Introduction
Breast cancer is one of the major causes of death in
women, ~350,000 women die from breast cancer each year (1). However, most mortality and morbidity
does not arise from the primary tumor, but from distant metastasis
(2). In order to metastasize a
cancer cell must shed many of its epithelial characteristics,
invade the surrounding tissue to enter the circulation,
subsequently survive in the circulation, extravasate and
proliferate in the metastatic niche (3). Invasion is therefore a key step in the
metastatic cascade. Targeted therapy for metastatic disease is
clinically unavailable because the molecular mechanism underlying
metastasis remains unclear (4).
Thus, identifying functional metastasis genes and their molecular
mechanisms underlying the metastatic process remains a top priority
in the cancer research field.
Recent research has demonstrated that
epithelial-to-mesenchymal transition (EMT) plays a key role in the
early process of metastasis of cancer cells (5). Greenburg and Hay (6) first described that epithelial cells
cultured in vitro might acquire mesenchymal features,
providing the proof of principle for the process of EMT. The
transition of epithelial cells into mesenchymal cells, known as
EMT, is a process during which cells undergo a morphological switch
from the epithelial polarized phenotype to a highly motile
fibroblastic or mesenchymal phenotype (7). In the EMT process, epithelial cells
lose their features, gain mesenchymal properties, and become motile
and invasive. The feature of EMT is the reduction of cell-cell
adhesion, especially the reduction of E-cadherin which is critical
to maintain the epithelial structure. It has been reported that the
loss of E-cadherin expression is correlated with tumor invasion and
metastasis (8). With the loss of
E-cadherin expression, the expression of mesenchymal markers,
vimentin and fibronectin, can be upregulated when EMT occurs
(7,9).
Transforming growth factor-β1 (TGF-β1) signals have
an important role in the metastatic spread of cancer cells, such as
migration, invasion, and EMT (7,10,11).
Overexpression of TGF-β1 is reported to be correlated with poor
prognosis of breast tumours, especially basal-like and luminal type
of cancers, suggesting that TGF-β signaling might have an important
role in the progression of breast cancer cells (12,13).
Therefore, inhibition of TGF-β signaling in breast carcinoma may
yield beneficial effects through inhibition of invasion and
metastasis of cancer. TGF-β1 mediates EMT by inducing Smad
signaling (7,14,15).
Smads are a group of intracellular proteins that are critical for
transmitting the TGF-β1 signals from the cell surface to the
nucleus in order to promote transcription of target genes (16). The role of Smad3 in the development
of EMT has been reported (17,18).
However, the potential role of Smad2 in the development of EMT is
unclear.
To understand the role of EMT in breast cancer
metastasis and its mechanism, we demonstrated in this study that
TGF-β1 induced a series of EMT-associated changes in breast cancer
is dependent on the Smad2 signaling and promoted tumor progression
and metastasis by means of EMT.
Materials and methods
Reagents
Total Smad2, phosphorylated Smad2, α-SMA, vimentin,
cytokeratin, TGF-β1 and E-cadherin antibodies, as well as secondary
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). The transwell chamber was obtained from
Corning Life Sciences (NY, USA). DMEM and fetal calf serum were
purchased from Gibco-BRL (Carlsbad, CA, USA). Human TGF-β1 was
obtained from Sigma (St. Louis, MO, USA). Other laboratory reagents
were obtained from Sigma.
Cell line and culture
Two human breast cancer cell lines, MCF-7 and
MDA-MB-435S, were obtained from the Cancer Research Institute of
Beijing, China. These cells were cultivated in T75 tissue culture
flasks in DMEM supplemented with 10% fetal calf serum, 100 IU/ml
penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, and 20 mM
hydroxyethyl piperazine ethanesulfonic acid, and incubated in a
humidified incubator containing 5% CO2 at 37°C.
Tissue immunohistochemistry and
scoring
Breast tumor samples from 128 patients who underwent
surgery in the Affiliated Hospital of Qingdao University Medical
College between March 2011 and October 2011, were studied. The
local institutional review board approved our protocol for use of
patient samples; all patients provided written informed consent
prior to participation in the study. Three 5-μm frozen sections
were taken from these specimens and stained with hematoxylin and
eosin (H&E). The original histologic diagnosis used for
clinical management was confirmed by three independent, expert
histopathologists. Cases were selected based on a confirmed
diagnosis of breast cancer and the presence of both an invasive
margin and a central tumor area on the same section. Several
criteria to verify the presence of an invasion front were assessed.
H&E was used to identify single cells, which appeared to be at
the tumor edge. Samples with invasion fronts identified on H&E
criteria were then subjected to immunohistochemistry using i)
MNF116, a pan cytokeratin marker (1:50) to identify epithelial
cells, and ii) CD45, a hematopoietic cell marker (1:100) to confirm
that cells identified as possibly invasive were not in fact
inflammatory.
Eighteen carcinoma samples from patients that
fulfilled these stringent criteria were selected, and a further
thirty 5-μm sections of these samples were then cut. An additional
H&E section was stained, and the remaining sections were used
for immunohistochemistry. Tissue was fixed with acetone for 10 min
and rehydrated with ethanol. Slides were blocked with 10% horse
serum for 1 h at room temperature and incubated with cytokeratin
(1:50), E-cadherin (1:100), vimentin (1:200), and TGF-β1 (1:50)
antibodies overnight at 4°C. Slides were incubated with anti-mouse
biotinylated secondary antibody (1:200). Finally, detection was
carried out with the DAB kit according to the manufacturer’s
instructions. Each slide had at least three replicate sections for
each antibody. Quantitative analysis of cytokeratin and vimentin
staining in the central tumor area compared with the invasive
margin was done by two independent observers. The invasive front
was identified and the area of central tumor most distant from this
invasive front was then selected, and three independent fields were
scored for staining intensity and for cellular localization of
cytokeratin staining. The intensity of staining was scored as 0
(none), 1 (weak), 2 (mild), 3 (moderate), and 4 (strong) compared
with a negative (no primary antibody) and positive control. The
cellular localization of cytokeratin was classified as membranous,
cytoplasmic or mixed.
