Introduction
Bronchopulmonary neuroendocrine tumours (BP-NETs)
comprise approximately 20% of lung cancers and represent a large
spectrum of tumours arising from neuroendocrine cells of the
bronchopulmonary epithelium. Although they share structural,
morphological, immunohistochemical and ultrastructural features,
BP-NETs exhibit considerably different biological characteristics
and clinical behaviours. Based on their increasing biologic
aggressiveness, BP-NETs are separated into four different
subgroups: low-grade typical carcinoids (TCs), intermediate-grade
atypical carcinoids (ACs) and two high-grade malignancies,
large-cell neuroendocrine carcinomas (LCNECs) and small-cell lung
carcinomas (SCLCs) (1–3).
TCs and ACs account for ~1–2% of primary lung
cancers. The slow-growing TCs exhibit a fairly good prognosis,
although ~10–23% of cases when diagnosed metastasize to the
regional lymph nodes, with a 5-year overall survival rate ranging
from 82 and 100% (2–5). In contrast, ~40–50% of ACs metastasize
to the regional lymph nodes when diagnosed, with a 5-year overall
survival rate of ~50% (2–5).
SCLCs are the most common BP-NETs and account for
15–20% of invasive lung malignancies, while LCNECs are a very rare
neoplasia and represent <1% of lung cancers. LCNECs are
currently considered a distinct subtype of non-small cell lung
cancer (NSCLC) and are classified as a variant of large-cell
carcinomas (LCCs), whose diagnosis may be difficult to establish
because of the difficulties in distinguishing LCNECs from poorly
differentiated adenocarcinomas, squamous cell carcinomas and
basaloid carcinomas. In spite of the several differences between
high-grade LCNECs and SCLCs, both progress rapidly, are
aggressively metastatic and have a very poor prognosis with a
5-year overall survival rate of 15 to 57% and <5%, respectively
(2–5).
The management of BP-NETs is largely dependent on
the grade of differentiation (low-to-intermediate grade vs.
high-grade) and clinical stage when diagnosed (localized vs.
metastatic disease). Surgical resection is the treatment of choice
for low-to-intermediate grade and localized tumours, whereas
chemotherapy is generally preferred for high-grade and/or
disseminated lesions. However, traditional therapies, including
radiotherapy and systemic chemotherapy with DNA-damaging cytotoxic
agents, are not effective and offer limited benefits to patients
with advanced disease (6,7). The lack of effectiveness of
traditional agents for BP-NETs has led to an extensive exploration
of the molecular profile of these tumours in order to clarify the
molecular mechanisms of BP-NET carcinogenesis and progression and
to identify new targets for innovative therapies.
The phosphatidylinositol-3-kinase (PI3K)/Akt
signalling pathway plays a key role in essential cellular
processes, such as cell proliferation, cell growth, apoptosis,
metabolism, transcription, protein synthesis, angiogenesis, and
tissue invasion, and is involved in the pathogenesis of several
types of human cancers (8–11).
Class IA PI3K is a heterodimeric lipid kinase
composed of a regulatory subunit (p85α, p85β, p50α, p55α, or p55γ,
collectively called p85) and a catalytic subunit (p110α, p110β, or
p110δ, collectively called p110). Upon activation by multiple
receptor kinase families, including receptor tyrosine kinases and G
protein-coupled receptors, PI3K phosphorylates
phosphatidylinositol-4,5-bisphosphate (PIP2) to produce
phosphatidylinositol-3,4,5-trisphosphate (PIP3), a process that is
reversed by the lipid and protein phosphatase PTEN (phosphatase and
tensin homologue deleted on chromosome 10). PIP3 acts as a docking
site and recruits pleckstrin homology-domain containing proteins to
the plasma membrane, such as the serine/threonine kinase Akt and
phosphoinositide-dependent kinase 1 (PDK1). Once localized to the
plasma membrane, Akt is activated by phosphorylation on threonine
308 in the kinase domain and on serine 473 in the regulatory domain
by PDK1 and PDK2, respectively. The active Akt phosphorylates
multiple downstream targets namely involved in cell survival, cell
cycle progression, cell motility and metabolism (8–11).
Several studies have reported
phosphatidylinositol-3-kinase α catalytic subunit (PIK3CA)
gene amplification, deletions and somatic missense mutations in
several types of human cancers, including colorectal, breast and
hepatocellular carcinomas where these mutations occur in up to 30%
of the tumours examined (12–14).
PIK3CA is a 34 kb gene located on chromosome
3q26.3 and consists of 20 exons encoding for the p110α catalytic
subunit of PI3K. A number of PIK3CA missense mutations are
clustered in two PIK3CA mutational hotspots and affect
conserved regions within the helical (exon 9) and catalytic (exon
20) domains of p110α. The crystal structure of the complex between
p110α and p85α has revealed that a number of the cancer-associated
PIK3CA mutations occur at residues lying at the interfaces
between p110α and p85α or between the kinase domain of p110α and
other domains within the catalytic subunit. In vitro and
in vivo studies show that these mutations lead to enhanced
enzymatic activity, upregulation of the signalling cascade and
oncogenic transformation (15–17).
