependent epithelial-mesenchymal
transition in cancer cells. Carcinogenesis. 29:2267–2278. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lluis JM, Buricchi F, Chiarugi P, Morales
A and Fernandez-Checa JC: Dual role of mitochondrial reactive
oxygen species in hypoxia signaling: activation of nuclear
factor-{kappa}B via c-SRC and oxidant-dependent cell death. Cancer
Res. 67:7368–7377. 2007.
|
|
3
|
Sansone P, Piazzi G, Paterini P, et al:
Cyclooxygenase-2/carbonic anhydrase-IX up-regulation promotes
invasive potential and hypoxia survival in colorectal cancer cells.
J Cell Mol Med. 13:3876–3887. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
To KK, Koshiji M, Hammer S and Huang LE:
Genetic instability: the dark side of the hypoxic response. Cell
Cycle. 4:881–882. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang LE, Bindra RS, Glazer PM and Harris
AL: Hypoxia-induced genetic instability - a calculated mechanism
underlying tumor progression. J Mol Med. 85:139–148. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bristow RG and Hill RP: Hypoxia and
metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev
Cancer. 8:180–192. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoo YG, Hayashi M, Christensen J and Huang
LE: An essential role of the HIF-1alpha-c-Myc axis in malignant
progression. Ann NY Acad Sci. 1177:198–204. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang LE, Arany Z, Livingston DM and Bunn
HF: Activation of hypoxia-inducible transcription factor depends
primarily upon redox-sensitive stabilization of its alpha subunit.
J Biol Chem. 271:32253–32259. 1996. View Article : Google Scholar
|
|
9
|
Maynard MA and Ohh M: The role of
hypoxia-inducible factors in cancer. Cell Mol Life Sci.
64:2170–2180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harris AL: Hypoxia - a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brahimi-Horn MC, Chiche J and Pouyssegur
J: Hypoxia and cancer. J Mol Med. 85:1301–1307. 2007. View Article : Google Scholar
|
|
12
|
Brahimi-Horn MC and Pouyssegur J: Hypoxia
in cancer cell metabolism and pH regulation. Essays Biochem.
43:165–178. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brahimi-Horn MC, Chiche J and Pouyssegur
J: Hypoxia signalling controls metabolic demand. Curr Opin Cell
Biol. 19:223–229. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brahimi-Horn MC and Pouyssegur J: HIF at a
glance. J Cell Sci. 122:1055–1057. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koukourakis MI, Giatromanolaki A,
Simopoulos C, Polychronidis A and Sivridis E: Lactate dehydrogenase
5 (LDH5) relates to up-regulated hypoxia inducible factor pathway
and metastasis in colorectal cancer. Clin Exp Metastasis. 22:25–30.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sivridis E, Giatromanolaki A and
Koukourakis MI: Proliferating fibroblasts at the invading tumour
edge of colorectal adenocarcinomas are associated with endogenous
markers of hypoxia, acidity, and oxidative stress. J Clin Pathol.
58:1033–1038. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Giles RH, Lolkema MP, Snijckers CM, et al:
Interplay between VHL/HIF1alpha and Wnt/beta-catenin pathways
during colorectal tumorigenesis. Oncogene. 25:3065–3070. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koukourakis MI, Giatromanolaki A,
Polychronidis A, et al: Endogenous markers of hypoxia/anaerobic
metabolism and anemia in primary colorectal cancer. Cancer Sci.
97:582–588. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lassalle P, Molet S, Janin A, et al: ESM-1
is a novel human endothelial cell-specific molecule expressed in
lung and regulated by cytokines. J Biol Chem. 271:20458–20464.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sarrazin S, Adam E, Lyon M, et al: Endocan
or endothelial cell specific molecule-1 (ESM-1): a potential novel
endothelial cell marker and a new target for cancer therapy.
Biochim Biophys Acta. 1765:25–37. 2006.PubMed/NCBI
|
|
21
|
Bechard D, Scherpereel A, Hammad H, et al:
Human endothelial-cell specific molecule-1 binds directly to the
integrin CD11a/CD18 (LFA-1) and blocks binding to intercellular
adhesion molecule-1. J Immunol. 167:3099–3106. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bechard D, Meignin V, Scherpereel A, et
al: Characterization of the secreted form of
endothelial-cell-specific molecule 1 by specific monoclonal
antibodies. J Vasc Res. 37:417–425. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Scherpereel A, Depontieu F, Grigoriu B, et
al: Endocan, a new endothelial marker in human sepsis. Crit Care
Med. 34:532–537. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grigoriu BD, Depontieu F, Scherpereel A,
et al: Endocan expression and relationship with survival in human
non-small cell lung cancer. Clin Cancer Res. 12:4575–4582. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abid MR, Yi X, Yano K, Shih SC and Aird
WC: Vascular endocan is preferentially expressed in tumor
endothelium. Microvasc Res. 72:136–145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leroy X, Aubert S, Zini L, et al: Vascular
endocan (ESM-1) is markedly overexpressed in clear cell renal cell
carcinoma. Histopathology. 56:180–187. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aitkenhead M, Wang SJ, Nakatsu MN, Mestas
J, Heard C and Hughes CC: Identification of endothelial cell genes
expressed in an in vitro model of angiogenesis: induction of ESM-1,
(beta)ig-h3, and NrCAM. Microvasc Res. 63:159–171. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song Q, Jing H, Wu H, Zhou G, Kajiyama T
and Kambara H: Gene expression analysis on a photodiode array-based
bioluminescence analyzer by using sensitivity-improved SRPP.
