Introduction
Lang-du (LD) is a naturally occurring product from
the Traditional Chinese Medicine (TCM) Euphorbia fischeriana
Steud. LD has been used in China to treat clinical patients with
cancer, edema, and ascites for many years. The discovery of novel
natural compounds with low toxicity and high selectivity of killing
various cancer cells, including MCF-7 cells, is an important area
in cancer research. Due to their wide range of biological
activities and low toxicity in animal models, some natural products
have been used as alternative treatments for cancer (1).
Jolkinolide B (JB) is a diterpenoid component
isolated from the dried roots of Euphorbia fischeriana
Steud. It has been reported that JB regulates proliferation and
induces apoptosis in human prostate LNCaP cancer, MDA-MB-231 and
K562 cells in vitro(2–4). The
purpose of this study was to investigate the effects of JB on the
in vitro and in vivo growth of MCF-7 cells and its
molecular mechanisms of action. Our results showed that JB
significantly suppressed the in vitro MCF-7 cell growth in a
dose- and time-dependent manner. We investigated the induction of
apoptosis in MCF-7 breast cancer cells and examined whether its
effects are associated with the PI3K/Akt/mTOR signaling pathway.
Apoptosis is associated with biochemical changes mediated by
various genes and cell signaling pathways, such as the
phosphoinositide 3-kinase (PI3K)/Akt survival pathway.
Materials and methods
Plant extracts and purification
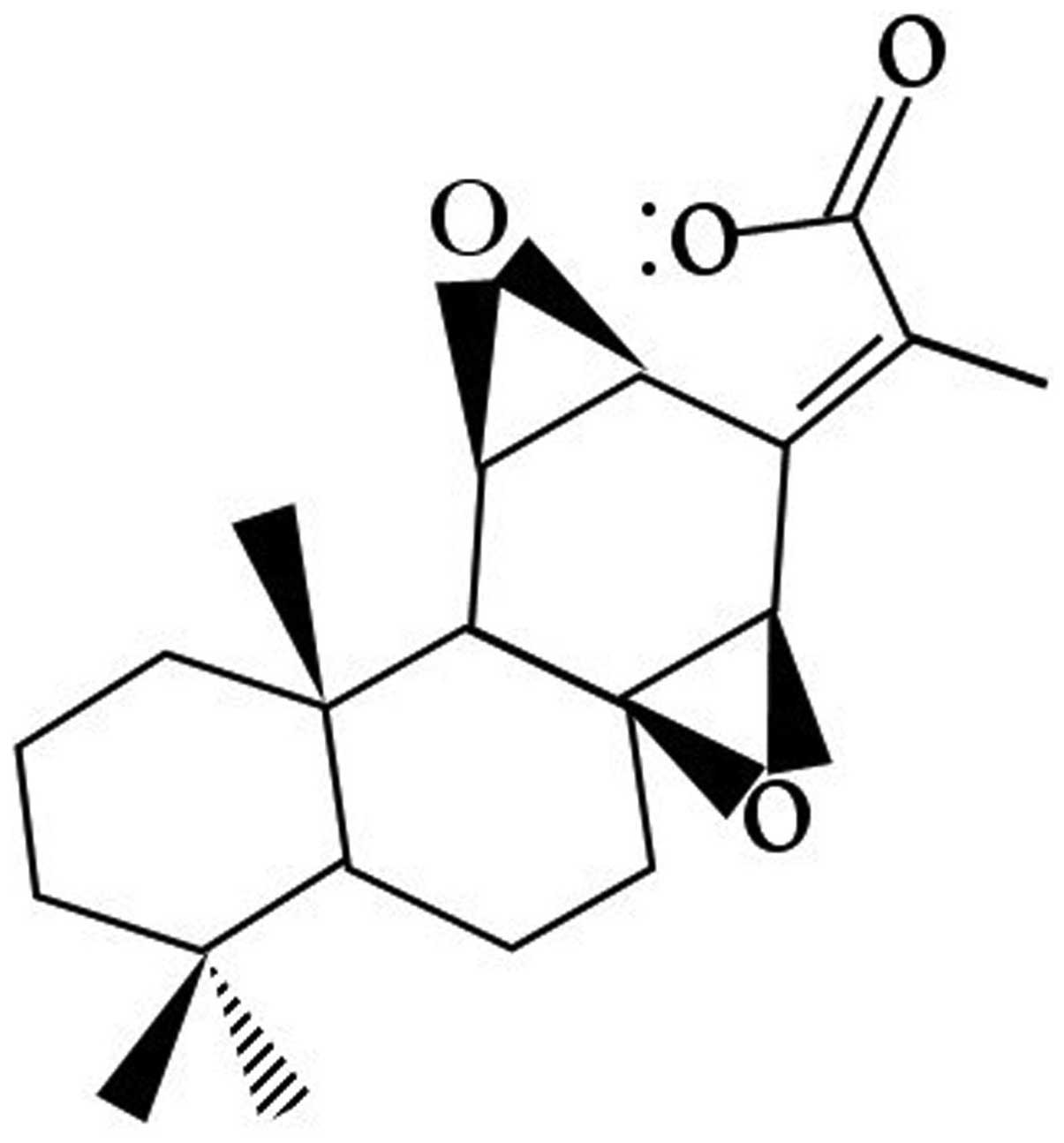
JB (molecular weight of 330.4 kDa, chemical formula
C28H26O4, purity >99%)
(Fig. 1) was kindly provided by
Professor Shujun Zhang (Chemical Engineering Institute, Qiqihar
University). JB was dissolved in dimethylsulfoxide (DMSO) to make a
stock solution at a concentration of 100 mM, which was further
diluted to the appropriate concentration with culture medium prior
to each experiment. Control experiments contained DMSO.
Cell line and cell culture
The human breast cancer cell line MCF-7 was obtained
from the Laboratory of Molecular Oncology, Beijing Cancer
Hospital/Institute. Cells were incubated in complete DMEM (Gibco
