Introduction
Apoptosis is an important continuous process of
destruction of undesirable cells during development or homeostasis
in multi-cellular organisms. This process is characterized by
distinct morphological changes, including membrane bleeding, cell
shrinkage, dissipation of mitochondrial membrane potential
(ΔΨm), chromatin condensation and DNA fragmentation
(1,2). The extrinsic and intrinsic pathways
are the two major pathways involved in the regulation of apoptosis
(3): the extrinsic pathway is
mediated via cell surface death receptor, leading to the activation
of caspase-8; the intrinsic pathway is dependent on various cell
stress stimuli, leading to altered ratio of Bcl-2 family members
which affect cytochrome c (cyt-c), Smac and apoptotic
protease activating factor-1 (Apaf-1) release that leads to
caspase-9 and -3 activation (4).
Several therapeutic agents eliminate tumor cells by inducing
apoptotic cell death (5), and some
natural plants have been investigated for their cytotoxicity in
cancer targeting apoptosis (6).
Flavonoids are a diverse family of natural phenolic
compounds commonly found in fruits and vegetables, such as
flavonols, flavonones and flavans. They have demonstrated
anticancer and chemopreventive properties in numerous
epidemiological studies (7), and
were able to inhibit the proliferation of tumor cells, such as
breast, prostate and lung cancer cells, both in vitro and
in vivo (8,9), although the exact mechanism is not yet
fully understood. The flavonoids are generally safe with low
toxicity, making them ideal candidates for cancer chemopreventive
agents. Chrysin (5,7-dihydroxyflavone) is a natural flavonoid
presented in many plant extracts, including blue passion flower
(Passiflora caerulea), honey and propolis (10). A number of studies have shown that
chrysin has multiple biological activities, such as
antiinflammation, antioxidation and anticancer effects (11–13).
Chrysin has been reported to induce apoptosis in a panel of cancer
cell lines, including HeLa cervical cancer cells, U937, HL-60 and
L1210 leukemia cells (14). Chrysin
was also able to inhibit tumor angiogenesis in vivo, which
is a key step in cancer cell metastasis (15,16).
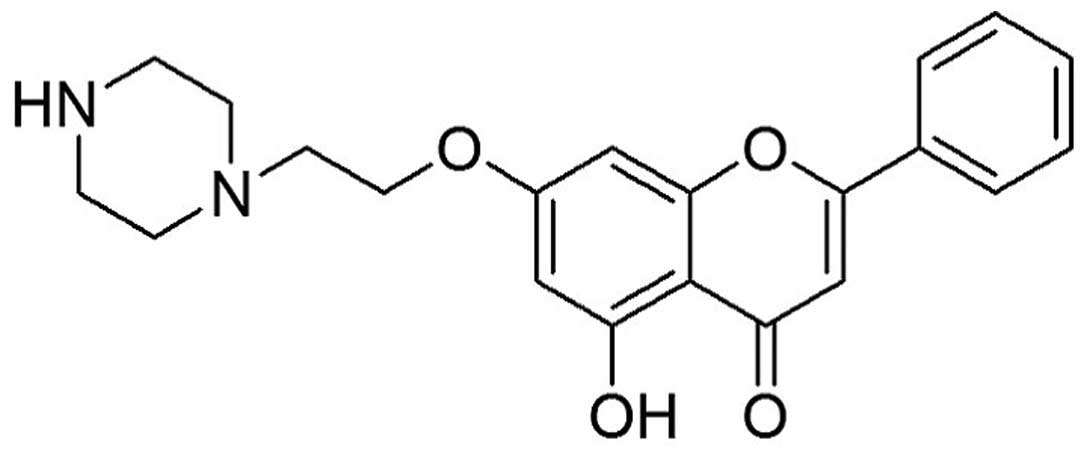
We previously reported that 7-piperazinethylchrysin (7-PEC)
(Fig. 1) significantly inhibited
the growth of various cancer cell lines such as HCT-116 cells
(17). The aim of the present study
was to elucidate the mechanisms of cell growth inhibition induced
by 7-PEC. Herein we report that 7-PEC can inhibit the proliferation
of HCT-116 cells in a time- and dose-dependent manner, including
the ΔΨm loss, elevating the ratio of Bax/Bcl-2, releasing
cyt-c to cell cytoplasm, activating caspase-9, -3 and p53,
followed by PARP cleavage and induction of apoptosis.
Materials and methods
Materials
7-PEC was synthesized according to the procedure
described in our previous report (17). 7-PEC (>95% purity) was dissolved
in DMSO and added to the experimental media to give the final
concentrations. Antibodies for detecting p-Akt, Akt, p53, Bcl-2,
Bax, cyt-c, pro-caspase-9, pro-caspase-3, PARP1 and β-actin
were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Ac-DEVD-CHO and Rhodamine 123 were purchased from the
Beyotime Institute of Biotechnology (Haimen, China). Hoechst 33258
and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
RPMI-1640 was purchased from Gibco (Invitrogen, Carlsbad, CA, USA).
Neonatal bovine serum (NBS) was purchased from Hangzhou Sijiqing
Biological Engineering Materials Co. (China).
Cell line and culture conditions
HCT-116 human colon cancer cells were kindly
provided by Shanghai Jiao Tong University. The cells were routinely
cultured in RPMI-1640 medium, supplemented with 10% NBS. The
culture was maintained at 37°C with a gas mixture of 5%
CO2/95% air. All media were supplemented with 100 U/ml
penicillin and 100 μg/ml streptomycin.
Cell viability assay
The cells were seeded in 96-well microtiter plates
(3×104/ml). After 12 h of incubation in the appropriate
medium, cells were treated with various concentrations (1, 10, 25
and 50 μM ) of 7-PEC for another 72 h (24 or 48 h). Subsequently,
10 μl of MTT stock solution was added to each well for an
additional 4 h of incubation. Then, 100 μl of DMSO was added to
each well and the absorbance at 570 nm was determined with a
microplate reader. Using the MTT method, cell numbers were obtained
as absorbance values. The results were expressed as viability
compared with that of control cells. Each treatment and time-point
had three independent wells. The representative data shown in this
study are the results of three independent experiments.
