Introduction
Interleukin-6 (IL-6) is a pleiotropic inflammatory
cytokine that induces the growth and differentiation of immune
cells as well as the expression of many cytokines. IL-6 is also a
representive marker of clinical correlation and prognostic factor
in patients with cancer (1,2). Angiogenesis is an essential process in
the progression and development of cancer. The association of IL-6
with angiogenesis depends on its ability to induce the production
of vascular endothelial growth factor (VEGF), which is a very
potent angiogenic agent (1).
Additionally, IL-6 activates the RhoA and phosphorylated-Src
protein, which is associated with aggressive lymph node metastasis
and poor survival in malignancy (3).
Nuclear factor-κB (NF-κB), a nuclear protein, was
first identified as a transcription factor in the nuclei of mature
B lymphocytes (4). It regulates the
expression of various genes, particularly those involved in the
inflammatory and immune responses (5). Recent evidence has revealed that the
activity of the NF-κB pathway is significantly involved in the
process leading from inflammation to carcinogenesis and tumor
development (6). NF-κB promotes the
overexpression of inflammatory cytokines that act as tumor growth
factors for colitis-associated cancer (7). IL-6, which is encoded by an NF-κB
target gene, is proposed to be one of these tumor growth factors.
Specific inactivation of IL-6 signaling by antagonistic anti-IL-6
antibodies inhibited tumor growth, similar to the inhibition of
TGF-β signaling in colorectal cancer (8). Furthermore, progression and
chemoresistance also appear to involve IL-6, NF-κB induced
expression of IL-6 by its regulation of the growth and survival of
tumor cells (9,10).
Gastric carcinoma is the fourth most frequent
malignancy worldwide and the second most common cause of mortality;
it is the result of accumulated genomic damage which is crucial for
cancer development (11,12). The high rates of gastric cancer
mortality may be related to direct invasion into the adjacent
organs, lymph node metastasis, and distant metastasis of gastric
cancer. IL-6 plays a positive role as a prognostic factor in lymph
node metastasis and advanced gastric cancer (13). However, whether the expression of
IL-6 correlates with the expression of NF-κB in patients suffering
from gastric cancer remains unclear. The aim of our study was to
investigate the protein and mRNA levels of IL-6 and NF-κB and to
analyze the correlation of these two proteins in gastric cancer
patients.
Materials and methods
Patients
Eligible patients were adults (18–75 years old), who
had been diagnosed with biopsy-confirmed gastric cancer. Fresh
cancer tissue samples and corresponding normal tissue samples from
areas adjacent to the tumor specimens (≥5 cm) were obtained from
the patients. All patients were screened and treated at Beijing
Luhe Hospital and samples for the current study were obtained with
the informed consent of the patients. Each tissue fragment was
divided into three parts; one portion was processed for
immunohistochemistry, the second portion for western blot analysis
freezing them in liquid nitrogen, and the third portion was for
reverse transcription (RT) quantitative PCR (RT-qPCR), freezing
them in liquid nitrogen.
Determination of serum cytokines
All blood samples without EDTA were centrifuged at
100,000 rpm for 15 min at 4˚C immediately, and the supernatant was
all stored at −80˚C until analysis. Enzyme-linked immunosorbent
assay (ELISA) (R&D Systems, USA) was used to detected the serum
level of human TNF-α, IL-6, according to the manufacturer’s
instructions.
Immunohistochemistry
Sections (5 μm) of formalin-fixed, paraffin-embedded
primary gastric specimens were prepared for immunohistochemical
analysis. The sections were stained with antibody (Santa Cruz
Biotechnology, USA). The expression levels of VEGF, NF-κB, and IL-6
in the experimental gastric samples were determined by an anti-VEGF
antibody (1:100 dilution), an anti-NF-κB antibody (1:100 dilution)
and an anti-IL-6 antibody (1:50 dilution). Non-specific IgG
antibody was used for negative control of tissue sections. Specific
antibody staining was visualized using a diaminobenzidine substrate
kit. All slides were observed under a bright-field microscope.
