Introduction
Retinoblastoma is a malignant tumor of the retina of
the eye and generally affects children under the age of six years.
Retinoblastoma is rare and it affects approximately 1 in 15,000
live births. Worldwide, approximately 5,000 new cases occur per
year, while in the US that incidence is 300 cases/year (1). It is the most common eye cancer in
children and is caused by mutation on chromosome 13, called the RB1
gene. The defective RB1 gene can be inherited from either of the
parents in some children; however, the mutation occurs in the early
stages of fetal development. Characterized by the typical cat’s eye
or the white pupil reflex (leukocoria) noted by parents,
approximately 63% of all retinoblastomas arise in the first two
years of life. In some cases, retinoblastoma metastasizes to
extraocular organs including bone, lung and brain. Although
non-metastatic tumors can be treated by enucleation (removal of the
eye), currently, there is no treatment for metastatic
retinoblastoma (1). Only 10% of
cases tend to have a family history. Retinoblastoma gene has been
identified as an abnormality on chromosome 13. Parents with a
familial bilateral retinoblastoma have 50% chance of passing it on
to their children. In addition, sporadic mutation in the gene can
still be passed on to the next generation even though the parent
did not inherit the gene or suffer any cancer because of it. Ninety
percent of the children who develop retinoblastomas are the first
ones in their families to have it. The survival rate drops with
each decade of life for patients with a genomic mutation (2,3).
Cancer cells from tumors spread by degrading the
extracellular matrix (ECM) with the use of a group of endopeptidase
enzymes, the matrix metalloproteinases (MMPs). Activity of these
enzymes correlates with the aggressiveness of tumor growth and
metastasis. In 1992, Rath and Pauling (4) postulated that nutrients such as lysine
and ascorbic acid act as natural inhibitors of ECM proteolysis and
as such have the potential to modulate tumor growth and metastasis.
These nutrients can exert their antitumor effect both through
inhibition of MMPs and strengthening the connective tissue
surrounding cancer cells (tumor encapsulating effect). In previous
in vitro and in vivo studies, we demonstrated the
antitumor potential of a nutrient mixture (NM) in a number of
cancer cell lines (5–7).
Considering the efficacy of NM on other cancer cell
lines, we investigated the effects of NM on the Y-79 retinoblastoma
cell line regarding cell proliferation, modulation of MMP
expression, cell invasive potential by Matrigel invasion and
apoptosis and cell morphological changes using the Live Green Poly
Caspase Detection kit and H&E staining, respectively.
Materials and methods
Composition of the nutrient mixture
(NM)
Stock solution of the NM prepared for testing was
composed of the following: vitamin C (as ascorbic acid and as
magnesium, calcium and palmitate ascorbate) 700 mg; L-lysine 1,000
mg; L-proline 750 mg; L-arginine 500 mg; N-acetylcysteine 200 mg;
standardized green tea extract 1,000 mg (green tea extract was
derived from green tea leaves obtained from US Pharma Lab). The
certificate of analysis indicates the following characteristics:
total polyphenol 80%, catechins 60%, epigallocatechin gallate
(EGCG) 35% and caffeine 1.0%; selenium 30 μg; copper 2 mg;
manganese 1 mg.
Cell culture
The retinoblastoma Y-79 cell line was obtained from
the American Type Culture Collection (ATCC, Manassas, VA). The
cells were cultured on Roswell Park Memorial Institute (RPMI)-1640
medium containing 20% fetal bovine serum and antibiotics. The cells
were grown in a humidified 5% CO2 atmosphere at 37°C and
later treated with the NM at 0, 10, 50, 100, 500 and 1,000 μg/ml in
triplicate at each dose. Cells were also treated with Phorbol
12-myristate 13-acetate (PMA) to induce MMP secretion. The plates
were then returned to the incubator.
Cell proliferation study
Cell proliferation was assessed by trypan blue dye
exclusion test after 24 h, as previously described (8). Viable cell count was expressed as a
function of the control.
Gelatinase zymography
MMP secretion in conditioned media was determined by
gelatinase zymography as previously described (9). In brief, gelatinase zymography was
performed in 10% polyacrylamide Novex® precast gel,
sodium dodecyl sulphate (SDS) (Invitrogen Corp.), in the presence
of 0.1% gelatin under non-reducing conditions. Culture medium (20
μl) was loaded and SDS-polyacrylamide gel electrophoresis
(SDS-PAGE) was performed with Tris-glycerine SDS buffer as
described by the manufacturer (Novex). Samples were not boiled
before electrophoresis. After electrophoresis, the gels were washed
with 5% Triton X-100 for 30 min at room temperature to remove SDS.