Cell morphological assessment
Cell morphological changes were assessed by Hoechst
33258 staining. Briefly, following exposure to 7- PEC for 48 h, the
cells were washed twice with PBS and fixed with 4% formaldehyde at
4°C for 10 min. The samples were then washed with PBS and stained
with Hoechst 33258 solution (0.5 μg/ml) for 10 min at room
temperature. Finally, the cells were observed under the
fluorescence microscope (Nikon Eclipse Ti-s, Nikon Corp., Tokyo,
Japan).
Detection of mitochondrial membrane
potential
ΔΨm was measured using Rhodamine 123.
Briefly, cells under different concentrations of 7-PEC treatment
were incubated with Rhodamine 123 (5 μg/ml) at 37°C for 30 min, and
washed with PBS. The cell pellet was collected by centrifugation
(1,500 × g, 3 min), and resuspended in 1 ml of PBS. Fluorescence
intensities of Rhodamine 123 in cells were analyzed by flow
cytometric analysis.
Cell cycle analysis
For cell cycle analysis, HCT-116 cells
(1×105 cells/ml, 3 ml) were cultured in 6-well plates,
with or without 7-PEC (1.25, 2.5 and 5 μM) for 48 h. Cells were
collected and resuspended in 500 μl of PBS containing 0.025 mg of
propidium iodide (PI) and 50 μg of RNase for 30 min at room
temperature in the dark. Flow cytometry was performed on Quanta SC
(Beckman Coulter, Fullerton, CA, USA).
Annexin V-FITC/PI assay of apoptotic
cells
Briefly, HCT-116 cells (1×105 cells/ml)
exposed to 7-PEC for 48 h were determined by flow cytometry (Quanta
SC, Beckman Coulter) using a detection kit. Following 7-PEC
treatment, cells were collected and washed twice in cold PBS and
resuspended in 200 μl of binding buffer (1×105
cells/ml). The samples were incubated with 5 μl of Annexin V-FITC
and 5 μl PI in the dark for 15 min at room temperature. Finally,
samples were analyzed by flow cytometry and evaluated based on the
percentage of cells for Annexin V-positive.
Western blot analysis
HCT-116 cells were treated with 7-PEC (1.25, 2.5 and
5 μM) for 48 h. Proteins were extracted with cell lysis buffer for
western and IP (Beyotime Institute of Biotechnology). Equal amounts
(40 μg/lane) of protein were separated on 10 or 15%
SDS-polyacrylamide gel electrophoresis, transferred to
polyvinylidene fluoride (PVDF) membranes (Millipore Corp., Bedford,
MA, USA) and blocked at room temperature for 1 h in 3% (w/v)
non-fat milk in TBST. The blots were incubated overnight at 4°C
with the primary antibodies diluted in TBST buffer. The membranes
were incubated with anti-Akt, p-Akt, Bcl-2, Bax, p53, cyt-c,
pro-casapse-3, pro-caspase-9, PARP1 and β-actin primary antibodies
(1:1000). After washing with TBST, the membranes were incubated
