Introduction
RON receptor tyrosine kinase is a member of the MET
proto-oncogene that induces cell dissociation, migration, and
matrix invasion (1–3). Receptor tyrosine kinases consist of a
large group of cell surface proteins with unique structure and
biological activities (4,5). Abnormal accumulation and activation of
receptor tyrosine kinases causes the initiation and progression of
a variety of malignancies including tumors derived from breast and
kidneys (6,7). The invasive growth features are
controlled by a genetic program which is conserved in MET family
members (8,9). Mature RON is a 180-kDa heterodimeric
protein that is composed of a 40-kDa extracellular α chain and 150
kDa transmembrane β chain with intrinsic protein tyrosine kinase
activity (10,11). α and β chains are proteolytic
products of a 180-kDa RON precursor (12). RON is activatedby
macrophage-stimulating protein (MSP), a serum protein that is
constitutively expressed by liver cells as an inactive form and
requires proteolytis conversion for receptor binding (13–15).
The binding of MSP to RON results in receptor autophosphorylation
and upregulation of RON kinase activity, which in turn stimulates a
number of intracellular pathways mediating MSP effects (16). In normal cells MSP-induced
activation is a transient event, whereas in tumor cells RON
activity is often constitutively upregulated (17). RON activation causes invasive growth
and motility of certain epithelial tumor cells (18,19). A
number off acts suggest that RON might be involved in the
progression of certain epithelial malignancies, particularly at the
stage of tumor metastasis (20).
It has been shown that receptor tyrosine kinases can
be activated by a variety of mechanisms, including mutation,
deletion, gene rearrangement and alternative splicing of pre-mRNA
(21). Various RON protein isoforms
are produced through alternative splicing of pre-mRNA (12). One of the protein variants is
RonΔ160, a naturally occurring oncogenic form of RON identified in
human colon cancers, which is produced from a splicing mRNA
transcript by exons 5 and 6. RonΔ160 causes structural changes that
lead to cellular transformation in vitro and tumor growth
in vivo (22,23). Another variant RonΔ170 is a 170-kDa
variant is generated by skipping of exon 19 in alternative splicing
(24). RonΔ170 is kinase defective
and acts as dominant negative agent that inhibits tumorigenic
activities mediated by oncogenic variant RONΔ160 in colon cancer
cells (24). RonΔ165 is produced by
an in-frame deletion of exon 11 that affects the proteolytic
process which results in the accumulation of single-chain
pro-RonΔ165 in the cytoplasm (25).
RonΔ155 is a derived from a combined deletion of exons 5, 6 and 11,
the function of RonΔ155 is similar to RonΔ160 (25,26).
Here we show two novel splicing variants of RON
proto-oncogene. From the sequence analysis it is proved they are
partially spliced during the splicing events of exons 5 and 6.
These two variants contain either intron 5, or introns 5 and 6, in
addition to exons 4–7. The proteins encoded by these variants are
not identified yet.
Materials and methods
Cell culture
HeLa and C33A cells were maintained in Dulbecco’s
modified Eagle’s medium (DMEM) and HCT116 and HT-29 cells were
maintained in RPMI-1640 medium supplemented with 10% of Fetal
Bovine Serum (FBS) at 37°C in a humidified 5% CO2
condition.
RT-PCR
Total RNA was extracted using RiboEx (GeneAll)
following the manufacturer’s instructions. Total RNA (1 μg)
was reverse transcribed with a RON exon 8 specific primer
(5′-TGGCACATAAAAGCTG-3′) using ImProm-II™ reverse transcriptase
(Promega) following the manufacturer’s instructions. RON cDNA was
amplified by PCR using RON exon 4-specific primer
(5′-GTTTTCCAGGTACCTATCCAAG-3′) and RON exon 7 specific primer
5′-CGTGCTAGCAGACACTCAGTC-3′). The PCR products were loaded onto 2%
agarose gel and visualized by staining with ethidium bromide
solution. RT-PCR bands were purified and sequenced.
