Introduction
Colorectal cancer (CRC) is the third most common
malignant neoplasm worldwide (1)
and the second leading cause of death due to cancer (2). To date, the prognosis of CRC patients
with liver metastasis remains poor. Therefore, it is necessary to
clarify the molecular mechanisms involved in metastasis and to
identify the specific biomarkers for predicting CRC liver
metastasis.
Octamer-binding transcription factor 4 (OCT4), one
of the key genes that induces pluripotent stem cells (3,4), has
also been implicated in several types of cancers including gastric
cancer (5–7), breast cancer (8), non-small cell lung carcinomas
(9), glioma (10–12)
and esophageal squamous cell carcinoma (13). In addition, Saigusa et
al(14) showed that OCT4
expression is associated with the distant recurrence of rectal
cancer after chemoradiotherapy. Chang et al(15) demonstrated that OCT4 promoted
tumorigenesis of CRC cells in both autocrine and paracrine manners.
Gazouli et al(16) found
that OCT4 expression levels were higher in CRC tissues compared to
adjacent non-cancerous tissues, and the OCT4 expression levels in
CRC tissues were correlated with tumor stage. Yet, to date the role
of OCT4 in CRC metastasis and the potential molecular basis is
unclear.
Epithelial-mesenchymal transition (EMT) is a
well-coordinated process that occurs during the progression of
cancers and is necessary for metastasis of epithelial cancer
(17–19). CRC cells at the invasive front
acquire mesenchymal properties such as high migratory potential,
poor differentiation, hyperproliferation and loss of cell-cell
contact-mediated growth inhibition (18). In this study, we investigated
whether OCT4 promotes CRC metastasis through the EMT process.
RNA interference is a powerful method for the
research of gene function. We applied lentivirus-mediated shRNA to
produce specific and stable silencing of OCT4 in SW620 CRC cells.
We designed our experiment as a loss-of-function study. Western
blot analysis was used to measure the extent and stability of OCT4
knockdown. We evaluated the metastatic phenotype of OCT4-silenced
SW620 cells using standard migration and invasion assays in
vitro and the commonly used mouse model for experimental
metastases in vivo. The results revealed that OCT4 enhanced
CRC cell metastasis by improving tumor cell invasion and migration.
We further demonstrated that OCT4 knockdown in CRC SW620 cells
induced MET (the reverse process of EMT) with characteristic
morphological changes and changes in expression of EMT-associated
key genes such as E-cadherin and vimentin. In addition, activity of
the WNT pathway was significantly inhibited by OCT4 silencing in
CRC SW620 cells. Finally, we showed that expression of OCT4 was
correlated with CRC liver metastasis in human CRC tissues. Our
study showed a novel role of OCT4 in regulating colorectal cancer
metastasis by the EMT process, and OCT4 expression was identified
as a promising biomarker for identifying colorectal cancer patients
at high risk for liver metastasis.
Materials and methods
Cell culture
The human colorectal cell line SW620 was obtained
from American Type Culture Collection (Manassas, VA, USA) and
maintained in RPMI-1640 medium supplemented with 10% fetal bovine
serum at 37°C in a 5% CO2 atmosphere at constant
humidity. The stable silencing of OCT4 in SW620 cells was generated
by transduction with home made lentiviral particles for human
OCT4 gene silencing.
Immunofluorescence cell staining
Cells were seeded on coverslips, incubated with
primary antibody overnight at 4°C, washed with PBS and then
incubated with secondary antibody conjugated with FITC (green) or
Cy3 (red) (Millipore, USA) for 1 h. Cells were imaged using a
confocal microscope (Leica, Germany). Primary antibodies,
anti-vimentin, anti-E-cadherin and anti-β-catenin, were purchased
from Abcam (Cambridge, MA, USA).
Western blot analysis
Cells were lysed in RIPA cell lysis buffer (25 mM
Tris-HCl, pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate,
0.1% SDS) containing Protease Inhibitor Cocktail kit (Pierce,
Rockford, IL, USA). Twenty micrograms of total cellular protein was
loaded per lane, separated by 4–12% SDS-polyacrylamide gel
electrophoresis and then transferred to nitrocellulose (Invitrogen,
Carlsbad, CA, USA) by electroblotting (20). The antibodies anti-E-cadherin,
anti-GAPDH, anti-β-catenin (1247-1, Epitomics), anti-α-tublin and
anti-TCF/LEF1 were purchased from Abcam.
RNA isolation and RT-PCR
Total RNA was extracted from cells and then reverse
transcribed. Quantitative real-time PCR reaction was performed in
Biosystems 7500 (ABI, USA). All samples were amplified in
triplicate using the following cycles: 95°C for 2 min, 35 cycles of
95°C for 15 sec, 60°C for 30 sec and 72°C for 20 sec.
Cell migration and invasion assays
Cell migration assay was performed in Transwells
(Corning Company). Cells in 0.5 ml serum-free medium were placed in
the upper chamber, whereas the lower chamber was loaded with 0.8 ml
medium containing 10% FBS. The total number of cells that migrated
to the lower chamber were counted after incubation for 48 h. Six
optional visual fields were chosen for counting. The Transwell cell
invasion assay was performed using Transwells that were pre-loaded
with a layer of Matrigel (Sigma-Aldrich) on the upper surface. The
rest of the experimental procedure was the same as with the cell
migration assay.