with horseradish peroxidase-conjugated goat anti-rabbit or goat
anti-mouse secondary antibodies (1:5000), and visualized with the
ECL detection kit (Thermo, USA), according to the manufacturer’s
instructions.
Measurement of ROS production
The elevations of intracellular ROS induced by 7-PEC
in HCT-116 cells were detected by DCFH-DA
(2′,7′-dichlorofluorescein diacetate) using flow cytometry. This
compound is a cell-permeant indicator for ROS that is
non-fluorescent until the acetate groups are removed by
intracellular esterases and oxidation occurs within the cell.
Briefly, cells were seeded at 1×105 cells/well in 6-well
plates, and treated with or without 7-PEC (5, 10 and 20 μM). At the
indicated times, cells were harvested and washed with PBS, then
resuspended in PBS containing DCFH-DA (10 μM) and incubated for 20
min at 37°C. After the inhibition, cells were washed twice by PBS
and then analyzed by flow cytometry.
Statistical analysis
Results are expressed as the mean ± SD for three
independent experiments. Statistical differences were evaluated
using Student’s t-test or one-way analysis of variance (ANOVA).
P<0.05 was considered to indicate statistically significant
differences.
Results
Effects of 7-PEC on cell viability
The cytotoxic effects of 7-PEC on five different
cell lines were examined by MTT assay. The results showed that the
cytotoxicity of 7-PEC on HCT-116 cells is most potent; it is
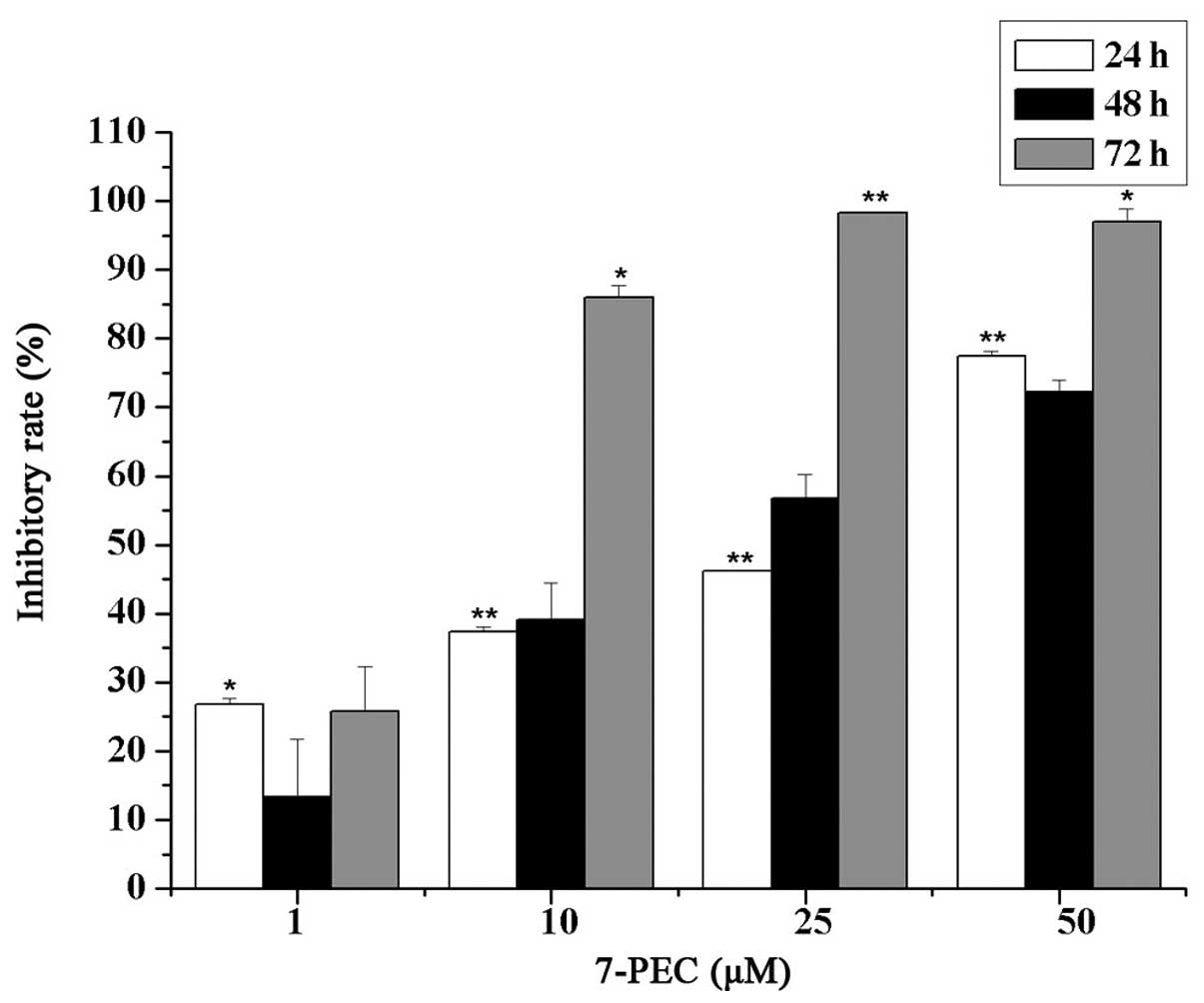
comparable with that of 5-FU as a positive control (Table I). HCT-116 cells in exponential
growth were treated with graded concentrations of 7-PEC (1, 10, 25
and 50 μM) for 24, 48 and 72 h. Under the experimental conditions,
7-PEC treatment exhibited strong inhibition on the survival of
HCT-116 cells in a time- and dose-dependent manner as shown in
Fig. 2. The IC50 values
were calculated as 16.25, 5.49 and 1.5 μM in cells treated for 24,
48 and 72 h, respectively.
 | Table IThe cytotoxicity of compound 7-PEC
against the DU-145, SGC-7901, HCT-116, HeLa and HEK-293 cell
lines. |
Table I
The cytotoxicity of compound 7-PEC
against the DU-145, SGC-7901, HCT-116, HeLa and HEK-293 cell
lines.
| Cytotoxicity
(IC50, μM)a |
|---|
|
|
|---|
| Compound | DU-145 | SGC-7901 | HCT-116 | HeLa | HEK-293 |
|---|
| 7-PEC | 3.08 | 2.78 | 1.50 | 2.46 | 41.90 |
| 5-FU | 2.95 | 2.19 | 1.93 | 9.70 | >100 |
Effects of 7-PEC on the morphology of
HCT-116 cells
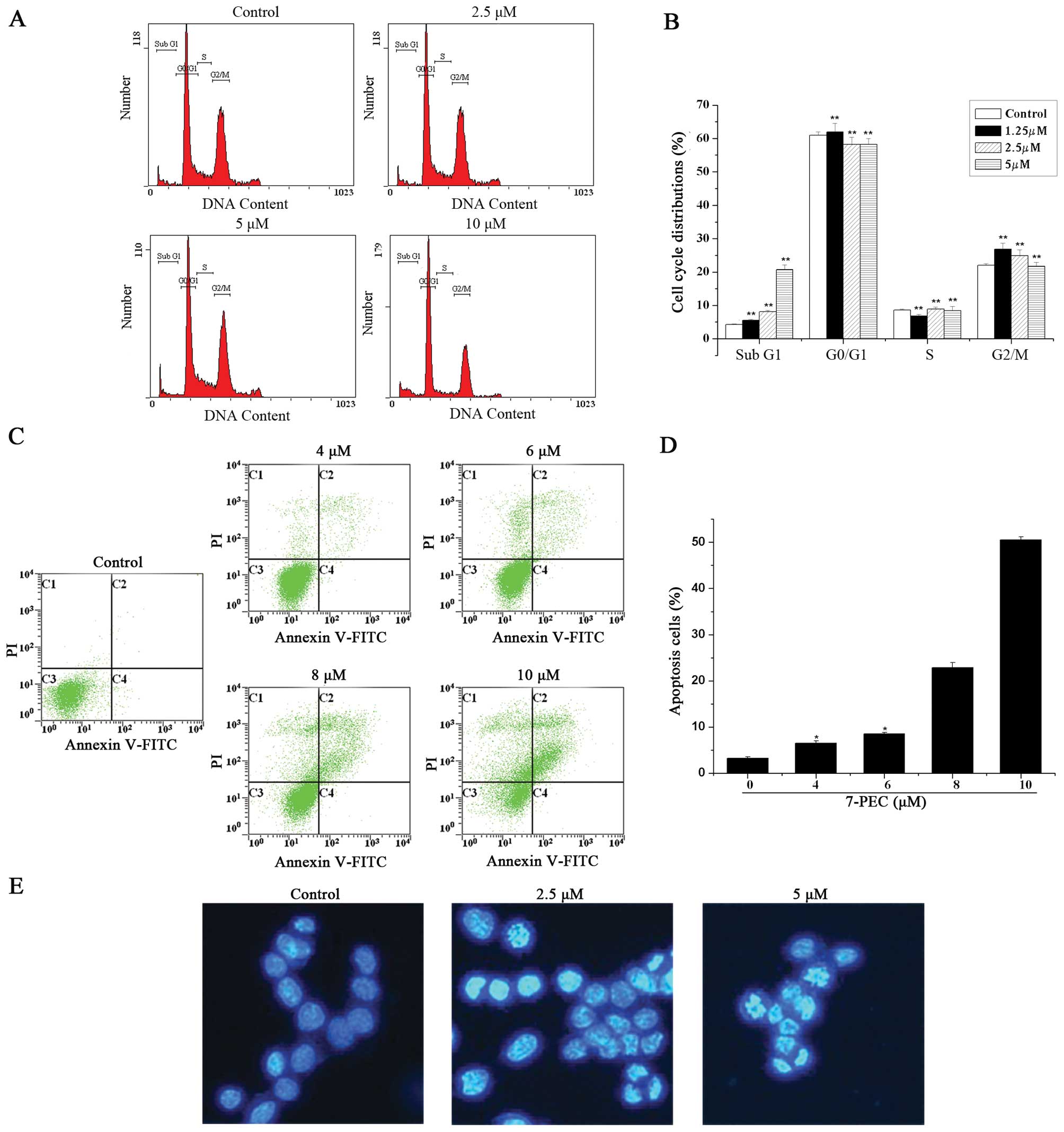
In order to elucidate whether the loss of HCT-116
cell viability induced by 7-PEC was associated with apoptosis, the
occurrence of apoptosis was identified with Hoechst 33258 staining.
