Introduction
Tumor metastasis occurs through a multistep process
(1). The interaction of circulating
tumor cells that have detached from the primary tumor with
structures of the tissue microvasculature is a crucial step
preceding the invasion of the target organ. The specific events
determining tumor cell interactions with endothelial cells during
hematogenous metastasis are well defined (2); the role of other cell types such as
platelets in this process, however, remains unclear.
Gangliosides, sialic acid-containing
glycosphingolipids found in all eukaryotic cell membranes, play a
role in cell-cell interaction, as well as in cell growth and
proliferation (3). Gangliosides
interact with a number of cell surface receptors (4–8)
including integrin receptors (3,9).
Neuroblastoma, an invasive, aggressive malignancy seen in children,
is metastatic in 80% of patients at the time of presentation.
Gangliosides are shed from the membrane of neuroblastoma cells into
the extracellular milieu in large quantities. Gangliosides also
affect platelet function. Gangliosides isolated from neuroblastoma
tumor cells enhance platelet aggregation and activation and also
promote platelet adhesion to extracellular matrix collagen
(9–11). These effects are mediated through
the integrin collagen receptor α2β1.
Gangliosides shed from the tumor may therefore represent a
paracrine factor which, by virtue of its platelet activating
activity, promotes metastasis.
Since gangliosides modify signals from receptor
tyrosine kinases (12), they
interact with integrins that signal via the tyrosine kinase
mechanism (13) and regulate
integrin-dependent cell adhesion and spreading by affecting
α5β1 signaling (14). One protein, focal adhesion kinase,
p125FAK (FAK) localizes to focal plaques following cell adhesion to
extracellular matrix (15). Its
catalytic activity increases following cell adhesion to collagen
(16). We speculated that tumor
gangliosides might also modify the p125FAK signals generated by
integrin in platelets. The present studies were undertaken to
examine the effects of neuroblastoma tumor gangliosides (NBTGs) on
α2β1-mediated p125FAK signaling in
platelets.
Materials and methods
Monoclonal antibodies
Murine anti-human monoclonal antibodies against
p125FAK and phosphotyrosine residues, and goat anti-murine
horseradish peroxidase-conjugated antibody used for immunoblotting
were purchased from Transduction Laboratories (San Diego, CA, USA).
For immunoprecipitation of p125FAK, murine anti-human monoclonal
antibody was purchased from Upstate Biotechnology (Lake Placid, NY,
USA).
Extraction and purification of tumor
gangliosides
Total gangliosides were isolated from the
neuroblastoma tumor cell line LAN-5 cells as previously described
(17). Total lipids were extracted
twice with 10 volumes of chloroform-methanol (1:1); the extracts
were then combined and dried under a stream of N2,
re-dissolved in a small volume of chloroform-methanol (1:1) and
stored overnight at −20°C. Insoluble glycoproteins were removed by
centrifugation (1,000 × g, 4°C) and the supernatant was dried under
a stream of N2. The gangliosides were isolated by
partitioning the dried total lipid extract in di-isopropyl
ether/1-butanol/water (6:4:5, v/v) (4), followed by Sephadex G-50 gel exclusion
chromatography to remove traces of salts and other low molecular
weight contaminants and further purified by normal phase high
pressure liquid chromatography. The GD2
(disialoganglioside) fraction was collected, lyophilized and
re-purified by gel exclusion chromatography to remove salts.
Gangliosides were quantified as nmol lipid bound sialic acid (LBSA)
as previously described (18),
separated by high performance thin layer chromatography (HPTLC) and
visualized as purple bands following staining with resorcinol
reagent (5).
Platelet isolation
Platelet donors abstained from all medications for a
minimum of 7 days, fasted overnight and provided written, informed
consent. Platelets were isolated from donors according to the
methods previously described (11).
In brief, blood was drawn into tubes containing acid-citrate
dextrose-A to which 35 U/ml preservative-free heparin was added.
Platelet-rich plasma was isolated by centrifugation (3,000 × g, 15
min at 22°C) and then passed over Sepharose 2B (Amersham, Uppsala,
Sweden) in modified Tyrode’s buffer (MTB) in the absence of
Ca2+ or Mg2+.
Platelet adhesion assay
Platelet adhesion was determined as previously
described (17). Collagen was
diluted with isotonic glucose (pH 2.7–2.9) to a concentration of 40
μg/ml. One hundred microliters of this suspension was used
to coat the wells of a polystyrene microtiter plate (Falcon 3915,
Becton-Dickinson) overnight at 22°C. The wells were aspirated and
blocked with 100 μl of 0.5% BSA solution for 1 h at 22°C and
then washed 3 times with MTB. Control wells were coated with BSA
alone. Gel-filtered platelets were adjusted to
105/μl in MTB without MgC12 and
incubated with purified tumor gangliosides at the specified
concentration for 30 min at 37°C with gentle mixing. The platelets
were washed once to remove unbound gangliosides and resuspended in
MTB with 2.56 mM MgC12, and 100 μl of the final
platelet suspension was added to the wells and incubated for 1 h at
37°C with gentle mixing. The wells were vigorously washed 5 times
with 100 μl MTB to remove non-adherent platelets and loose
aggregates. The number of adherent platelets was determined using
the BCA assay (Pierce, Rockford, IL, USA) as previously described
(17). Absorbance was measured at
570 nm (OD570) with a microtiter plate reader (BioTek
Instruments, Winooski, VT, USA). In each experiment, a standard
curve of OD570 and platelet number was constructed by
adding platelets (103–106 per well) to
collagen-coated, BSA-blocked wells as described above. An average
OD570 value was calculated from triplicate wells over
the range of platelet concentrations and related to direct
phase-contrast microscopy counts by linear regression analysis. In
all experiments, a direct correlation was observed between measured
OD570 and platelet number (r2>0.95).