Xenograft studies
All animal experiments were performed strictly in
accordance with the related ethics regulations of our university.
Ten six-week-old female nude mice were each injected with
SW620-OCT4-shRNA (OCT4-knockdown cells) or SW620-mock-shRNA (mock
control cells) (5×106) into the spleen. Mice were
sacrificed 30 days after injection. The liver was embedded in
paraffin and sectioned for H&E staining to examine for
metastasis.
Immunohistochemistry
Anti-human OCT4 antibody from Abcam was used for
immunohistochemical (IHC) staining. Colorectal cancer tissue from
our hospital was used. Tissues were formalin fixed, paraffin
embedded (PPFE) slides. Each tissue was confirmed by experienced
pathologists. Primary antibodies for OCT4 were diluted at 1:250 for
IHC. Secondary antibody was used at a 1:200 dilution. The
percentage of the positive cell population was categorized for
scoring: 0, 0% of the cell population was positive; 1, 1–25% of the
cell population was positive; 2, 26–50% of the cell population was
positive; 3, 51–75% of the cell population was positive; 4, 76–100%
of the cell population was positive. The staining intensities were
scored as: −, negative staining; +, weak staining intensity; ++,
medium staining intensity; +++, strong staining intensity.
Statistical analysis
The results are reported as means ± SD. Statistical
analysis was performed using the Student’s t-test or Chi-square
test as appropriate. P<0.05 was considered to indicate a
statistically significant difference.
Results
OCT4 knockdown inhibits cell migration
and invasiveness in vitro
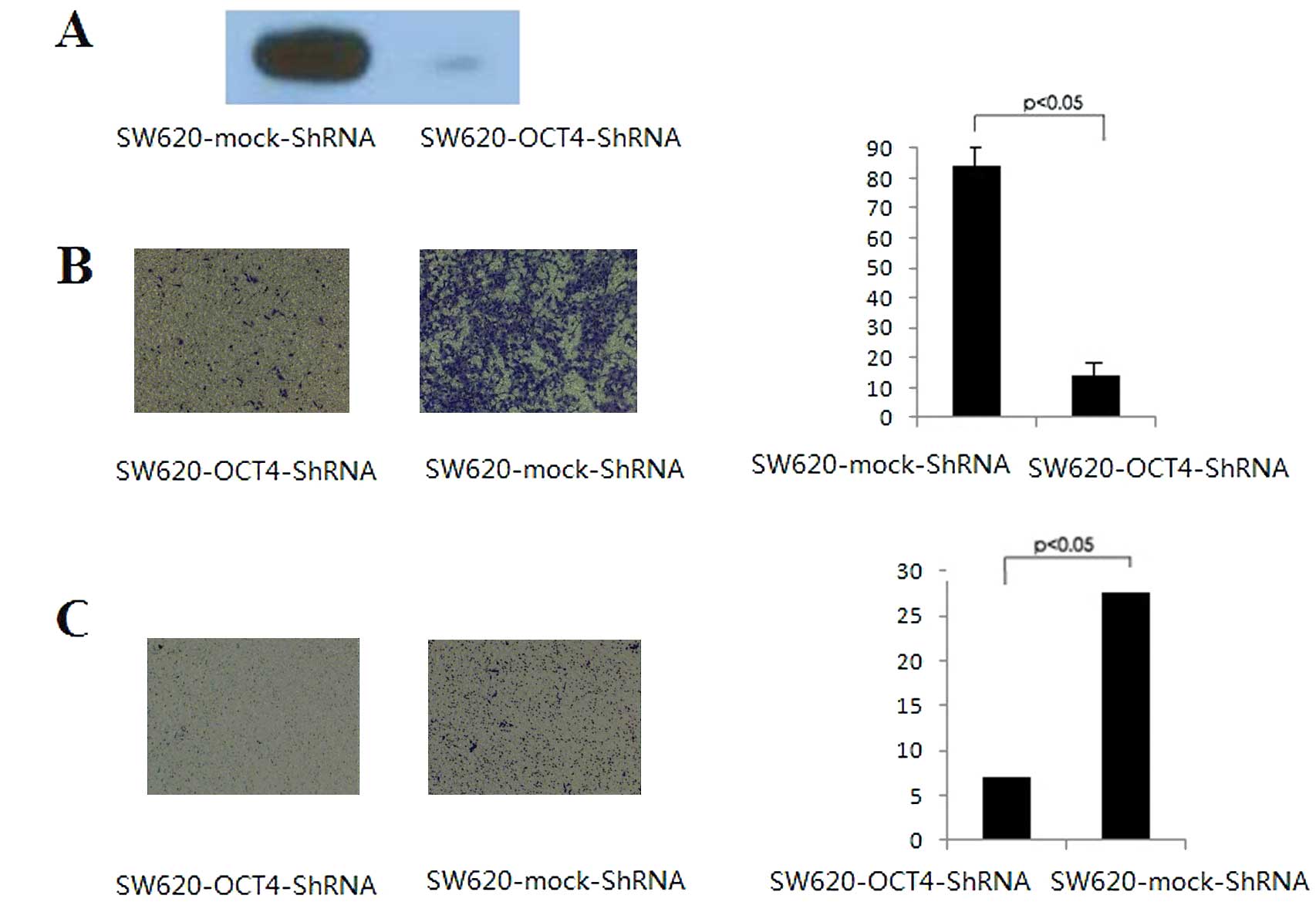
The protein levels of OCT4 were evaluated in the
mock control and OCT4-knockdown cells by western blotting. As shown
