Introduction
Colorectal carcinoma (CRC) is one of the most
commonly diagnosed cancers which remains a leading cause of
cancer-related death and ~50% of patients with colorectal cancer
develop synchronous or metachronous liver metastases (1). The 5-year survival rate of colorectal
cancer patients with metastatic disease is <10% (2). Understanding the biological mechanism
of metastasis is important for development of new treatment
strategies and markers predictive of metastasis. Several recent
studies have implied that targeted therapies in conjunction with
chemo- or radiotherapy is a potential approach for a rational
molecular-based tumor therapy in colorectal cancer (3,4).
PrPc is a ubiquitous glycoprotein highly expressed
in neurons, mostly anchored at the cell surface via a C-terminal
glycosylphosphatidylinositol (GPI) moiety. The best described
property of PrPc is its high affinity for Cu(II), which suggests
that PrPc could participate in copper metabolism and protection
against oxidative stress (5).
Indeed, primary cultures of neurons from PrP−/− mice
exhibit increased susceptibility to oxidative stress (6). Also, PrPc has been proposed to be
involved in neurite outgrowth and neuronal survival in cell
cultures of primary neurons (7).
Recently, emerging evidence have indicated that
cellular prion protein (PrPc) may be implicated in tumor cell
biology (8–11). PrPc overexpression is correlated to
the acquisition by tumor cells of a phenotype for resistance to
cell death induced by antitumor drugs. Upon temozolomide treatment
in gliomas, PrPc exerts its antiapoptotic activity by inhibiting
PKA-mediated par-4 phosphorylation at the T155 residue that are
important for par-4 activation, nuclear entry and initiation of
apoptosis (12). Also, PrPc
significantly promoted the adhesive, invasive, and in vivo
metastatic capacities of the gastric cancer cells (11). Moreover, overexpression of PrPc
accelerated the proliferation of gastric cancer cells through
transcriptional activation of cyclin D3 to faciliate G1/S-phase
transition (13). Therefore, PrPc
seems to serve as a promising target for novel anti-cancer
therapies.
We demonstrate that PrPc expression is associated
with metastatic potential of colorectal cancer cells since it
mediates invasive and metastatic capacities by regulating SATB1
expression via epigenetic activation of Fyn-SP1 pathway. PrPc
depletion inhibits tumor metastasis of colorectal cancer both in
vivo and in vitro. Based on above, these studies suggest
PrPc as a promising therapeutic target against advanced metastatic
human CRCs.
Materials and methods
Cell lines
The human colorectal carcinoma cell line SW480 was
purchased from American Type Culture Collection (Manassas, VA,
USA). LIM 2405 cells, which derive from a poorly differentiated
primary human colon adenocarcinoma, were obtained from Dr R.
Whitehead (Ludwig Institute, Melbourne, Australia). Cells were
maintained in growth medium at 37°C in a humidified incubator.
Immunoblotting
Total protein was extracted from cells using RIPA
lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Protein extract (50 μg/lane) was electrophoresed, transferred to
PVDF membranes, and incubated overnight with primary antibodies
against PrPc, E-cadherin, N-cadherin (all Santa Cruz
Biotechnology), SATB1 and β-actin (both Sigma-Aldrich, St. Louis,
MO, USA). Membranes were then treated with the appropriate
HRP-conjugated secondary antibodies (Invitrogen, Carlsbad, CA,
USA). Detection was carried out using the reagents provided in the
ECL Plus kit (GE Healthcare, Milwaukee, WI, USA).
Quantitative real-time PCR
qPCR was performed using TaqMan probes from Applied
Biosystems, according to the manufacturer’s instructions. Reactions
were carried out in an ABI 7000 sequence detector (Perkin-Elmer)
and results were expressed as fold change calculated by the ΔΔCt
method relative to the control sample or to the first sample
quantified. GAPDH or β-actin was used as internal normalization
controls.
In vitro migration and invasion
assays
A bioassay for in vitro cell
migration/invasion using Matrigel Invasion Chambers (Corning
Costar, Corning, NY, USA) was performed as described previously
(14).
Chromatin immunoprecipitation
LIM2405 cells were transfected with PrPc siRNA for
72 h prior to carrying out ChIP using antibodies against SP1. A
total of 1.0–2.5% of each immunoprecipitation was assayed by PCR
using primers specific for a region of interest.