Reverse transcription quantitative
PCR
Samples (including gastric cancer tissue and the
tissue of corresponding normal areas) were treated with the TRIzol
reagent (Invitrogen, USA) for total-RNA extraction. The potentially
contaminated genomic DNA was removed by treating 10 mg of the RNA
sample at 37˚C for 30 min with 1 ml of TURBO DNase (Ambion, USA)
followed by extraction with phenol:chloroform:isoamyl alcohol
(25:24:1). Real-time PCR analysis was carried out on the ABI PrismH
7300 Sequence Detection System (Applied Biosystems, USA).
Expression of IL-6, NF-κB and VEGF were analyzed using the TaqMan
PCR Master Mix Reagents kit (Applied Biosystems). The TaqMan probe
and primers for human IL-6, NF-κB and VEGF designed using the
Primer Express 2.0 version were: NF-κB forward
5′-gaaccacacccctgcatatag-3′, reverse 5′-gcattttcccaagagtcatcc-3′
and probe 5′-agaggcta aagttctccaccagg-3′; IL-6 forward
5′-ccactcacctcttcagaacg-3′, reverse 5′-catctttggaaggttcaggttg-3′
and probe 5′-aaattcggta catcctcgacggcatc-3′; VEGF forward
5′-agtccaacatcaccatgcag-3′, reverse 5′-ttccctttcctcgaactgattt-3′
and probe 5′-tcaccaaggccag cacataggag-3′. The cDNA was synthesized
from 500 ng of RNA using the TaqMan RT Reagents kit (Applied
Biosystems). The optimized concentrations for real-time PCR were
0.4 μM for both primers, 0.2 μM for the probe and 5 ng cDNA in a 20
μl reaction volume. Human actin primers (forward 5′-tgcagaaag
agatcaccgc-3′, reverse 5′-ccgatccacaccgagtatttg-3′) were used as an
internal control. Each sample was tested in triplicate. Cycle
threshold (Ct) values were obtained graphically for IL-6, NF-κB,
VEGF and actin. The difference in Ct values between actin and IL-6,
NF-κB, VEGF are presented as ΔCt values. The ΔΔCt values were
obtained by subtracting the ΔCt values of the control samples from
those of the treated samples. Relative fold change in gene
expression was calculated as 2−ΔΔCt.
Western blot analysis
Whole tissue lysates were prepared from human
gastric tissue specimens. Standard western blotting was performed
using anti-IL-6 and anti-NF-κB, anti-VEGF antibodies (Santa Cruz
Biotechnology). Simultaneous determination of the expression level
of β-actin was carried out as an internal control. Proteins were
detected using the enhanced chemiluminescence system in accordance
with the manufacturer’s instructions (Tanon 4500, Shandong Aibo
Technology Co., China). Separate analyses were performed for each
sample and the experiment was repeated three times.
Statistical analysis
Data were expressed as the mean ± SD. Values were
performed with a one-way analysis using SPSS15.0 software, followed
by a student’s two-tailed test, and comparison between groups was
performed using an analysis of variance (ANOVA) or through a
non-parametric test. P<0.05 was considered to indicate
statistically significant differences.
Results
Determination of serum samples
quantifying IL-6, TNF-α
Ninety-eight patients were enrolled in this study.
Patient plasma samples were collected to determine cytokine levels
prior to and following surgery. IL-6 and TNF-α were examined to
conform any differences in plasma pre-and post-operatively.
Cytokine concentration (P<0.005) of IL-6, TNF-α decreased in
post-operative plasma samples (Table
I). Two-paired t-test was used by observing a significant
difference in pre-operative, post-operative and normal serum
samples.
 | Table IComparison of plasma cytokine levels
of IL-6 and TNF-α in gastric cancer patients. |
Table I
Comparison of plasma cytokine levels
of IL-6 and TNF-α in gastric cancer patients.