Due to the importance of the PI3K/Akt pathway in tumourigenesis and
the high frequency of PIK3CA gene mutations in human
cancers, small PI3K inhibitors are regarded as a promising strategy
for cancer treatment.
To date, the mutational status of the PIK3CA
gene in BP-NETs remains unknown. The aim of this study was to
analyse the mutational profile of the PIK3CA gene in a large
series of BP-NETs and to correlate the PIK3CA gene status
with the main clinicopathological parameters.
Materials and methods
Patient selection and tumour
characteristics
One hundred and ninety consecutive BP-NETs were
retrospectively collected from patients who had undergone surgery
at the Department of Cardio-Thoracic Surgery of the University of
Pisa between 2000 and 2009. Patients enrolled in this study did not
receive chemotherapy and/or radiotherapy before the surgery.
Histological diagnoses and pathological features
were reviewed independently by two pathologists (G.A. and G.F.) and
formulated according to the 2004 World Health Organization (WHO)
classification. Discrepant diagnoses were re-evaluated jointly and
discussed until an agreement was reached. Neuroendocrine
differentiation was detected by a positive immunohistochemical
staining for chromogranin A, synaptophysin and/or CD56 markers.
The selection of patients did not require approval
by the Institutional Ethics Committee since all samples were coded
and the names of the patients were not revealed.
DNA isolation
Genomic DNA was isolated from 10 μm sections of
formalin-fixed and paraffin-embedded tissues. Tissue digestion was
preceded by xylene treatment to remove paraffin, rehydration
through a graded series of alcohol and manual macrodissection of
the tumour area to obtain at least 70% of the tumour cells. Then,
genomic DNA was extracted using the QIAamp DNA mini kit (Qiagen)
according to the manufacturer’s instructions for paraffin-embedded
tissues. The DNA quality and quantity were evaluated using a
NanoDrop ND-1000 spectrophotometer.
Mutational analysis of the PIK3CA
gene
Mutational analysis of the PIK3CA gene
(Reference sequence ENSG00000121879) was performed by PCR
amplification and direct gene sequencing of the helical and kinase
domains of PI3K encoded by exons 9 and 20, respectively.
Primer pairs flanking PIK3CA exons 9 and 20
were selected to avoid the frequent cross-amplification of
chromosome 22q (a known PIK3CA pseudogene) using the
software Primer3 (http://frodo.wi.mit.edu/primer3/). The PIK3CA
gene was amplified for exon 9 with the primers 5′-ATCATCTGTG
AATCCAGA-3′ (forward) and 5′-TTAGCACTTACCTGTG AC-3′ (reverse) and
for the exon 20 with the primers 5′-TGAC ATTTGAGCAAAGACC-3′
(forward) and 5′-GTGTGGAAT CCAGAGTGA-3′ (reverse).
PCR amplification was performed in a total volume of
25 μl containing 100 ng of genomic DNA, 12.5 μl of HotStarTaq
master mix (Qiagen), 0.5 μl of each primer (20 μM), and water as
follows. After an initial denaturation step of 15 min at 95°C, the
reaction mixture was run for 40 cycles at 95°C for 30 sec, 50°C for
30 sec, and 72°C for 1 min, followed by a final elongation step at
72°C for 10 min. The efficiency and the quality of the PCR
amplification were confirmed by running the PCR products on a 1.5%
agarose gel.
The PCR products were subsequently subjected to a
purification procedure to remove primers, nucleotides, enzymes and
salts using the QIAquick PCR Purification kit (Qiagen).
Cycle sequencing analysis of the purified PCR
products was performed with the ABI BigDye Terminator version 3.1
Cycle Sequencing kit (Applied Biosystems) according to the
manufacturer’s instructions using the PCR amplification primers for
bidirectional sequencing, and the reaction products were purified
by ethanol precipitation. Finally, sequence determination was
performed using an ABI PRISM 3130 Genetic Analyzer (Applied
Biosystems) and data were analysed using the Sequencing Analysis
5.0 Software (Applied Biosystems).
Statistical analysis
Statistical analyses were performed using the
StatView 5.0 Software (Abacus Concepts, Inc., Berkeley, CA). The
contingency tables and the Chi-square test were used to analyse the
association between the different biological parameters. All the
tests were two-tailed and a P-value <0.05 was considered to
indicate a statistically significant difference.
