Introduction
Recently, molecular-targeted drugs including
bevacizumab (1), cetuximab
(2) and panitumumab (3) have attracted increased attention for
improvement of survival outcome following conventional chemotherapy
in refractory colorectal cancer. A number of patients receiving
chemotherapy gain the additional benefit of conversion therapy when
the chemotherapeutic effect is observed remarkably in refractory
colorectal cancer patients. However, 40–50% of patients who receive
chemotherapy may experience adverse events. Regarding anti-EGFR
antibodies, the efficacy is limited to colorectal patients with
K-ras wild-type (4). Therefore, the
development of predictive markers for efficacy of chemotherapy and
novel molecular targets are required for colorectal cancer
treatment.
It has been reported that ubiquitin-like with PHD
and ring-finger domain 1 (UHRF1) expression, also known as ICBP90
and Np95, is upregulated in various types of cancers, including
breast, lung, pancreatic, astrocytomas, cervical and bladder cancer
(5–12). These common alterations in cancer
cells imply the biological functions of UHRF1 that are associated
with rapid cellular progression and DNA repair processes (13). In fact, decreasing UHRF1 expression
suppresses cellular proliferation by inhibiting G1-S transition
(7,14,15).
Thus, UHRF1 is considered to play an essential role in cellular
proliferation.
UHRF1 plays a pivotal role in carcinogenesis via
gene silencing mechanism. UHRF1 possesses several domains, which
are associated with DNA methylation and histone methylation by
recognizing hemi-methylated DNA and recruiting DNA
methyltransferase 1 (DNMT1) (16–20).
UHRF1 co-operates with histone deacetylase 1 (HDAC1) and
methyltransferase G9a, which methylates histone H3K9, and induces
heterochromatin formation through these interactions (21). In cancer cells, UHRF1 localizes on
methylated promoters of a number of tumor-suppressor genes,
including p16INK4A, p14ARF and BRCA, through
complexes with HDAC1 and DNMT1 (7,21,22). A
previous report showed that UHRF1 is involved in de novo DNA
methyltransferases, DNMT3a and DNMT3b (23). Therefore, UHRF1 may recruit these
de novo DNA methyltransferases to methylated
tumor-suppressor promoters further suppressing the expression of
tumor-suppressor genes, resulting in the contribution to
carcinogenesis.
Regarding the regulation of UHRF1 expression, E2F-1
induces the expression of UHRF1 through binding to the UHRF1
promoter (6,7,24).
Moreover, the tumor-suppressor p53 has been revealed to suppress
UHRF1 expression through the inactivation of E2F-1 (14). Thus, the overexpression of UHRF1 in
various types of cancers is considered to be associated with the
p53-E2F-1 pathway.
Although enhanced UHRF1 expression has been
demonstrated in various types of cancers, there is no evidence to
demonstrate the relationship between UHRF1 expression and
gastrointestinal cancer. In the present study, UHRF1 expression was
evaluated using an immunohistochemical method in 231 colorectal
cancer cases and 40 colonic adenomas. The relationship between
UHRF1 expression and clinicopathological factors was analyzed. We
further examined the correlation between UHRF1 and E2F-1
expression.
Materials and methods
Tissue samples
A total of 231 surgical specimens of colorectal
cancer, obtained from the Fukushima Medical University Hospital
from January 1990 to March 2007, were used for immunohistochemical
experiments. The clinical characteristics are shown in Table I. The carcinomas at the time of the
primary tumor resection were staged according to the UICC
classification. An additional 40 adenoma samples were obtained from
the Fukushima Medical University Hospital from January 2007 to
March 2008. This study was performed in accordance with the Ethical
Guidelines for Clinical Research with the approval of the
Institutional Ethics Committee. Informed consent was obtained from
the individuals included in the study.
 | Table IClinicopathological findings of 231
patients with colon cancer. |
Table I
Clinicopathological findings of 231
patients with colon cancer.