OD570 values of 0.1, 0.2, 0.3 and 0.4 represent platelet
numbers of 1.76×103, 1.57×104,
1.39×105 and 1.26×106, respectively.
Effects of NBTGs on protein tyrosine
phosphorylation of platelets adherent to collagen
The effects of NBTGs on protein tyrosine
phosphorylation signaling through α2β1
integrin were examined in platelets adherent to immobilized
collagen. Collagen was diluted in isotonic glucose (pH 2.7–2.9) to
a concentration of 100 μg/ml and 600 μl of this
suspension was used to coat the surface of 6-well plates (Falcon
1143, Becton-Dickinson, Oxnard, CA, USA) overnight at 22°C. Wells
were blocked with 0.1% BSA prior to adding platelets. Gel-filtered
platelets (105/ml) were incubated with 1 μmol
NBTGs or GD2 dissolved in MTB with 2.56 mM
MgCl2 and 100 mM Na3VO4 at 37°C
for 30 min. Then, 1.2 ml of platelet suspension was added to the
wells and incubated for 1 h at 37°C with gentle mixing.
Non-adherent platelets were removed by aspiration, concentrated by
centrifugation and lysed with ice cold lysis buffer (50 mM Tris, pH
8.0, 2.0 mM EDTA, 0.15 M NaCl, 1% Triton X-100, 200 mM
Na3VO4, 1 mM PMSF, 1 μg/ml aprotinin
and 1 μg/ml leupeptin) at 4°C. Residual adherent platelets
were lysed directly in the wells.
Immunoprecipitation
Protein lysates were incubated with washed Pansorbin
cells (Calbiochem, La Jolla, CA, USA) for 30 min at 4°C with
mixing. The sample was clarified (12,000 × g for 15 min at 4°C) and
the protein recovered, then incubated overnight at 4°C with
monoclonal antibody against p125FAK (2 mg/ml). To precipitate the
p125FAK antibody complex, Protein G Plus-Agarose beads were added
and the lysate was incubated for 4 h at 4°C. The complex was then
recovered and washed thrice with Triton X-100 lysis buffer. Immune
complexes were boiled in equal volume of 2X loading buffer for 2
min and the supernatant was recovered and immunoblotted.
Immunoblotting