Results
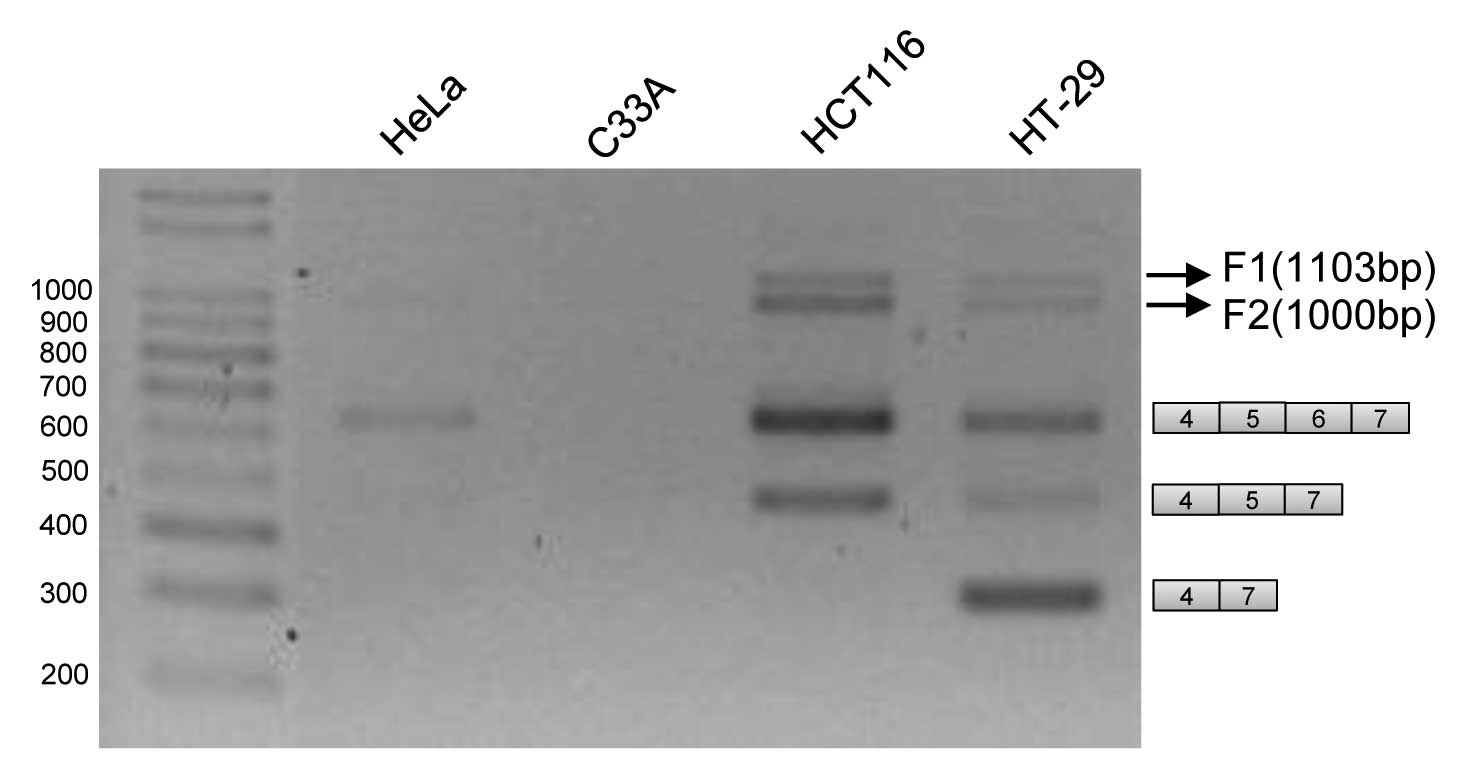
Novel RT-PCR products are produced from
RNA fragment covering exons 4–7
In the pre-mRNA splicing of RON gene, RonΔ160 is
produced due to skipping of exons 5 and 6. In order to compare the
skipping/inclusion of exons 5 and 6 in different cell lines, we
performed RT-PCR reaction using primers from exons 4 and 7. As
expected we observed several splicing variants that had been
previously described to include or exclude exons 5 and 6 in human
colon cancer HT-29 and HCT116 cells (23). In addition to these two isoforms, a
variant which includes exon 6, but not exon 5, is also produced in
these two cell lines, consistent with previous results (27). To our surprise, we also found that
two other PCR products which we name as F1 and F2, are also
produced, as shown in Fig. 1. As
compared with size markers on the agarose gel, the length of F1 is
~1,100 bp; the length of F2 is ~1,000 bp. On the other hand, in
cervix cancer cell C33A, none of any RT-PCR product RON RNA is
produced; consistent with the fact that RON is not expressed in
cervix cells. In HeLa cells, the only RT-PCR products we have
observed are variants that have exon 5 and 6 inclusion. F1 and F2
products are not found in HeLa and C33A cells.