Luciferase reporter assays
Cells in 24-well plates were transiently transfected
with different SATB1 promoter reporter constructs and pRLTK
Renilla luciferase plasmid (Promega) using FuGENE 6 (Roche). Cell
extracts were prepared 48 h after transfection, and luciferase
activity was measured using the dual-luciferase reporter assay
system (Promega).
Immunofluorescence microscopy
analysis
Immunofluorescence microscopy analysis using
antibodies against β-catenin (Sigma) was carried out as described
previously (14).
In vitro kinase assays
For studies of Fyn activation in the presence and
absence of PrPc, in vitro kinase assays were carried out on
Fyn, as previously described (15).
Animal studies
Indicated tumor cells were orthotopically inoculated
into the serosa of the intestine after the laparotomy. The growth
and metastasis of the tumors were monitored by weekly
bioluminescence imaging using intravital imaging system (Nikon).
End-point assays were conducted 7 weeks after the inoculation
unless animal sacrifice was required because of significant
morbidity. The animal welfare guidelines of the Huashan Hospital’s
Research Institute Institutional Animal Use and Care Committee were
followed and the experimental protocol was approved by the
committee.
Patient study
Patients were identified from the HuaShan Hospital
records. The ethics committees of the participating hospital
approved the written informed consent for the collection of tumor
tissue obtained from each patient. Histological classification of
colorectal carcinomas proposed by the World Health Organization was
adopted. Three-micrometer sections were cut from paraffin blocks
onto silanized slides, and the sections were immunostained using