Analyst. 135:1315–1319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maurage CA, Adam E, Mineo JF, et al:
Endocan expression and localization in human glioblastomas. J
Neuropathol Exp Neurol. 68:633–641. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kang YH, Ji NY, Lee CI, et al: ESM-1
silencing decreased cell survival, migration, and invasion and
modulated cell cycle progression in hepatocellular carcinoma. Amino
Acids. 40:1003–1013. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ji NY, Kim YH, Jang YJ, et al:
Identification of endothelial cell-specific molecule-1 as a
potential serum marker for colorectal cancer. Cancer Sci.
101:2248–2253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar
|
|
33
|
Chen LY, Liu X, Wang SL and Qin CY:
Over-expression of the endocan gene in endothelial cells from
hepatocellular carcinoma is associated with angiogenesis and tumour
invasion. J Int Med Res. 38:498–510. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rennel E, Mellberg S, Dimberg A, et al:
Endocan is a VEGF-A and PI3K regulated gene with increased
expression in human renal cancer. Exp Cell Res. 313:1285–1294.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bechard D, Gentina T, Delehedde M, et al:
Endocan is a novel chondroitin sulfate/dermatan sulfate
proteoglycan that promotes hepatocyte growth factor/scatter factor
mitogenic activity. J Biol Chem. 276:48341–48349. 2001.
|
|
36
|
Chomczynski P and Sacchi N: Single-step
method of RNA isolation by acid guanidinium
thiocyanate-phenol-chloroform extraction. Anal Biochem.
162:156–159. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hockel M and Vaupel P: Biological
consequences of tumor hypoxia. Semin Oncol. 28:36–41. 2001.
View Article : Google Scholar
|
|
38
|
Foo SS, Abbott DF, Lawrentschuk N and
Scott AM: Functional imaging of intratumoral hypoxia. Mol Imaging
Biol. 6:291–305. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mabjeesh NJ and Amir S: Hypoxia-inducible
factor (HIF) in human tumorigenesis. Histol Histopathol.
22:559–572. 2007.PubMed/NCBI
|
|
40
|
Filep JG: Endocan or endothelial
cell-specific molecule-1: a novel prognostic marker of sepsis? Crit
Care Med. 34:574–575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sarrazin S, Lyon M, Deakin JA, et al:
Characterization and binding activity of the chondroitin/dermatan
sulfate chain from endocan, a soluble endothelial proteoglycan.
Glycobiology. 20:1380–1388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zuo L, Zhang SM, Hu RL, et al: Correlation
between expression and differentiation of endocan in colorectal
cancer. World J Gastroenterol. 14:4562–4568. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ryan HE, Poloni M, McNulty W, et al:
Hypoxia-inducible factor-1alpha is a positive factor in solid tumor
growth. Cancer Res. 60:4010–4015. 2000.PubMed/NCBI
|
|
44
|
Swami M: Hypoxia: the HIF2alpha puzzle.
Nat Rev Cancer. 10:6032010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gu YZ, Moran SM, Hogenesch JB, Wartman L
and Bradfield CA: Molecular characterization and chromosomal
localization of a third alpha-class hypoxia inducible factor
subunit, HIF3alpha. Gene Expr. 7:205–213. 1998.PubMed/NCBI
|
|
46
|
Kietzmann T, Cornesse Y, Brechtel K,
Modaressi S and Jungermann K: Perivenous expression of the mRNA of
the three hypoxia-inducible factor alpha-subunits, HIF1alpha,
HIF2alpha and HIF3alpha, in rat liver. Biochem J. 354:531–537.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Clottes E: Hypoxia-inducible factor 1:
regulation, involvement in carcinogenesis and target for anticancer
therapy. Bull Cancer. 92:119–127. 2005.(In French).
|
|
48
|
Zhong H, De Marzo AM, Laughner E, et al:
Overexpression of hypoxia-inducible factor 1alpha in common human
cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
49
|
Baba Y, Nosho K, Shima K, et al: HIF1A
overexpression is associated with poor prognosis in a cohort of 731
colorectal cancers. Am J Pathol. 176:2292–2301. 2010. View Article : Google Scholar : PubMed/NCBI
|