Phase contrast microscopy
The phenotypic changes of breast cancer cells were
determined by phase contrast microscopy. The cancer cells in
cultures treated with recombinant TGF-β1 and left untreated
(control) for 72 h and morphological changes were visualized by
phase contrast microscopy. The images were collected using Nikon
inverted microscope.
Western blot analysis
Breast cancer cells were cultured on a 6-well tissue
culture plate to confluence. The cell were treated with recombinant
human TGF-β1 at the time of switching to serum-free medium, at a
final concentration of 5 ng/ml. The cancer cells cultured without
TGF-β1 were considered as control. The cells were harvested at 72
h. Total cellular protein was extracted using a lysis buffer and
quantified using protein quantification reagents from Bio-Rad.
Next, 60 μg of the protein was suspended in 5× reducing sample
buffer, boiled for 5 min, electrophoresed on 10% SDS-PAGE gels, and
then transferred to polyvinylidene difluoride membrane by
electroblotting. The membrane was blocked in 1% BSA/0.05% Tween/PBS
solution overnight at 4°C, followed by incubation with the primary
antibody (mouse monoclonal antibodies to either human α-SMA,
vimentin, cytokeratin, E-cadherin, phosphorylated-Smad2, or Smad2)
for 24 h. A horseradish peroxidase-labelled goat anti-mouse IgG was
used as the secondary antibody. The blots were then developed by
incubation in a chemiluminescence substrate and exposed to X-ray
film.
Small interfering-RNA (siRNA)
treatment
The breast cancer cells were grown to a 70%
confluence on culture dishes and the transient transfection was
performed with specific stealth small interference RNA against
Smad2, or control siRNA over- night using Lipfectamine-2000,
according the manufacturer’s instructions. The total of two siRNA
sequences for Smad2 and control-siRNA were designed and synthesized
from Invitrogen using RNAi designer software program. The
concentration of 300 nM was determined to be the most effective
siRNA concentration for Smad2 silencing. The transfection medium
was changed with culture medium containing 5% FBS for 24 h. TGF-β1
at final concentration of 5 ng/ml was added to the cell cultures in
serum-free medium or without TGF-β1 (control). The cells were
harvested at 4, 24 and 72 h for further experiments.
Invasion assay
In vitro invasiveness was measured by the
method described in the study by Albini et al(19), with some modifications. We used
chemotaxis chambers with a 8 μm-pore membrane filter coated with 50
mg of matrigel in a 24-well culture plate. MDA-MB-435S cells were