antibodies against PrPc (Sigma), E-cadherin (BioGenex, San Ramon,
CA, USA), and vimentin (Sigma) respectively.
Statistical analyses
Statistical analyses were calculated by SPSS
software (SPSS, Chicago, IL, USA). The results are presented as the
mean ± SEM. ANOVA, Student’s t-test analysis, and Dunnett’s
multiple comparison tests were used to compare mean values.
P<0.05 was consiedered statistically significant.
Results
PrPc expression in colorectal carcinoma
tissues
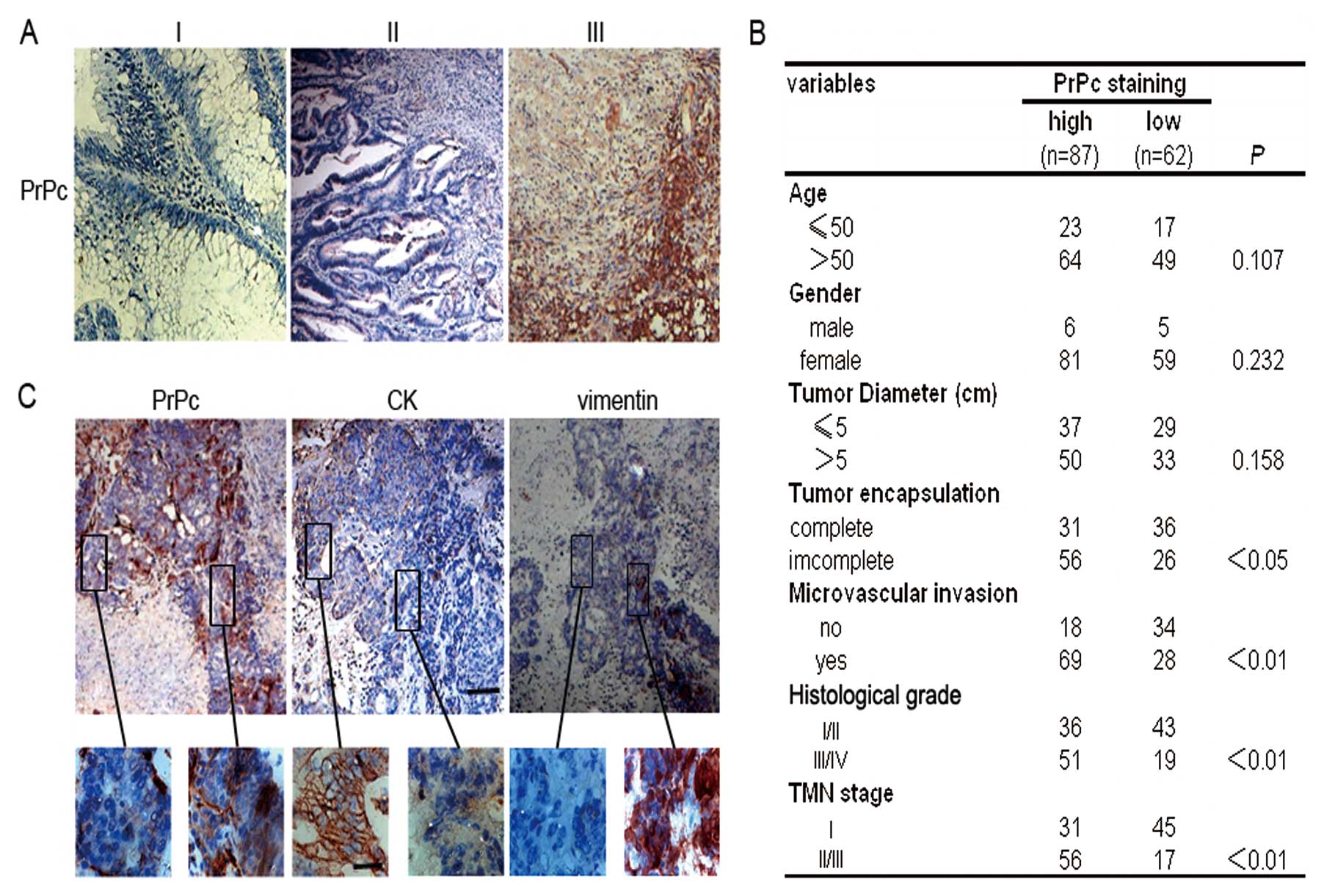
Histological analysis revealed that PrPc, primarily
located at the membrane/cytoplasm, were significantly increased in
tumor tissues compared with those in adjacent normal mucosal
tissues (Fig. 1A). The Pearson’s
χ2 test indicated that PrPc expression was closely
associated with tumor encapsulation, microvascular invasion,
histological grade and TNM stage. Furthermore, we found that the
expression of PrPc did not correlate with other clinicopathological
characteristics such as age, gender or tumor diameter (Fig. 1B). Interestingly, all samples from
CRC patients reproducibly demonstrated that PrPc-positive cells
were sporadically distributed within the tumor parenchyma, but were
arranged in dense clusters at the invasive front of the tumor.
PrPc-positive cells were often found in more differentiated
epithelial cells but were essentially negative for the epithelial
marker cytokeratin and positive for the mesenchymal marker vimentin
(Fig. 1C).
PrPc promotes metastatic potential of CRC
cells
It has been reported that EMT-like cell
dedifferentiation takes place at the invasive front of CRCs
(16). Combined with the fact that
higher expression of PrPc at the invasive front was noted in CRC
tissues, we hypothesized that PrPc might be associated with the