F1 and F2 fragments are partially spliced
product of RON pre-mRNA
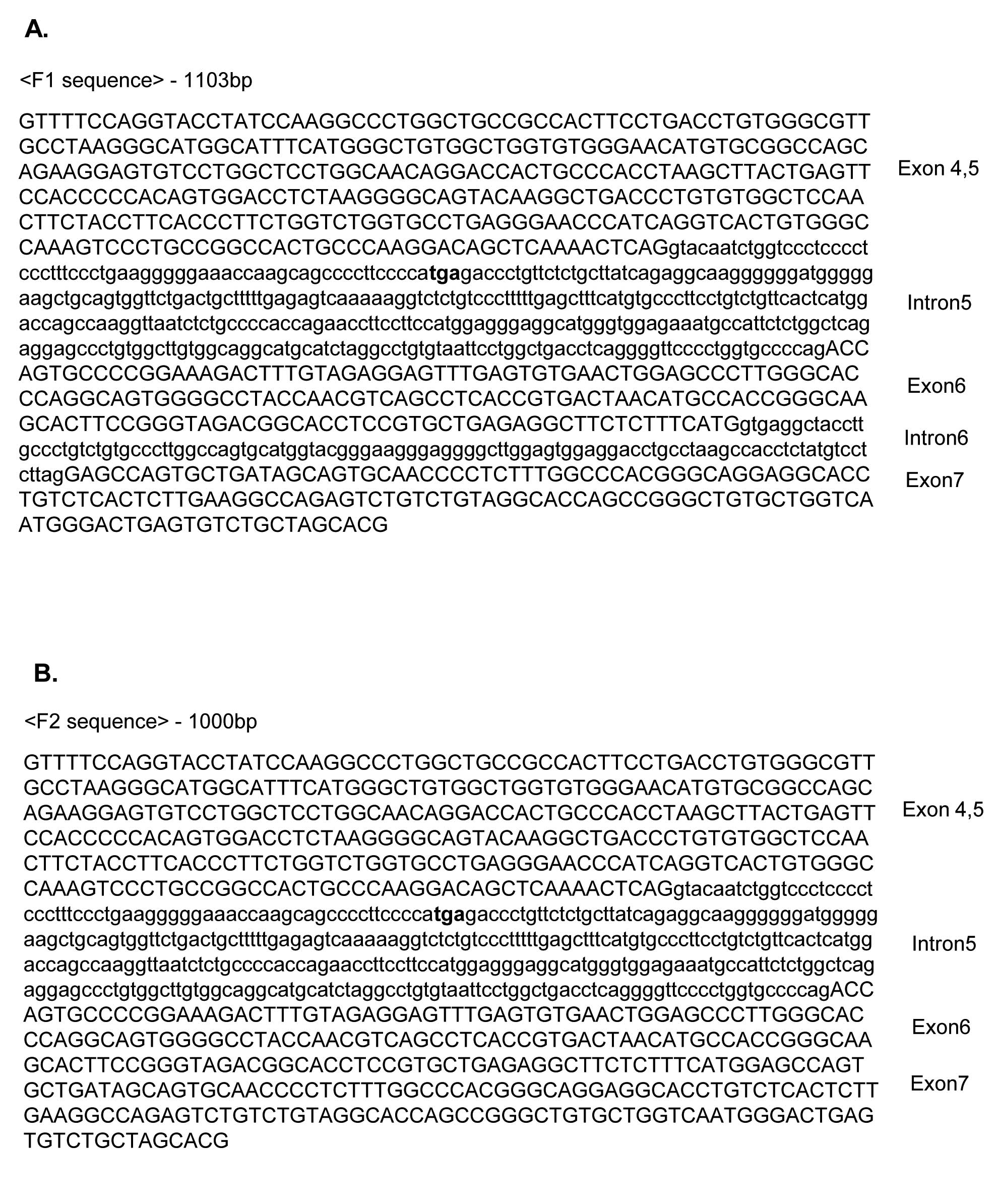
In order to characterize two unknown pre-mRNA
splicing products from exons 4 and 7 of RON, we performed sequence
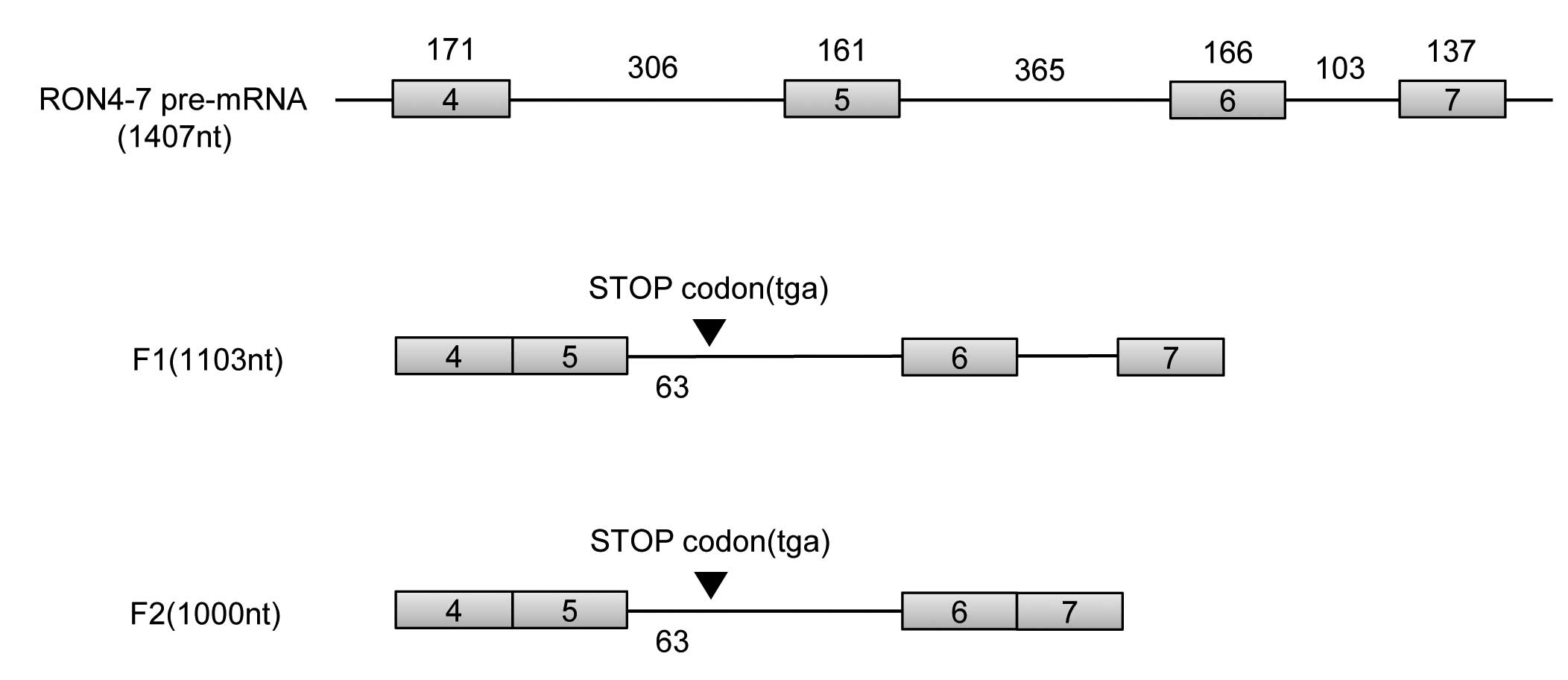
analysis. As shown in Fig. 2, we
found that the RNA fragment, which corresponds to F1 RT-PCR
product, contains exons 4 and 5, intron 5, exon 6, intron 6 and
exon 7 (1103Base). Another RNA fragment, which corresponds to F2
RT-PCR product, contains exons 4 and 5, intron 5, exons 6 and 7
(1000Base). Therefore F1 is a partial splicing product in which
only intron 4 is spliced while F2 is a product in which both
introns 4 and 6 are spliced as shown in Fig. 3. F1 and F2 intron 5 are not spliced
in either F1 or F2 products. We conclude that F1 and F2 are
partially spliced product of RON pre-mRNA.