pretreated with TGF-β1 or Smad2-siRNA, and plated at a
concentration of 3×105/ml per upper well in 200 μl of
serum-free medium. As a chemoattractant, 10% fetal calf serum
medium was used in the lower chamber. After being recultured with
5% CO2 at 37°C for 24 h, the filters were removed, fixed
in 95% alcohol, and stained with trypan blue. The cells remaining
on the top surface of the membrane were completely removed with a
cotton swab, and the membrane was removed from the chamber and
mounted on a glass slide. The number of infiltrating cancer cells
were counted in five regions selected at random, and the extent of
invading cancer cells was determined by the mean count.
Statistical analysis
All values in the text and figures are presented as
mean ± SD. In univariate analysis, 2-tailed χ2 tests for
categorical variables and 2-tailed t-test for continuous variables
were used for statistical comparisons. Values of P<0.05 were
taken to show a significant difference between means.
Results
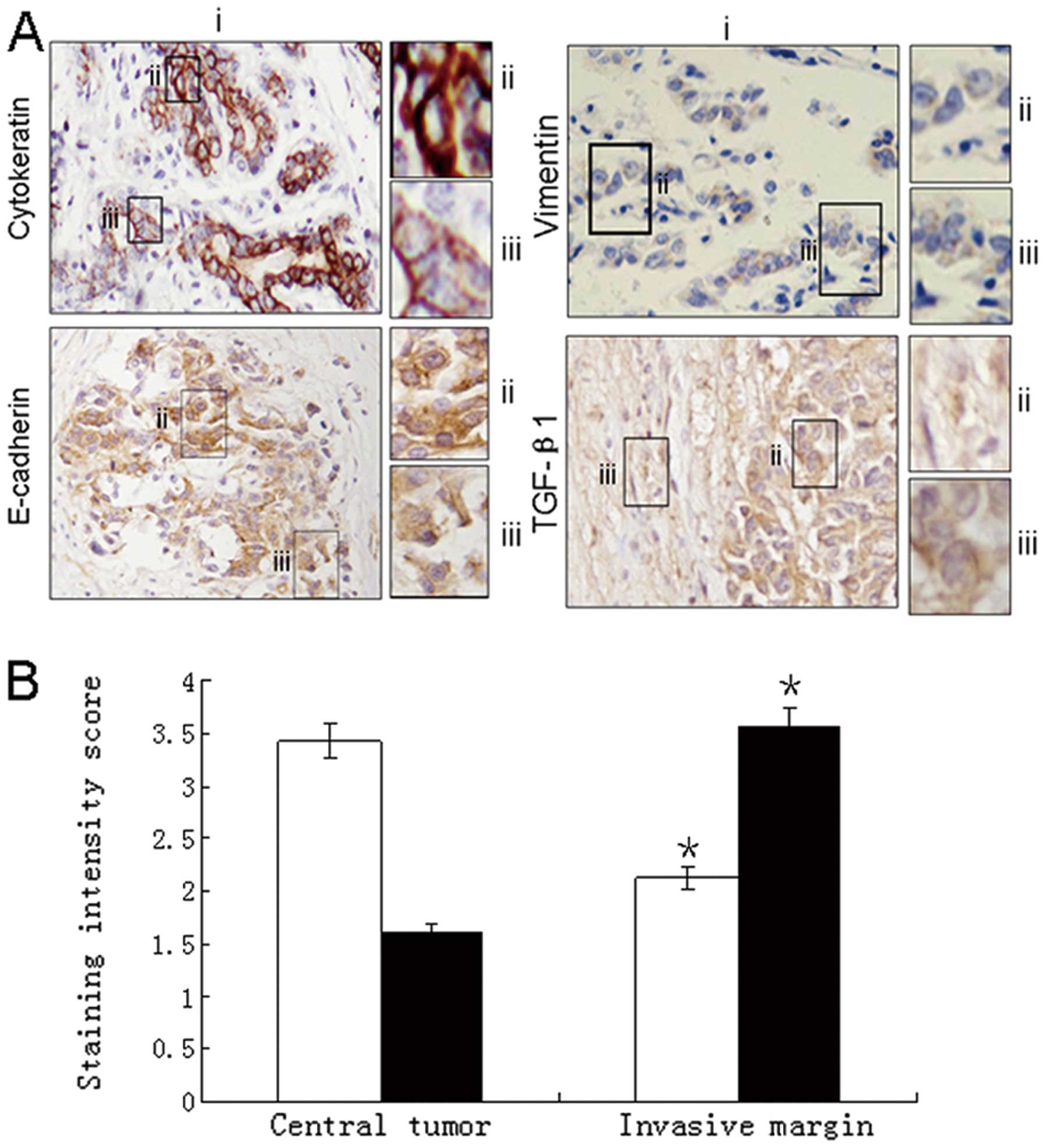
Increased expression of mesenchymal
markers at invasion front
Immunohistochemical analysis of the invasive
component of carcinoma specimens was compared with the central area
of the tumor. There was downregulation of the intensity of the
epithelial staining with E-cadherin and cytokeratin at the invasive
tumor margin. Furthermore, there was a redistribution of E-cadherin
staining from a predominantly membranous pattern in the central
area to a predominantly cytoplasmic pattern at the margin. In
contrast, a low intensity of vimentin staining was observed in the
center of the tumor compared with increased intensity of expression
at the invasive margin. Quantification of cytokeratin and vimentin
staining intensity showed downregulation of cytokeratin at the
invasive margin (P<0.005) and a contrasting upregulation of
vimentin staining (P<0.05). Staining for TGF-β1 showed a
predominantly stromal expression pattern in both the central and
invasive tumor components with foci of increased uptake in the
invasive front (Fig. 1).
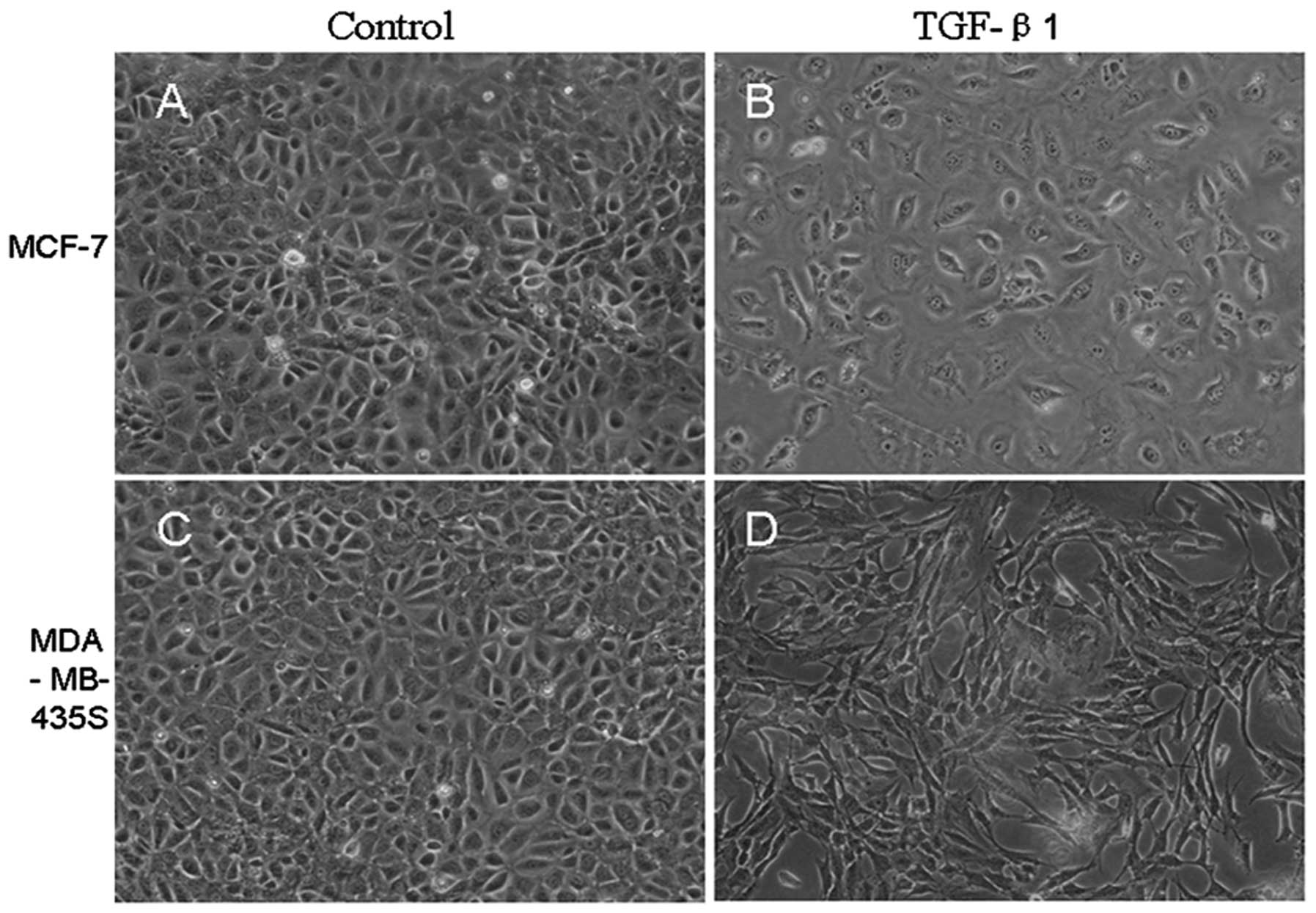
Morphological changes of breast cancer
cells
In the absence of TGF-β1, small portions of cell
morphology were somewhat mesenchymal, but most breast cancer cells
showed pebble-like shape and tight cell-cell adhesion. However,
after TGF-β1 treatment for 72 h, MCF-7 and MDA-MB-435S cells showed
morphological changes assessed by phase contrast microscopy. Many
cells assumed more elongated shape and lost contact with their
neighbor, displaying fibroblast-like appearance compared to the
untreated cells (Fig. 2).