HCT-116 cells were incubated with various concentrations of 7-PEC
(2.5 and 5 μM) for 48 h and stained by Hoechst 33258 for
observation of the morphology. As is clearly shown in Fig. 3E, significant nuclear condensation
and morphological changes for HCT-116 cells were observed, whereas
in the control group, the cells without 7-PEC treatment
demonstrated normal nuclear morphology. These data confirmed that
7-PEC could induce apoptosis in HCT-116 cells.
Effects of 7-PEC on cell cycle and
apoptosis
To investigate the effects of 7-PEC on apoptosis and
the cell cycle of HCT-116 cells, sub-diploid DNA-content and
phosphatidylserine (PS) externalization were measured by FACS after
PI and Annexin V-FITC/PI staining. For the cell cycle study,
HCT-116 cells were treated with 7-PEC (1.25, 2.5 and 5 μM) for 48
h, and the DNA content of 10, 000 events was analyzed by flow
cytometry. Fig. 3A and B show a
dose-dependent increase of apoptosis induction which is indicated
by percentage of sub-diploid DNA content. Apoptotic cells reached
~8.53 and 22.27% when the cells were exposed to 2.5 and 5 μM of
7-PEC, respectively. For the apoptosis study, HCT-116 cells were
incubated with different concentrations of 7-PEC (4, 6, 8 and 10
μM) for 48 h, and then the cells were subjected to Annexin
V-FITC/PI staining and analyzed by flow cytometry. Significant
apoptosis for HCT-116 cells is observed in Fig. 3C and D. Upon treatment with 2 and 10
μM of 7-PEC, the percentage of apoptotic cells increased from 3.09
to 50.03%. These results suggest that the Annexin-V-FITC assay is
more sensitive than sub-diploid DNA-content measurement for the
evaluation of apoptosis.
Effects of 7-PEC on caspase-3
activity
Caspase, a family of cysteine proteases, is known to
form integral parts of the apoptotic pathway (18). Caspase-3 activation is considered
the central and final apoptotic marker enzyme for both
mitochondrial intrinsic and death-domain receptor-dependent
extrinsic pathways. Poly(ADP-ribose) polymerase (PARP), an enzyme
involved in DNA repair, is a substrate for caspase-3 (19). Therefore, we investigated the
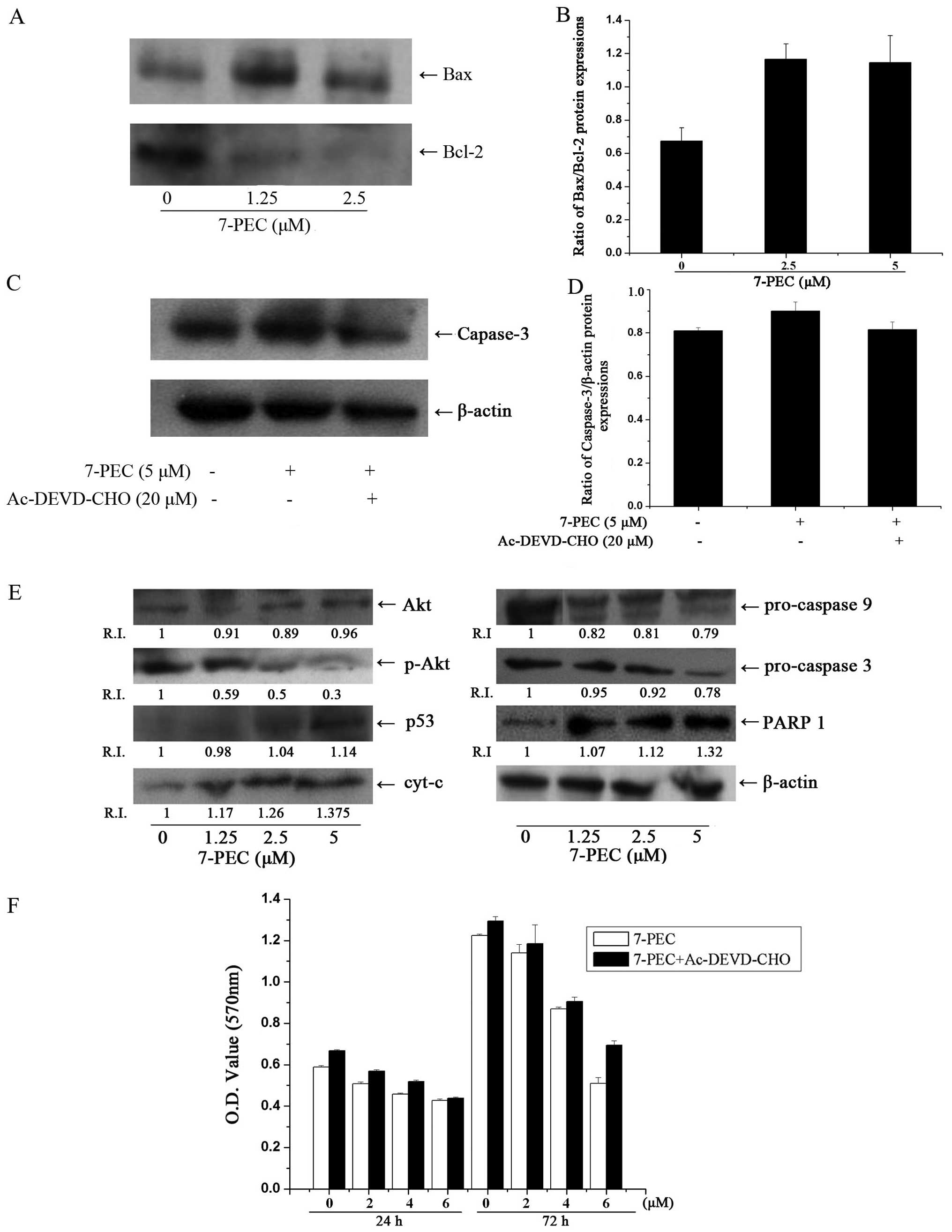
protein levels and activity of caspase-3. As is shown in Fig. 4E, the pro-caspase-9 and -3 protein
levels were significantly decreased and cleavage of PARP1 was
detected in 7-PEC-treated HCT-116 cells.