Results
PI3KCA gene mutation in BP-NETs
To assess the frequency of PIK3CA gene
mutations in BP-NETs we sequenced genomic DNA isolated from
formalin-fixed and paraffin-embedded tissues in a large series of
lung neuroendocrine tumours, including 75 low-grade TCs, 23
intermediate-grade ACs, 17 high-grade LCNECs and 75 high-grade
SCLCs. Clinical and pathological characteristics of the patients,
including patient age, gender, histological type, tumour size, and
lymph node status were recorded whenever available and summarized
in Table I.
 | Table ICharacteristics of the BP-NET
patients. |
Table I
Characteristics of the BP-NET
patients.
| Clinicopathological
features | TC | AC | LCNEC | SCLC |
|---|
| Age (years) |
| Median
(range) | 61 (24–82) | 58 (23–82) | 68 (45–84) | 69 (49–83) |
| Gendera | n=75 | n=23 | n=17 | n=75 |
| Male | 31 (41.3) | 6 (26.0) | 13 (76.5) | 60 (80.0) |
| Female | 44 (58.7) | 17 (74.0) | 4 (23.5) | 15 (20.0) |
| Tumour sizea | n=74 | n=23 | n=16 | n=65 |
| T1 (T1a-T1b) | 50 (67.6) | 9 (39.1) | 4 (25.0) | 21 (32.3) |
| T2 (T2a-T2b) | 19 (25.7) | 11 (47.8) | 7 (43.7) | 29 (44.6) |
| T3 | 5 (6.7) | 3 (13.1) | 5 (31.3) | 15 (23.1) |
| Lymph node
statusa | n=65 | n=19 | n=13 | n=56 |
| Negative | 62 (95.4) | 13 (68.4) | 11 (84.6) | 34 (60.7) |
| Positive | 3 (4.6) | 6 (31.6) | 2 (15.4) | 22 (39.3) |
Targeted sequencing of the helical and kinase
domains of PI3K revealed that PIK3CA gene mutations were
present in 44 of the 190 analysed tumours (23.2%). The distribution
of mutations between the kinase and the helical domains of PI3K
showed that the mutation frequency of the kinase domain (34/190,
17.9%) was approximately three times the mutation frequency of the
helical domain (10/190, 5.3%) (Table
II).
 | Table IIPIK3CA gene status in BP-NET
patients. |
Table II
PIK3CA gene status in BP-NET
patients.
| Exon | PIK3CA
Domain | ID Sample | Histology | Nucleotide
substitution | Amino acid
substitution | No. of cases |
|---|
| 9 | Helical | N145 | SCLC | c.1573 G>A | p.E525K | 1 |
| 9 | Helical | N56 | TC | c.1576 A>G | p.N526D | 1 |
| 9 | Helical | N170 | SCLC | c.1624 G>A | p.E542K | 1 |
| 9 | Helical | N20, N46, N50 | TC | c.1639 G>A | p.E547K | 3 |
| 9 | Helical | N34 | AC | c.1639 G>A | p.E547K | 1 |
| 9 | Helical | N18, N33, N59 | SCLC | c.1639 G>A | p.E547K | 3 |
| 20 | Kinase | N188 | SCLC | c.2944 G>C | p.E982Q | 1 |
| 20 | Kinase | N101 | SCLC | c.2949 G>A | p.M983I | 1 |
| 20 | Kinase | N172 | TC | c.2993 T>C | p.F998S | 1 |
| 20 | Kinase | N173 | SCLC | c.2998 A>G | p.N1000D | 1 |