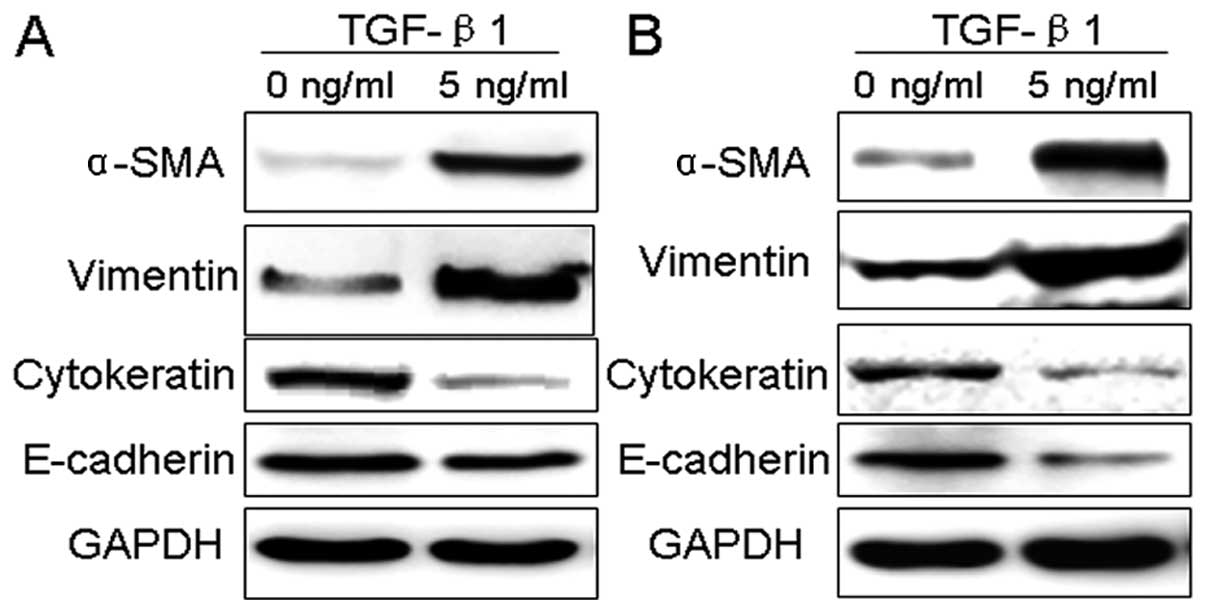
TGF-β1 induces EMT marker changes in
breast cancer cells
To better confirm morphological changes in breast
cancer cells, MCF-7 and MDA-MB-435S cells, represent EMT, western
blotting was used to examine the changes of EMT-related protein
markers. The results indicated that the expression of cytokeratin
and E-cadherin, the epithelial phenotype marker, was significantly
decreased in MDA-MB-435S cells, while TGF-β1 did not affect the
E-cadherin expression levels in MCF-7. Those of mesenchymal
phenotype markers, α-SMA and vimentin, were greatly increased in
MCF-7 and MDA-MB-435S cells (Fig.
3).