in Fig. 1A, OCT4 proteins were
strongly expressed in the mock control cells. However, as expected,
SW620-OCT4-shRNA cells demonstrated a decreased OCT4 protein
expression level. We first assessed the effects of OCT4 expression
on CRC cell migration and invasion with Transwell migration and
invasion assays. OCT4-knockdown cells (SW620-OCT4-shRNA) exhibited
a significant decrease in migration. The number of SW620-OCT4-shRNA
cells migrating to the lower chamber was ~6 times lower than the
number of migrating SW620 mock control cells (Fig. 1B). An in vitro cell invasion
assay was performed based on the principle of the Boyden chamber
assay, and Matrigel matrix was used as a reconstituted basement
membrane in vitro. The number of cells migrating through the
Matrigel matrix was counted and the result is presented in Fig. 1C. The OCT4-knockdown cells showed
significantly reduced invasiveness compared to the mock control
cells (P<0.05). These data indicate that the inhibition of OCT4
expression in SW620 cells is associated with reduced invasive
ability.
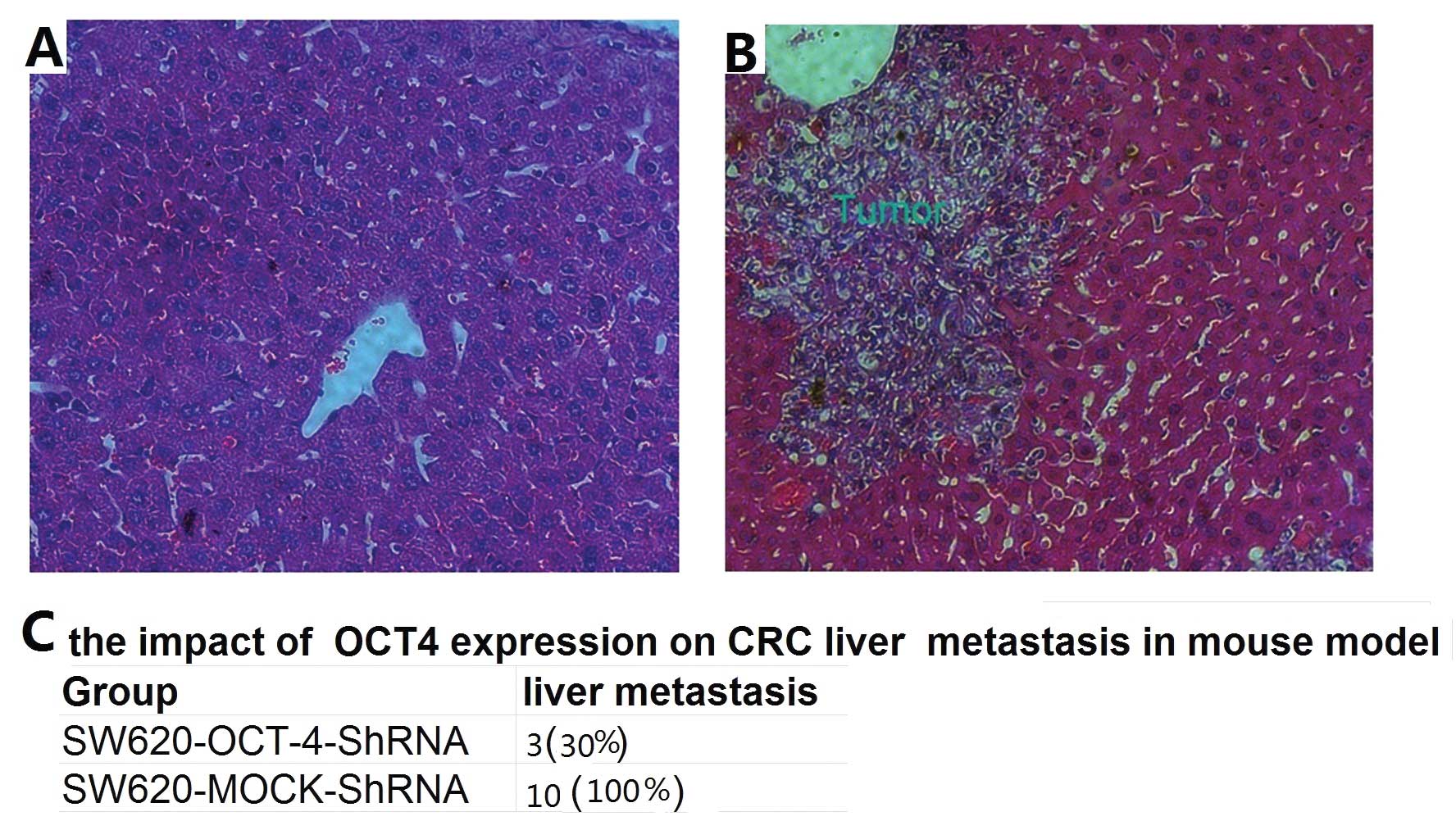
OCT4 knockdown reduces liver metastasis
of SW620 cells in a mouse model
To investigate whether OCT4 silencing suppresses
colorectal cancer liver metastasis, a hepatic metastasis mouse
model was used. SW620-OCT4-shRNA and mock control cells were
locally injected to the spleen, respectively. All of the mice
subjected to tumor cell injection survived until sacrifice on Day
30. All of the mice in the mock-control group (10/10, 100%)
demonstrated the formation of metastatic foci in the liver
(Fig. 2B). The incidence of liver
metastasis was significantly decreased in the OCT4 shRNA
group (3/10, 30%) (Fig. 2A).