Lysates were clarified by centrifugation (12,000 × g
for 15 min at 4°C) and the supernatant was collected. Protein
content in each sample was determined using the BCA assay. Western
blotting of platelet lysates was performed with equal amounts of
protein mixed with 1:1 vol of 2X loading buffer, then boiled at
100°C for 2 min and analyzed by 6 or 7.5% SDS-polyacrylamide gel
electrophoresis. Proteins were transferred to a nitrocellulose
membrane (Amersham) using a wet transfer module (Bio-Rad
Laboratories, Hercules, CA, USA). Membranes were blocked with
buffer A (1% BSA or 5% non-fat milk, 50 mM Tris, 0.1% Tween-20, 100
mM NaCl, pH 7.5) for 1 h. The immunoblots were probed with
anti-phosphotyrosine monoclonal antibody (PY-20) or anti-p125FAK
monoclonal antibody (1/1,000) in buffer A for 1 h, washed and then
incubated with HRP-conjugated secondary antibody (1/1,000) for 1 h.
Proteins were visualized by enhanced chemiluminescence (ECL) and
exposed to Hyperfilm ECL (Amersham).
Results
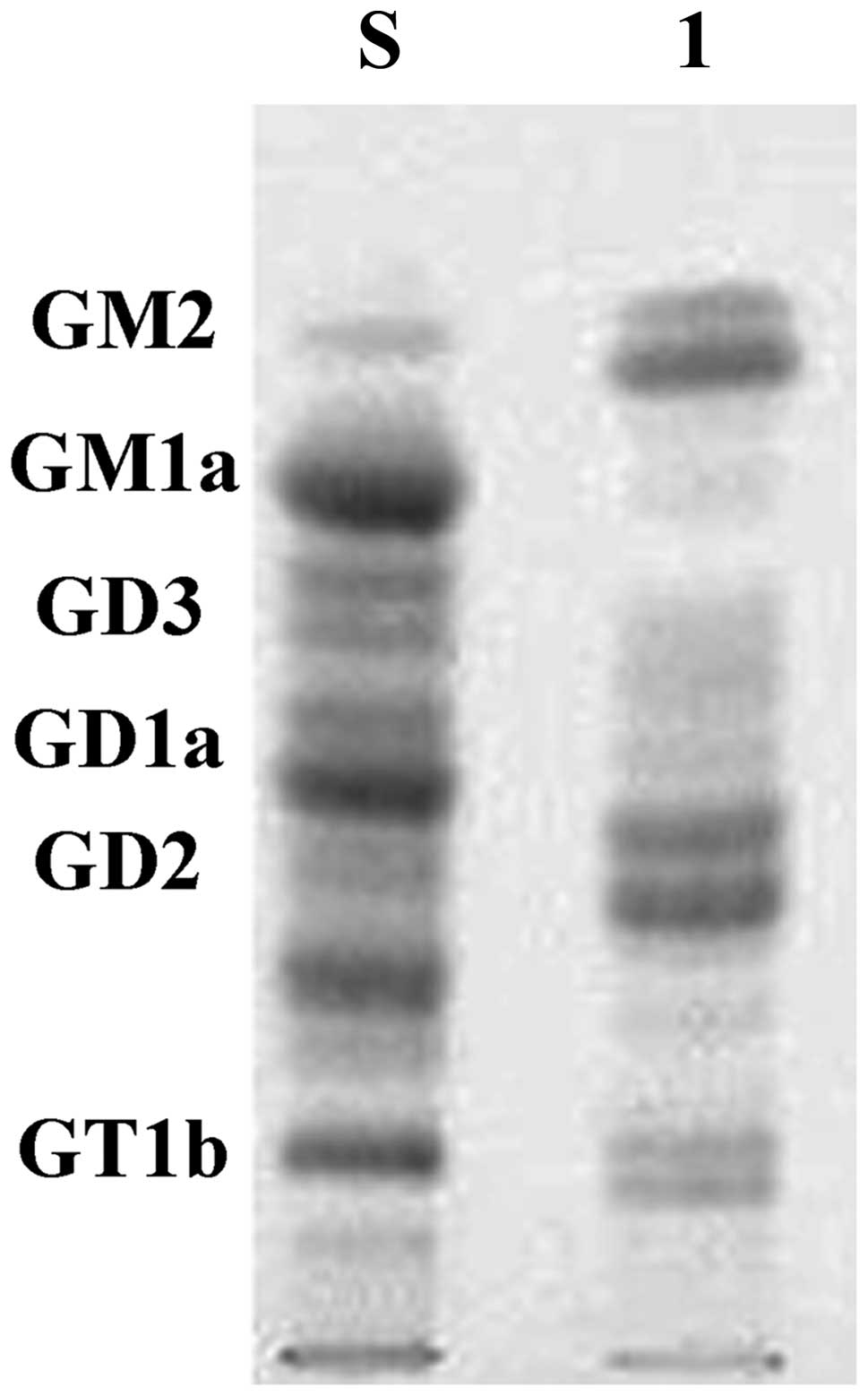
Ganglioside compositions in LAN-5
neuroblastoma cell lines
The chromatographic profile of the LAN-5 cell line
gangliosides is shown in Fig. 1.
Human brain gangliosides (gift from Dr Zhengmei Zhu) were used as a
standard. The predominance of GD2 is evident in the cell
line, the other major composition is GM2, fewer
compositions include GD3, GT1b.
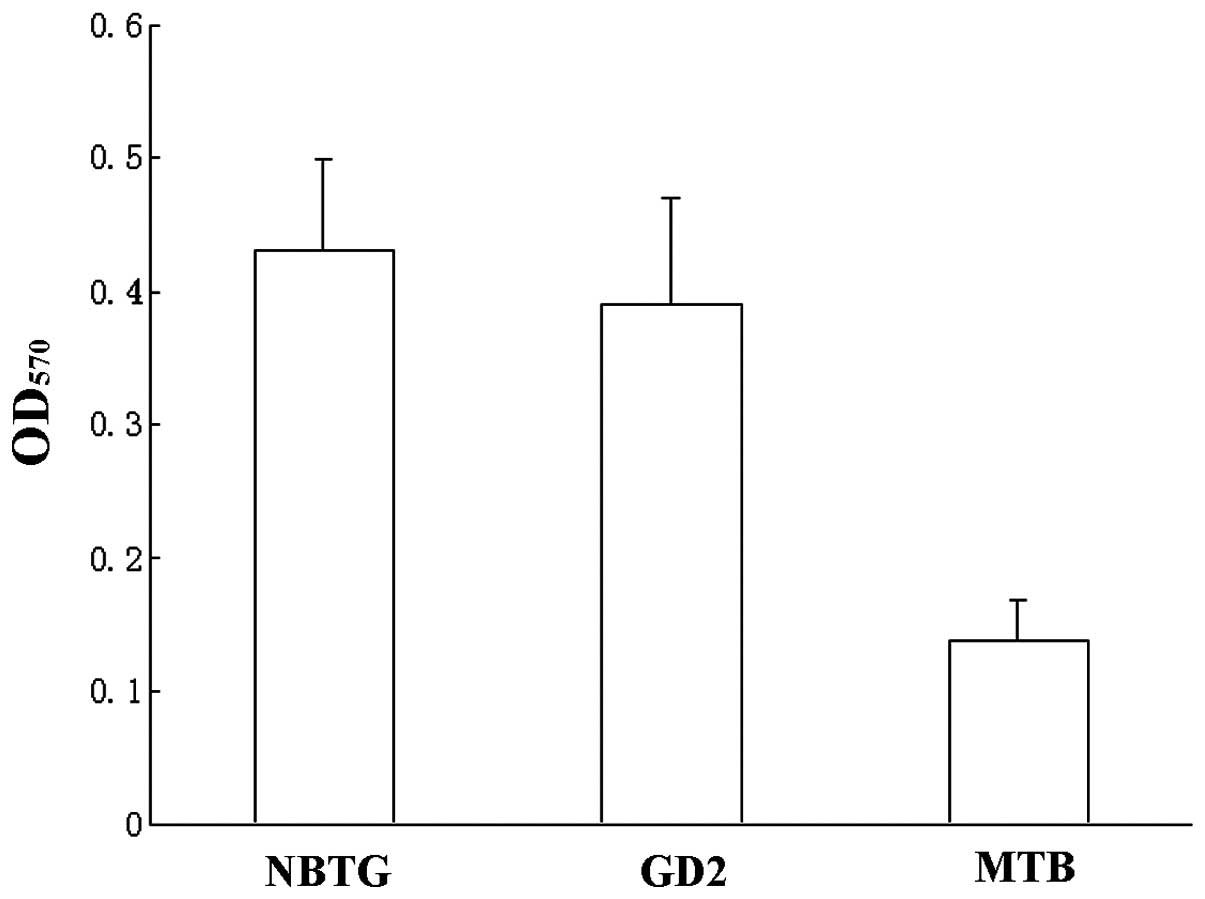
Neuroblastoma tumor gangliosides enhance
α2β1 integrin-mediated platelet adhesion to
collagen
In Fig. 2, platelets
pre-incubated with 1 μmol total NBTG or the major individual
ganglioside GD2 were more adherent to immobilized
collagen (OD570 0.43±0.12, 0.39±0.13) compared to
platelets pre-incubated with MTB (0.14±0.06, P<0.001). Adhesion
was maximal by 30 min, and no further increase was observed with
incubation duration up to 120 min (not shown). No effect of MTB on
platelet adhesion to collagen was observed.