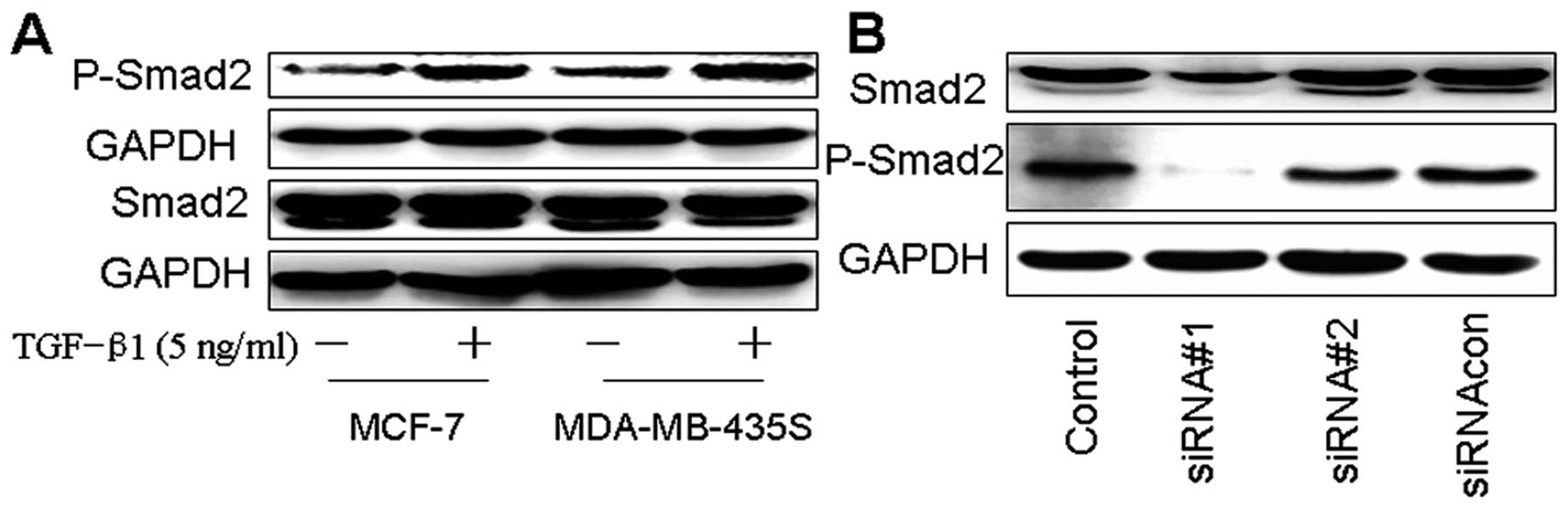
Effects of TGF-β1 or siRNAi-Smad2 on
Smad2 phosphorylation of breast cancer cells
Here we showed that Smad2 phosphorylation was
increased by TGF-β1 in MCF-7 and MDA-MB- 435S, while TGF-β1 did not
affect the total Smad2 expression levels. In order to confirm
whether Smad2 is involved in TGF-β1 mediated EMT of breast cancer,
siRNAs were used to knockdown the Smad2 gene in MDA-MB-435S. As
shown in Fig. 4B, siRNAi-Smad2#1
highly significant knockdown for Smad2 and phosphorylated Smad2
when compared to the other siRNAs or the control.
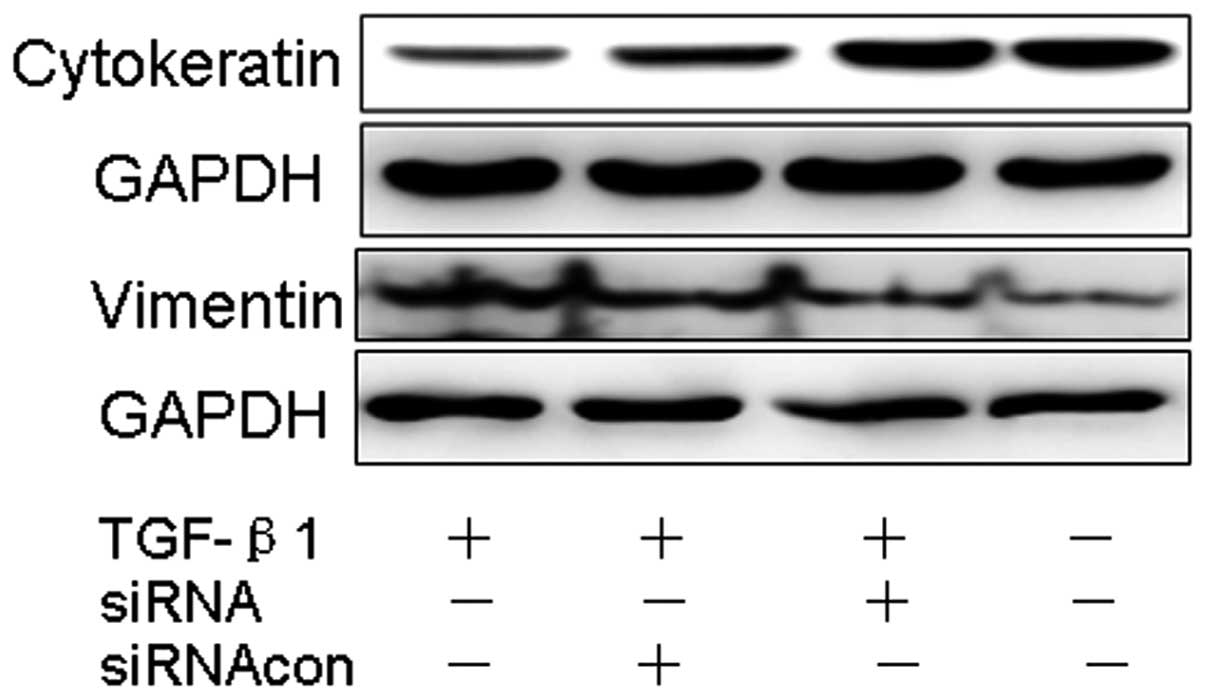
Silencing Smad2 signaling blocks
TGF-β1-induced mesenchymal transformation in MDA-MB-435S
After silencing Smad2 by using siRNA-Smad2 or
control-siRNA in cancer cells treated with TGF-β1, we noted a
remarkably reduced expression of vimentin, and most importantly a
significant restoration of the junctional protein cytokeratin
suggesting a role for Smad2 signaling in EMT of cancer cells
(Fig. 5).