| Characteristics | n |
|---|
| Gender |
| Male | 136 |
| Female | 95 |
| Median age
(years) | 66 (24–89) |
| Tumor location |
| Right hemicolon | 79 |
| Left hemicolon | 152 |
| Tumor
differentiation | |
| Differentiated | 201 |
|
Undifferentiated | 30 |
| Depth of
invasion |
| T1 | 8 |
| T2 | 45 |
| T3 | 162 |
| T4 | 16 |
| Lymph node
metastasis |
| NX | 7 |
| N0 | 138 |
| N1 | 58 |
| N2 | 21 |
| N3 | 7 |
| UICC stage |
| 0 | 5 |
| I | 36 |
| II | 85 |
| III | 67 |
| IV | 38 |
Immunohistochemical staining and
evaluation of UHRF1 and E2F-1 expression
Immunohistochemical staining of UHRF1 and E2F-1 was
performed as previously described (12). Briefly, paraffin sections were
incubated at 4°C overnight with mouse monoclonal anti-human UHRF1
antibody (BD Transduction Laboratories, San Jose, CA, USA) and
mouse monoclonal anti-E2F-1 antibody (Santa Cruz Biotechnology,
Santa Cruz, CA) at room temperature for 1 h. These proteins were
visualized by EnVision System-HRP (Dako). Immunohistochemical
evaluations were performed by two investigators (Y.K. and K.K.)
independently without prior information of the clinicopathological
features. For the microscopic analysis, at least 200 cancer cells
were examined to determine whether the cells were positive for
UHRF1 and E2F-1 at high power (×200) after screening for the areas
with the highest intensity of staining at lower power (×40). A high
expression was defined when positive staining cancer cells were
observed in >20% of at least 200 cancer cells, and a low
expression was defined when <20% of at least 200 cancer cells
were stained.
Cell culture
Twelve human colon cancer cell lines, Colo 201, Colo
205, HCT15, HCT116, LS174T, LS180, LoVo, RKO, SW48, SW480, SW620
and SW837, were originally obtained from the American Type Culture
Collection (Rockville, MD, USA). These cells were grown at 37°C
under the presence of 5% CO2 in the recommended culture
media.
Western blotting
Protein lysates were prepared in ice cold lysis
buffer (50 mM Tris HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 50 mM
NaF, 10 mM sodium pyrophosphate, 25 mM β-glycerophosphate, protease
and phosphatase inhibitors). Protein aliquots were separated by
Tris-Glycine gels, and transferred onto nitrocellulose membranes.
The protein blots were incubated with a primary antibody for 1 h at
room temperature, and then incubated with a horseradish
peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology)
for 30 min at room temperature. Bound antibodies were detected by
enhanced chemiluminescence (ECL) detection reagents (Thermo Fisher
Scientific Inc., Waltham, MA, USA) and visualized by
autoradiography. The primary antibodies used for the western blot
analysis were mouse monoclonal anti-UHRF1 antibody (BD Transduction
Laboratories) and mouse monoclonal anti-β-actin antibody (Santa
Cruz Biotechnology).
Real-time reverse transcription-PCR
(RT-PCR) analysis of mRNA expression
Total RNA from cells was harvested using TRIzol
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s
instructions. Total RNA (5 μg) was used for the synthesis of
first-strand cDNA using SuperScript III First-strand cDNA Synthesis
kit (Invitrogen) following the manufacturer’s instructions. RT-PCR
analysis was performed using ABI PRISM 7500 (Applied Biosystems,
Foster City, CA, USA) with TaqMan probes provided by the
manufacturer. TaqMan probes of UHRF1 (Hs00273589_m1) and β-actin
(Hs99999903_m1) were used for RT-PCR analysis. The relative amount
of UHRF1 transcripts was normalized by the amount of β-actin
transcripts used as the internal control in the same sample, and
described as the ratio of UHRF1/β-actin.
siRNA experiments
The siRNA oligos (Invitrogen) were designed as
follows: UHRF1 siRNA, 5′-GCGCAATGTCAA GGGTGGCAAGAAT-3′; scrambled
siRNA, 5′-GCGTATG ACGAGGGTCGAAGACAAT-3′. Each siRNA oligos was
transfected into colon cancer cells using the RNAiMAX reagent
(Invitrogen) according to the manufacturer’s instructions. Briefly,
cells were resuspended to an appropriate concentration
(1×105 cells/ml). The cell suspensions (2 ml) were
dispensed on a 6-well culture plate. Cells were the treated with 40
nM UHRF1 siRNA or 40 nM scrambled siRNA, and harvested at 48 h
after treatment of siRNAs.
Cell counting
Cell counting was performed using a Cell Counting
kit-8 (Dojindo, Kumamoto, Japan). Approximately 5,000 cells, which
were treated with 40 nM UHRF1 siRNA or 40 nM scrambled siRNA, were
dispensed onto a well of 96-well plate. After 48 h of incubation,
the absorbance (450 nm) was measured using a microplate
spectrophotometer (Bio-Rad Laboratories; Benchmark Plus).