To confirm whether 7-PEC specifically triggers
caspase-3 expression, caspase-3 protein expression was investigated
in HCT-116 cells by treating with 5 μM of 7-PEC for 48 h in the
presence or absence of caspase-3 inhibitor, Ac-DEVD-CHO. As shown
in Fig. 4C, activation of caspase-3
induced by 7-PEC is blocked in the presence of Ac-DEVD-CHO. MTT
results demonstrated that the cell growth inhibition activity of
7-PEC was also weakened by Ac-DEVD-CHO (Fig. 4F). These data suggest that
7-PEC-induced apoptosis might engage caspase-3 dependent signaling
cascades. Taken together, our results indicate that 7-PEC-induced
apoptosis is possibly via the caspase-dependent apoptotic pathway
in HCT-116 cells.
Effects of 7-PEC on
p53/mitochondria-related apoptotic markers
The expression of Akt, p-Akt, p53, pro-caspase-3,
pro-caspase-9, PARP1 and cyt-c was measured in HCT-116 cells
treated with 7-PEC (1.25, 2.5 and 5 μM). As is shown in Fig. 4, 7-PEC treatment resulted in the
decrease of antiapoptotic protein Bcl-2 and increase of the Bax
(Fig. 4A), with an increase in the
Bax/Bcl-2 ratio (Fig. 4B). In
addition, upregulation of p53, cyt-c, pro-caspase-3,
pro-caspase-9 and subsequent cleavage of PARP1 were detected in
7-PEC-treated HCT-116 cells. The exposure to 7-PEC had no effects
on steady-state levels of total Akt protein, whereas p-Akt levels
were decreased significantly in a dose-dependent manner (Fig. 4E). These findings suggest the
activation of the mitochondria-based intrinsic apoptosis in HCT-116
cells after 7-PEC treatment.
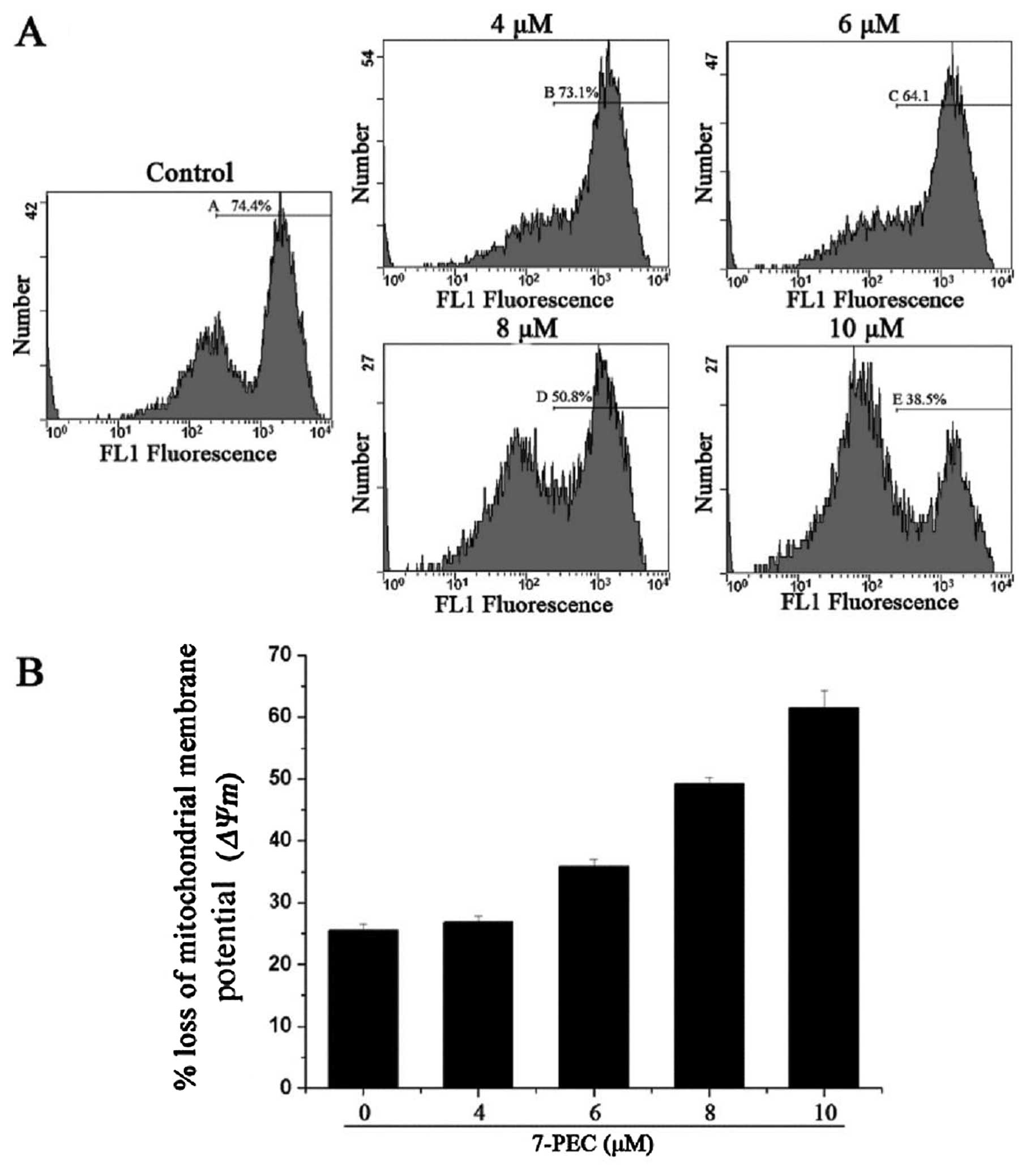
Effects of 7-PEC on mitochondrial
membrane potential
Early apoptosis is always accompanied by the
disruption of the mitochondrial membrane, resulting in a rapid
collapse in the electrochemical gradient (20). In this study, we explored the
effects of 7-PEC on the loss of ΔΨm using a cationic dye
Rhodamine 123, which can diffuse into the mitochondria matrix and
reflect the change of ΔΨm (21). Thus, HCT-116 cells were incubated
with different concentrations of 7-PEC (4, 6, 8 and 10 μM) for 24
h, and then incubated with Rhodamine 123 dye for another 30 min.