Moreover, the average number of metastatic foci in the
OCT4-knockdown group was significantly lower than that in the
mock-control group (Fig. 2C). These
data suggest that OCT4 knockdown inhibits the ability of colorectal
cancer cells to form metastasis.
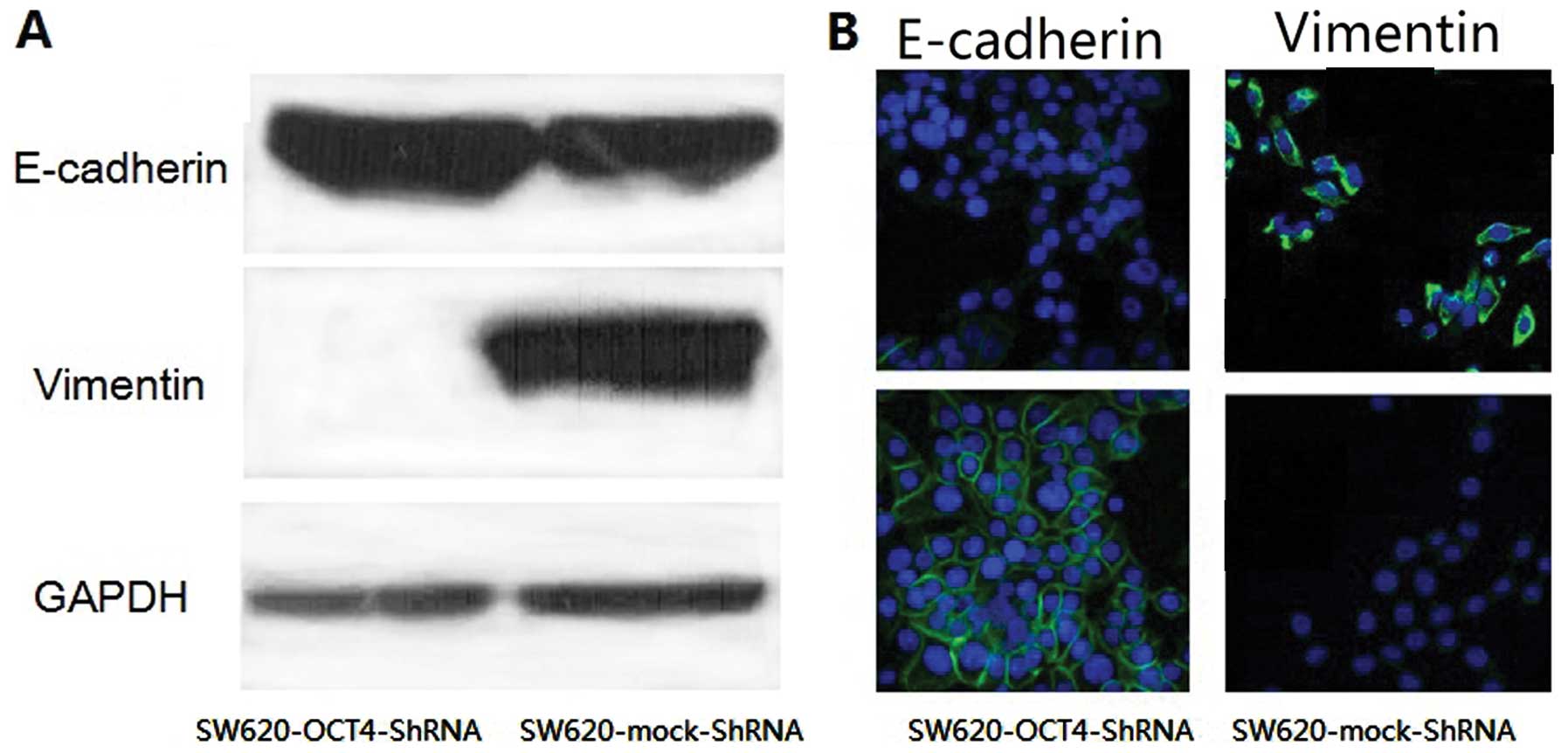
OCT4 induces epithelial-mesenchymal
transition in SW620 cells
EMT is important for epithelial cancer metastasis.