epithelial-mesenchymal transition (EMT). To confirm the
relationship between them, we investigated the effects of PrPc
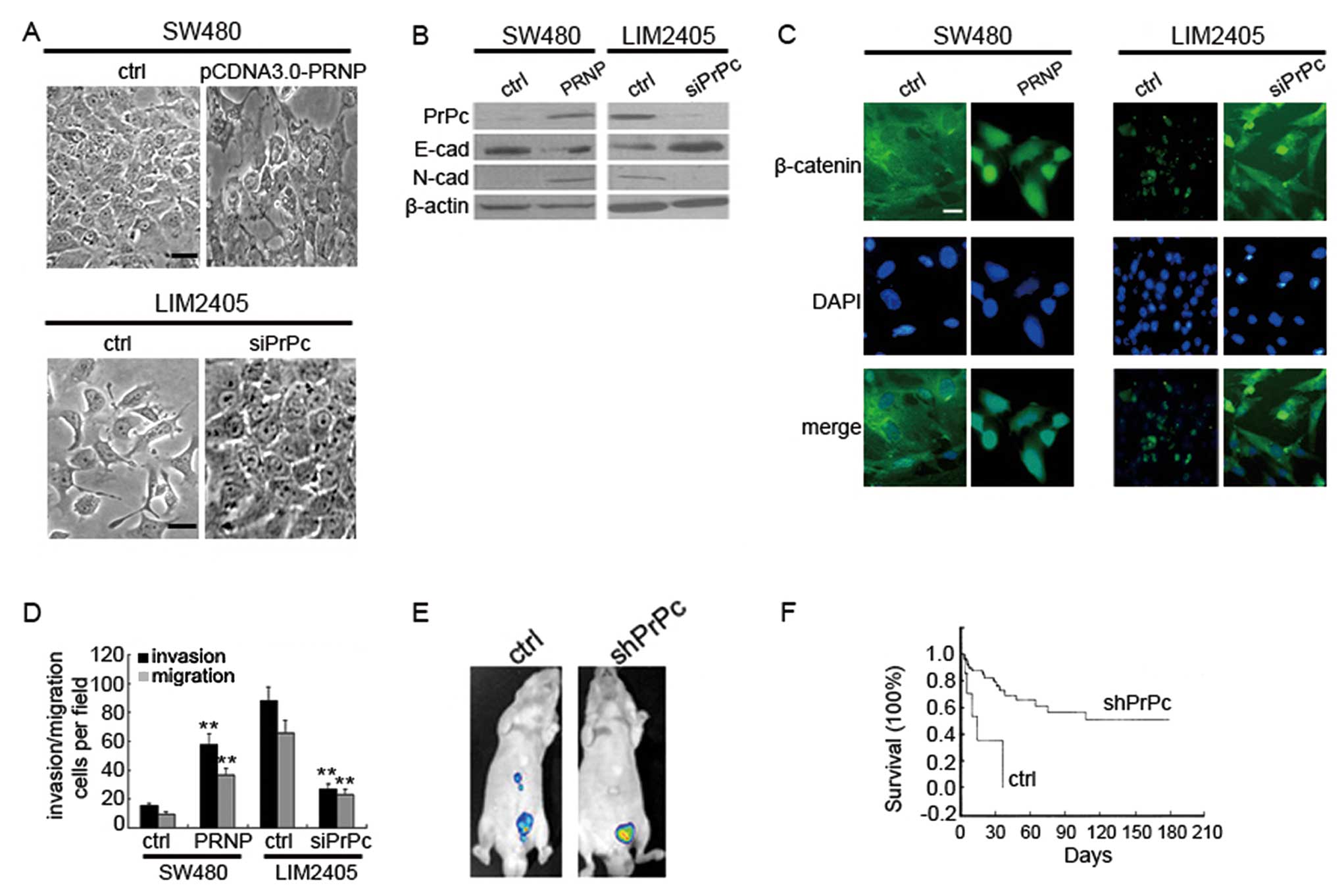
expression on the phenotypic changes of CRC cells. We observed that
while SW480 cells grew as tightly packed colonies characteristic of
epithelial cells, cells treated with pCDNA3.0-PRNP appeared
actively spreading and had lost the majority of their cell-cell
contacts (Fig. 2A). These phenomena
were associated with decreased expression of E-cadherin,
up-regulation of N-cadherin and translocation of β-catenin from
membrane to nucleus (Fig. 2B and
C). Conversely, mesenchymal-like LIM2405 cells transfected with
PrPcsiRNA obtained a characteristic epithelial porphology (Fig. 2A) and displayed downregulated
N-cadherin, upregulated E-cadherin and cytoplasmic restoration of
nuclear β-catenin (Fig. 2B and C).
Thus, it can be concluded that PrPc induces EMT in epithelial CRC
cells.
EMT contributes to invasive and metastatic tumor
growth. In vitro transwell migration assays showed that PrPc
promoted the migration of SW480 cells, while inhibition of PrPc
reduced the motility of LIM2405 cells (Fig. 2D). We also orthotopically implanted
GFP-expressing and shPRNP-treated cells in SCID mice. Although no
significant growth-inhibitory effect was observed in the treated
groups as compared with the control groups, LIM2405 cells formed a
greater number of metastases than LIM2405-shRNA cells in the liver
(Fig. 2E). Seventeen weeks after
treatment, the Kaplan-Meier plot assessment showed a significant
prolongation of surviving mice bearing shPRNP-treated colorectal
tumors (Fig. 2F). These experiments
confirmed the role of PrPc in the promotion of CRC metastasis.