Statistical analysis
The statistical analysis of the relationship between
UHRF1 expression and clinicopathological findings was performed
using Chi-square test, t-test, or Mann-Whitney test. The Chi-square
test was performed to evaluate the relationship between UHRF1 and
E2F-1 expression. The cell viability tests were performed in
triplicate. Results are presented as the means ± standard
deviation. P<0.05 were considered to indicate a statistically
significant difference. Statistical analysis was performed with
Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA).
Results
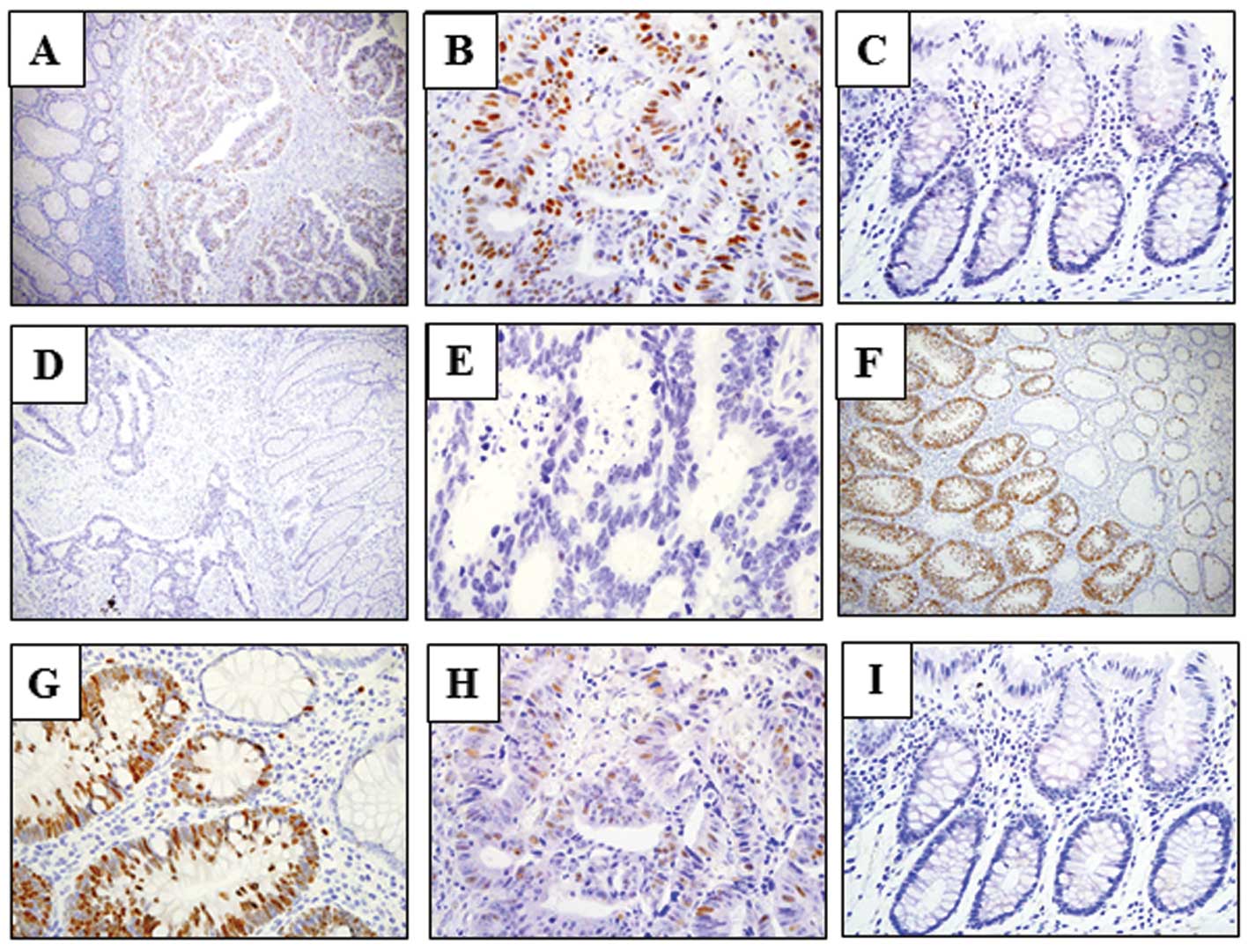
UHRF1 expression was upregulated in colon
cancer
Of 231 colon cancer cases, high UHRF1 expression was
detected in 152 (65.8%) by immunohistochemical evaluation. UHRF1
expression was detected in cancer cells, while no expression was
observed in normal mucosa (Fig.