The characteristics of EMT include a switch from the expression of
E-cadherin to vimentin and typical cell morphological changes. To
examine whether knockdown of OCT4 alters the expression pattern of
these two markers and cellular shape in SW620 cells, we used
loss-of-function approaches in SW620 cells. We found that upon
knockdown of OCT4, the level of E-cadherin was decreased whereas
the level of vimentin was increased (Fig. 3A), consistent with altered staining
patterns and intensities of these two markers (Fig. 3B). We also noted that the
SW620-mock-shRNA cells maintained mesenchymal cell morphology with
spindle- and fibroblastoid-shape cells, while the SW620-OCT4-shRNA
cells showed epithelial cell morphology with epithelioid spreading
cells (Fig. 3B). These results
indicate that OCT4 is involved in the EMT process in colorectal
cancer cells.
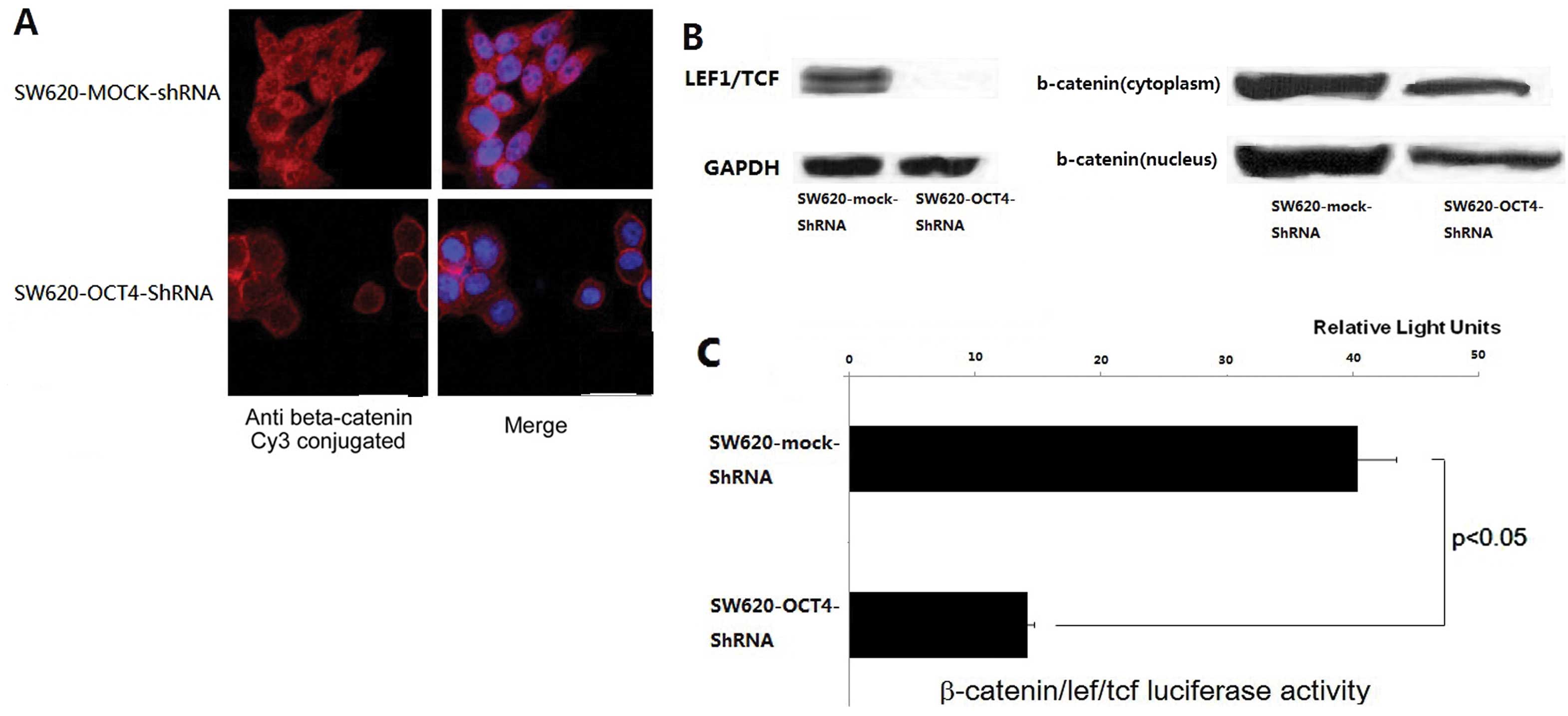
Knockdown of OCT4 leads to reduction in
β-catenin/TCF/LEF signaling in SW620 cells
In colorectal cancer, EMT is companied by nuclear
translocation of β-catenin and the activation of the WNT/β-catenin
signaling pathway (18–20). Following cell staining with the
β-catenin antibody, we found that OCT4 knockdown induced the
accumulation of β-catenin in the cell membrane and the reduction in
cytoplasmic and nuclear β-catenin in SW620 cells compared to the
mock-control (Fig. 4A). Western
blot analysis further confirmed the results (Fig. 4B). β-catenin serves as an activator
of T-cell factor (TCF) and activates transcription of downstream
targets in the canonical WNT pathway (21). To evaluate the effect of OCT4
expression on the WNT pathway, LEF/TCF activity was assessed using
an LEF/TCF reporter luciferase assay. We found that OCT4 knockdown
led to an ~3-fold decrease in β-catenin/TCF/LEF signaling activity
(Fig. 4C). OCT4 knockdown resulted
in upregulated β-catenin membrane translocation and reduced
canonical WNT pathway activities.