PrPc regulates SATB1 expression via
Fyn-SP1 pathway
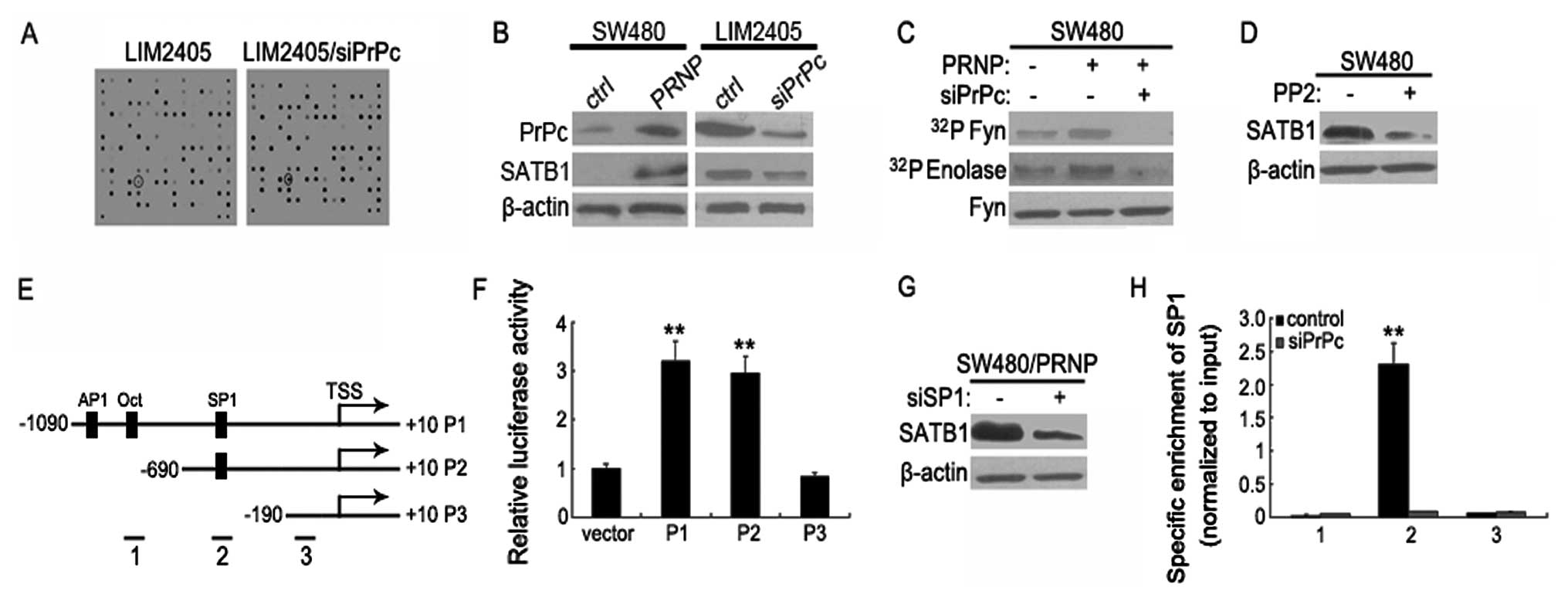
To find genes involved in the observed PrPc-mediated
CRC metastasis, we performed transcriptome profiling. After 72 h of
PrPc depletion in LIM2405 cells, analysis of array hybridization
revealed 89 differentially expressed genes. SATB1 was further
investigated because it was the most significantly changed
metastasis-related gene in PrPc-depleted cells (Fig. 3A). As shown in Fig. 3B, SATB1 was downregulated in LIM2405
cells 72 h after PrPc silencing, according with the results
obtained from immunoblot analysis. On the contrary, SATB1
expression was significantly enhanced upon pCDNA3.0-PrPc
transfection into SW480 cells in which PrPc is negligibly
expressed.
Among the potential signaling pathways which are
modulated by PrPc (PI3K/Akt, cAMP/PKA, PKC, Fyn, and Erk1/2), only
Fyn pathway is constitutively activated in PrPc-overexpressing
SW480 cells, which could not be observed after PrPc siRNA treatment
(Fig. 3C). SATB1 expression was
primarily abolished by exposure of the cells to PP2 (a specific Fyn
inhibitor), even in the presence of PrPc (Fig. 3D). To further determine which
response element participates in the regulation of SATB1 promoter
activity in response to PrPc, we constructed a series of SATB1
promoter reporter mutation vectors (P1 to P3). Using MatInspector
software, an algorithmic prediction of potential transfactor
binding sites identified putative-binding sites for AP1, Oct and
SP1 (Fig. 3E). P1 to P2 were
significantly activated in LIM2405 cells. Deletion of the putative
SP1 site in the SATB1 promoter (P3) strongly diminished the SATB1
promoter activity (Fig. 3F).