| 20 | Kinase | N116 | SCLC | c.3007 T>C | p.S1003P | 1 |
| 20 | Kinase | N129 | AC | c.3007 T>C | p.S1003P | 1 |
| 20 | Kinase | N97 | SCLC | c.3012 G>T | p.M1004I | 1 |
| 20 | Kinase | N8 | AC | c.3017 T>C | p.L1006P | 1 |
| 20 | Kinase | N35 | SCLC | c.3016 C>T | p.L1006F | 1 |
| 20 | Kinase | N167 | SCLC | c.3022 T>C | p.S1008P | 1 |
| 20 | Kinase | N176 | AC | c.3022 T>C | p.S1008P | 1 |
| 20 | Kinase | N125 | SCLC | c.3032 C>T | p.P1011L | 1 |
| 20 | Kinase | N26 | TC | c.3034 G>A | p.E1012K | 1 |
| 20 | Kinase | N95 | AC | c.3041 A>G | p.Q1014R | 1 |
| 20 | Kinase | N182 | AC | c.3050 A>T | p.D1017V | 1 |
| 20 | Kinase | N67 | AC | c.3062 A>G | p.Y1021C | 1 |
| 20 | Kinase | N143 | SCLC | c.3062 A>T | p.Y1021F | 1 |
| 20 | Kinase | N88, N157 | SCLC | c.3061 T>A | p.Y1021N | 2 |
| 20 | Kinase | N159 | TC | c.3061 T>A | p.Y1021N | 1 |
| 20 | Kinase | N17 | SCLC | c.3068 G>A | p.R1023Q | 1 |
| 20 | Kinase | N119 | AC | c.3068 G>A | p.R1023Q | 1 |
| 20 | Kinase | N138 | SCLC | c.3074 C>T | p.T1025I | 1 |
| 20 | Kinase | N115 | SCLC | c.3085 G>C | p.D1029H | 1 |
| 20 | Kinase | N14 | SCLC | c.3110 A>G | p.E1037G | 1 |
| 20 | Kinase | N11 | SCLC | c.3115 T>C | p.F1039L | 1 |
| 20 | Kinase | N152 | TC | c.3133 G>A | p.D1045N | 1 |
| 20 | Kinase | N45 | SCLC | c.3140 A>G | p.H1047R | 1 |
| 20 | Kinase | N102 | LCNEC | c.3140 A>G | p.H1047R | 1 |
| 20 | Kinase | N62, N82 | TC | c.3145 G>A | p.G1049S | 2 |
| 20 | Kinase | N114 | LCNEC | c.3145 G>A | p.G1049S | 1 |
| 20 | Kinase | N121 | SCLC | c.3145 G>A | p.G1049S | 1 |
| 20 | Kinase | N186 | AC | c.3155 C>T | p.T1052I | 1 |
| Total PIK3CA
gene mutationsa | 44/190 (23.2%) |
| Total mutations in
the helical domain of the PIK3CA genea | 10/190 (5.3%) |
| Total mutations in
the kinase domain of the PIK3CA genea | 34/190 (17.9%) |
All the mutations identified in our series of
BP-NETs were single nucleotide substitutions that lead to
non-synonymous mutations, including 36 transversions (G↔A or C↔T)
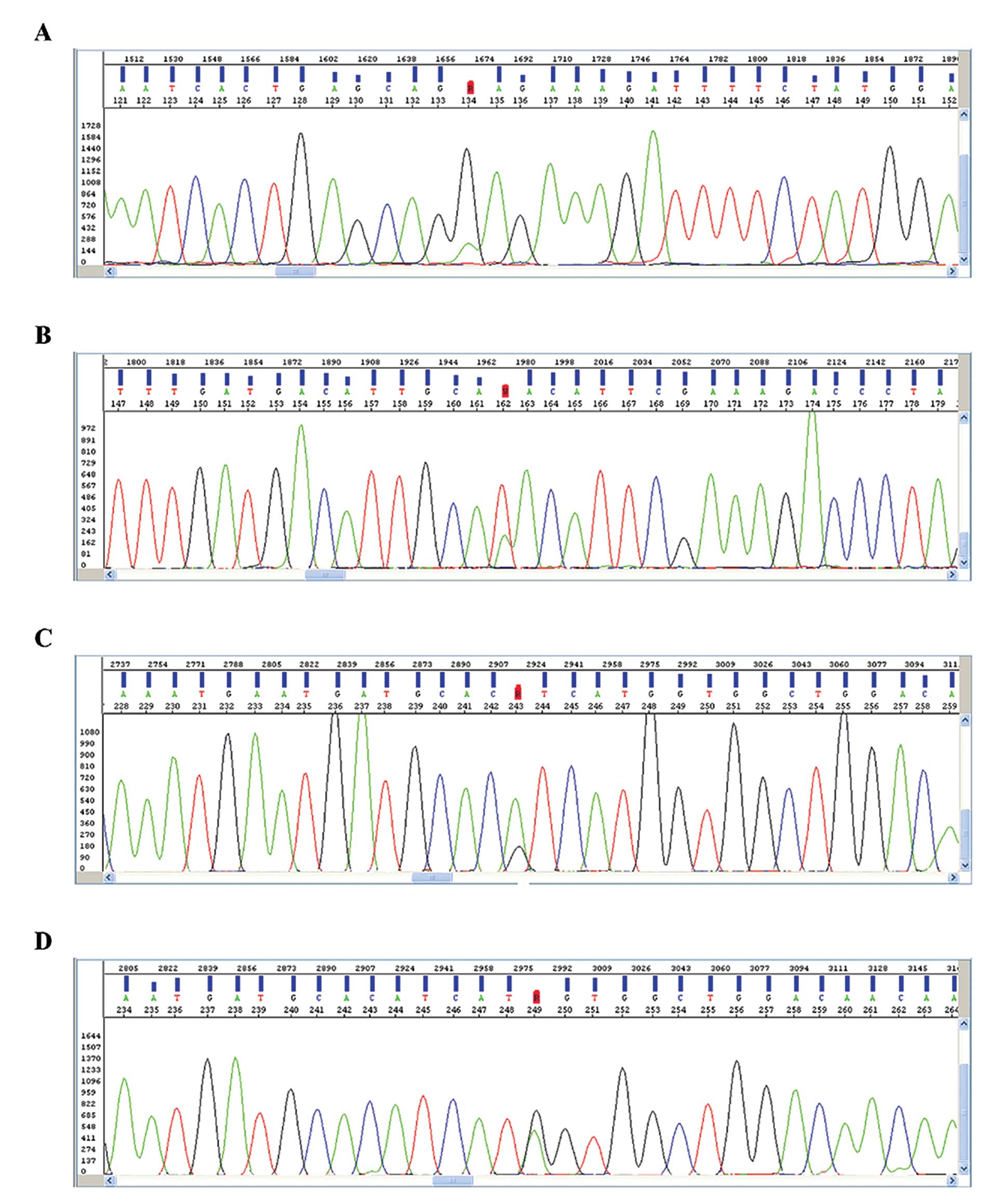
and 8 transitions (A↔T, G→T or G→C) (Table II). The most frequent genetic
alteration was the E547K mutation in the helical domain accounting
for 15.9% (7/44) of the PIK3CA gene mutations identified in
our study (Table II and Fig. 1A). In the kinase domain we
identified 3 different mutational hotspots at codons 1021, 1047,
and 1049. The most prevalent genetic anomalies observed in these
codons were the mutations Y1021N/F/C (5/44, 11.4%), H1047R (2/44,
4.5%) and G1049S (4/44, 9.1%) (Table
II and Fig. 1B-D).