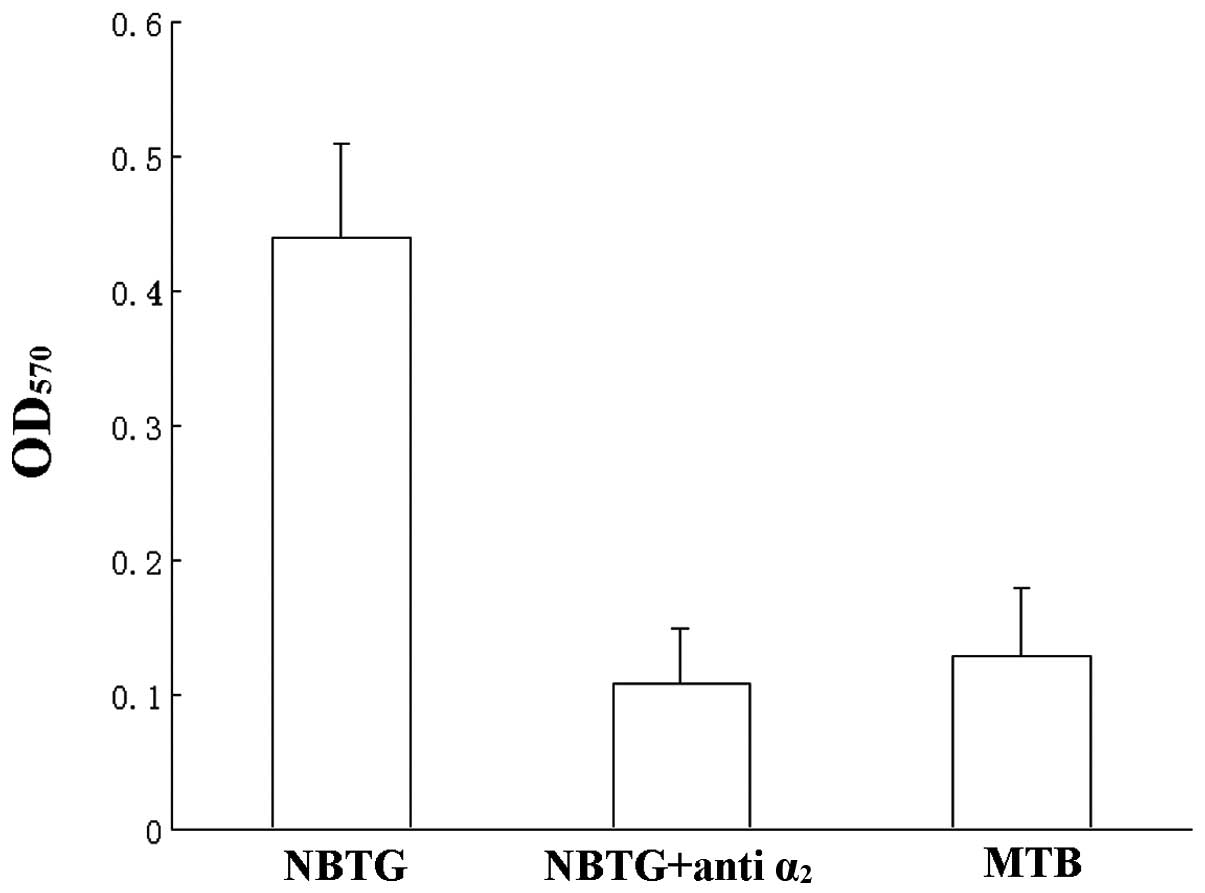
α2β1 integrin is the major collagen receptor
on platelets, adhesion experiments were performed with
anti-α2 monoclonal antibody to block the
α2β1 receptor. In Fig. 3 adhesion of NBTG-pre-incubated
platelets was reduced to control levels by F-17 anti-α2
antibody (OD570 0.11±0.05 vs. 0.13±0.06 P>0.05).