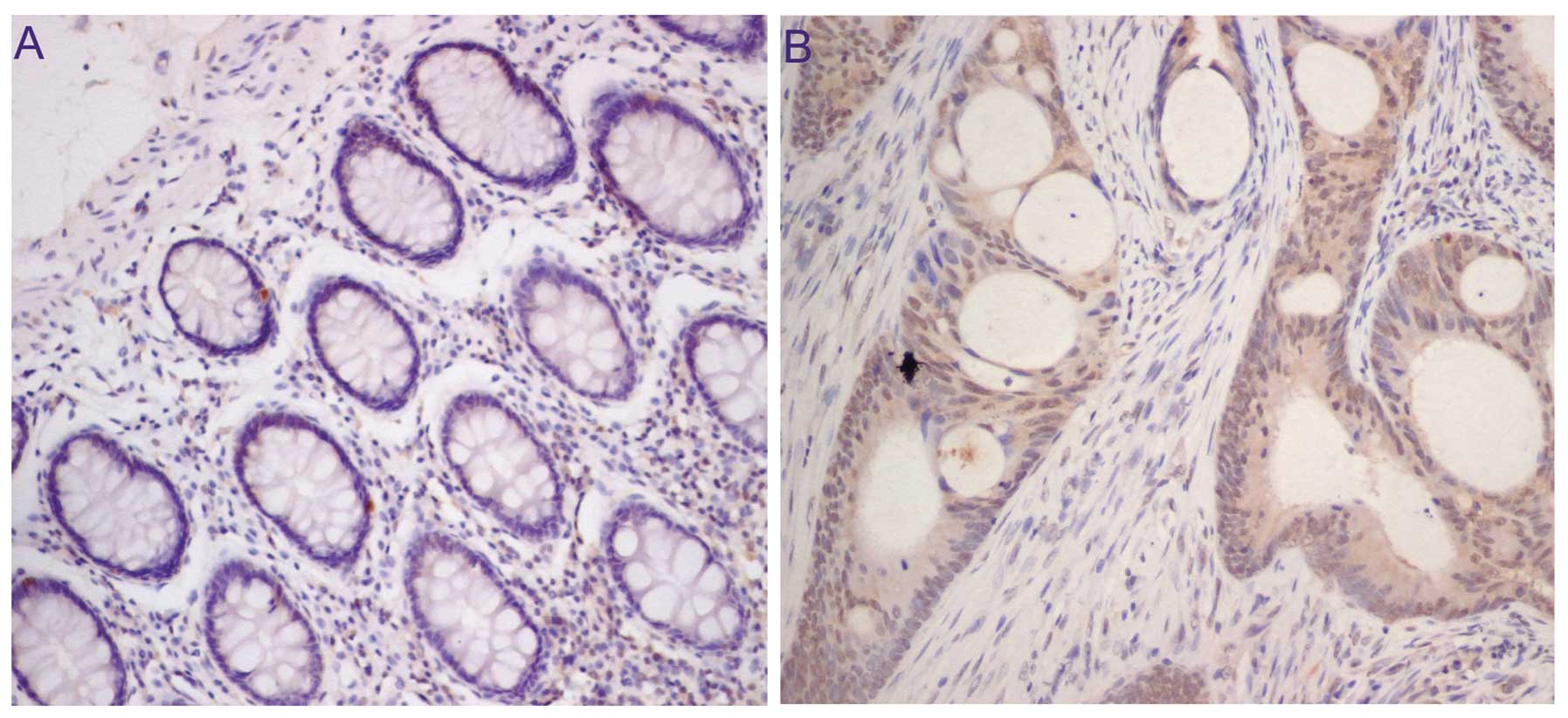
High expression of OCT4 is correlated
with liver metastasis in human colorectal cancer
We next investigated the levels of OCT4 expression
in human colorectal cancers using IHC methods and aimed to
ascertain whether the OCT4 expression level in primary colorectal
cancer is associated with tumor liver metastasis in CRC patients.
OCT4 expression was examined in paraffin-embedded primary tumor
tissues from 50 CRC patients. We found that weak OCT4 expression
was detected in every sample of normal colorectal tissue (Fig. 5A), and 46% of CRC cases (23/50)
exhibited high expression of OCT4 (Fig.