Correlation between the presence of
PIK3CA gene mutations and clinical and pathological parameters
The mutational status of the PIK3CA gene was
compared with the main clinical and pathological characteristics of
the BP-NET patients. The PIK3CA gene mutations did not
correlate with the clinical and pathological characteristics of the
patients, such as age, gender, or lymph node status (Table III). However, statistical analysis
showed a significant association between the PIK3CA gene
mutations in the helical and kinase domains and BP-NET histology
(Chi-square test, P=0.011). Our results showed a relatively lower
prevalence of PIK3CA gene mutations in the low-grade TCs
(10/75, 13.3%) compared to the intermediate-grade ACs (9/23, 39.1%)
and high-grade SCLCs (23/75, 30.7%). In contrast to the SCLCs, the
high-grade LCNECs showed a lower frequency of PIK3CA gene
mutations (2/17, 11.8%) compared with the other types of BP-NETs
(Table III).
 | Table IIICorrelations between PIK3CA
gene status and clinicopathological characteristics of patients
with BP-NETs. |
Table III
Correlations between PIK3CA
gene status and clinicopathological characteristics of patients
with BP-NETs.
| Clinicopathological
features | PIK3CA
wild-typea | PIK3CA
mutanta | P-valueb |
|---|
| Gender |
| Male | 84 (76.4) | 26 (23.6) | 0.992 |
| Female | 62 (77.5) | 18 (22.5) | |
| Age (years) |
| <66 | 69 (77.5) | 20 (22.5) | 0.979 |
| ≥66 | 72 (76.5) | 22 (23.5) | |
| Tumour size |
| T1 (T1a-T1b) | 67 (79.8) | 17 (20.2) | 0.972 |
| T2 (T2a-T2b) | 50 (75.7) | 16 (24.3) | |
| T3 | 21 (75.0) | 7 (25.0) | |
| Lymph node
status |
| Negative | 95 (79.2) | 25 (20.8) | 0.641 |
| Positive | 24 (72.7) | 9 (27.3) | |
| Histology |
| TC | 65 (66.6) | 10 (13.3) | |
| AC | 14 (60.9) | 9 (39.1) | 0.011 |
| LCNEC | 15 (88.2) | 2 (11.8) | |
| SCLC | 52 (69.3) | 23 (30.7) | |
Discussion
BP-NETs comprise a large spectrum of lung cancers
ranging from low-grade TCs, to intermediate-grade ACs, to
high-grade LCNECs and SCLCs that exhibit considerably different
biological aggressiveness and clinical behaviour. At present, the
only curative treatment for BP-NETs is radical surgery since
traditional therapies are not effective (1,2,5,7).
Recently, successful clinical trials of imatinib,
gefitinib/erlotinib and trastuzumab, which are specific for
BCR/ABL translocations (18), epidermal growth factor receptor
(EGFR) mutations (19,20)
and HER-2/neu amplifications (21), respectively, have illustrated the
ability to develop drugs that target genetic abnormalities and lead
to potential streamlined therapies based on the genomic landscape
of an individual’s cancer.
Several studies have shown that the dysregulation of
the PI3K/Akt pathway is involved in cancer pathogenesis and
prognosis, and PIK3CA gene mutations have been reported in
several types of human cancers, including colorectal, breast and
hepatocellular carcinomas (10–14).
The functional activation of the PI3K/Akt pathway
has been investigated in the spectrum of pulmonary or other
neuroendocrine tumours only by the indirect evidence of the
expression of functionally related molecules, such as PTEN
(22), tuberous sclerosis complex
(TSC) (23) and mammalian target of
rapamycin (mTOR) (24,25). However, the mutational status of the
PIK3CA gene in BP-NETs remains unknown. This study aimed to
explore the mutational profile of the PIK3CA gene in a large
series of BP-NETs and to determine the correlation of the
PIK3CA status with the main clinico-pathological
parameters.