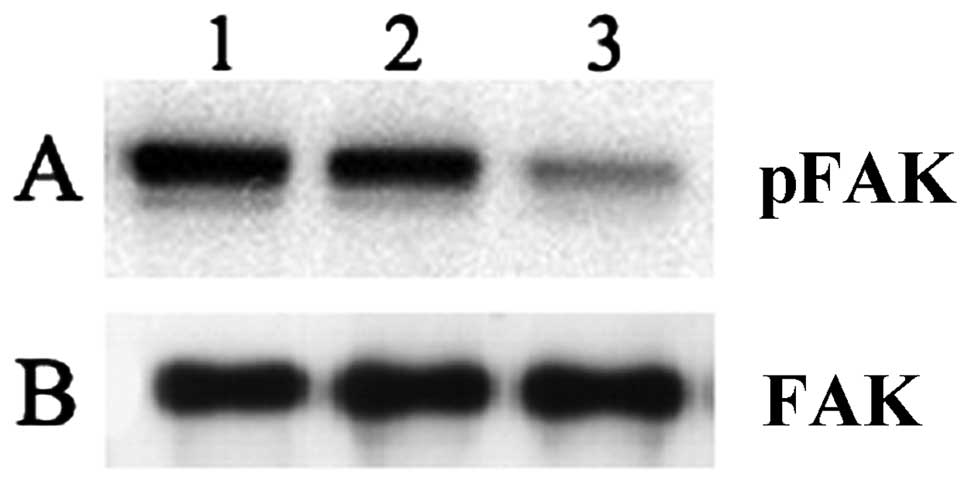
Effects of neuroblastoma tumor
gangliosides on tyrosine phosphorylation of platelet adhesion to
collagen
We examined the effects of NBTGs on phosphotyrosine
signaling following platelet adhesion to immobilized collagen
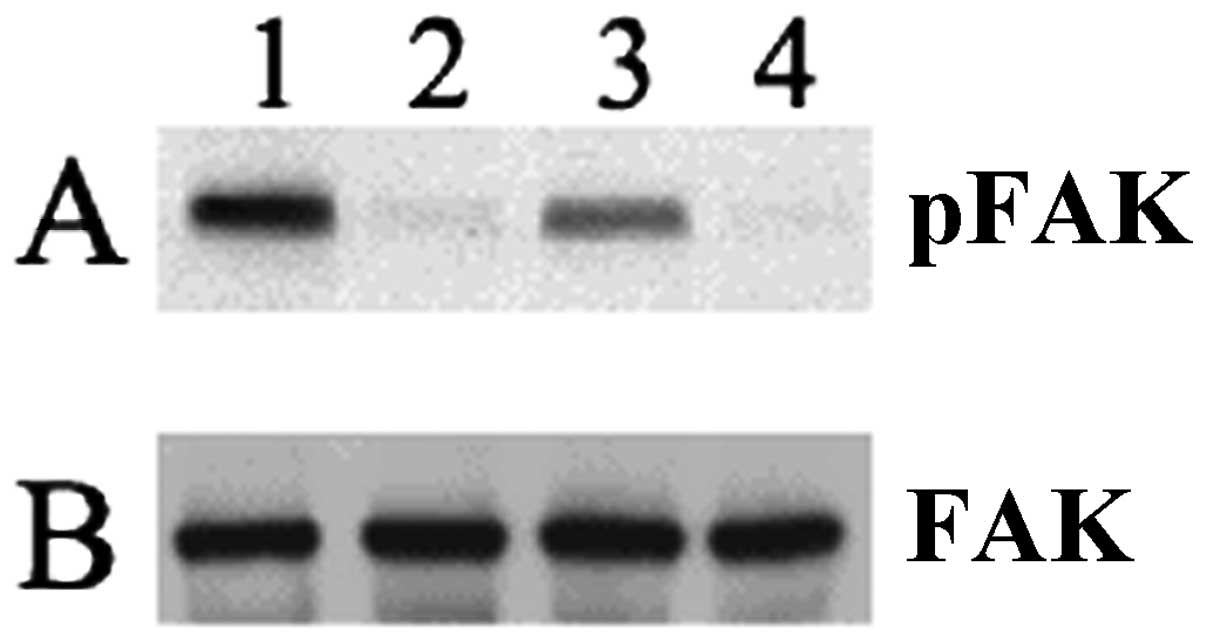
(Fig. 4). Adherent platelets were
immunoprecipitated with anti-p125FAK antibody, then examined with
an antiphosphotyrosine (Fig. 4A) or
anti-p125FAK (Fig. 4B) antibody.
The phosphotyrosine intensity of the p125FAK band (Fig. 4A) in platelets pre-incubated with
NBTGs (lane 1) or GD2 (lane 2) was increased compared to
MTB buffer-pre-incubated adherent platelets (lane 3). Total p125FAK
protein content in each sample is comparable (Fig. 4B) and is not affected by
gangliosides.
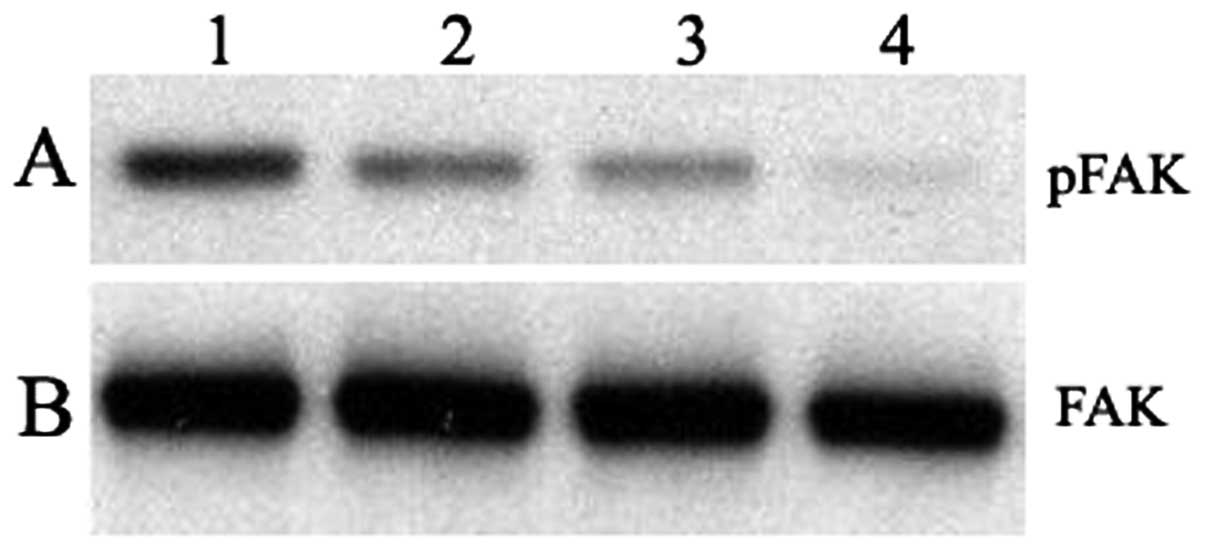
The effects of the gangliosides on tyrosine
phosphorylation were explored in platelets adherent to immobilized
collagen and in the non-adherent platelets (Fig. 5). Pre-incubation of platelets with 1
μM NBTGs (lane 1) resulted in marked increases in the
phosphotyrosine content of p125FAK (Fig. 5A) in the adherent platelets compared
to the MTB-pre-incubated adherent platelets (lane 2); a similar,
but less striking, effect of NBTGs on non-adherent platelets (lane
3) was observed, compared to MTB buffer-pre-incubated non-adherent
platelets (lane 4). Total p125FAK protein content in each sample is
comparable (Fig. 5B) and is not
affected by the gangliosides.