The gels were then incubated at 37°C overnight in the presence of
50 mM Tris-HCl, 5 mM CaCl2, 5 μM ZnCl2 at pH
7.5, stained with Coomassie Blue R 0.5% for 30 min and destained.
Protein standards were run concurrently, and approximate molecular
weights were determined by plotting the relative mobilities of
known proteins.
Matrigel invasion studies
Invasion studies were conducted using Matrigel
(Becton-Dickinson) inserts in 24-well plates (9). In brief, the malignant retinoblastoma
Y-79 cells suspended in medium were supplemented with nutrient, as
specified in the design of the experiment and seeded on the insert
in the well. Thus, both the medium on the insert and in the well
contained the same supplements. The plates with the inserts were
then incubated in a culture incubator equilibrated with 95% air and
5% CO2 for 24 h. After incubation, the media from the
wells were withdrawn. The outer surface of the insert was washed
gently and the media and washing were collected in the well. The
media were spun, and the cells were counted.
Assessment of cell morphology
Cell morphology of the cells cultured for 24 h in
the test concentrations of NM was evaluated by H&E staining and
observed for apoptotic changes and images were captured.
Analysis of apoptosis
Apoptosis was determined by the method described in
the Live Green Poly Caspase Detection kit at different doses of NM.
Cells were challenged with NM at concentrations of 0, 50, 100, 250,
500 and 1,000 μg/ml and incubated for 24 h. The culture was washed
with PBS and treated with caspase reagent as specified in the
manufacturer’s protocol (Molecular Probes Image-IT™ Live Green Poly
Caspase Detection kit 135104; Invitrogen Corp.). Cells were
photographed under a fluorescence microscope and counted.
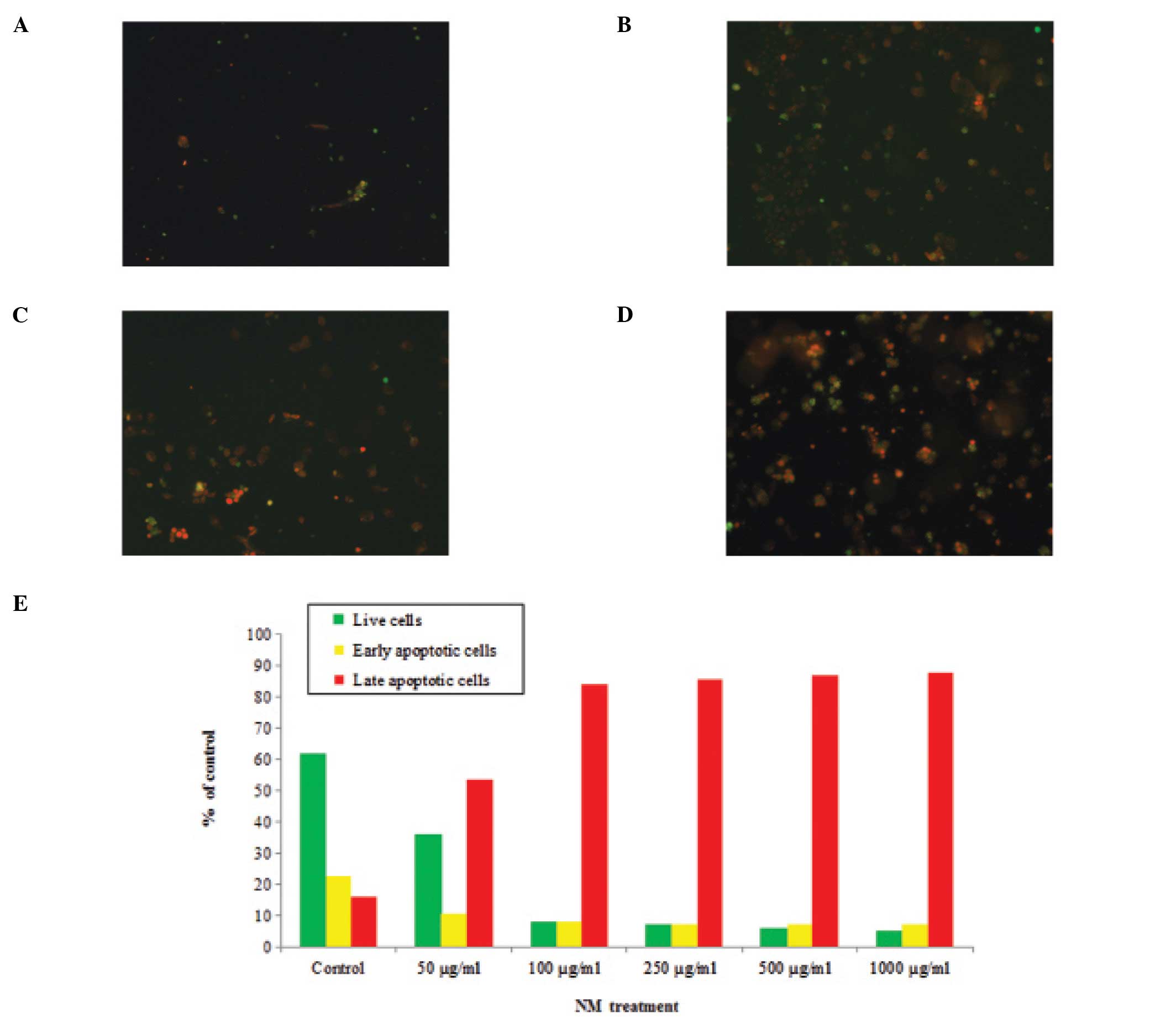
Green-colored cells represented viable cells, while
yellow-orange-colored cells represented cells undergoing early