A number of the somatic mutations involving the
PIK3CA gene are clustered in exons 9 and 20, which encodes
for PI3K helical and kinase domains, respectively (16,17).
Previous studies have analysed PIK3CA gene mutation in NSCLC
and have demonstrated that its frequency is relatively low
(3.4–4.3%) compared to that observed in other tumours, such as
breast, colon and ovarian cancers. (12–14).
To the best of our knowledge, we demonstrated for
the first time a high prevalence of somatic missense mutations
(23.2%) in the PIK3CA gene in human BP-NETs that is
comparable to the mutational frequency of the PIK3CA gene in
other types of human cancers (12–14,26,27).
Moreover, our data are in agreement with the results obtained by
Shibata et al(28) who have
shown three mutations of the PIK3CA gene in 13 SCLC cell
lines (23%) in an extensive mutational screening. The high
frequency of the PIK3CA gene mutation in our series of
BP-NETs as in several other aggressive human tumours, highlights
that somatic mutations of this gene are an important genetic event
in BP-NET tumourigenesis and may represent a potentially effective
therapeutic target for these types of tumours.
The analysis of the distribution of mutations
between the kinase and the helical domains of PI3K in our series of
BP-NETs revealed that the frequency of the kinase domain mutations
(17.9%) was approximately three times the frequency of mutations in
the helical domain (5.3%) according to the findings reported by
other authors in different types of human cancers (12–14).
We identified four different mutational hotspots: the codon 547 in
the helical domain accounting for 15.9% of the PIK3CA gene
mutations identified in our study and codons 1021, 1047 and 1049 in
the kinase domain that together represent 25% of the identified
mutations. Several studies have demonstrated a direct connection
between mutations in the helical and kinase domain of PI3K and
carcinogenesis as well as the prognosis of colorectal and breast
cancers (29,30). The probable mechanisms for the
oncogenicity of these mutations are the disruption of an inhibitory
charge-charge interaction between p110α and the N-terminal SH2
domain of the p85 regulatory subunit and the increased binding
affinity of p110α for the negatively charged phosphatidylinositol
substrate, as it has been demonstrated by crystallographic and
biochemical studies (17,31).
In our current study, a statistically significant
correlation was not observed between PIK3CA mutations and
the main clinical and pathological characteristics of the patients,
such as age, gender or lymph node status. However, we found that
the frequency of PIK3CA gene mutations was significantly
associated with BP-NET histology (P=0.011). Interestingly, the
prevalence of PIK3CA gene mutations increases parallel to
the biological aggressiveness of the BP-NETs, since it was
relatively lower in the low-grade TCs (13.3%) compared to the
intermediate-grade ACs (39.1%) and high-grade SCLCs (30.7%).
Notably, the high-grade LCNECs demonstrated an unexpected lower
frequency of PIK3CA gene mutations (11.8%) compared with the
other types of lung neuroendocrine tumours. LCNECs are relatively
uncommon tumours, accounting for ~1% of the resected primary lung
cancers and represent a controversial entity from both the
diagnostic and clinical point of view (2–5). The
diagnosis of high-grade neuroendocrine tumours of the lung requires
to demonstrate the histopathologic neuroendocrine morphology and
the neuroendocrine differentiation using immunohistochemistry or
electron microscopy. However, LCNECs are a poorly recognized and
underdiagnosed entity as a result of the difficultly in recognizing
neuroendocrine morphology and are frequently mistaken for poorly
differentiated NSCLCs, ACs and intermediate cell-type SCLCs
(5,32,33).
Furthermore, although a previous retrospective report demonstrated
that the survival rate of patients with surgically resected LCNECs
and SCLCs were closely related to each other and inferior to that
of patients with TCs and ACs (5),
there is no clear-cut evidence concerning the optimal treatment for
LCNECs, and therapeutic approaches adopted for SCLCs are not
considered effective for patients with LCNEC, thus questioning
whether LCNECs are best classified and treated as SCLC or LCC
patients (5,32,33).
Our results demonstrated that PIK3CA gene
mutations were more common in ACs and SCLCs than in LCNECs,
suggesting that LCNECs, athough sharing several similarities with
SCLCs on morphological, immunohistochemical and molecular grounds,
represent a distinct biological entity. In a wide retrospective
study, Varlotto et al, demonstrated that clinical as well as
histopathologic and biological characteristics of LCNECs more
closely resemble LCCs than SCLCs (33). The differences in the PIK3CA
gene mutational status between LCNECs and ACs or SCLC observed in
our study may suggest that a different signalling pathway such as
the MAPK pathway or other receptor tyrosine kinases (RTKs), but not
PI3K, may play a key role in the tumourigenesis of the former type
of BP-NETs. In support of this hypothesis, Rossi et al
demonstrated that LCNECs overexpress several RTKs, including KIT,
the platelet-derived growth factor receptors α (PDGFRα) and β
(PDGFRβ), and MET in a number of patients, whereas they failed to
find a significant expression in other NSCLCs and carcinoids
(32,34).