| Variable | No. | IL-6 (ng/l) | TNF-α (ng/l) |
|---|
| Pre-operative | 30 |
279.2±56.7a |
315.4±60.7a |
| Post-operative | 33 |
183.2±39.5a,b |
236.5±31.8a,b |
| Normal | 35 | 38.9±11.2 | 53.5±17.6 |
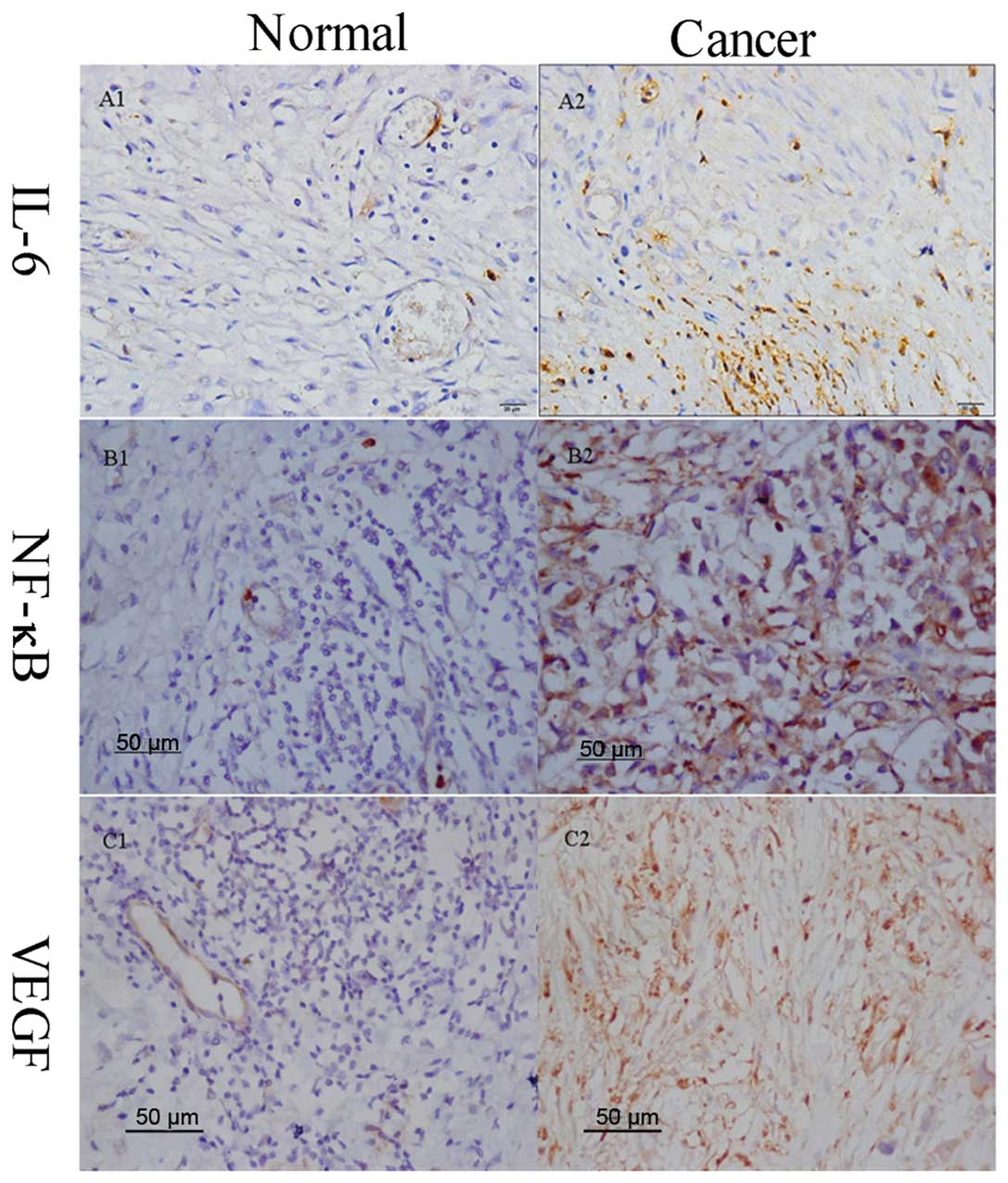
Immunohistochemical expression and
cellular distribution of IL-6, NF-κB, and VEGF
The production of IL-6, NF-κB and VEGF in human
gastric tissue and adjacent normal mucosa were all examined using
immunohistochemical staining. The findings of the
immunohistochemical staining confirmed a weak expression of NF-κB,