NBTGs enhance the phosphotyrosine
signaling through α2β1 integrin following
platelet adhesion to collagen
As we have shown NBTGs enhance
α2β1 integrin-mediated platelet adhesion to
collagen, we further assessed this receptor’s role in the
phosphotyrosine signaling of NBTG-enhanced platelet adhesion to
collagen by performing experiments in the presence or absence of an
α2-blocking antibody F-17. In Fig. 6, F-17 anti-α2 antibody
decreased protein tyrosinephosphorylation of NBTG-incubated
platelets adherent to collagen (Fig.
6A). Adherent platelets pre-incubated with NBTGs and F-17
anti-α2 antibody (lane 2) had significantly reduced
phosphorylation of p125FAK protein compared to adherent platelets
pre-incubated only with NBTGs (lane 1). Total p125FAK protein
content in each sample is comparable (Fig. 6B) and is not affected by the
gangliosides.
Discussion
Both clinical and experimental evidence point to a
role of platelets in the spread of cancer. Patients with metastatic
disease reveal increased platelet counts and significantly elevated
numbers of activated platelets (19). Depending on the type of tumor,
various aspects of cancer progression may be affected by platelets,
including tumor cell proliferation (20), tumor angiogenesis (21), vessel stability within tumors
(22) or immune evasion (23,24).
Integrins, a widely expressed family of transmembrane adhesion
receptors, represent a central determinant for physiological
platelet function. GPIIb/IIIa is involved in both cell-cell
adhesion and thrombus formation at the vascular wall, establishing
it as a therapeutic target in vascular diseases (25). Pharmacological inhibition of
GPIIb/IIIa has been demonstrated to reduce tumor cell metastasis,
although the underlying mechanisms remain elusive (26). Similarly to GPIIb/IIIa results
(14), Shield et al(27) reported that enhanced expression of
α2β1 integrin may influence spheroid
disaggregation and proteolysis responsible for the peritoneal
dissemination of ovarian carcinoma. Their findings raise the
possibility that α2β1 integrin may represent
a valuable therapeutic target in the suppression of
intra-peritoneal spread associated with the progression of ovarian
cancer. The expression of α2β1-integrin in
peritoneal lesions was significantly increased compared with its
expression in the primary lesion in the same individual. Peritoneal
implantation of gastric carcinoma might be closely associated with
α2β1-integrin (28). Increasing evidence has implicated
gangliosides, sialic acid-containing cell surface
glycosphingolipids, in the biological and clinical behavior of many
types of human tumor. Gangliosides are overexpressed and actively
shed by tumor cells; they can bind to normal cells in the tumor
microenvironment and have a number of biological properties that
could conceivably alter tumor-host interactions to influence the
survival of the malignant cells that carry these molecules.
Collectively, these diverse observations reported in the literature
have prompted us to investigate the modulation of platelet
signaling by tumor gangliosides.
Gangliosides increase platelet aggregation,
secretion (11) and adhesion
(10). These activities are
mediated through the collagen-binding integrin
α2β1(9).
NBTGs increase α2β1-dependent platelet
activation and adhesion (17). In
this study, we also showed that NBTGs increase
α2β1-dependent platelet adhesion to
immobilized collagen (Figs. 2 and
3) and increase intracellular
phosphotyrosine signals following integrin ligation by immobilized
ligand (Figs. 4–6).
Integrins, a widely expressed family of
transmembrane adhesion receptors, represent a central determinant
for physiological platelet function. A unique feature of integrins
is the ability to regulate adhesive competence. The ability to bind
ligand may be due to clustering into focal adhesions (29–31).
An early event during integrin signaling is tyrosine
phosphorylation. Several protein tyrosine kinases have been
implicated in integrin signaling events by virtue of their integrin
dependent activation or their localization into focal contacts.
p125FAK appears to play a central role in integrin-mediated signal
transduction. This kinase is tyrosine phosphorylated and its
tyrosine kinase activity is enhanced upon integrin-mediated
engagement (32). Blood platelets
contain high levels of tyrosine kinases (33,34)
and activation by various agonists including collagen leads to
tyrosine phosphorylation of many proteins (16,35–37).