Fluorescence emission was measured by flow cytometry. As shown in
Fig. 5, the ΔΨm was
significantly decreased by 7-PEC in a dose-dependent manner.
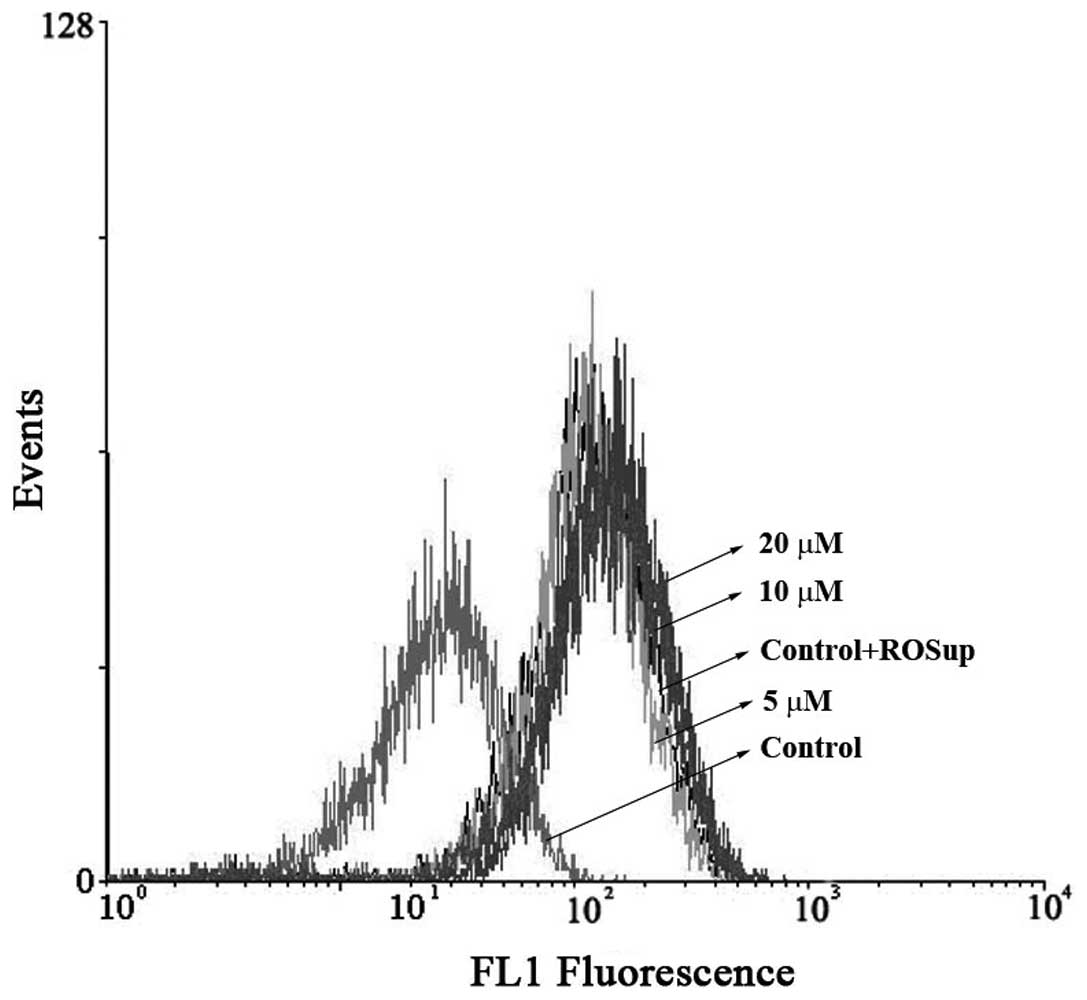
Effects of 7-PEC on cellular reactive
oxygen species production
Increased production of reactive oxygen species
(ROS) triggers cytotoxicity and cell death by increasing oxidative
stress. The intracellular production of ROS in HCT-116 cells was
measured while treating with 7-PEC (5, 10 and 20 μM) and using
DCFH-DA staining. The 7-PEC treatment of HCT-116 cells induced a
dose-dependent increase of ROS production. Fig. 6 shows an example of FACS analysis of
DCFH-DA-stained HCT-116 cells after 7-PEC treatment. The
experiments were triplicated and similar results were obtained.
Discussion
MTT assay revealed that 7-PEC significantly exerts
growth inhibitory effects on various cell lines, particularly on
HCT-116 human colon cancer cells with IC50 at 1.5 μM
after treating with 7-PEC for 72 h. We speculated that apoptosis
may be the main mechanism for 7-PEC-induced growth inhibitory
effects on HCT-116 cells. Previous studies have shown that a number
of anticancer drugs induce apoptosis through the activation of the
caspase pathways and the mitochondrial membrane dysfunction.