IL-6 and VEGF in adjacent normal mucosa but a strong expression in
gastric cancer tissue (Figs. 1 and
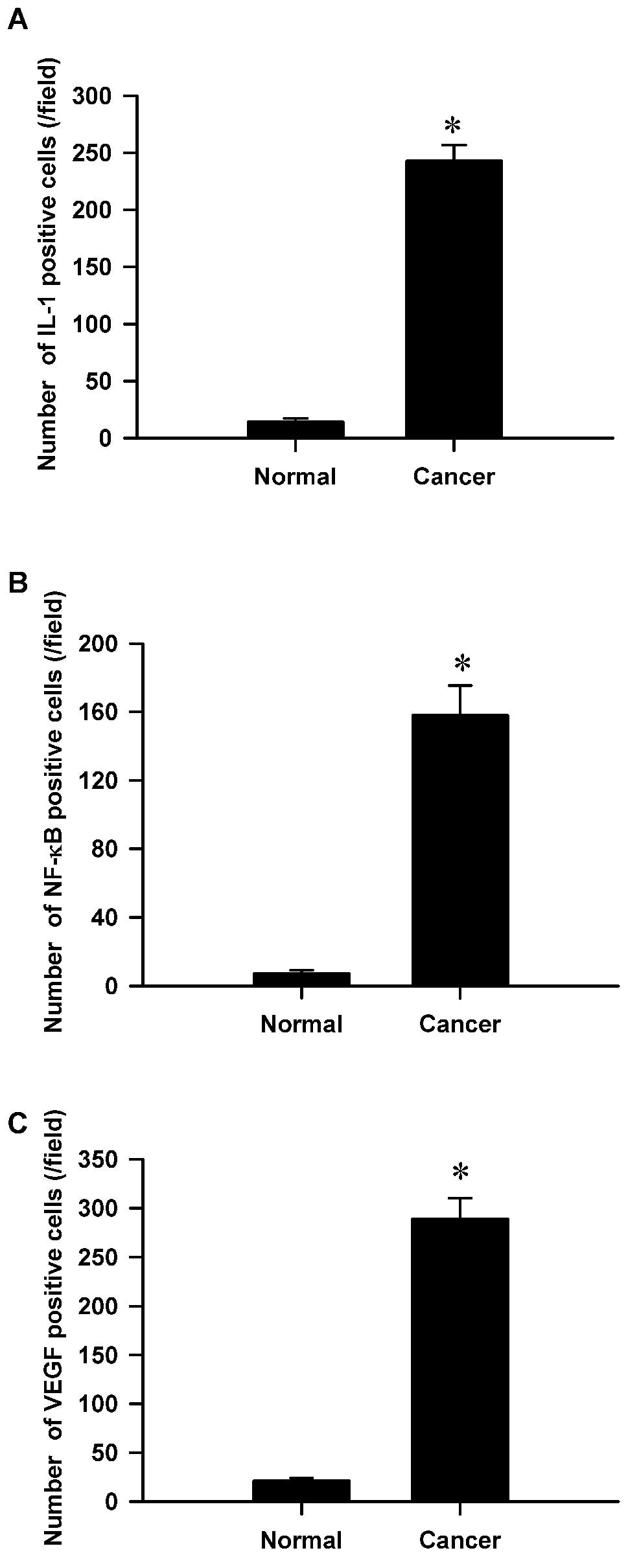
2A–C). The overexpression of IL-6
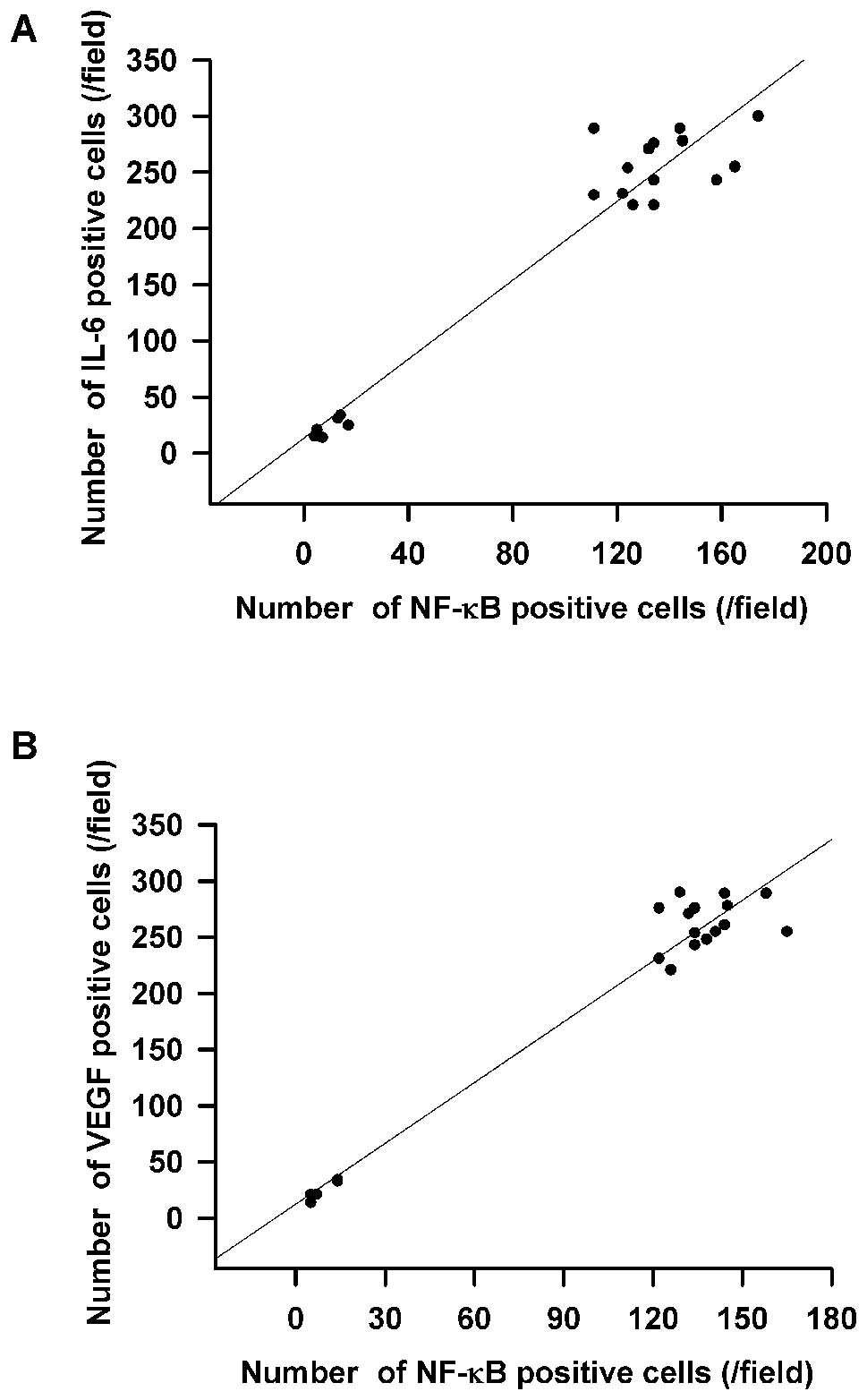
was directly associated with NF-κB activation (Fig. 3A). Overexpression of VEGF was also
directly associated with NF-κB activation according to further
correlation analysis (Fig. 3B).
Moreover, we found an association of increased IL-6, VEGF and NF-κB
expression in the clinicopathological characteristics of gastric