5B). Among the CRC cases with high expression of OCT4, the
percentage of liver metastasis was 82.6%. However, in the cases
with low expression, the percentage of liver metastasis was 22.0%
(11/50 cases). The difference achieved statistical significance
(P<0.05).
Discussion
OCT4, a member of the POU family, is an octamer
motif-binding transcription factor. It is highly expressed in stem
cells and has been considered as a key molecule which could induce
somatic cell pluripotency. OCT4 is essential for maintaining
undifferentiated and pluripotent populations of cells. Kim et
al(21,22) reported that OCT4 can directly
reprogram adult mouse and human neural stem cells to induced
pluripotent stem (iPS) cells. These studies showed that OCT4 plays
an important role in conferring the stemness of cells. In general,
cancer cells are similar to early embryonic cells exhibiting the
properties of immortality, undifferentiation and invasion (23). Thus, it is vital to study genes
associated with embryogenesis and tumorigenesis. In recent years,
there have been some reports on the regulated expression of
OCT4 in several cancer types (12–16).
Metastasis is the neoplastic process responsible for most deaths
from cancer as primary tumors can usually be surgically removed.
Liver metastasis is the main reason for death among colorectal
cancer patients. However, the specific molecular changes in CRC
cells that promote the metastatic process are largely unclear. Some
recent studies suggest that OCT4 may be involved in the metastasis
of CRC (14,16). This prompted us to ascertain whether
OCT4 has a relationship with CRC metastasis. In the present study,
we investigated the role of OCT4 in metastasis-related activities
in human colorectal cancer and its effects on the EMT process and
WNT pathway activity. To our knowledge, this is the first report to
study the role of OCT4 in a human colon cancer cell line by
inhibiting endogenous OCT4 expression using an RNA interference
technique. Metastatic cells undergo loss of adhesion and enhanced
motility and degradation of the basement membrane. Therefore, we
detected these capabilities of OCT4 in CRC SW620 cells. Knockdown
of OCT4 by specific shRNA inhibited SW620 cell migration and
invasion in a Transwell-based assay. After showing that knockdown
of OCT4 reduced cell migration and invasion in vitro, we
conducted an in vivo analysis. We compared the abilities of
the OCT4 knockdown and the mock control cells to form liver
metastasis in a mouse model. We found that the incidence of liver
metastasis and the average number of metastatic foci were
significantly decreased in the OCT4 shRNA group. These data
suggested that OCT4 knockdown reduces the ability of colorectal
cancer cells to form metastasis.
Epithelial-mesenchymal transition (EMT) is an
important process for epithelial cancer and is often activated
during cancer invasion and metastasis. During EMT, cancer cells
undergo morphological changes from an epithelial polarized
morphology to a mesenchymal fibroblastoid morphology and achieve
increased migratory and invasive capabilities. Our present research
demonstrated that OCT4 silencing induced typical cell morphological
changes and EMT marker expression alteration. These results
indicate that OCT4 is involved in metastasis through the EMT
process in colorectal cancer cells. The WNT pathway is widely
regarded as the crucial pathway for colorectal carcinogenesis
(24,25) and regulates EMT process to promote
cancer metastasis. β-catenin forms complexes in the adhesion
complex at the plasma membrane and the signaling complex in the
nucleus (26). We observed that
OCT4 knockdown induced relocalization of β-catenin from the
cytoplasm/nucleus to the membrane and reduced LEF/TCF activity.
Finally, in human tissue samples, increased levels of OCT4
expression in primary cancer were significantly correlated with
liver metastases.
Our research for the first time demonstrated a
relation between OCT4 and the EMT process. OCT4 may be involved in
metastasis of CRC in a Wnt/β-catenin signaling-dependent manner.
OCT4 expression in primary cancer could be used as a marker to
predict liver metastasis in colorectal cancer patients.
Abbreviations:
|
OCT4
|
octamer-binding transcription factor
4
|
|
CRC
|
colorectal cancer
|
|
EMT
|
epithelial-mesenchymal transition
|
References
|
1
|
Shike M, Winawer SJ, Greenwald PH, Bloch
A, Hill MJ and Swaroop SV: Primary prevention of colorectal cancer.
The WHO Collaborating Center for the Prevention of Colorectal
Cancer. Bull World Health Organ. 68:377–385. 1990.PubMed/NCBI
|
|
2
|
Winawer SJ, Fletcher RH, Miller L, et al:
Colorectal cancer screening: clinical guidelines and rationale.
Gastroenterology. 112:594–642. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takahashi K, Tanabe K, Ohnuki M, Narita M,
Ichisaka T, Tomoda K and Yamanaka S: Induction of pluripotent stem
cells from adult human fibroblasts by defined factors. Cell.