Accumulating evidence indicates that mitochondria play a pivotal
role in the apoptotic process in mammalian cells (22–24).
Disruption of mitochondrial ΔΨm is considered to be an
indicator of mitochondria damage and is generally defined as an
early stage of apoptosis, preceding efflux of small molecules from
the mitochondria (including cytochrome c, apoptosis-inducing
factor) and followed by caspase-9/-3 cascade activation (25–28).
In the present study, we found the marked decrease of pro-caspases
(pro-caspase-3 and -9) by 7-PEC after the breakdown of ΔΨm,
suggesting that the mitochondria-mediated pathway is involved in
7-PEC-triggered apoptosis. Sequential disruption of ΔΨm,
increased Bax/Bcl-2 ratio and activation of caspases-9 and -3 was
involved in 7-PEC-induced apoptosis. We showed that 7-PEC treatment
activated caspase-3 in a dose-dependent manner and resulted in the
cleavage of PARP1, a well-known caspase-3 substrate. A more
significant accumulation of the p53 protein in HCT-116 cells was
also observed after 7-PEC treatment, and this result indicates that
the 7-PEC-induced apoptosis could be p53-dependent.
Akt, a serine/threonine protein kinase, is activated
by phosphorylation and protects cells from apoptosis (29), and this protection is the result of
the fact that p-Akt increases expression of the FLICE inhibitory
protein (FLIP), which inhibits caspase-8 activity (30). We found that 7-PEC induced
downregulation/dephosphorylation of p-Akt.
The overexpression and integration of Bax in the
mitochondrial membrane were responsible for the commitment of the
cells to apoptosis (31). Bcl-2 is
localized in the mitochondria, endoplasmic reticulum, and nuclear
membranes, where most of the oxygen-free radicals are generated and
where the free radicals exert their apoptotic effects. Bcl-2
possibly acts to prevent apoptosis by scavenging oxygen derived
free radicals inside the cells (32,33).
The increase of the Bax/Bcl-2 ratio could induce cell apoptosis
(34,35). Treatment of HCT-116 cells with 7-PEC
decreased the Bcl-2 and increased the Bax protein levels. We
speculate that ROS might modulate the cellular distribution and
content of Bcl-2. Generation of ROS may contribute to mitochondrial
damage and lead to cell death by acting as apoptotic signaling
molecules (36–39). In the present study, we found that
in addition to its effect on ΔΨm, 7-PEC caused an increase
in ROS production in HCT-116 cells. The 7-PEC-mediated disruption
of ΔΨm and apoptosis in HCT-116 cells are apparently
dependent on ROS generation.
In conclusion, the present study demonstrates that
the significant growth inhibitory effects of 7-PEC on HCT-116 human
colon cancer cells is associated with induction of apoptosis,
involving sequential events, such as ROS production, reducing the
mitochondrial membrane potential (ΔΨm), and increasing the
Bax/Bcl-2 protein ratio.
Acknowledgements
This study was financially supported by the 2010
Industry for Attracting PhD Scientists Program of Jiangsu Province,
Changzhou Key Technology R&D Program (social development) and
the Priority Academic Program Development (PAPD) of Jiangsu Higher
Education Institutions.
References
|
1
|
Kaufmann SH and Hengartner MO: Programmed
cell death: alive and well in the new millennium. Trends Cell Biol.
11:526–534. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reed JC: Apoptosis-regulating proteins as
targets for drug discovery. Trends Mol Med. 7:314–319. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Earnshaw WC, Martins LM and Kaufmann SH:
Mammalian caspases: structure, activation, substrates, and
functions during apoptosis. Annu Rev Biochem. 68:383–424. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun XM, MacFarlane M, Zhuang J, Wolf BB,
Green DR and Cohen GM: Distinct caspase cascades are initiated in
receptor-mediated and chemical-induced apoptosis. J Biol Chem.
274:5053–5060. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thompson CB: Apoptosis in the pathogenesis
and treatment of disease. Science. 267:1456–1462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee KH: Anticancer drug design based on
plant-derived natural products. J Biomed Sci. 6:236–250.
1999.PubMed/NCBI
|
|
7
|
Arts IC: A review of the epidemiological
evidence on tea, flavonoids, and lung cancer. J Nutr.
138:S1561–S1566. 2008.PubMed/NCBI
|
|
8
|
Kupeli E, Sahin FP, Yesilada E, Calis I
and Ezer N: In vivo anti-inflammatory and antinociceptive activity
evaluation of phenolic compounds from Sideritis stricta. J Biosci.
62:519–525. 2007.PubMed/NCBI
|
|
9
|
Terao J: Dietary flavonoids as
antioxidants. Forum Nutr. 61:87–94. 2009. View Article : Google Scholar
|
|
10
|
Sobocanec S, Sverko V, Balog T, et al:
Oxidant/antioxidant properties of Croatian native propolis. J Agric
Food Chem. 54:8018–8026. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dhawan K, Kumar S and Sharma A: Beneficial
effects of chrysin and benzoflavone on virility in 2-year-old male
rats. J Med Food. 5:43–48. 2002.PubMed/NCBI
|
|
12
|
Lapidot T, Walker MD and Kanner J:
Antioxidant and prooxidant effects of phenolics on pancreatic
beta-cells in vitro. J Agric Food Chem. 50:7220–7225. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schnitzler P, Neuner A, Nolkem per S,
Zundel C, Nowack H, Sensch KH and Reichling J: Antiviral activity
and mode of action of propolis extracts and selected compounds.
Phytother Res. 24:S20–S28. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khoo BY, Chua SL and Balaram P: Apoptotic
effects of chrysin in human cancer cell lines. Int J Mol Sci.