apoptosis and red-colored cells represented those undergoing late
apoptosis.
Statistical analysis
The results are expressed as means ± standard
deviation (SD) for the groups. Data was analyzed by the independent
sample t-test.
Results
MTT study
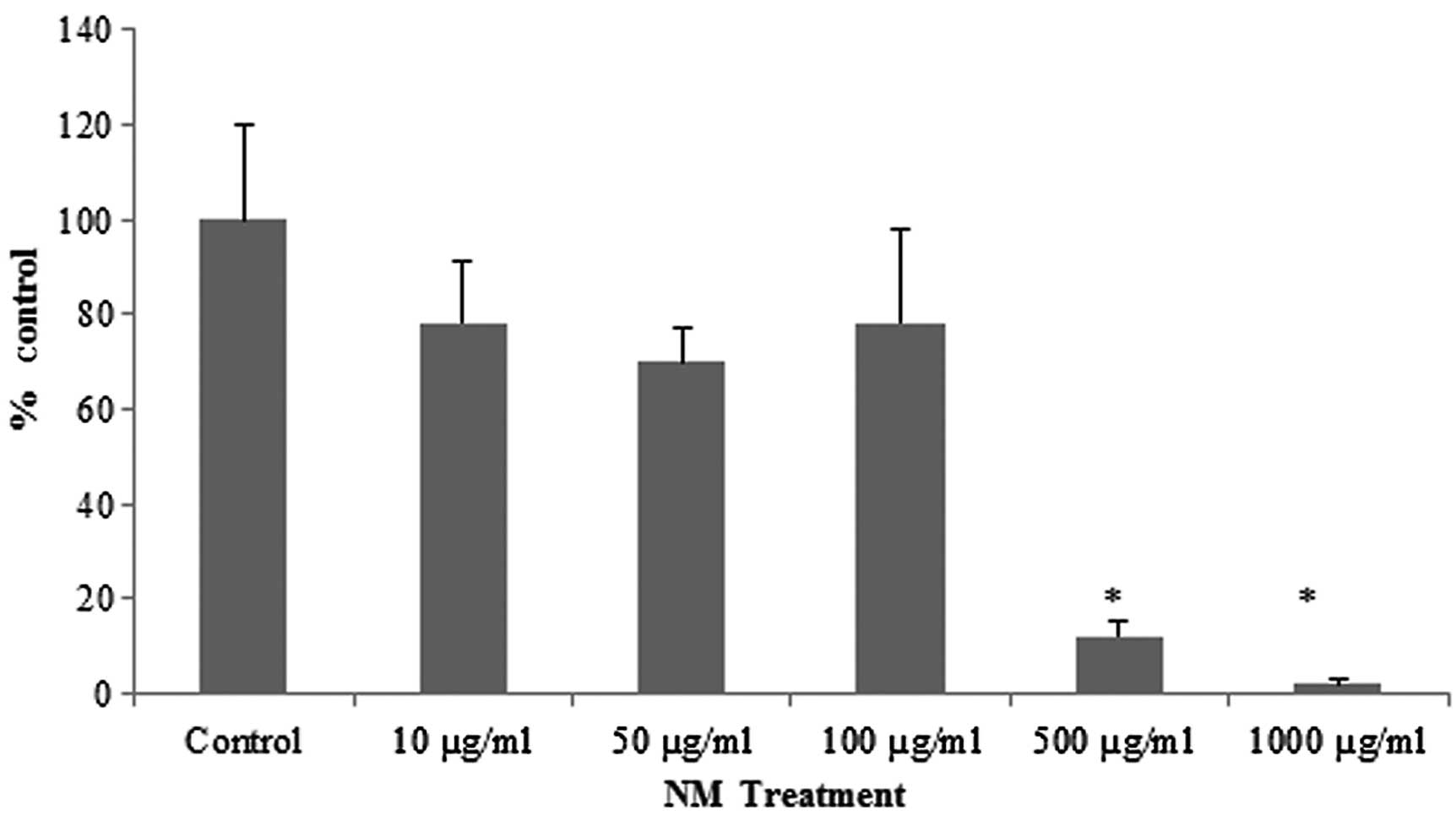
NM treatment of the retinoblastoma Y-79 cells
resulted in 25% toxicity at NM doses of 10–100 μg/ml. However,
significant toxicity was observed in the cells exposed to
concentrations of 500 and 1,000 μg/ml NM (Fig. 1).
Gelatinase zymography study
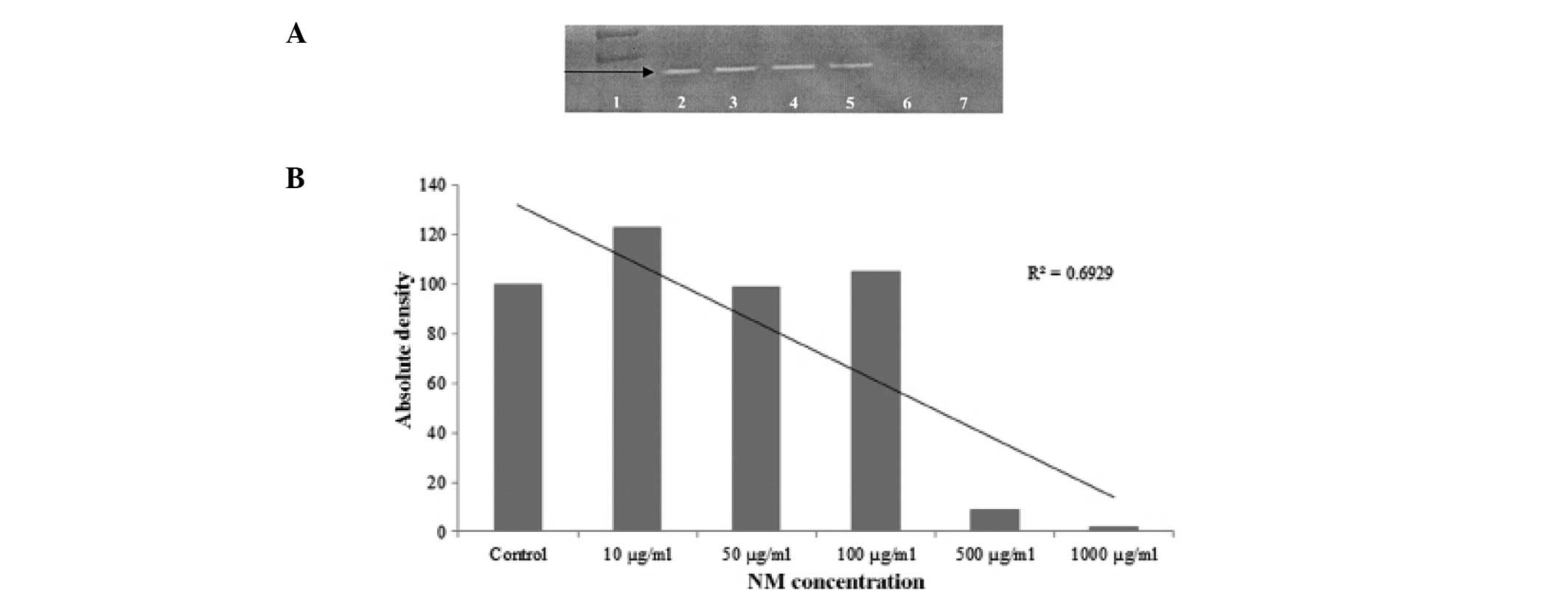
Gelatinase zymography study revealed only one band
corresponding to MMP-2. PMA treatment did not induce MMP-9
expression. The expression of MMP-2 was not affected by NM up to
100 μg/ml. However, it was significantly inhibited at an NM
concentration of 500 μg/ml with virtually total inhibition at 1000
μg/ml (Fig. 2A). This was further
confirmed by densitometric analysis as shown in Fig. 2B, with the R2 value being
0.6929.