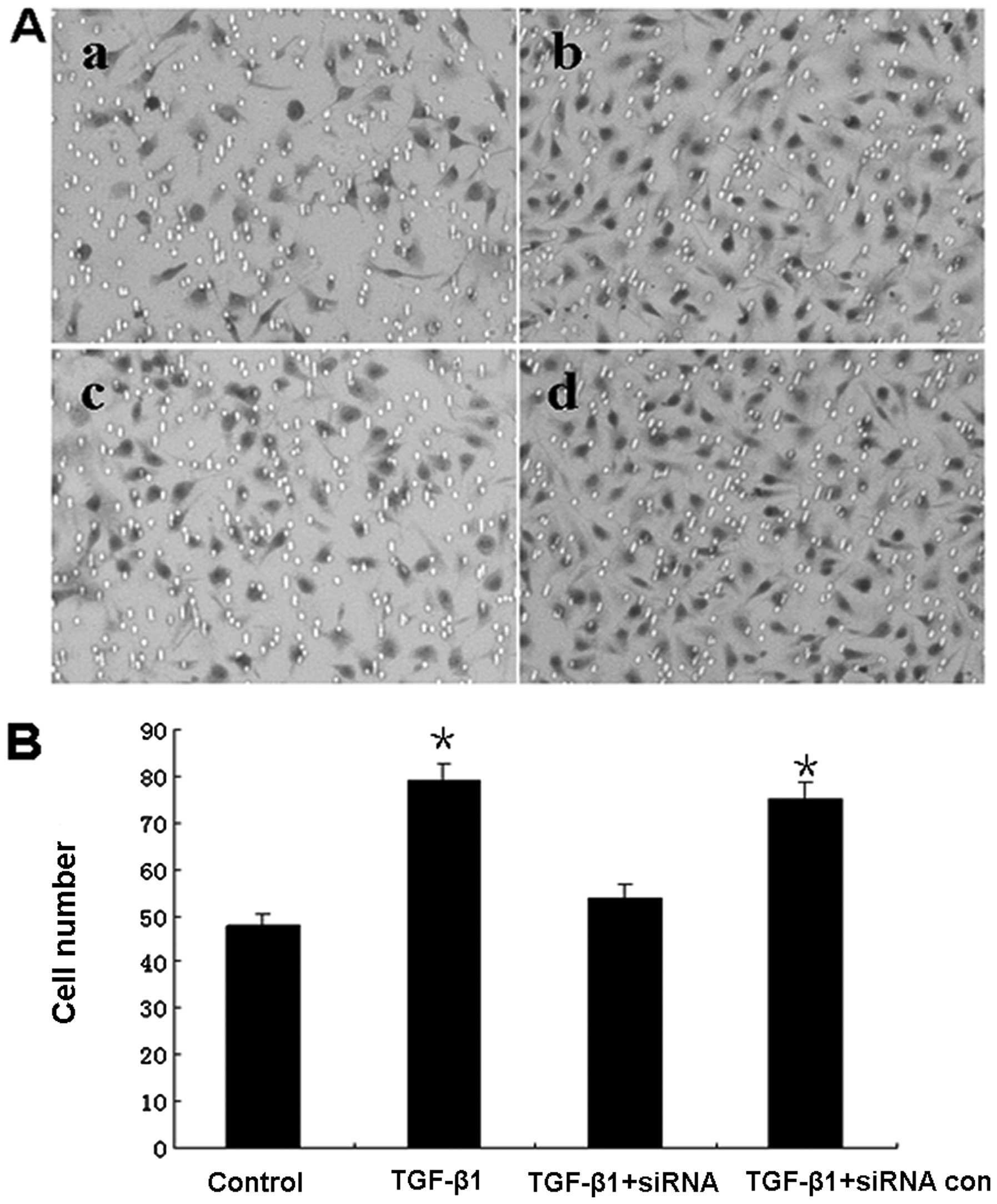
Promotion of cell invasion induced by
TGF-β1
We analyzed the invasion capability of the highly
metastatic MDA-MB-435S cells using the methods described above. The
results showed that TGF-β1 significantly promoted the invasiveness
of cells when compared to control (P<0.05), While Smad2-siRNA
significant decreased the number of invasive cells under TGF-β1
stimulation (P<0.05). These results suggested EMT of breast
cancer induced by TGF-β1 promote cancer cells metastasis, but
knockdown of the Smad2 gene, by silencing siRNA reduced the
invasion of gastric cancer cells can partially inhibit these
effects (Fig. 6).
Discussion
In this study, we showed immunohistochemical
evidence for EMT, which is associated with TGF-β1 expression at the
invasion front of breast cancer in vivo. A TGF-β1-induced
in vitro model of EMT showed morphologic, molecular, and
functional evidence for this process that was reversible by Smad2
RNAi.
The differential expression of epithelial and
mesenchymal markers has been recognized in several rare tumors of
mixed phenotype (20,21). More recently, this differential
expression of epithelial and mesenchymal markers at the invasive
margin has been described in colorectal and hepatocellular tumors
(22,23), suggesting that EMT might be a
feature of the invasive characteristics of epithelial tumors. In
addition, changes suggestive of EMT have been described in cell
lines, such as nonmalignant Madin-Darby canine kidney cells, and
cell lines derived from the pancreas cancers (24).
The molecular and phenotypic changes from an
epithelial to a mesenchymal cell type seem to be functionally
relevant because several studies have shown that EMT is important
in cancer progression (25,26). During EMT, epithelial cell-cell
contact is decreased by the downregulation of cytoskeletal
components and the cell morphology becomes more fibroblast-like
with upregulation of mesenchymal markers, including α-SMA and
vmentin (27,28). Loss of the classic epithelial marker
E-cadherin is associated with poor outcome in several tumor sites,
including invasive ductal breast carcinoma (29), and gastric adenocarcinoma (30). A reduction in E-cadherin level is
considered as a hallmark of EMT. E-cadherin plays a key role in
maintaining the epithelial structural integrity and polarization,
loss of which consequently destabilizes the structural integrity of
epithelium and makes cells dissociate from their neighbors
(31). Our data demonstrated that
breast cancer cells undergo transition from the epithelial to the
mesenchymal phenotype upon activation with TGF-β1, with the
induction of the transcription factor Smad2 and the expression
changes of EMT-related proteins occurred in MDA-MB-435S. However,
the E-cadherin expression did not change in MCF-7. We concluded
that MDA-MB-435S, but not MCF-7, was prone to undergo a complete
EMT. EMT may contribute to greater motility and higher invasiveness
of tumor cells.