Tyrosine phosphorylation of p125FAK has been demonstrated during
thrombin or collagen-induced aggregation mediated by the αIIbβ3
integrin (38). Platelet activation
leads to the upregulation of tyrosine kinases, including FAK
(39). FAK, a 125-kDa cytosolic
non-receptor tyrosine kinase, is associated with focal adhesion
plaques of adherent cells such as fibroblasts and platelets
(40). FAK is of particular
interest, as it is considered a key intermediary of signaling
through integrins (31,41). The present study addresses the role
of the collagen receptor α2β1 in the
regulation of FAK. Collagen activates FAK, as indicated by its
tyrosine phosphorylation state. The antibodies against the
α2 integrin, which prevent adhesion to collagen, block
FAK activation, the results demonstrate that
α2β1 occupancy by collagen fibers regulate
FAK. Our data suggest that α2β1 ligation by
immobilized ligand is capable of inducing phosphotyrosine
signals.
Neuroblastoma, a neoplasm originating from neural
crest cells, is the most common extracranial solid tumor of
childhood. Gangliosides shed from neuroblastoma tumors are capable
of enhancing platelet secretion, aggregation (9,10),
adhesion (42) and promote tumor
cell migration and invasion (43).
Our data show that neuroblastoma tumor gangliosides enhance
integrin α2β1 mediated platelet adhesion to
type I collagen and the phosphorylation of pFAK125 provide an
argument for a role of tumor gangliosides in metastasis. Jabbar
et al(44) presented a
9-step model to explain the role of gangliosides in metastasis:
gangliosides shed from tumor cells incorporate into the platelet
membrane, then induce α2β1 integrin
clustering, clustered integrins interact with soluble collagen or
with extracellular matrix collagen, intracellular signals (such as
activation of focal adhesion kinase) are maximally generated when
integrins are clustered and ligand is bound. These signals lead to
cell-platelet adhesion, secretion, aggregation and promote tumor
metastasis. Our results provide indirect evidence to confirm the
model.
Acknowledgements
This study was supported by the grant S2011010004349
from the Natural Science Foundation of Guangdong Province and the
Medical Key Subject of Health, Population and Family Planning
Commission of Shenzhen Municipality (2011026).
References
|
1
|
Brooks SA, Lomax-Browne HJ, Carter TM,
Kinch CE and Hall DM: Molecular interactions in cancer cell
metastasis. Acta Histochem. 112:3–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sahai E: Illuminating the metastatic
process. Nat Rev Cancer. 7:737–749. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu RK, Tsai YT, Ariga T and Yanagisawa M:
Structures, biosynthesis, and functions of gangliosides-an
overview. J Oleo Sci. 60:537–544. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yates AJ, Saqr HE and Van Brocklyn J:
Ganglioside modulation of the PDGF receptor. A model for
ganglioside functions. J Neurooncol. 24:65–73. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duchemin AM, Ren Q, Mo L, Neff NH and
Hadjiconstantinou M: GM1 ganglioside induces phosphorylation and
activation of Trk and Erk in brain. J Neurochem. 81:696–707. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garofalo T, Misasi R, Mattei V, et al:
Association of the death-inducing signaling complex with
microdomains after triggering through CD95/Fas. Evidence for
caspase-8-ganglioside interaction in T cells. J Biol Chem.
278:8309–8315. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang XQ, Sun P and Paller AS: Ganglioside
induces caveolin-1 redistribution and interaction with the
epidermal growth factor receptor. J Biol Chem. 277:47028–47034.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singleton DW, Lu CL, Colella R and Roisen
FJ: Promotion of neurite outgrowth by protein kinase inhibitors and
ganglioside GM1 in neuroblastoma cells involves MAP kinase ERK1/2.
Int J Dev Neurosci. 18:797–805. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Valentino LA and Ladisch S: Tumor
gangliosides enhance alpha2 beta1 integrin-dependent platelet
activation. Biochim Biophys Acta. 1316:19–28. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fang LH, Lucero M, Kazarian T, Wei Q, Luo
FY and Valentino LA: Effects of neuroblastoma tumor gangliosides on
platelet adhesion to collagen. Clin Exp Metastasis. 15:33–40. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Valentino LA and Ladisch S: Circulating
tumor gangliosides enhance platelet activation. Blood.
83:2872–2877. 1994.PubMed/NCBI
|
|
12
|
Hakomori S and Igarashi Y: Functional role
of glycosphingolipids in cell recognition and signaling. J Biochem.
118:1091–1103. 1995.PubMed/NCBI
|
|
13
|
Hynes RO: Integrins: bidirectional,
allosteric signaling machines. Cell. 110:673–687. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang XQ, Sun P and Paller AS: Ganglioside
modulation regulates epithelial cell adhesion and spreading via
ganglioside-specific effects on signaling. J Biol Chem.
277:40410–40419. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hildebrand JD, Schaller MD and Parsons JT:
Identification of sequences required for the efficient localization
of the focal adhesion kinase, pp125FAK, to cellular focal
adhesions. J Cell Biol. 123:993–1005. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lipfert L, Haimovich B, Schaller MD, Cobb
BS, Parsons JT and Brugge JS: Integrin-dependent phosphorylation
and activation of the protein tyrosine kinase pp125FAK in
platelets. J Cell Biol. 119:905–912. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wen FQ, Jabbar AA, Patel DA, Kazarian T
and Valentino LA: Atherosclerotic aortic gangliosides enhance
integrin-mediated platelet adhesion to collagen. Arterioscler
Thromb Vasc Biol. 19:519–524. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu G, Lu ZH, Wei TJ, Howells RD,
Christoffers K and Ledeen RW: The role of GM1 ganglioside in
regulating excitatory opioid effects. Ann NY Acad Sci. 845:126–138.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wiesner T, Bugl S, Mayer F, Hartmann JT
and Kopp HG: Differential changes in platelet VEGF, Tsp, CXCL12,
and CXCL4 in patients with metastatic cancer. Clin Exp Metastasis.