1A-E). The cellular localization of UHRF1 was nuclear. We
further examined UHRF1 expression in 40 colonic adenomas. UHRF1
expression was positively detected in 35 out of 40 samples (87.5%)
(Fig. 1F and G). The analysis of
the relationship between UHRF1 expression and clinicopathological
features is shown in Table II.
UHRF1 expression was correlated with the anatomical location of the
tumor. The positive cases of UHRF1 expression in right hemicolon
cancer were significantly more frequent than those in left
hemicolon cancer (P=0.008). Moreover, high UHRF1 expression showed
an association with depth of invasion (P=0.051). Correlations
between UHRF1 expression and other factors, including gender, age,
lymphatic invasion, venous invasion, lymph node metastasis or
stage, were not observed. The 5-year survival outcome did not
differ between patients with a high UHRF1 expression compared to
those with low UHRF1 expression (data not shown).
 | Table IIRelationship between UHRF1 expression
and clinicopathological findings. |
Table II
Relationship between UHRF1 expression
and clinicopathological findings.
| High expression
(n=152)
n (%) | Low expression
(n=79)
n (%) | P-value |
|---|
| Gender |
| Male | 84 (61.8) | 52 (38.2) | 0.12 |
| Female | 68 (71.6) | 27 (28.4) | |
| Median age
(years) | 66.9 (range
33–89) | 64.3 (range
24–85) | 0.12 |
| Tumor location | | | 0.008 |
| Right
hemicolon | 61 (77.2) | 18 (22.8) | |
| Left
hemicolon | 91 (59.9) | 61 (40.1) | |
| Tumor
differentiation | | | 0.47 |
|
Differentiated | 134 (66.6) | 67 (33.4) | |
|
Undifferentiated | 18 (60) | 12 (40) | |
| Depth of
invasion | | | 0.051 |