Smads are a group of intracellular proteins that are
critical for transmitting the TGF-β1 signals from the cell surface
to the nucleus to promote transcription of target genes (14,16).
Accumulating evidence indicates that TGF-β stimulates cancer cell
EMT and metastasis through a combination of Smad2/3-dependent and
-independent signaling systems. Engineering metastatic human
MCF10ACA1a breast cancer cells to express a dominant-negative
Smad3, or a TβR-I mutant incapable of activating Smad2/3,
significantly reduced the ability of MCF10ACA1a cells to colonize
the lung (32). In the present
study, we demonstrated the role of Smad2 in TGF-β1 mediated EMT in
MDA-MB-435S. The significance of the present study is that breast
cancer cells undergo the process of EMT via expression of the
mesenchymal markers vimentin and α-SMA, and siRNA-Smad2
significantly blocked the expression of vimentin in cancer cells
activated with TGF-β1 and prevented EMT.
In the context of epithelial cancer, EMT provides a
mechanism for tumor cells to leave the primary tumor and invade
into the local tissue and blood vessels, setting the stage for
metastatic spread (33). Therefore,
EMT is hypothesized to contribute to tumor progression, and indeed
clinical evidence suggests that regulators of EMT, such as TGF-β1,
in cancer cells correlate with poor patient outcomes and tumor
aggressiveness (13,34). To evaluate these biological
functions of the cells undergoing EMT and if Smad2 siRNA could
reduce this abilty of invasion, we used invasion assays in our
study. Our results showing cells undergoing EMT by the stimulation
with TGF-β1 were more invasive. Consistently, the inhibition of
TGF/Smad2 pathway by siRNA led to a signifcant decrease the abilty
of invasion.
In summary, we showed immunohistochemical evidence
for EMT, which is associated with TGF-β1 expression at the invasion
front of breast cancer in vivo. Furthermore, the exposure of
breast cancer cell lines to TGF-β1 results in EMT, marked by
changes in cell morphology, cell behavior, and expression of
EMT-related protein markers, whereas knockdown of the Smad2 gene by
silencing siRNA partially inhibited these effects. Collectively,
our current data demonstrated that EMT of breast cancer induced by
TGF-β1 is dependent on Smad2 signaling and promotes breast cancer
cell metastasis.
References
|
1
|
Porter PL: Global trends in breast cancer
incidence and mortality. Salud Publica Mex. 51:141–146. 2009.
View Article : Google Scholar
|
|
2
|
Naber HP, Wiercinska E, Pardali E, van
Laar T, Nirmala E, Sundqvist A, van Dam H, van der Horst G, van der
Pluijm G, Heckmann B, Danen EH and Ten Dijke P: BMP-7 inhibits
TGF-β-induced invasion of breast cancer cells through inhibition of
integrin β(3) expression. Cell Oncol. 35:19–28. 2012.
|
|
3
|
Ding Z, Wu CJ, Chu GC, Xiao Y, Ho D, Zhang
J, Perry SR, Labrot ES, Wu X, Lis R, Hoshida Y, Hiller D, Hu B,
Jiang S, Zheng H, Stegh AH, Scott KL, Signoretti S, Bardeesy N,
Wang YA, Hill DE, Golub TR, Stampfer MJ, Wong WH, Loda M, Mucci L,
Chin L and DePinho RA: SMAD4-dependent barrier constrains prostate
cancer growth and metastatic progression. Nature. 470:269–273.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gu Y, Mi W, Ge Y, Liu H, Fan Q, Han C,
Yang J, Han F, Lu X and Yu W: GlcNAcylation plays an essential role
in breast cancer metastasis. Cancer Res. 70:6344–6351. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: malignant and stem
celltraits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Greenburg G and Hay ED: Epithelia
suspended in collagen gels can lose polarity and express
characteristics of migrating mesenchymal cells. J Cell Biol.
95:333–339. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lv ZD, Na D, Ma XY, Zhao C, Zhao WJ and Xu
HM: Human peritoneal mesothelial cell transformation into
myofbroblasts in response to TGF-β1 in vitro. Int J Mol Med.
27:187–193. 2011.PubMed/NCBI
|
|
8
|
Uchikado Y, Okumura H, Ishigami S,
Setoyama T, Matsumoto M, Owaki T, Kita Y and Natsugoe S: Increased
Slug and decreased E-cadherin expression is related to poor
prognosis in patients with gastric cancer. Gastric Cancer.
14:41–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yanez-Mo M, Lara-Pezzi E, Selgas R,
Ramirez-Huesca M, Dominguez-Jimenez C, Jimenez Heffernan JA,
Aguilera A, Sanchez-Tomero JA, Bajo MA, Alvarez V, Castro MA, del
Peso G, Cirujeda A, Gamallo C, Sanchez-Madrid F and Lopez-Cabrera
M: Peritoneal dialysis and epithelial-to-mesenchymal transition of
mesothelial cells. N Engl J Med. 348:403–413. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang Y, Siegel PM, Shu W, Drobnjak M,
Kakonen SM, Cordón-Cardo C, Guise TA and Massagué J: A multigenic
program mediating breast cancer etastasis to bone. Cancer Cell.
3:537–549. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Katsuno Y, Hanyu A, Kanda H, Ishikawa Y,
Akiyama F, Iwase T, Ogata E, Ehata S, Miyazono K and Imamura T:
Bone morphogenetic protein signaling enhances invasion and bone
metastasis of breast cancer cells through Smad pathway. Oncogene.