11:2188–2199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weng MS, Ho YS and Lin JK: Chrysin induces
G1 phase cell cycle arrest in C6 glioma cells through inducing
p21Waf1/Cip1 expression: involvement of p38
mitogen-activated protein kinase. Biochem Pharmacol. 69:1815–1827.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Huang Q, Ong CN, Yang XF and Shen
HM: Chrysin sensitizes tumor necrosis factor-alpha-induced
apoptosis in human tumor cells via suppression of nuclear
factor-kappaB. Cancer Lett. 293:109–116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu K, Wang W, Cheng H, Pan SS and Ren J:
Synthesis and cytotoxicity of novel chrysin derivatives. Med Chem
Res. 20:838–846. 2011. View Article : Google Scholar
|
|
18
|
Thornberry NA and Lazebnik Y: Caspase:
enemies within. Science. 281:1308–1312. 1998. View Article : Google Scholar
|
|
19
|
Choi BH, Kim W, Wang QC, et al: Kinetin
riboside preferentially induces apoptosis by modulating Bcl-2
family proteins and caspase-3 in cancer cells. Cancer Lett.
261:37–45. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ly JD, Grubb DR and Lawen A: The
mitochondrial membrane potential (deltapsi(m)) in apoptosis; an
update. Apoptosis. 8:115–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou PH, Liu SQ and Peng H: The effect of
hyaluronic acid on IL-1beta-induced chondrocyte apoptosis in a rat
model of osteoarthritis. J Orthop Res. 26:1643–1648. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ling YH, Liebes L, Zou Y and Perez-Soler
R: Reactive oxygen species generation and mitochondrial dysfunction
in the apoptotic response to Bortezomib, a novel proteasome
inhibitor, in human H460 non-small cell lung cancer cells. Biol
Chem. 278:33714–33723. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ryan L, O’Callaghan YC and O’Brien NM: The
role of the mitochondria in apoptosis induced by
7β-hydroxycholesterol and cholesterol-5β, 6β-epoxide. Br J Nutr.
94:519–525. 2005.
|
|
24
|
Tang L and Zhang Y: Mitochondria are the
primary target in isothiocyanate-induced apoptosis in human bladder
cancer cells. Mol Cancer Ther. 4:1250–1259. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Doi S, Soda H, Oka M, et al: The histone
deacetylase inhibitor FR901228 induces caspase-dependent apoptosis
via the mitochondrial pathway in small cell lung cancer cells. Mol
Cancer Ther. 3:1397–1402. 2003.PubMed/NCBI
|
|
26
|
Ogbourne SM, Suhrbier A, Jones B, et al:
Antitumor activity of 3-ingenylangelate: plasma membrane and
mitochondrial disruption and necrotic cell death. Cancer Res.
64:2833–2839. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rotem R, Heyfets A, Fingrut O, Blickstein
D, Shaklai M and Flescher E: Jasmonates: novel anticancer agents
acting directly and selectively on human cancer cell mitochondria.
Cancer Res. 65:1984–1993. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu CC, Chan ML, Chen WY, Tsai CY, Chang FR
and Wu YC: Pristimerin induces caspase-dependent apoptosis in
MDA-MB-231 cells via direct effects on mitochondria. Mol Cancer
Ther. 4:1277–1285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Franke TF, Hornik CP, Segev L, Shostak GA
and Sugimoto C: PI3K/Akt and apoptosis: size matters. Oncogene.
22:8983–8998. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Panka DJ, Mano T, Suhara T, Walsh K and
Mier JW: Phosphatidylinositol 3-kinase/Akt activity regulates
c-FLIP expression in tumor cells. J Biol Chem. 276:6893–6896. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hayward RL, Macpherson JS, Cummings J,
Monia BP, Smyth JF and Jodrell DI: Enhanced oxaliplatin-induced
apoptosis following antisense Bcl-xl down-regulation is p53 and Bax
dependent: genetic evidence for specificity of the antisense
effect. Mol Cancer Ther. 3:169–178. 2004.
|
|
32
|
Sinicrope FA and Penington RC: Sulindac
sulfide-induced apoptosis is enhanced by a small-molecule Bcl-2
inhibitor and by TRAIL in human colon cancer cells over-expressing
Bcl-2. Mol Cancer Ther. 4:1475–1483. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamanaka K, Rocchi P, Miyake H, Fazli L,
Vessella B, Zangemeister-Wittke U and Gleave ME: A novel antisense
oligonucleotide inhibiting several antiapoptotic Bcl-2 family
members induces apoptosis and enhances chemosensitivity in
androgen-independent human prostate cancer PC3 cells. Mol Cancer
Ther. 4:1689–1698. 2005. View Article : Google Scholar
|
|
34
|
Childs AC, Phaneuf SL, Dirks AJ, Phillips
T and Leeuwenburgh C: Doxorubicin treatment in vivo causes
cytochrome C release and cardiomyocyte apoptosis, as well as
increased mitochondrial efficiency, superoxide dismutase activity,
and Bcl-2/Bax ratio. Cancer Res. 62:4592–4598. 2002.
|
|
35
|
Katiyar SK, Roy AM and Baliga MS:
Silymarin induces apoptosis primarily through a p53-dependent
pathway involving Bcl-2/Bax, cytochrome c release, and caspase
activation. Mol Cancer Ther. 4:207–216. 2005.PubMed/NCBI
|
|
36
|
Batra S, Reynolds CP and Maurer BJ:
Fenretinide cytotoxicity for Ewing’s sarcoma and primitive
neuroectodermal tumor cell lines is decreased by hypoxia and
synergistically enhanced by ceramide modulators. Cancer Res.
64:5415–5424. 2004.
|
|
37
|
Wang CC, Liu TY, Cheng CH and Jan TR:
Involvement of the mitochondrion-dependent pathway and oxidative
stress in the apoptosis of murine splenocytes induced by areca nut
extract. Toxicol In Vitro. 23:840–847. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xiao D, Powolny AA, Antosiewicz J, et al:
Cellular responses to cancer chemopreventive agent D,L-sulforaphane
in human prostate cancer cells are initiated by mitochondrial
reactive oxygen species. Pharm Res. 26:1729–1738. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang H, Kong X, Kang J, Su J, Li Y, Zhong
J and Sun L: Oxidative stress induces parallel autophagy and
mitochondria dysfunction in human glioma U251 cells. Toxicol Sci.
110:376–388. 2009. View Article : Google Scholar : PubMed/NCBI
|