cancer (Fig. 3A and B).
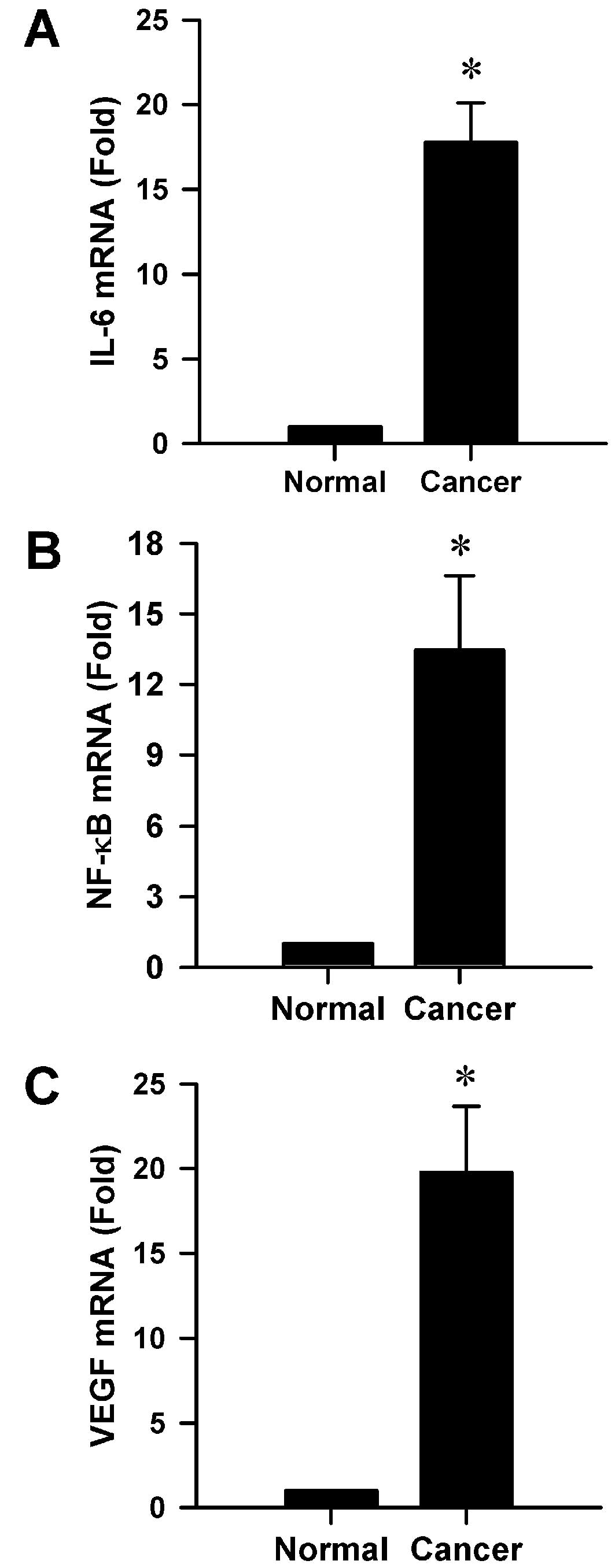
mRNA levels are significantly increased
in gastric cancer tissue
We investigated the mRNA levels of IL-6, NF-κB and
VEGF in gastric cancer tissue according to RT-qPCR. As we expected,
mRNA levels of IL-6, NF-κB and VEGF in human gastric cancer tissue
were all significantly increased compared to those in adjacent
normal mucosa tissue samples (all P<0.001, Fig. 4), suggesting that high NF-κB mRNA
levels might be positively correlated with IL-6 mRNA levels.