An important implication of this study is the
possibility of applying our results in the clinics. PIK3CA
mutations have been associated with paclitaxel resistance in breast
epithelial cells and the PI3K/Akt pathway has been linked with
resistance to a number of other cancer therapies (35). In experimental models of human
pulmonary carcinoid and SCLC cells, the inhibition of the PI3K/Akt
pathway by LY294002 and Tricribine, respectively, significantly
reduced cellular growth and neuroendocrine marker expression in
vitro and increased apoptosis and sensitivity to
chemotherapeutic treatments (28,36).
Since mutations in the PIK3CA gene result in constitutively
active PI3K activity, the presence of PIK3CA mutations may
allow for the selection of patients with a high response rate to
novel and targeted strategies of treatment based on the development
of compounds designed to target PI3K or more feasibly downstream
effectors within the PI3K/Akt signalling pathway.
In conclusion, our results strongly suggest that
PIK3CA genetic alterations may play an extensive and
fundamental role in the tumourigenesis and aggressiveness of
BP-NETs and specific-based targeting at the PI3K/Akt signalling
pathway may be an effective therapeutic strategy for BP-NET
treatment.
References
|
1
|
Travis WD, Brambilla E, Muller-Hermelink
HD and Harris CC: World Health Organization classification of
tumors. Pathology and Genetics of Tumors of the Lung, Pleura,
Thymus and Hearth. IARC Press; Lyon: 2004
|
|
2
|
Righi L, Volante M, Rapa I, Scagliotti GV
and Papotti M: Neuro-endocrine tumours of the lung. A review of
relevant pathological and molecular data. Virchows Arch.
451:S51–S59. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gustafsson BI, Kidd M, Chan A,
Malfertheiner MV and Modlin IM: Bronchopulmonary neuroendocrine
tumors. Cancer. 113:5–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cooper WA, Thourani VH, Gal AA, Lee RB,
Mansour KA and Miller JI: The surgical spectrum of pulmonary
neuroendocrine neoplasms. Chest. 119:14–18. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asamura H, Kameya T, Matsuno Y, Noguchi M,
Tada H, Ishikawa Y, Yokose T, Jiang SX, Inoue T, Nakagawa K, Tajima
K and Nagai K: Neuroendocrine neoplasms of the lung: a prognostic
spectrum. J Clin Oncol. 24:70–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
García-Yuste M, Matilla JM and
González-Aragoneses F: Neuroendocrine tumors of the lung. Curr Opin
Oncol. 20:148–154. 2008.
|
|
7
|
Srirajaskanthan R, Toumpanakis C,
Karpathakis A, Marelli L, Quigley AM, Dusmet M, Meyer T and Caplin
ME: Surgical management and palliative treatment in bronchial
neuroendocrine tumours: a clinical study of 45 patients. Lung
Cancer. 65:68–73. 2009. View Article : Google Scholar
|
|
8
|
Katso R, Okkenhaug K, Ahamdi K, White S,
Timms J and Waterfield MD: Cellular function of phosphoinositide
3-kinases: implications for development, homeostasis, and cancer.
Annu Rev Cell Dev Biol. 17:615–675. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vivanco I and Sawyers CL: The
phosphatidylinositol-3-kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fresno Vara JA, Casado E, De Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004.PubMed/NCBI
|
|
12
|
Samuels Y, Wang ZH, Bardelli A, Silliman
N, Ptak J, Szabo S, Yan H, Gazdar A, Powell DM, Riggins GJ, et al:
High frequency of mutations of the PIK3CA gene in human cancers.
Science. 304:5542004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bachman KE, Argani P, Samuels Y, Silliman
N, Ptak J, Szabo S, Konishi H, Karakas B, Blair BG, Lin C, et al:
The PIK3CA gene is mutated with high frequency in human breast
cancers. Cancer Biol Ther. 3:772–775. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee JW, Soung YH, Kim SY, Lee HW, Park WS,
Nam SW, Kim SH, Lee JY, Yoo NJ and Lee SH: PIK3CA gene is
frequently mutated in breast carcinomas and hepatocellular
carcinomas. Oncogene. 24:1477–1480. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bader AG, Kang S, Zhao L and Vogt PK:
Oncogenic PI3K deregulates transcription and translation. Nat Rev
Cancer. 5:921–929. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Karakas B, Bachman KE and Park BH:
Mutation of the PIK3CA oncogene in human cancers. Br J Cancer.