131:861–872. 2007. View Article : Google Scholar
|
|
5
|
Zhang Y, Zhang X, Wang X, Gan L, Yu G,
Chen Y, Liu K, Li P, Pan J, Wang J and Qin S: Inhibition of LDH-A
by lentivirus-mediated small interfering RNA suppresses
intestinal-type gastric cancer tumorigenicity through the
downregulation of Oct4. Cancer Lett. 32:45–54. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Asadi MH, Mowla SJ, Fathi F, Aleyasin A,
Asadzadeh J and Atlasi Y: OCT4B1, a novel spliced variant of OCT4,
is highly expressed in gastric cancer and acts as an antiapoptotic
factor. Int J Cancer. 128:2645–2652. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Z, Xu WR, Qian H, Zhu W, Bu XF, Wang
S, Yan YM, Mao F, Gu HB, Cao HL and Xu XJ: Oct4, a novel marker for
human gastric cancer. J Surg Oncol. 99:414–419. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim RJ and Nam JS: OCT4 expression
enhances features of cancer stem cells in a mouse model of breast
cancer. Lab Anim Res. 27:147–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iida H, Suzuki M, Goitsuka R and Ueno H:
Hypoxia induces CD133 expression in human lung cancer cells by
up-regulation of OCT3/4 and SOX2. Int J Oncol. 40:71–79.
2012.PubMed/NCBI
|
|
10
|
Guo Y, Liu S, Wang P, Zhao S, Wang F, Bing
L, Zhang Y, Ling EA, Gao J and Hao A: Expression profile of
embryonic stem cell-associated genes Oct4, Sox2 and Nanog in human
gliomas. Histopathology. 59:763–775. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ikushima H, Todo T, Ino Y, Takahashi M,
Saito N, Miyazawa K and Miyazono K: Glioma-initiating cells retain
their tumorigenicity through integration of the Sox axis and Oct4
protein. J Biol Chem. 286:41434–41441. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fang XF, Zhang WY, Zhao N, Yu W, Ding D,
Hong X, Li LS, Zhang HR, Zheng S and Lin BY: Genome-wide analysis
of OCT4 binding sites in glioblastoma cancer cells. J Zhejiang Univ
Sci B. 12:812–819. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He W, Li K, Wang F, Qin YR and Fan QX:
Expression of OCT4 in human esophageal squamous cell carcinoma is
significantly associated with poorer prognosis. World J
Gastroenterol. 18:712–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saigusa S, Tanaka K, Toiyama Y, Yokoe T,
Okugawa Y, Ioue Y, Miki C and Kusunoki M: Correlation of CD133,
OCT4, and SOX2 in rectal cancer and their association with distant
recurrence after chemoradiotherapy. Ann Surg Oncol. 16:3488–3498.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang CJ, Chien Y, Lu KH, Chang SC, Chou
YC, Huang CS, Chang CH, Chen KH, Chang YL, Tseng LM, Song WS, Wang
JJ, Lin JK, Huang PI and Lan YT: Oct4-related cytokine effects
regulate tumorigenic properties of colorectal cancer cells. Biochem
Biophys Res Commun. 415:245–251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gazouli M, Roubelakis MG, Theodoropoulos
GE, Papailiou J, Vaiopoulou A, Pappa KI, Nikiteas N and Anagnou NP:
OCT4 spliced variant OCT4B1 is expressed in human colorectal
cancer. Mol Carcinog. 51:165–173. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bates RC and Mercurio AM: The
epithelial-mesenchymal transition (EMT) and colorectal cancer
progression. Cancer Biol Ther. 4:365–370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brabletz T, Hlubek F, Spaderna S,
Schmalhofer O, Hiendlmeyer E, Jung A and Kirchner T: Invasion and
metastasis in colorectal cancer: epithelial-mesenchymal transition,
mesenchymal-epithelial transition, stem cells and beta-catenin.
Cells Tissues Organs. 179:56–65. 2005. View Article : Google Scholar
|
|
20
|
Floor S, van Staveren WC, Larsimont D,
Dumont JE and Maenhaut C: Cancer cells in epithelial-to-mesenchymal
transition and tumor-propagating-cancer stem cells: distinct,
overlapping or same populations. Oncogene. 30:4609–4621. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim JB, Greber B, Arauzo-Bravo MJ, Meyer
J, Park KI, Zaehres H and Scholer HR: Direct reprogramming of human
neural stem cells by OCT4. Nature. 461:649–653. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim JB, Sebastiano V, Wu G, Arauzo-Bravo
MJ, Sasse P, Gentile L, Ko K, Ruau D, Ehrich M, van den Boom D, et
al: Oct4-induced pluripotency in adult neural stem cells. Cell.
136:411–419. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Monk M and Holding C: Human embryonic
genes re-expressed in cancer cells. Oncogene. 20:8085–8091. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gottardi CJ and Gumbiner BM: Adhesion
signaling: how beta-catenin interacts with its partners. Curr Biol.
11:R792–R794. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim K, Lu Z and Hay ED: Direct evidence
for a role of beta-catenin/LEF-1 signaling pathway in induction of
EMT. Cell Biol Int. 26:463–476. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kolligs FT, Bommer G and Goke B:
Wnt/beta-catenin/tcf signaling: a critical pathway in
gastrointestinal tumorigenesis. Digestion. 66:131–144. 2002.
View Article : Google Scholar : PubMed/NCBI
|