Cell invasion studies
The malignant retinoblastoma Y-79 cells did not
exhibit invasiveness through Matrigel (data not shown).
Analysis of cell morphology (H&E
staining)
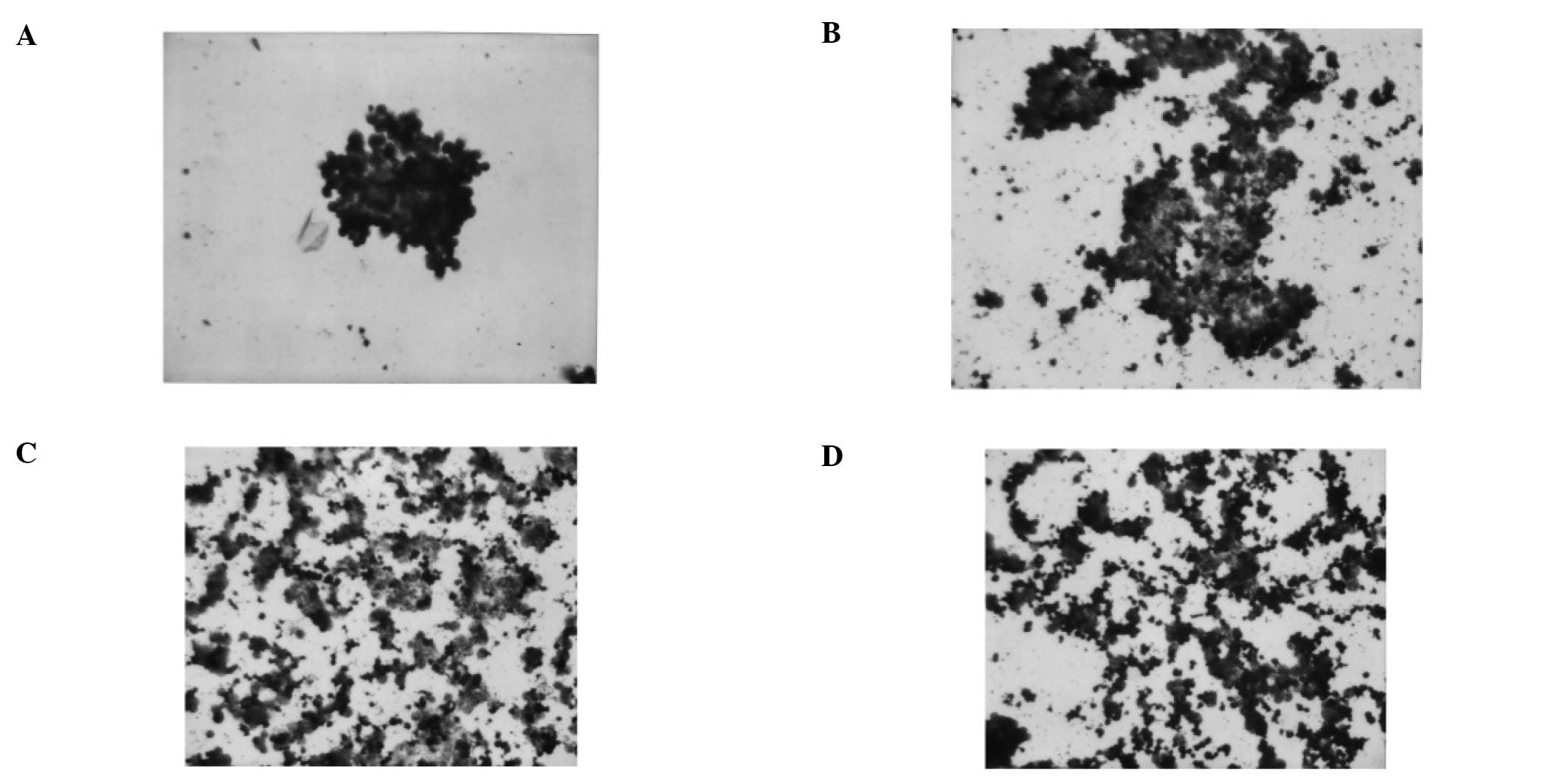
H&E staining demonstrated obvious cell
apoptosis. Apoptotic cells showed shrinkage with deeply stained and
condensed nuclei and strongly acidophilic cytoplasm (Fig. 3A-D).