Moreover, PrPc-mediated SATB1 expression was primarily abrogated by
treatment with SP1siRNA in PrPc-overexpressed cells, suggesting
that the PrPc-SP1 axis is required for SATB1 expession (Fig. 3G). In agreement with this, a
chromatin immunoprecipitation (ChIP) assay revealed that the
promoter region containing the putative SP1 site was specifically
co-immunoprecipitated by anti-SP1 antibody, whereas PrPc knockdown
significantly attenuated this effect (Fig. 3H). Thus, these studies demonstrate
that the Fyn-SP1 signaling pathway plays a critical role in the
PrPc-mediated upregulation of SATB1.
PrPc-SATB1 axis is essential for CRC
metastasis
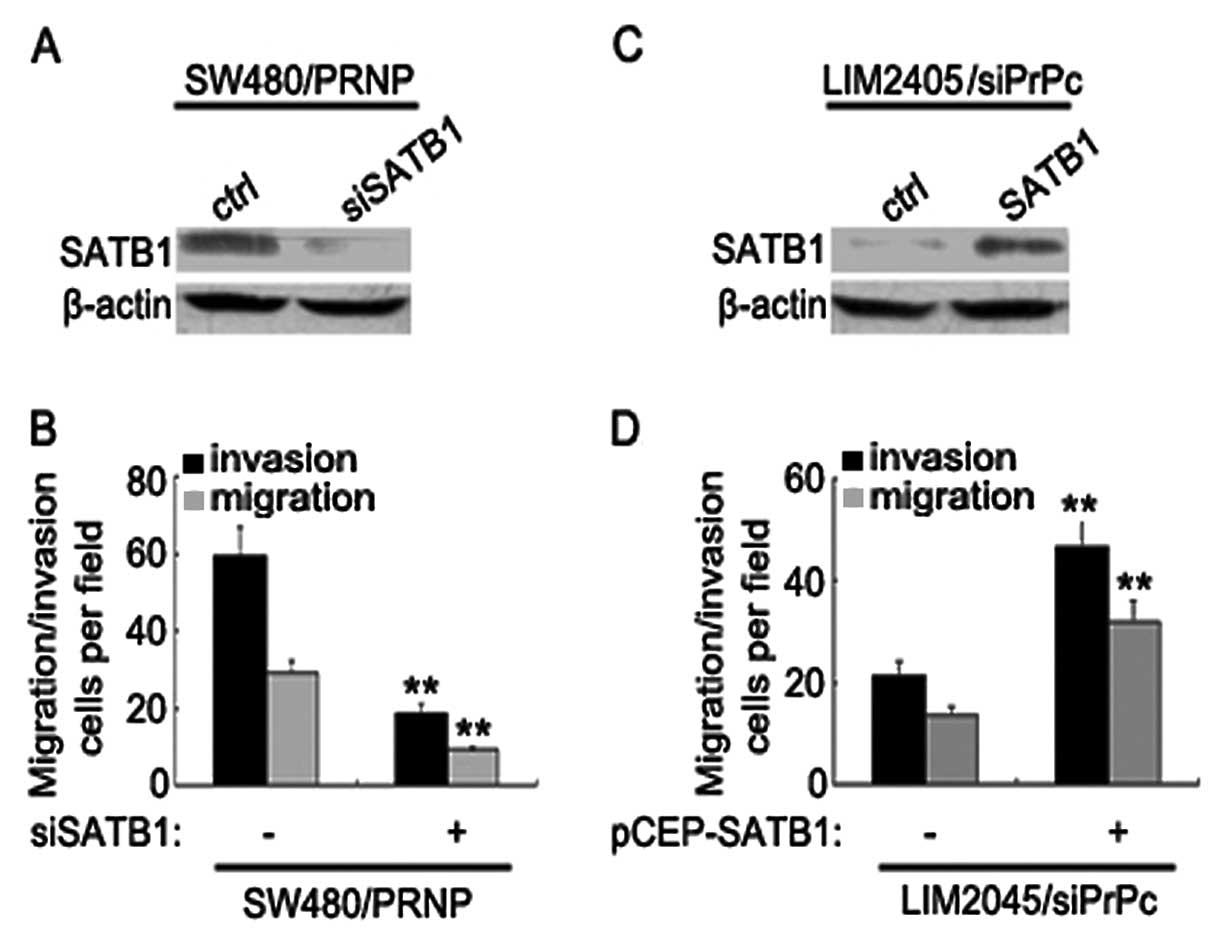
To evaluate whether SATB1 mediates the increase of
CRC metastasis by PrPc, we used siRNAs to specifically block
PrPc-induced SATB1 expression (Fig.