| T1 | 4 (50) | 4 (50) | |
| T2 | 26 (57.8) | 19 (42.2) | |
| T3 | 109 (67.3) | 53 (32.7) | |
| T4 | 13 (81.3) | 3 (18.7) | |
| Lymph node
metastasis | | | 0.36 |
| N0 | 88 (63.8) | 50 (36.2) | |
| N1 | 41 (70.7) | 17 (29.3) | |
| N2 | 12 (57.1) | 9 (42.9) | |
| N3 | 7 (100) | 0 (0) | |
| UICC stage | | | 0.45 |
| 0 | 2 (40) | 3 (60) | |
| I | 22 (61.1) | 14 (38.9) | |
| II | 57 (67.1) | 28 (32.9) | |
| III | 46 (68.7) | 21 (31.3) | |
| IV | 25 (65.8) | 13 (34.2) | |
Correlation between UHRF1 and E2F-1
expression
High E2F-1 expression was detected
immunohistochemically in 99 (42.9%) colorectal cancer specimens
(Fig. 1H and I). When the
association between UHRF1 and E2F-1 expression was analyzed, a
statistically significant difference was noted (P<0.0001).
(Table III).
 | Table IIIRelationship between UHRF1 and E2F-1
expression in 231 colorectal cancer patients. |
Table III
Relationship between UHRF1 and E2F-1
expression in 231 colorectal cancer patients.
| E2F-1 |
|---|
|
|
|---|
| High | Low |
|---|
| UHRF1 |
| High | 85 | 67 |
| Low | 14 | 65 |
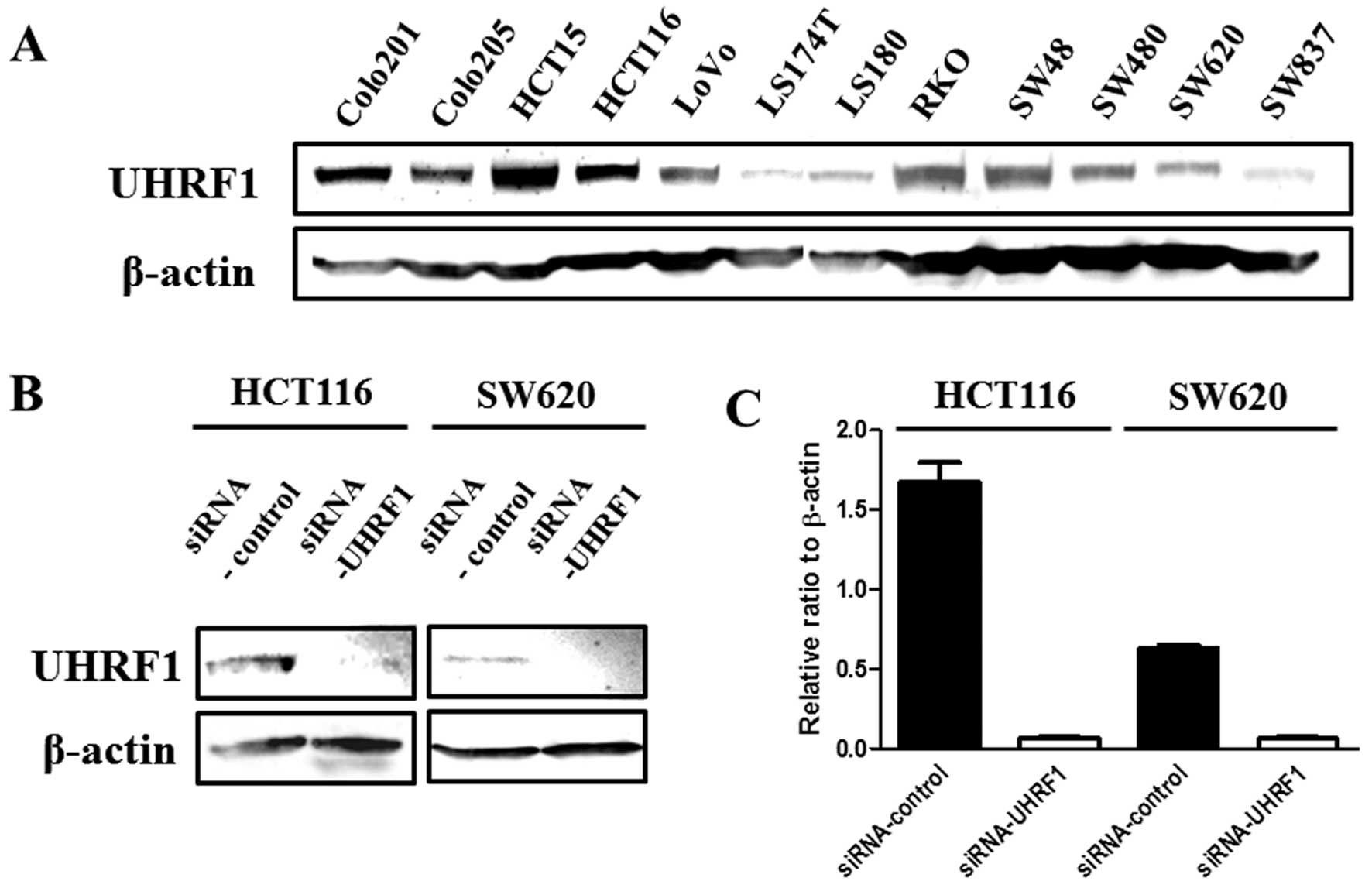
Knockdown of UHRF1 induces cellular
growth inhibition
UHRF1 expression was examined by western blotting in
12 human colon cancer cell lines (Fig.
2A). Of these cell lines, the HCT116 and SW620 cells, which
express UHRF1 at relatively high and low levels respectively, were
used for knockdown experiments. First, the effect of UHRF1 siRNA
was validated. The expression level of UHRF1 was significantly
decreased by UHRF1 siRNA treatment compared with scrambled siRNA at
both the protein and mRNA levels (Fig.