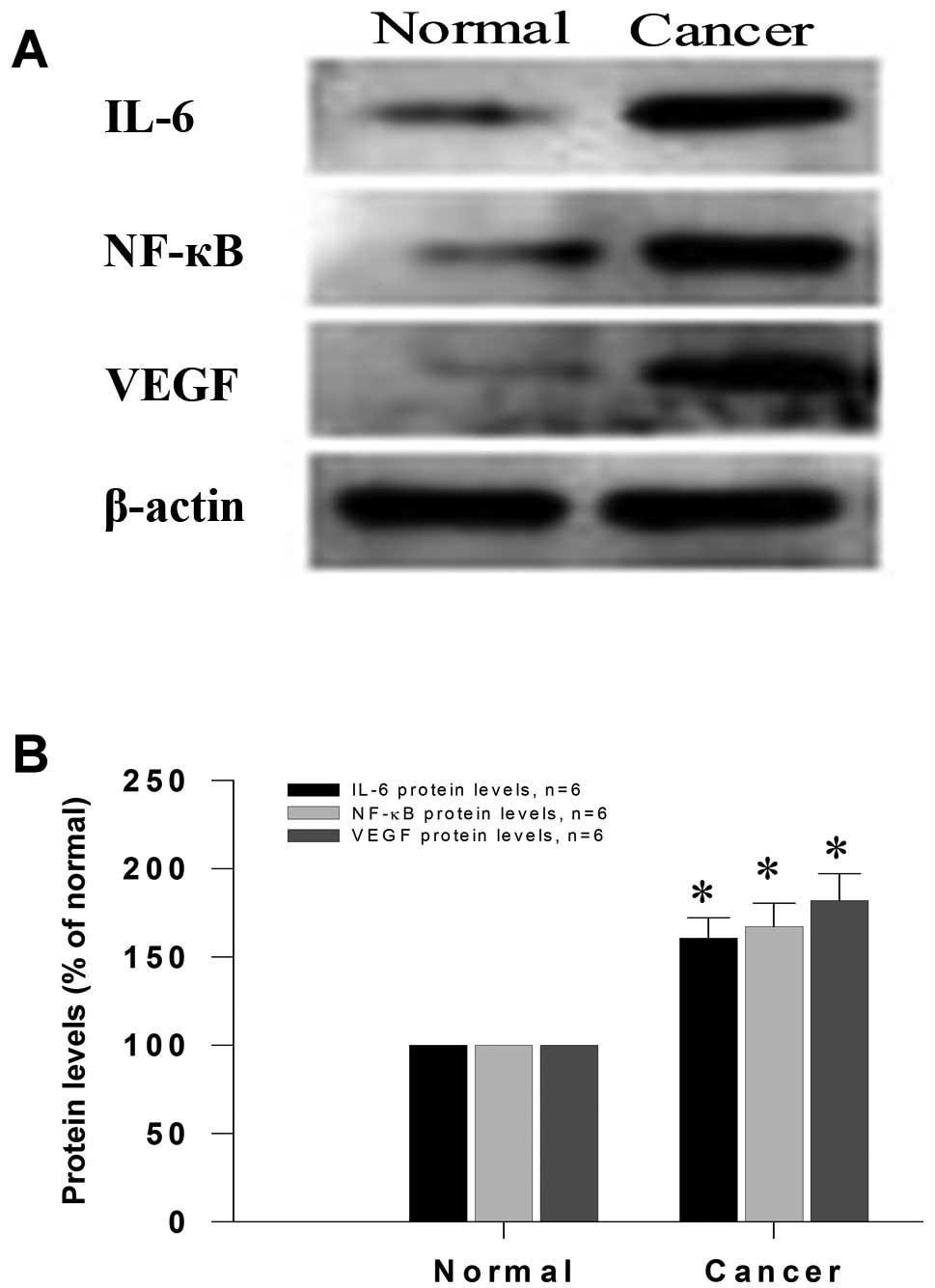
Protein levels are markedly upregulated
in gastric cancer tissue
We further explored the protein expression of IL-6,
NF-κB and VEGF in gastric cancer tissue according to western
blotting. As we expected, a single band was performed using each
antibody (Fig. 5A) and the protein
production levels of IL-6, NF-κB and VEGF in human gastric cancer
tissue were all clearly upregulated compared to those in the
adjacent normal mucosa tissue (all P<0.001, Fig. 5B). IL-6, NF-κB and VEGF increased
significantly in gastric cancer tissue, suggesting that high
protein levels of NF-κB might be positively correlated with IL-6
protein levels.