Intron 5 includes a stop codon
Both novel RNA variants which correspond to F1 and
F2 contain intron 5. We analyzed intron 5 sequence. We found that
intron 5 contains a stop codon at the position 63-nt from 5′ splice
site of exon 5 in frame with exons 1–4. Therefore it is likely that
F1 and F2 will encode truncated RON isoforms from exons 1–4, but
not beyond exon 4.
Discussion
The full length RON precursor is 180 kDa. But it has
been well-documented that RON has several isoforms that are
produced from alternative splicing or protein truncation. RonΔ160
without exons 5 and 6 is oncogenic while RonΔ170 is a dominant
negative isoform of RON protein. The different effects of these two
isoforms suggest that it is important to investigate how many
isoforms and what isoforms are present in particular cells. In this
study, we used RT-PCR and identified two additional RON RNA
variants both of which include intron 5. The intron 5 sequence
contains stop codons that prevent these two RON RNA variants to
translate proteins beyond exon 4, leading to potential truncated
isoforms of RON protein (from exons 1–4). The existence and
possible functions of these isoforms should be investigated in the
future.
Acknowledgements
This study was supported by Mid-career Researcher
Program through National Research Foundation (NRF) grant
(20110000188 and 2011-0016757) funded by the Ministry of Education,
Science, and Technology (MEST), Korea; a Systems Biology
Infrastructure Establishment Grant provided by GIST in 2011.
References
|
1
|
Ronsin C, Muscatelli F, Mattei MG and
Breathnach R: A novel putative receptor protein tyrosine kinase of
the met family. Oncogene. 8:1195–1202. 1993.PubMed/NCBI
|
|
2
|
Trusolino L and Comoglio PM:
Scatter-factor and semaphorin receptors: cell signalling for
invasive growth. Nature reviews Cancer. 2:289–300. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang MH, Wang D and Chen YQ: Oncogenic and
invasive potentials of human macrophage-stimulating protein
receptor, the RON receptor tyrosine kinase. Carcinogenesis.
24:1291–1300. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van der Geer P, Hunter T and Lindberg RA:
Receptor protein-tyrosine kinases and their signal transduction
pathways. AnnuRev Cell Biol. 10:251–337. 1994.PubMed/NCBI
|
|
5
|
Robertson SC, Tynan J and Donoghue DJ: RTK
mutations and human syndromes: when good receptors turn bad. Trends
Genet. 16:265–271. 2000. View Article : Google Scholar
|
|
6
|
Birchmeier W, Brinkmann V, Niemann C, et
al: Role of HGF/SF and c-Met in morphogenesis and metastasis of
epithelial cells. Ciba Found Symp. 212:230–240. 1997.PubMed/NCBI
|
|
7
|
Niranjan B, Buluwela L, Yant J, et al:
HGF/SF: a potent cytokine for mammary growth, morphogenesis and
development. Development. 121:2897–2908. 1995.PubMed/NCBI
|
|
8
|
Vande Woude GF, Jeffers M, Cortner J,
Alvord G, Tsarfaty I and Resau J: Met-HGF/SF: tumorigenesis,
invasion and metastasis. Ciba Found Symp. 212:119–154.
1997.PubMed/NCBI
|
|
9
|
Comoglio PM, Tamagnone L and Boccaccio C:
Plasminogen-related growth factor and semaphorin receptors: a gene
superfamily controlling invasive growth. Exp Cell Res. 253:88–99.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu XM, Zhou YQ and Wang MH: Mechanisms of
cytoplasmic{beta}-catenin accumulation and its involvement in
tumorigenic activities mediated by oncogenic splicing variant of
the receptor originated from Nantes tyrosine kinase. J Biol Chem.