4A). Knockdown of SATB1 resulted in drastic reduction of
invasive and metastatic capacity of LIM2405 cells (Fig. 4B). We also overexpressed SATB1 in a
PrPc knockdown clone of LIM2405 cells (Fig. 4C). PrPc-depleted cells exhibited
increased metastatic capacities upon ectopic SATB1 expression,
whereas the untreated cells yielded lower metastasis (Fig. 4D). These results provide direct
evidence that SATB1 plays an essential role in regulating CRCs
metastasis by PrPc.
Discussion
PrPc has emerged as an important molecule to
modulate cancer cell proliferation (13), drug resistance (12,17)
and metastasis (11). Our study
used an extensive collection of CRC samples to show that PrPc
expression was clearly associated with the differentiation and TNM
stage of CRCs, which were similar to previous reports in other
malignancies such as breast cancer (9) and gastric cancer (12). Indeed, several pieces of evidence in
this study showed a close association between PrPc expression and
CRC metastasis. First, we observed that PrPc was specifically
expressed at the invasive front of CRCs, where carcinoma cells gain
the characteristics of EMT and facilitate tumor invasion. Second,
functional assays showed that ectopic PrPc expression promoted
in vitro metastatic potential of CRC cells, whereas
inhibition of PrPc significantly reduced the motility of cancer
cells. Consistently, using an orthotopic xenograft model, we found
that knockdown of PrPc in the implanted CRC cells remarkeably
abolished the number of distant metastases. These results lend
further credence to the notion that PrPc plays a crucial role in
regulating CRC progression and metastasis.
The mechanisms for the functional role of PrPc in
promoting tumor malignancy still remain poorly understood. The
present results characterized a novel PrPc-dependent pathway, where
PrPc accelerated tumor metastasis by upregulating SATB1 expression.
SATB1 is a matrix attachment region (MARAR)-binding protein that
participates in higher-order chromatin organization and
tissue-specific gene expression. SATB1 has recently been shown to
be the ‘master regulator’ of global gene expression (18,19).
SATB1 markedly altered the gene expression profile of cancer cells
to induce an aggressive phenotype that promotes tumor metastasis.
Strikingly, depletion of SATB1 results in reduced cancer
progression and the reversion of metastatic cells to normal
appearance (18). Therefore, a
positive regulatory relationship between PrPc and SATB1 may provide
an explanation for the action of PrPc in CRC malignancy.
Although the biological function and clinical
significance of SATB1 in cancers are well established, the detailed
mechanism regulating SATB1 expression is still not clear. We showed
here that SP1 is an important downstream target of PrPc explaining
the mode of regulation of SATB1 expression at the molecular level
in CRC cells. A direct and specific binding of SP1 to the SATB1
promoter was detected, which was largely abolished by PrPc
depletion. PrPc is a membranous protein which functions as a
receptor or co-receptor for extracellular matrix proteins such as
laminin (20,21) and vitronectin (22), as well as the secreted co-chaperone
stressinducible protein 1 (STI1) (23) to promote intracellular
signaling-mediated downstream effetcs. In the present study, we
demonstrated that the Fyn pathway lies downstream of PrPc.
Inhibition of Fyn pathway abolished the molecular events downstream
of PrPc, suggesting that the Fyn pathway is required for
PrPc-mediated SATB1 expression. Since inhibition of SP1 also
reduced SATB1 expression and metastatic capacity in colorectal
cancer cells, PrPc-Fyn-SP1-SATB1 axis is a relevant molecular
mechanism that links PrPc expression to poor prognosis in
malignancies depending on a high rate of metastasis.
In conclusion, this study showed that PrPc
expression in CRC confers a significant metastatic advantage for
CRCs by a mechanism that involves the function of PrPc as an
ancillary protein required for the function and expression of
SATB1. Thus, PrPc could be a strong indicator for more aggressive
tumors and possible poorer clinical outcome. Targeting pathways
involved in metastasis such as PrPc-Fyn-HIF2α-SATB1 axis represents
a promising avenue to inhibit maglinancy of colorectal cancer
cells.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics. CA Cancer J Clin.