2B and C). A morphological change was not observed in all of
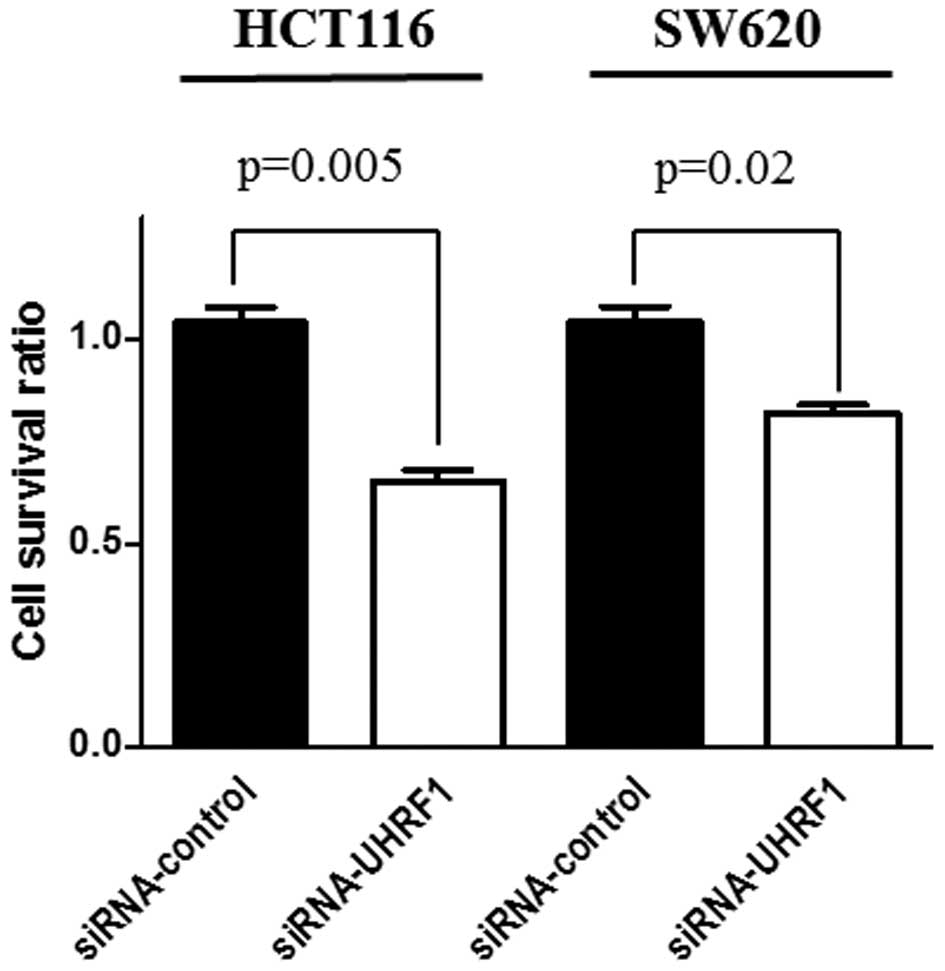
the knocked-down UHRF1 cells. Significant suppression of cellular
proliferation was observed in both HCT116 and SW620 cells treated
with UHRF1 siRNA compared with scrambled siRNA (P=0.005, P=0.02,
respectively) (Fig. 3).
Discussion
Previous studies have shown that UHRF1 expression is
constantly upregulated in proliferating cultured cancer cells and
various types of cancer (5–12). Therefore, enhanced UHRF1 expression
in cancer cells is considered to be associated with cellular
proliferation. To the best of our knowledge, we demonstrated for
the first time that UHRF1 expression was upregulated in
approximately two-thirds of all colon cancer cases. Moreover,
increased UHRF1 expression was observed in a number of specimens of
adenoma, but not in colonic normal mucosa. Our findings indicate
that UHRF1 expression is upregulated at an early stage of
colorectal carcinogenesis and may be involved in cellular
proliferation.
Regarding clinical significance of enhanced UHRF1
expression in colorectal cancer, the frequency of high UHRF1
expression in cancer tissue in the right hemicolon was higher than
that in the left hemicolon. It has been implicated that cancer of
the right (RCC) and left colon (LCC) has a differing prevalence at
varying ages, in high- and low-incidence regions, as well as in
males and in females. There is also a difference in clinical
presentation, prognosis and possibly in genetic and environmental
epidemiology (25,26). For examples, RCC is more common in
females, LCC in males (25),
response to 5-fluorouracil treatment is significantly better in RCC
(27), nuclear β-catenin and p53
are expressed to a greater extent in rectal compared with proximal
cancer (28), expression of
cytoplasmic c-erbB2, epidermal growth factor receptor (EGFR),
proliferating cell nuclear antigen (PCNA), and dipeptidylpeptidase
IV (DPP IV) is higher in RCC compared with LCC (28). Distal tumors display a higher
frequency of 17p and 18q allelic loss, p53 accumulation, c-myc
expression and aneuploidy compared to proximal tumors (29). Since the expression of UHRF1 was
especially high in right hemicolon cancer, UHRF1 may be one of the
factors that determine these features of RCC. Furthermore,
upregulated UHRF1 tended to be involved with deeper invasion,
suggesting that UHRF1 may be involved in malignant transformation
of colon cancer. Statistical correlations were not observed for
gender, age, lymph duct invasion, vessel invasion, lymph node
metastasis and stage in colorectal cancer. Survival outcome for
each stage had no significance between UHRF1- positive and
-negative cases. These results suggest that UHRF1 expression did
not directly affect the metastasis and prognosis of colon cancer,
but UHRF1 may increase the possiblity of DNA damages or replication
errors, which cause the malignant transformation, by promoting
speed of cell cycles.