7:6322–6333. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Padua D, Zhang XH, Wang Q, Nadal C, Gerald
WL, Gomis RR and Massagué J: TGFbeta primes breast tumors for lung
metastasis seeding through angiopoietin-like 4. Cell. 133:66–77.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Benson JR: Role of transforming growth
factor beta in breast carcinogenesis. Lancet Oncol. 5:229–239.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu J, Lamouille S and Derynck R:
TGF-beta-induced epithelial to mesenchymal transition. Cell Res.
19:156–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shibata S, Marushima H, Asakura T,
Matsuura T, Eda H, Aoki K, Matsudaira H, Ueda K and Ohkawa K:
Three-dimensional culture using a radial flow bioreactor induces
matrix metalloprotease 7-mediated EMT-like process in tumor cells
via TGFβ1/ mad pathway. Int J Oncol. 34:1433–1448. 2009.PubMed/NCBI
|
|
16
|
Heldin CH, Miyazono K and ten Dijke P:
TGF-β signalling from cell membrane to nucleus through SMAD
proteins. Nature. 390:465–471. 1997.
|
|
17
|
Liu Q, Mao H, Nie J, Chen W, Yang Q, Dong
X and Yu X: Transforming growth factor beta1 induces
epithelial-mesenchymal transition by activating the JNK-Smad3
pathway in rat peritoneal mesothelial cells. Perit Dial Int.
3:88–95. 2008.PubMed/NCBI
|
|
18
|
Yoo YA, Kang MH, Kim BS, Kim JS and Seo
JH: Sustained co-cultivation with human placenta-derived MSCs
enhances ALK5/Smad3 signaling in human breast epithelial cells,
leading to EMT and differentiation. Differentiation. 77:450–461.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Albini A and Benelli R: The chemoinvasion
assay: a method to assess tumor and endothelial cell invasion and
its modulation. Nat Protoc. 2:504–511. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wick MR and Swanson PE: Carcinosarcomas:
current perspectives and an historical review of nosological
concepts. Semin Diagn Pathol. 10:118–127. 1993.PubMed/NCBI
|
|
21
|
Thompson L, Chang B and Barsky SH:
Monoclonal origins of malignant mixed tumors (carcinosarcomas).
Evidence for a divergent histogenesis. Am J Surg Pathol.
20:277–285. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brabletz T, Herrmann K, Jung A, Faller G
and Kirchner T: Expression of nuclear h-catenin and c-myc is
correlated with tumor size but not with proliferative activity of
colorectal adenomas. Am J Pathol. 156:865–870. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Giannelli G, Bergamini C, Fransvea E,
Sgarra C and Antonaci S: Laminin-5 with transforming growth
factor-β1 induces epithelial to mesenchymal transition in
hepatocellular carcinoma. Gastroenterology. 129:1375–1383.
2005.
|
|
24
|
Tojo M, Hamashima Y, Hanyu A, Kajimoto T,
Saitoh M, Miyazono K, Node M and Imamura T: The ALK-5 inhibitor
A-83-01 inhibits Smad signaling and epithelial-to-mesenchymal
transition by transforming growth factor-beta. Cancer Sci.
96:791–800. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Scheel C and Weinberg RA: Phenotypic
plasticity and epithelial-mesenchymal transitions in cancer and
normal stem cells? Int J Cancer. 129:2310–2314. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang J, Tang YL and Liang XH: EMT: a new
vision of hypoxia promoting cancer progression. Cancer Biol Ther.
11:714–723. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vered M, Dayan D, Yahalom R, Dobriyan A,
Barshack I, Bello IO, Kantola S and Salo T: Cancer-associated
fibroblasts and epithelial-mesenchymal transition in metastatic
oral tongue squamous cell carcinoma. Int J Cancer. 127:1356–1362.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jin H, Morohashi S, Sato F, Kudo Y,
Akasaka H, Tsutsumi S, Ogasawara H, Miyamoto K, Wajima N, Kawasaki
H, Hakamada K and Kijima H: Vimentin expression of esophageal
squamous cell carcinoma and its aggressive potential for lymph node
metastasis. Biomed Res. 31:105–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
ElMoneim HM and Zaghloul NM: Expression of
E-cadherin, N-cadherin and snail and their correlation with
clinicopathological variants: an immunohistochemical study of 132
invasive ductal breast carcinomas in Egypt. Clinics. 66:1765–1771.
2011.
|
|
30
|
Mimata A, Fukamachi H, Eishi Y and Yuasa
Y: Loss of E-cadherin in mouse gastric epithelial cells induces
signet ring-like cells, a possible precursor lesion of diffuse
gastriccancer. Cancer Sci. 102:942–950. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gavert N and Ben-Ze’ev A:
Epithelial-mesenchymal transition and the invasive potential of
tumors. Trends Mol Med. 14:199–209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ge R, Rajeev V, Ray P, Lattime E, Rittling
S, Medicherla S, Protter A, Murphy A, Chakravarty J, Dugar S,
Schreiner G, Barnard N and Reiss M: Inhibition of growth and
metastasis of mouse mammary carcinoma by selective inhibitor of
transforming growth factor-beta type I receptor kinase in vivo.
Clin Cancer Res. 12:4315–4330. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ansieau S, Caron de Fromentel C, Bastid J,
Morel AP and Puisieux A: Role of the epithelial-mesenchymal
transition during tumor progression. Bull Cancer. 97:7–15. 2010.(In
French).
|
|
34
|
Buck MB, Fritz P, Dippon J, Zugmaier G and
Knabbe C: Prognostic significance of transforming growth factor
beta receptor II in estrogen receptor-negative breast cancer
patients. Clin Cancer Res. 10:491–498. 2004. View Article : Google Scholar : PubMed/NCBI
|