280:25087–25094. 2005.
|
|
11
|
Chen YQ, Zhou YQ, Angeloni D, Kurtz AL,
Qiang XZ and Wang MH: Overexpression and activation of the RON
receptor tyrosine kinase in a panel of human colorectal carcinoma
cell lines. Exp Cell Res. 261:229–238. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu Y, Yao HP and Wang MH: Multiple
variants of the RON receptor tyrosine kinase: biochemical
properties, tumorigenic activities, and potential drug targets.
Cancer Lett. 257:157–164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gaudino G, Follenzi A, Naldini L, et al:
RON is a heterodimeric tyrosine kinase receptor activated by the
HGF homologue MSP. EMBO J. 13:3524–3532. 1994.PubMed/NCBI
|
|
14
|
Skeel A, Yoshimura T, Showalter SD, Tanaka
S, Appella E and Leonard EJ: Macrophage stimulating protein:
purification, partial amino acid sequence, and cellular activity. J
Exp Med. 173:1227–1234. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bezerra JA, Witte DP, Aronow BJ and Degen
SJ: Hepatocyte-specific expression of the mouse hepatocyte growth
factor-like protein. Hepatology. 18:394–399. 1993.PubMed/NCBI
|
|
16
|
Danilkovitch A and Leonard EJ: Kinases
involved in MSP/RON signaling. J Leukoc Biol. 65:345–348.
1999.PubMed/NCBI
|
|
17
|
Danilkovitch-Miagkova A and Zbar B:
Dysregulation of Met receptor tyrosine kinase activity in invasive
tumors. J Clin Invest. 109:863–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang MH, Montero-Julian FA, Dauny I and
Leonard EJ: Requirement of phosphatidylinositol-3 kinase for
epithelial cell migration activated by human macrophage stimulating
protein. Oncogene. 13:2167–2175. 1996.PubMed/NCBI
|
|
19
|
Santoro MM, Collesi C, Grisendi S, Gaudino
G and Comoglio PM: Constitutive activation of the RON gene promotes
invasive growth but not transformation. Mol Cell Biol.
16:7072–7083. 1996.PubMed/NCBI
|
|
20
|
Camp ER, Liu W, Fan F, Yang A, Somcio R
and Ellis LM: RON, a tyrosine kinase receptor involved in tumor
progression and metastasis. Ann Surg Oncol. 12:273–281. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rodrigues GA and Park M: Oncogenic
activation of tyrosine kinases. Curr Opin Genet Dev. 4:15–24. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang MH, Lee W, Luo YL, Weis MT and Yao
HP: Altered expression of the RON receptor tyrosine kinase in
various epithelial cancers and its contribution to tumourigenic
phenotypes in thyroid cancer cells. J Pathol. 213:402–411. 2007.
View Article : Google Scholar
|
|
23
|
Wang MH, Kurtz AL and Chen Y:
Identification of a novel splicing product of the RON receptor
tyrosine kinase in human colorectal carcinoma cells.
Carcinogenesis. 21:1507–1512. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang MH, Lao WF, Wang D, Luo YL and Yao
HP: Blocking tumorigenic activities of colorectal cancer cells by a
splicing RON receptor variant defective in the tyrosine kinase
domain. Cancer Biol Ther. 6:1121–1129. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Collesi C, Santoro MM, Gaudino G and
Comoglio PM: A splicing variant of the RON transcript induces
constitutive tyrosine kinase activity and an invasive phenotype.
Mol Cell Biol. 16:5518–5526. 1996.
|
|
26
|
Zhou YQ, He C, Chen YQ, Wang D and Wang
MH: Altered expression of the RON receptor tyrosine kinase in
primary human colorectal adenocarcinomas: generation of different
splicing RON variants and their oncogenic potential. Oncogene.
22:186–197. 2003. View Article : Google Scholar
|
|
27
|
Eckerich C, Schulte A, Martens T, Zapf S,
Westphal M and Lamszus K: RON receptor tyrosine kinase in human
gliomas: expression, function, and identification of a novel
soluble splice variant. J Neurochem. 109:969–980. 2009. View Article : Google Scholar : PubMed/NCBI
|