Detection of apoptosis
Apoptosis was assessed using the Live Green Poly
Caspase Detection kit. Dose-dependent apoptosis of Y-79 cells was
evident following NM challenge (Fig.
4A–D). A moderate amount of cell apoptosis was observed in
cells exposed to 50 μg/ml NM. However, the extent of apoptosis
increased significantly with increasing doses of NM up to 1,000
μg/ml. Quantitative analysis of living, early and late apoptotic
cells is shown (Fig. 4E). Following
exposure to 50 μg/ml NM, the percentage of viable Y-79 cells was
observed to be 36% and that of late apoptotic cells was 53%. With
increasing concentrations of NM, the percentage of late apoptotic
cells gradually increased up to 88%, the percentage of early
apoptotic cells was 7%, while the percentage of living cells
decreased to 5% following treatment with 1,000 μg/ml of NM.
Discussion
In the present study, we investigated the effects of
NM on malignant retinoblastoma cell line Y-79. The results
indicated that NM had a profound inhibitory effect on the
proliferation of these cells and inhibition of MMP secretion. NM
also induced apoptosis. These are the most important steps in
cancer metastasis.
Free radical injury plays a key role in cancer
initiation and progression. During the multistep process, the
degradation of ECM by MMPs is a critical step in tumor growth,
invasion and metastasis. It is important to restrict this step to
halt tumor progression. Ascorbic acid, a potent antioxidant, used
alone has been shown to have a cytotoxic effect on Y-79 cells
(10,11).
Aggressiveness of retinoblastoma is highly
correlated with the expression of MMPs, which by degrading
surrounding ECM contributes to the invasiveness of cancer. Although
the relationship of MMP enzymes to cancer progression and
metastasis has been studied in many types of cancer, their
importance in retinoblastoma has not been established until
recently. Researchers have recently demonstrated that MMP-2
activity is directly involved in the differentiation of
retinoblastoma cells. They concluded that, ‘therapeutics targeting
to MMP-2 may prove useful for reducing malignancy through the
differentiation of retinoblastoma cells’ (12). In our study, we demonstrated that
the expression of MMP-2 enzymes can be completely blocked by NM at
a concentration of 500 μg/ml. Although in our study the Y-79 cells
did not secrete MMP-9, the expression of this enzyme has been
considered to directly contribute to the cellular proliferative
process in retinoblastoma. Based on the recent understanding of the
importance of MMP enzymes in retinoblastoma it has also been
suggested that differential expression of MMP-9 and MMP-2 could be
a significant pathologic factor reflecting the biology of
retinoblastoma and may also be used as a monitoring test.
The results of our MTT assay and apoptosis studies
demonstrated that NM has profoundly toxic effects on Y-79 cells.
Many of our previous studies with various cancer cell lines have
shown the anticancer effects of NM to be mediated through ECM
stability. This study indicates that the NM effectiveness in Y-79
cells was through a pro-apoptotic effect. This effect appears to be
cancer-specific since our previous studies demonstrated no NM
toxicity to a variety of normal cells, such as fibroblasts, smooth
muscle cells and endothelial cells (13,14).
In the attempt to understand the etiology of
retinoblastoma, researchers have explored factors such as parental
age, occupation, exposure to toxins and maternal nutrition. The
etiology of sporadic retinoblastoma linked to the maternal diet and
nutrition has been suggested (15).
Deficiencies in nutrients in the first year of life also appear to
contribute to the genetic mutation in retinoblastoma. Based on our
current results we believe that the dietary supplementation of NM
should be explored further both in preventive and therapeutic
aspects of retinoblastoma and its metastasis.