It has been reported that E2F-1 upregulates UHRF1
transcription through binding to E2F-1 binding consensus sequences
found on the UHRF1 promoter (6,7,24). We
then investigated the relationship between the expression levels of
UHRF1 and E2F-1 in clinical cases. Our results showed that the
number of patients with high UHRF1 and high E2F-1 expression is
larger than that of others. Therefore, UHRF1 expression was
significantly associated with E2F-1 expression in colorectal
cancer.
UHRF1 plays an essential role in G1/S transition,
and knockdown of UHRF1 suppresses cellular proliferation (7,14). We
also demonstrated that knockdown of UHRF1 inhibited cellular
proliferation in colon cancer cell lines. These facts suggest that
UHRF1 may be a therapeutic target for colorectal cancer patients
with high UHRF1 expression. Knockdown of E2F-1 has also been
reported to decrease UHRF1 expression (14). Considering clinical cases, UHRF1 can
be considered a better therapeutic target of colon cancer than
E2F-1 because the rate of UHRF1-positive cases in colon cancer was
higher compared to E2F-1 (65.8 and 42.9%, respectively).
Furthermore, UHRF1 interacting proteins such as DNMTs and HDACs are
satisfactory targets of anticancer drugs (13) and a combination therapy of an UHRF1
inhibitor with these drugs may be more effective.
In conclusion, we found that UHRF1 expression was
upregulated and involved in the cellular proliferation of
colorectal cancer. Particularly, UHRF1 expression appears to be
associated with carcinogenesis of the right hemicolon. Based on our
novel findings, UHRF1 is a therapeutic target for colorectal
cancer.
References
|
1
|
Saltz LB, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Bevacizumab in combination with oxaliplatin-based
chemotherapy as first-line therapy in metastatic colorectal cancer:
a randomized phase III study. J Clin Oncol. 26:2013–2019. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bokemeyer C, Bondarenko I, Makhson A,
Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G,
Stroh C, et al: Fluorouracil, leucovorin, and oxaliplatin with and
without cetuximab in the first-line treatment of metastatic
colorectal cancer. J Clin Oncol. 27:663–671. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Douillard JY, Siena S, Cassidy J,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Randomized, phase III trial of panitumumab with
infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4)
versus FOLFOX4 alone as first-line treatment in patients with
previously untreated metastatic colorectal cancer: the PRIME study.
J Clin Oncol. 28:4697–4705. 2010. View Article : Google Scholar
|
|
4
|
Karapetis CS, Khambata-Ford S, Jonker DJ,
O’Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD,
Robitaille S, et al: K-ras mutations and benefit from cetuximab in
advanced colorectal cancer. N Engl J Med. 359:1757–1765. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hopfner R, Mousli M, Jeltsch JM, Voulgaris
A, Lutz Y, Marin C, Bellocq JP, Oudet P and Bronner C: ICBP90, a
novel human CCAAT bonding protein, involved in the regulation of
topoisomerase II alpha expression. Cancer Res. 60:121–128.
2000.PubMed/NCBI
|
|
6
|
Mousli M, Hopfner R, Abbady AQ, Monté D,
Jeanblanc M, Oudet P, Louis B and Bronner C: ICBP90 belongs to a
new family of proteins with an expression that is deregulated in
cancer cells. Br J Cancer. 89:120–127. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Unoki M, Nishidate T and Nakamura Y:
ICBP90, an E2F-1 target, recruits HDAC1 and binds to methyl-CpG
through its SRA domain. Oncogene. 23:7601–7610. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Unoki M, Daigo Y, Koinuma J, Tsuchiya E,
Hamamoto R and Nakamura Y: UHRF1 is a novel diagnostic marker of
lung cancer. Br J Cancer. 103:217–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Crnogorac-Jurcevic T, Gangeswaran R,
Bhakta V, Capurso G, Lattimore S, Akada M, Sunamura M, Prime W,
Campbell F, Brentnall TA, et al: Proteomic analysis of chronic
pancreatitis and pancreatic adenocarcinoma. Gastroenterology.
129:1454–1463. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oba-Shinjo SM, Bengtson MH, Winnischofer
SM, Colin C, Vedoy CG, de Mendonça Z, Marie SK and Sogayar MC:
Identification of novel differentially expressed genes in human
astrocytomas by cDNA representational difference analysis. Brain
Res Mol Brain Res. 140:25–33. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lorenzato M, Caudroy S, Bronner C, Evrard
G, Simon M, Durlach A, Birembaut P and Clavel C: Cell cycle and/or
proliferation markers: what is the best method to discriminate
cervical high-grade lesions? Hum Pathol. 36:1101–1107. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Unoki M, Kelly JD, Neal DE, Ponder BA,
Nakamura Y and Hamamoto R: UHRF1 is a novel molecular marker for
diagnosis and the prognosis of bladder cancer. Br J Cancer.