Discussion
NF-κB, discovered in 1986, binds to the enhancer
region of the κB chain of immunoglobulin as a nuclear factor in B
cells. Constitutive activation of NF-κB has been found in the
majority of tumor cell lines, including solid and hematologic
tumors (14). Proliferation of most
tumor cells depends on constitutive activation of NF-κB, as
inhibition of NF-κB leads to abrogation of proliferation (15). Pro-inflammatory cytokines such as
TNF, IL-1 and IL-6, all regulated by the NF-κB pathway, have been
shown to be overexpressed in colitis, gastritis, or hepatitis.
IL-6, whose expression is regulated by NF-κB, has been implicated
in the oncogenesis process by inducing proliferation of multiple
myeloma cells (16). In gastric
carcinoma, IL-6 induces VEGF expression by increasing angiogenesis
(17), and may be a marker of tumor
angiogenesis and disease status (18,19).
In the present study, we showed that activation of
NF-κB correlates with IL-6 in human gastric cancer tissues. These
data indicating a role for NF-κB and IL-6 are supported by studies
in patients with gastric cancer. In particular, expression of IL-6
mRNA in gastric mucosa related to the level of gastric mucosal
inflammation cytokine (20,21). Serum levels of IL-6 and TNF-α were
significantly higher in patients with gastric cancer than gastritis
(22). IL-6 plays a key role as a
prognostic factor in gastric cancer invasion and lymph and/or
hepatic node metastasis (13), and
consistent with our results, in a series of gastric cancer
patients, high IL-6 serum levels predict a shorter survival.
NF-κB mediates the expression of most gene products
that play key roles in cell survival, angiogenesis and immune
responses. One of the gene targets of NF-κB is IL-6. The IL-6
promoter involves at least four transcription factor binding sites,
the IL-6-NF-κB regulatory site is one of them. Although the
transcriptional regulation of IL-6 expression appears to be very
complex, including multiple transcription factors and signaling
pathways, NF-κB may play a crucial role in the expression of IL-6
in gastric cancer. In numerous cells, activation of NF-κB is
responsible for the inducible production of the proinflammatory
cytokines, involving IL-1b, IL-6, IL-8, and TNF-α (23,24).
These studies are consistent with our results.
In the present study, we found that IL-6 expression
was significantly associated with NF-κB, and both were found
overexpressed in human gastric cancer; a weak expression was found
in adjacent normal mucosa. Since our results indicate a
differential expression of IL-6 and NF-κB in gastric cancer tissue
and adjacent normal mucosa, this may suggest that the expression
pattern of the IL-6-NF-κB signal pathway may be linked to the
development of gastric cancer.
NF-κB inhibition does not completely prevent cancer
pathogenesis, as cytokines could also promote tumorigenesis via
alternative pathways (25).
Therefore, investigations of other molecular pathways may provide
further insights into chronic inflammation-induced tumorigenesis
and targeted cancer therapy.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (81071586).
References
|
1
|
Cohen T, Nahari D, Cerem LW, Neufeld G and
Levi BZ: Interleukin 6 induces the expression of vascular
endothelial growth factor. J Biol Chem. 271:736–741. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thong-Ngam D, Tangkijvanich P, Lerknimitr
R, Mahachai V, Theamboonlers A and Poovorawan Y: Diagnostic role of
serum interleukin-18 in gastric cancer patients. World J
Gastroenterol. 12:4473–4477. 2006.PubMed/NCBI
|
|
3
|
Lin MT, Lin BR, Chang CC, Chu CY, Su HJ,
Chen ST, Jeng YM and Kuo ML: IL-6 induces AGS gastric cancer cell
invasion via activation of the c-Src/RhoA/ROCK signaling pathway.
Int J Cancer. 120:2600–2608. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sen R and Baltimore D: Inducibility of
kappa immunoglobulin enhancer-binding protein NF-kappa B by a
posttranslational mechanism. Cell. 47:921–928. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karin M and Delhase M: The I kappa B
kinase (IKK) and NF-kappa B: key elements of proinflammatory
signaling. Semin Immunol. 12:85–98. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karin M, Cao Y, Greten FR and Li ZW:
NF-kappaB in cancer: from innocent bystander to major culprit. Nat
Rev Cancer. 2:301–310. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Greten FR, Eckmann L, Greten TF, Park JM,
Li ZW, Egan LJ, Kagnoff MF and Karin M: IKKbeta links inflammation
and tumorigenesis in a mouse model of colitis-associated cancer.