BRL; Grand Island, NY, USA) supplemented with 5% heat-inactivated
fetal bovine serum (FBS; Gibco BRL) in a humidified atmosphere at
37°C and 5% CO2.
Cell viability analysis
Cells (1×104/ml) were cultured in 96-well
chamber slides for 24 h prior to use in the experiment. Culture
mdium was replaced with fresh medium containing the appropriate
concentration of JB ranging from 0 to 80 μg/ml, and fresh medium
with the drug was added from 24 to 72 h. MTT (Sigma-Aldrich; St.
Louis, MO, USA) was added into each well and cultured for another 4
h, and the supernatant was discarded and supplemented with 100 μl
DMSO. When the crystals were dissolved, the absorbance values were
read on the enzyme-labeled mini reader II (Bio-Rad; Hercules, CA,
USA) at the wave length of 570 nm. The procedure was repeated three
times.
Cell cycle analysis
Cells were seeded in 60-mm culture dishes and grown
to 50% confluence. Subsequently, the cells were cultured in
serum-free medium for 24 h and then incubated with 0, 10, 20, 40
μg/ml of JB for 24 h in complete medium. The cells were harvested
by trypsinization, centrifuged at 2,000 rpm for 5 min, washed in
PBS, and resuspended in cold 70% ethanol. Finally, 1 ml propidium
iodide stain solution (20 μg/ml PI, 100 μg/ml DNase free RNase A)
was added to the samples which were analyzed on a FACScan
(Becton-Dickinson; San Francisco, CA, USA) within 30 min. Data on
20,000 cells were acquired and processed using Lysis II software
(Becton-Dickinson).
Flow cytometric analysis of
apoptosis
Cells were seeded in 6-well plates at a density of
1.2×106 cells/well. After 24 h of JB treatment at a
concentration of 0, 10, 20, 40 μg/ml, cells were collected, washed
twice with cold PBS and 1×106 cells were resuspended in
500 ml 1X binding buffer. Subsequently, 100 μl of cell suspension
was transferred to a 5 ml culture tube and incubated with 10 μl of
Annexin V antibodies and 10 μl of propidium iodide containing 300
μg/ml RNase (Sigma). The cells were vortexed gently and incubated
for 15 min at room temperature (RT) in the dark. 1X binding buffer
(400 μl) was added to each tube and the cells were analyzed with
flow cytometry within 1 h.