101:98–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Unoki M, Brunet J and Mousli M: Drug
discovery targeting epigenetic codes: the great potential of UHRF1,
which links DNA methylation and histone modifications, as a drug
target in cancers and toxoplasmosis. Biochem Pharmacol.
78:1279–1288. 2009. View Article : Google Scholar
|
|
14
|
Arima Y, Hirota T, Bronner C, Mousli M,
Fujiwara T, Niwa S, Ishikawa H and Saya H: Down-regulation of
nuclear protein ICBP90 by p53/p21Cip1/WAF1-dependent DNA-damage
checkpoint signals contributes to cell cycle arrest at G1/S
transition. Genes Cells. 9:131–142. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Trotzier MA, Bronner C, Bathami K, Mathieu
E, Abbady AQ, Jeanblanc M, Muller CD, Rochette-Egly C and Mousli M:
Phosphorylation of ICBP90 by protein kinase A enhances
topoisomerase II alpha expression. Biochem Biophys Res Commun.
319:590–595. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bostick M, Kim JK, Estève PO, Clark A,
Pradhan S and Jacobsen SE: UHRF1 plays a role in maintaining DNA
methylation in mammalian cells. Science. 317:1760–1764. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arita K, Ariyoshi M, Tochio H, Nakamura Y
and Shirakawa M: Recognition of hemi-methylated DNA by the SRA
protein UHRF1 by a base-flipping mechanism. Nature. 455:818–821.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Avvakumov GV, Walker JR, Xue S, Li Y, Duan
S, Bronner C, Arrowsmith CH and Dhe-Paganon S: Structural basis for
recognition of hemi-methylated DNA by the SRA domain of human
UHRF1. Nature. 455:822–825. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hashimoto H, Horton JR, Zhang X, Bostick
M, Jacobsen SE and Cheng X: The SRA domain of UHRF1 flips
5-methylcytosine out of the DNA helix. Nature. 455:826–829. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sharif J, Muto M, Takebayashi S, Suetake
I, Iwamatsu A, Endo TA, Shinga J, Mizutani-Koseki Y, Toyoda T,
Okamura K, et al: The SRA protein Np95 mediates epigenetic
inheritance by recruiting Dnmt1 to methylated DNA. Nature.
450:908–912. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim JK, Estève PO, Jacobsen SE and Pradhan
S: UHRF1 binds G9a and participates in p21 transcriptional
regulation in mammalian cells. Nucleic Acids Res. 37:493–505. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jin W, Chen L, Chen Y, Xu SG, Di GH, Yin
WJ, Wu J and Shao ZM: UHRF1 is associated with epigenetic silencing
of BRCA1 in sporadic breast cancer. Breast Cancer Res Treat.
123:359–373. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meilinger D, Fellinger K, Bultmann S,
Rothbauer U, Bonapace IM, Klinkert WE, Spada F and Leonhardt H:
Np95 interacts with de novo DNA methyltransferases, Dnmt3a and
Dnmt3b, and mediates epigenetic silencing of the viral CMV promoter
in embryonic stem cells. EMBO Rep. 10:1259–1264. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abbady AQ, Bronner C, Bathami K, Muller
CD, Jeanblanc M, Mathieu E, Klein JP, Candolfi E and Mousli M: TCR
pathway involves ICBP90 gene down-regulation via E2F binding sites.
Biochem Pharmacol. 70:570–579. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Distler P and Holt PR: Are right- and
left-sided colon neoplasms distinct tumors? Dig Dis. 15:302–311.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Birkenkamp-Demtroder K, Olesen SH,
Sørensen FB, et al: Differential gene expression in colon cancer of
the caecum versus the sigmoid and rectosigmoid. Gut. 54:374–384.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Elsaleh H, Joseph D, Grieu F, Zeps N, Spry
N and Iacopetta B: Association of tumour site and sex with survival
benefit from adjuvant chemotherapy in colorectal cancer. Lancet.
355:1745–1750. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kapiteijn E, Liefers GJ, Los LC, et al:
Mechanisms of oncogenesis in colon versus rectal cancer. J Pathol.
195:171–178. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fric P, Sovová V, Sloncová E, Lojda Z,
Jirásek A and Cermák J: Different expression of some molecular
markers in sporadic cancer of the left and right colon. Eur J
Cancer Prev. 9:265–268. 2000. View Article : Google Scholar : PubMed/NCBI
|