27:141–149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y and Zhang H: Platelet-induced
inhibition of tumor cell growth. Thromb Res. 123:324–330. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zaslavsky A, Baek KH, Lynch RC, et al:
Platelet-derived thrombospondin-1 is a critical negative regulator
and potential biomarker of angiogenesis. Blood. 115:4605–4613.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ho-Tin-Noe B, Goerge T, Cifuni SM,
Duerschmied D and Wagner DD: Platelet granule secretion
continuously prevents intratumor hemorrhage. Cancer Res.
68:6851–6858. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kopp HG, Placke T and Salih HR:
Platelet-derived transforming growth factor-beta down-regulates
NKG2D thereby inhibiting natural killer cell antitumor reactivity.
Cancer Res. 69:7775–7783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nieswandt B, Hafner M, Echtenacher B and
Mannel DN: Lysis of tumor cells by natural killer cells in mice is
impeded by platelets. Cancer Res. 59:1295–1300. 1999.PubMed/NCBI
|
|
25
|
Gawaz M and Geisler T: Coronary artery
disease: platelet activity: an obstacle for successful PCI. Nat Rev
Cardiol. 6:391–392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Amirkhosravi A, Mousa SA, Amaya M, et al:
Inhibition of tumor cell-induced platelet aggregation and lung
metastasis by the oral GpIIb/IIIa antagonist XV454. Thromb Haemost.
90:549–554. 2003.PubMed/NCBI
|
|
27
|
Shield K, Riley C, Quinn MA, Rice GE,
Ackland ML and Ahmed N: Alpha2beta1 integrin affects metastatic
potential of ovarian carcinoma spheroids by supporting
disaggregation and proteolysis. J Carcinog. 6:112007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matsuoka T, Yashiro M, Nishimura S, et al:
Increased expression of α2β1-integrin in the
peritoneal dissemination of human gastric carcinoma. Int J Mol Med.
5:21–25. 2000.
|
|
29
|
Clark EA and Brugge JS: Integrins and
signal transduction pathways: the road taken. Science. 268:233–239.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shattil SJ, Kashiwagi H and Pampori N:
Integrin signaling: the platelet paradigm. Blood. 91:2645–2657.
1998.PubMed/NCBI
|
|
31
|
Wang XQ, Sun P and Paller AS: Inhibition
of integrin-linked kinase/protein kinase B/Akt signaling: mechanism
for ganglioside-induced apoptosis. J Biol Chem. 276:44504–44511.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Burridge K, Turner CE and Romer LH:
Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell
adhesion to extracellular matrix: a role in cytoskeletal assembly.
J Cell Biol. 119:893–903. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Golden A, Nemeth SP and Brugge JS: Blood
platelets express high levels of the pp60c-src-specific tyrosine
kinase activity. Proc Natl Acad Sci USA. 83:852–856. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Golden A, Brugge JS and Shattil SJ: Role
of platelet membrane glycoprotein IIb-IIIa in agonist-induced
tyrosine phosphorylation of platelet proteins. J Cell Biol.
111:3117–3127. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Haimovich B, Lipfert L, Brugge JS and
Shattil SJ: Tyrosine phosphorylation and cytoskeletal
reorganization in platelets are triggered by interaction of
integrin receptors with their immobilized ligands. J Biol Chem.
268:15868–15877. 1993.
|
|
36
|
Polanowska-Grabowska R, Geanacopoulos M
and Gear AR: Platelet adhesion to collagen via the alpha 2 beta 1
integrin under arterial flow conditions causes rapid tyrosine
phosphorylation of pp125FAK. Biochem J. 296:543–547.
1993.PubMed/NCBI
|
|
37
|
Smilowitz HM, Aramli L, Xu D and Epstein
PM: Phosphotyrosine phosphatase activity in human platelets. Life
Sci. 49:29–37. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nakamura S and Yamamura H: Thrombin and
collagen induce rapid phosphorylation of a common set of cellular
proteins on tyrosine in human platelets. J Biol Chem.
264:7089–7091. 1989.PubMed/NCBI
|
|
39
|
Gilmore AP and Burridge K: Molecular
mechanisms for focal adhesion assembly through regulation of
protein-protein interactions. Structure. 4:647–651. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Frangioni JV, Oda A, Smith M, Salzman EW
and Neel BG: Calpain-catalyzed cleavage and subcellular relocation
of protein phosphotyrosine phosphatase 1B (PTP-1B) in human
platelets. EMBO J. 12:4843–4856. 1993.
|
|
41
|
Sun P, Wang XQ, Lopatka K, Bangash S and
Paller AS: Ganglioside loss promotes survival primarily by
activating integrin-linked kinase/Akt without phosphoinositide 3-OH
kinase signaling. J Invest Dermatol. 119:107–117. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Meuillet E, Cremel G, Dreyfus H and Hicks
D: Differential modulation of basic fibroblast and epidermal growth
factor receptor activation by ganglioside GM3 in cultured retinal
Muller glia. Glia. 17:206–216. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zou CY, Wen FQ, Chen YX, Liu ZP and Zhang
ZX: Effect of integrin alpha2beta1 on invasion and migration of
neuroblastoma cells. Zhongguo Dang Dai Er Ke Za Zhi. 10:386–390.
2008.(In Chinese).
|
|
44
|
Jabbar AA, Kazarian T, Hakobyan N and
Valentino LA: Gangliosides promote platelet adhesion and facilitate
neuroblastoma cell adhesion under dynamic conditions simulating
blood flow. Pediatr Blood Cancer. 46:292–299. 2006. View Article : Google Scholar : PubMed/NCBI
|