Transmission electron microscopy (TEM)
analysis
In brief, small sections of tumor tissue (1
mm3) from control and treated mice were fixed with 4%
paraformaldehyde and 2% glutaraldehyde in 0.1 M sodium phosphate
buffer (pH 7.4) for 4 h at RT (24°C). This was followed by washing
the tissue samples in 0.1 M sodium phosphate buffer (pH 7.4) and
then placing them in 2% osmium tetroxide in 0.1 M sodium phosphate
buffer (pH 7.4) for 2 h at room temperature. Dehydration was
carried out in an ascending grade of ethanol, followed by embedding
in Epon 812 and polymerization at 60°C for 48 h. Ultrathin sections
(50–70 nm) were obtained using an Ultracut Ultra microtome (Leica
Microsystems GmbH; Wetzlar, Germany) and picked up onto 200-mesh
copper grids. The sections were double-stained with uranyl acetate
and lead citrate and then analyzed under an FEI Tecnai-12 twin
transmission electron microscope equipped with an SIS Mega View II
CCD camera at 80 kV (FEI Co.; Hillsboro, OR, USA).
Western blot analysis
Western blot analysis was performed as previously
described. Proteins were transferred to a nitrocellulose membrane
and blots were probed with anti- cyclinD1, cyclinE, p-PI3K, p-Akt,
PTEN, mTOR, p-eIF4E and β-actin followed by incubation with
horseradish peroxidase-conjugated mouse or rabbit immunoglobulin.
Blots were then developed using the West Pico Chemiluminescent
substrate (Pierce; Woburn, MA, USA).
Xenograft tumor in nude mice
All animal procedures were approved by the Committee
on Animal Experimentation of Beijing Cancer Hospital and the
procedures complied with the NIH Guide for the Care and Use of
Laboratory Animals. Female Balb/c nude mice (4–5 weeks of age),
obtained from the Beijing Vital River Experimental Animals
Technology (Beijing, China), were used in all experiments.
Suspensions of 5×105 cells for MCF-7 in 100 μl PBS were
subcutaneously inoculated into the right and left flank of the nude
mice (n=3 in each group). JB (40 mg/kg) was administered
intraperitoneally every three days for one month. Tumor growth was
measured with a caliper every three days using the formula, tumor
volume V (mm3) = height × length × depth. All of the
nude mice were fed for one month. At the end of the experiment,
tumor tissues were weighed and harvested for additional analyses as
described below.
Immunocytochemistry
All tumor tissues were fixed with 4%
paraformaldehyde (pH 7.4) and embedded in paraffin. All were plated
on coverslips and fixed with cold acetone for 10 min. Following
washing with PBS, coverslips were treated with methanol-hydrogen
peroxide (97 ml:3 ml) for 30 min at RT. All coverslips were washed
in PBS and treated with 1:100 dilution of antibody (p-PI3K, p-Akt,
PTEN, mTOR, IgG conjugated with HRP) for 1 h at 37°C. Coverslips
were washed thoroughly and treated with 0.05% DBA containing 0.05%
fresh hydrogen peroxide. Coverslips were counter-stained with
haematoxylin and observed under a light microscope.
Statistical analysis
Each experiment was performed at least three times.
All data were expressed as the mean value ± standard deviation
(SD). The means of the different groups were compared using one-way
of variance (ANOVA). All statistical analyses were performed with
the SPSS17.0 software (SPSS; Chicago, IL, USA). Differences were
considered statistically significant at p<0.05, p<0.01,
p<0.001. Log IC50 calculation was performed using the
built-in algorithms for dose-response curve with variable slope. A
fixed maximum value of the dose-response curve was set to maximum
obtained value for each drug.