Acknowledgements
Dr Rath Health Foundation, a non-profit
organization, provided research funding for the present study.
References
|
1
|
American Cancer Society. Learn about
cancer. Retinoblastoma. http://www.cancer.org/Cancer/Retinoblastoma/DetailedGuide/retinoblastoma-risk-factors.
Accessed November 2011
|
|
2
|
Young JL Jr, Smith MA, Roffers SD, Liff JM
and Bunin GR: National Cancer Institute-SEER Pediatric Monograph.
Retinoblastoma. www.seer.cancer.gov/publications/childhood/retinoblastoma.pdfhttps://.seer.cancer.gov/publications/childhood/retinoblastoma.pdf.
|
|
3
|
Carlos Rodriguez-Galindo and Wilson MW:
Retinoblastoma-Pediatric Oncology. 1st edition. Springer; New York,
NY: pp. 1–10. 2010
|
|
4
|
Rath M and Pauling L: Plasmin-induced
proteolysis and the role of apoprotein(a), lysine and synthetic
analogs. J Orthomol Med. 7:17–23. 1992.
|
|
5
|
Netke SP, Roomi MW, Roomi NW, Ivanov V,
Niedzwiecki A and Rath M: A specific combination of ascorbic acid,
lysine, proline and epigallocatechin gallate inhibits proliferation
and extracellular matrix invasion of various human cancer cell
lines. Res Commun Pharmacol Toxicol Emerging Drugs. 2:37–50.
2003.
|
|
6
|
Roomi MW, Ivanov V, Kalinovsky T,
Niedzwiecki A and Rath M: Synergistic effect of combination of
lysine, proline, arginine, ascorbic acid, and epigallocatechin
gallate on colon cancer cell line HCT116. JANA. 7:40–43. 2004.
|
|
7
|
Roomi MW, Ivanov V, Kalinovsky T,
Niedzwiecki A and Rath M: In vivo antitumor effect of ascorbic
acid, lysine, proline and green tea extract on human prostate
cancer PC-3 xenografts in nude mice: evaluation of tumor growth and
immunohistochemistry. In Vivo. 19:179–183. 2005.
|
|
8
|
Roomi MW, Bhanap BA, Roomi NW, Rath M and
Niedzwiecki A: Antineoplastic effects of nutrient mixture on Raji
and Jurkat T cells: the two highly aggressive non Hodgkin’s
lymphoma cell lines. Exp Oncol. 31:149–155. 2009.PubMed/NCBI
|
|
9
|
Roomi MW, Roomi NW, Kalinovsky T, Rath M
and Niedzwiecki A: Marked inhibition of growth and invasive
parameters of head and neck squamous carcinoma FaDu by a nutrient
mixture. Integr Cancer Ther. 8:168–176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Medina MA and Schweigerer L: A plasma
membrane redox system in human retinoblastoma cells. Biochem Mol
Biol Int. 29:881–887. 1993.PubMed/NCBI
|
|
11
|
Medina MA, García de Veas R and
Schweigerer L: Ascorbic acid is cytotoxic for pediatric tumor cells
cultured in vitro. Biochem Mol Biol Int. 34:871–874.
1994.PubMed/NCBI
|
|
12
|
Kim JH, Kim JH, Cho CS, et al:
Differential roles of matrix metalloproteinase-9 and -2, depending
on proliferation or differentiation of retinoblastoma cells. Invest
Ophthalmol Vis Sci. 51:1783–1788. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ivanov VO, Ivanova SV and Niedzwiecki A:
Ascorbate affects proliferation of guinea-pig vascular smooth
muscle cells by direct and extracellular matrix-mediated effects. J
Mol Cell Cardiol. 29:3293–3303. 1997. View Article : Google Scholar
|
|
14
|
Ivanov V, Ivanova S, Roomi MW, et al:
Naturally produced extracellular matrix inhibits growth rate and
invasiveness of human isteosarcoma cancer cells. Med Oncol.
24:209–217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Orjuela MA, Titievsky L, Liu X, et al:
Fruit and vegetable intake during pregnancy and risk for
development of sporadic retinoblastoma. Cancer Epidemiol Biomarkers
Prev. 14:1433–1440. 2005. View Article : Google Scholar : PubMed/NCBI
|