Cell. 118:285–296. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Becker C, Fantini MC, Schramm C, Lehr HA,
Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, et al:
TGF-beta suppresses tumor progression in colon cancer by inhibition
of IL-6 trans-signaling. Immunity. 21:491–501. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kawano M, Hirano T, Matsuda T, Taga T,
Horii Y, Iwato K, Asaoku H, Tang B, Tanabe O and Tanaka H:
Autocrine generation and requirement of BSF-2/IL-6 for human
multiple myelomas. Nature. 332:83–85. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Klein B, Zhang XG, Lu ZY and Bataille R:
Interleukin-6 in human multiple myeloma. Blood. 85:863–872.
1995.PubMed/NCBI
|
|
11
|
Oue N, Sentani K, Sakamoto N, Motoshita J,
Nishisaka T, Fukuhara T, Matsuura H, Sasaki H, Nakachi K and Yasui
W: Characteristic gene expression in stromal cells of gastric
cancers among atomic-bomb survivors. Int J Cancer. 124:1112–1121.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar
|
|
13
|
Ashizawa T, Okada R, Suzuki Y, Takagi M,
Yamazaki T, Sumi T and Aoki T, Ohnuma S and Aoki T: Clinical
significance of interleukin-6 (IL-6) in the spread of gastric
cancer: role of IL-6 as a prognostic factor. Gastric Cancer.
8:124–131. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sethi G, Sung B and Aggarwal BB: Nuclear
factor-kappaB activation: from bench to bedside. Exp Biol Med.
233:21–31. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bargou RC, Emmerich F, Krappmann D,
Bommert K, Mapara MY, Arnold W, Royer HD, Grinstein E, Greiner A,
Scheidereit C and Dörken B: Constitutive nuclear factor-kappaB-RelA
activation is required for proliferation and survival of Hodgkin’s
disease tumor cells. J Clin Invest. 100:2961–2969. 1997.PubMed/NCBI
|
|
16
|
Akira S and Kishimoto T: The evidence for
interleukin-6 as an autocrine growth factor in malignancy. Semin
Cancer Biol. 3:17–26. 1992.PubMed/NCBI
|
|
17
|
Huang SP, Wu MS, Shun CT, Wang HP, Lin MT,
Kuo ML and Lin JT: Interleukin-6 increases vascular endothelial
growth factor and angiogenesis in gastric carcinoma. J Biomed Sci.
11:517–527. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim DK, Oh SY, Kwon HC, Lee S, Kwon KA,
Kim BG, Kim SG, Kim SH, Jang JS, Kim MC, et al: Clinical
significances of preoperative serum interleukin-6 and C-reactive
protein level in operable gastric cancer. BMC Cancer. 9:1552009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liao WC, Lin JT, Wu CY, Huang SP, Lin MT,
Wu AS, Huang YJ and Wu MS: Serum interleukin-6 level but not
genotype predicts survival after resection in stages II and III
gastric carcinoma. Clin Cancer Res. 14:428–434. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Harris PR, Smythies LE, Smith PD and
Dubois A: Inflammatory cytokine mRNA expression during early and
persistent Helicobacter pylori infection in nonhuman
primates. J Infect Dis. 181:783–786. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamaoka Y, Kita M, Kodama T, Sawai N and
Imanishi J: Helicobacter pylori cagA gene and expression of
cytokine messenger RNA in gastric mucosa. Gastroenterology.
110:1744–1752. 1996. View Article : Google Scholar
|
|
22
|
Crabtree JE, Shallcross TM, Heatley RV and
Wyatt JI: Mucosal tumour necrosis factor alpha and interleukin-6 in
patients with Helicobacter pylori associated gastritis. Gut.
32:1473–1477. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kopp EB and Ghosh S: NF-κB and Rel
proteins in innate immunity. Adv Immunol. 58:1–27. 1995.
|
|
24
|
Busam K, Gieringer C, Freudenberg M and
Hohmann HP: Staphylococcus aureus and derived exotoxins
induce nuclear factor κB-like activity in murine bone marrow
macrophages. Infect Immun. 60:2008–2015. 1992.
|
|
25
|
Dranoff G: Cytokines in cancer
pathogenesis and cancer therapy. Nat Rev Cancer. 4:11–22. 2004.
View Article : Google Scholar
|