Results
Effect of JB on the proliferation of
MCF-7 cells
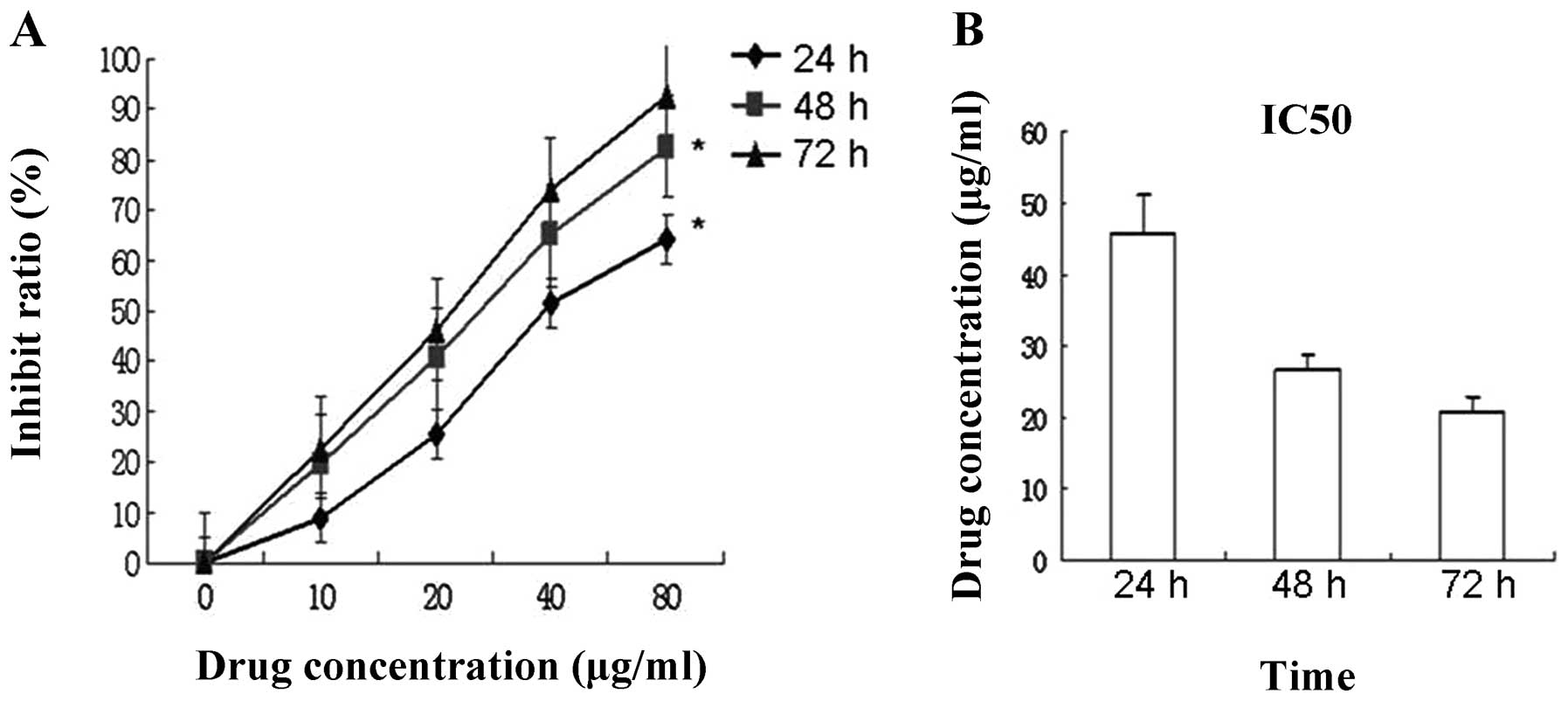
Cell viability was measured by MTT analysis. The
treatment of MCF-7 cells for 24–72 h with 0–80 μg/ml resulted in a
dose-dependent decrease in cell proliferation (Fig. 2A). Following treatment with the
different concentrations of JB for 24, 48, and 72 h, the growth
activities of drug treatment groups were reduced in a
time-dependent manner by IC50 (Fig. 2B), (p<0.05, p<0.01).
JB causes apoptosis and induces S arrest
in MCF-7 cells
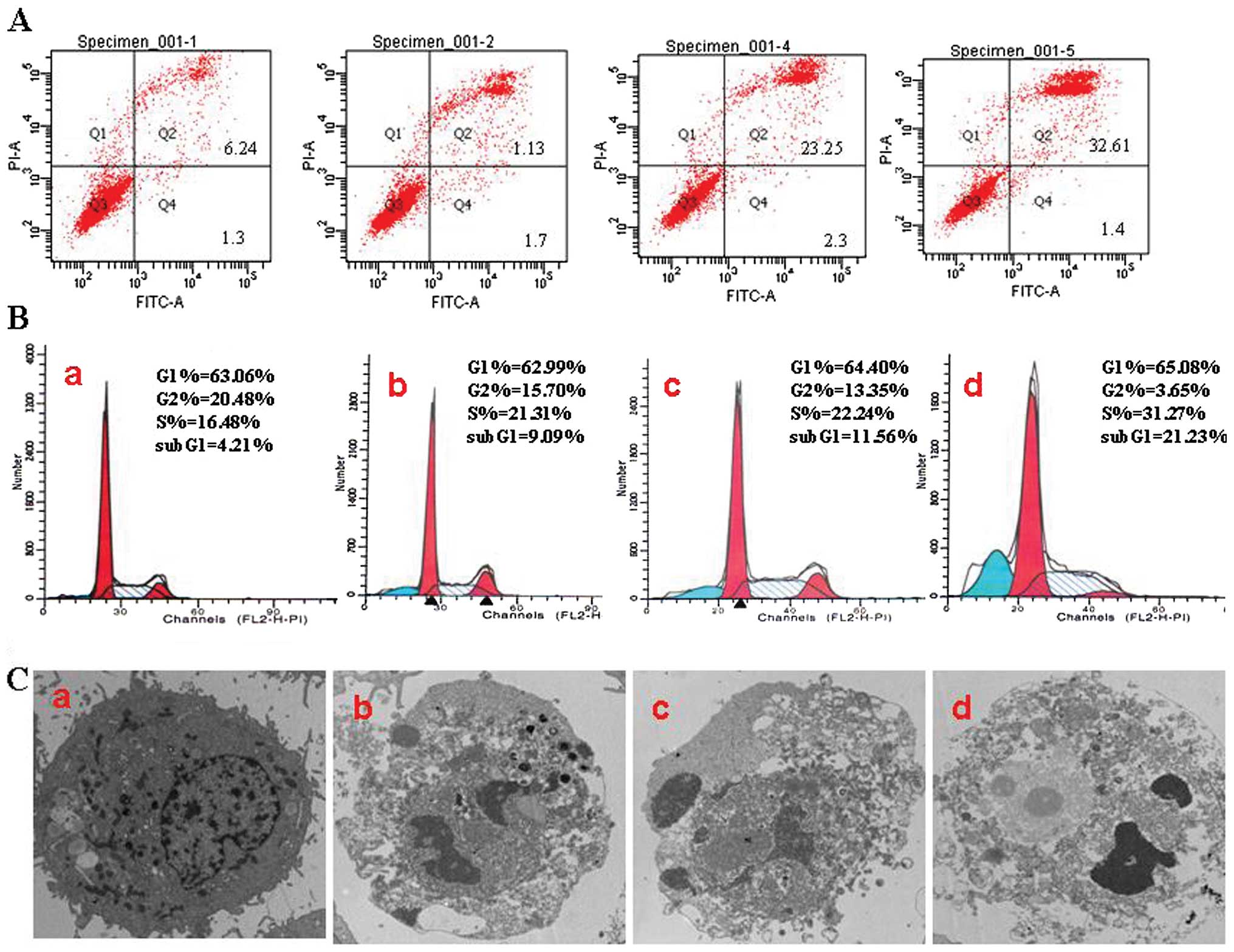
The percentages of G1, S, and G2/M cells were
evaluated by PI staining and flow cytometric analysis. The
apoptotic rates (APO) of the experimental groups (9.09±1.13% and
11.56±2.25%) were higher than those of the untreated control group
(4.21±0.54%). Together with the prolongation of time, the
experimental group of JB (40 μg/ml) showed typical apoptosis peak
(21.23±4.25%). Meanwhile, the cell cycle also showed notable change
with the appearance of S peak and increased percentage of S cells
(Fig. 3B). When cells were treated
with 40 μg/ml JB for 24 h, we found a significant number of cells
that was more prominently arrested at the S phase of the cell cycle