58:71–96. 2008.
|
|
2
|
Boyle P and Ferlay J: Cancer incidence and
mortality in Europe, 2004. Ann Oncol. 16:481–488. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bardelli A, Parsons DW, Silliman N, et al:
Mutational analysis of the tyrosine kinome in colorectal cancers.
Science. 300:949–950. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stahl N, Borchelt DR, Hsiao K and Prusiner
SB: Scrapie prion protein contains a phosphatidylinositol
glycolipid. Cell. 51:229–249. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brown DR, Qin K, Herms JW, et al: The
cellular prion protein binds copper in vivo. Nature. 390:684–687.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brown DR, Nicholas RS and Canevari L: Lack
of prion protein expression results in a neuronal phenotype
sensitive to stress. J Neurosci Res. 67:211–224. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen S, Mangé A, Dong L, Lehmann S and
Schachner M: Prion protein as trans-interacting partner for neurons
is involved in neurite outgrowth and neuronal survival. Mol Cell
Neurosci. 22:227–233. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kikuchi Y, Kakeya T, Yamazaki T, et al:
G1-dependent prion protein expression in human glioblastoma cell
line T98G. Biol Pharm Bull. 25:728–733. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meslin F, Conforti R, Mazouni C, et al:
Efficacy of adjuvant chemotherapy according to Prion protein
expression in patients with estrogen receptor-negative breast
cancer. Ann Oncol. 18:1793–1798. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ochel HJ, Gademann G, Trepel J and Neckers
L: Modulation of prion protein structural integrity by
geldanamycin. Glycobiology. 13:655–660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pan Y, Zhao L, Liang J, et al: Cellular
prion protein promotes invasion and metastasis of gastric cancer.
FASEB J. 20:1886–1888. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhuang D, Liu Y, Mao Y, et al: TMZ-induced
PrPc/par-4 interaction promotes the survival of human glioma cells.
Int J Cancer. 130:309–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liang J, Pan Y, Zhang D, et al: Cellular
prion protein promotes proliferation and G1/S transition of human
gastric cancer cells SGC7901 and AGS. FASEB J. 21:2247–2256. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li QQ, Chen ZQ, Cao XX, et al: Involvement
of NF-κB/miR-448 regulatory feedback loop in chemotherapy-induced
epithelial-mesenchymal transition of breast cancer cells. Cell
Death Differ. 18:16–25. 2011.
|
|
15
|
Boussiotis VA, Barber DL, Lee BJ, Gribben
JG, Freeman GJ and Nadler LM: Differential association of protein
tyrosine kinases with the T cell receptor is linked to the
induction of anergy and its prevention by B7 family-mediated
costimulation. J Exp Med. 184:365–376. 1996. View Article : Google Scholar
|
|
16
|
Natalwala A, Spychal R and Tselepis C:
Epithelial-mesenchymal transition mediated tumourigenesis in the
gastrointestinal tract. World J Gastroenterol. 14:3792–3797. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Du J, Pan Y, Shi Y, et al: Overexpression
and significance of prion protein in gastric cancer and
multidrug-resistant gastric carcinoma cell line SGC7901/ADR. Int J
Cancer. 113:213–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han HJ, Russo J, Kohwi Y and
Kohwi-Shigematsu T: SATB1 reprogrammes gene expression to promote
breast tumour growth and metastasis. Nature. 452:187–193. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kumar PP, Purbey PK, Sinha CK, Notani D,
Limaye A, Jayani RS and Galande S: Phosphorylation of SATB1, a
global gene regulator, acts as a molecular switch regulating its
transcriptional activity in vivo. Mol Cell. 22:231–243. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Graner E, Mercadante AF, Zanata SM, et al:
Cellular prion protein binds laminin and mediates neuritogenesis.
Mol Brain Res. 76:85–92. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Graner E, Mercadante AF, Zanata SM,
Martins VR, Jay DG and Brentani RR: Laminin-induced PC-12 cell
differentiation is inhibited following laser inactivation of
cellular prion protein. FEBS Lett. 482:257–260. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hajj GN, Lopes MH, Mercadante AF, et al:
Cellular prion protein interaction with vitronectin supports axonal
growth and is compensated by integrins. J Cell Sci. 120:1915–1926.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zanata SM, Lopes MH, Mercadante AF, et al:
Stress-inducible protein 1 is a cell surface ligand for cellular
prion that triggers neuroprotection. EMBO J. 21:3307–3316. 2002.
View Article : Google Scholar : PubMed/NCBI
|