94:455–459. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang CH, Mandelker D, Gabelli SB and
Amzel LM: Insights into the oncogenic effects of PIK3CA mutations
from the structure of p110alpha/p85alpha. Cell Cycle. 7:1151–1156.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maekawa T, Ashihara E and Kimura S: The
Bcr-Abl tyrosine kinase inhibitor imatinib and promising new agents
against Philadelphia chromosome-positive leukemias. Int J Clin
Oncol. 12:327–340. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Paez JG, Janne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Romond EH, Perez EA, Bryant J, Suman VJ,
Geyer CE, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA,
et al: Trastuzumab plus adjuvant chemotherapy for operable
HER2-positive breast cancer. N Engl J Med. 353:1673–1684. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang L, Ignat A and Axiotis CA:
Differential expression of the PTEN tumor suppressor protein in
fetal and adult neuroendocrine tissues and tumors: progressive loss
of PTEN expression in poorly differentiated neuroendocrine
neoplasms. Appl Immunohistochem Mol Morphol. 10:139–146. 2002.
View Article : Google Scholar
|
|
23
|
Yao JC: Neuroendocrine tumors. Molecular
targeted therapy for carcinoid and islet-cell carcinoma. Best Pract
Res Clin Endocrinol Metab. 21:163–172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Righi L, Volante M, Rapa I, Tavaglione V,
Inzani F, Pelosi G and Papotti M: Mammalian target of rapamycin
signalling activation patterns in neuroendocrine tumors of the
lung. Endocr Relat Cancer. 17:977–987. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alì G, Boldrini L, Capodanno A,
Pelliccioni S, Servadio A, Crisman G, Picchi A, Davini F, Mussi A
and Fontanini G: Expression of p-AKT and p-mTOR in a large series
of bronchopulmonary neuroendocrine tumors. Exp Ther Med. 2:787–792.
2011.PubMed/NCBI
|
|
26
|
Campbell IG, Russell SE, Choong DYH,
Montgomery KG, Ciavarella ML, Hooi CSF, Cristiano BE, Pearson RB
and Phillips WA: Mutation of the PIK3CA gene in ovarian and breast
cancer. Cancer Res. 64:7678–7681. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li VSW, Wong CW, Chan TL, Chan ASW, Zhao
W, Chu KM, So S, Chen X, Yuen ST and Leung SY: Mutations of PIK3CA
in gastric adenocarcinoma. BMC Cancer. 5:292005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shibata T, Kokubu A, Tsuta K and Hirohashi
S: Oncogenic mutation of PIK3CA in small cell lung carcinoma: a
potential therapeutic target pathway for chemotherapy-resistant
lung cancer. Cancer Lett. 283:203–211. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Farina Sarasqueta A, Zeestraten EC, van
Wezel T, van Lijnschoten G, van Eijk R, Dekker JW, Kuppen PJ,
Goossens-Beumer IJ, Lemmens VE, van de Velde CJ, et al: PIK3CA
kinase domain mutation identifies a subgroup of stage III colon
cancer patients with poor prognosis. Cell Oncol. 34:523–531.
2011.PubMed/NCBI
|
|
30
|
Ellis MJ, Lin L, Crowder R, Tao Y, Hoog J,
Snider J, Davies S, DeSchryver K, Evans DB, Steinseifer J, et al:
Phosphatidyl-inositol-3-kinase alpha catalytic subunit mutation and
response to neoadjuvant endocrine therapy for estrogen receptor
positive breast cancer. Breast Cancer Res Treat. 119:379–390. 2010.
View Article : Google Scholar
|
|
31
|
Miled N, Yan Y, Hon WC, Perisic O,
Zvelebil M, Inbar Y, Schneidman-Duhovny D, Wolfson HJ, Backer JM
and Williams RL: Mechanism of two classes of cancer mutations in
the phosphoinositide 3-kinase catalytic subunit. Science.
317:239–242. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rossi G, Cavazza A, Marchioni A, Longo L,
Migaldi M, Sartori G, Bigiani N, Schirosi L, Casali C, Morandi U,
et al: Role of chemotherapy and the receptor tyrosine kinases KIT,
PDGFRalpha, PDGFRbeta, and Met in large-cell neuroendocrine
carcinoma of the lung. J Clin Oncol. 23:8774–8785. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Varlotto JM, Medford-Davis LN, Recht A,
Flickinger JC, Schaefer E, Zander DS and DeCamp MM: Should large
cell neuroendocrine lung carcinoma be classified and treated as a
small-cell lung cancer or with other large cell carcinomas? J
Thorac Oncol. 6:1050–1058. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rossi G, Cavazza A, Marchioni A, Migaldi
M, Bavieri M, Facciolongo N, Petruzzelli S, Longo L, Tamberi S and
Crinò L: Kit expression in small cell carcinomas of the lung:
effects of chemotherapy. Mod Pathol. 16:1041–1047. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gustin JP, Cosgrove DP and Park BH: The
PIK3CA gene as a mutated target for cancer therapy. Curr Cancer
Drug Targets. 8:733–740. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pitt SC, Chen H and Kunnimalaiyaan M:
Phosphatidylinositol 3-kinase-Akt signalling in pulmonary carcinoid
cells. J Am Coll Surg. 209:82–88. 2009. View Article : Google Scholar : PubMed/NCBI
|