(31.27±3.91%) than in the JB 0 μg/ml group (16.48±1.7%) (Fig. 3B) Furthermore, we performed
phosphatidylserine externalization (Annexin V binding) and cell
membrane integrity (PI staining) binding assay to detect the
redistribution of phosphatidylserine, which is a hallmark for early
apoptotic cells.
Annexin V-FITC/PI dual labeling further showed that
after treatment with JB (10, 20 and 40 μg/ml for 24 h), the maximum
concentration of JB induced a higher percentage of apoptosis cells;
JB 40 μg/ml (32.61±5.87%) compared to JB 0 μg/ml (6.24±1.24%)
(Fig. 3A). The representative
morphological changes in MCF-7 cell were observed by transmission
electron microscope (original magnification, ×10,000). The JB 0
μg/ml group of cells cultured membrane showed normal, the nuclei
were round and homogeneous, the structure displayed after staining.
With the increasing concentration of JB, apoptotic cells appeared
more and more in the JB-treated group. The typical characteristics
of apoptosis were cytoplasmic edema and nuclei condensed, the
fragmented chromatin arranged against the nuclear membrane when
MCF-7 cells were treated with JB (10, 20, 40 μg/ml) for 24 h. The
JB 40 μg/ml group of cells presented necrosis (Fig. 3C).
JB inhibits tumor growth in nude
mice
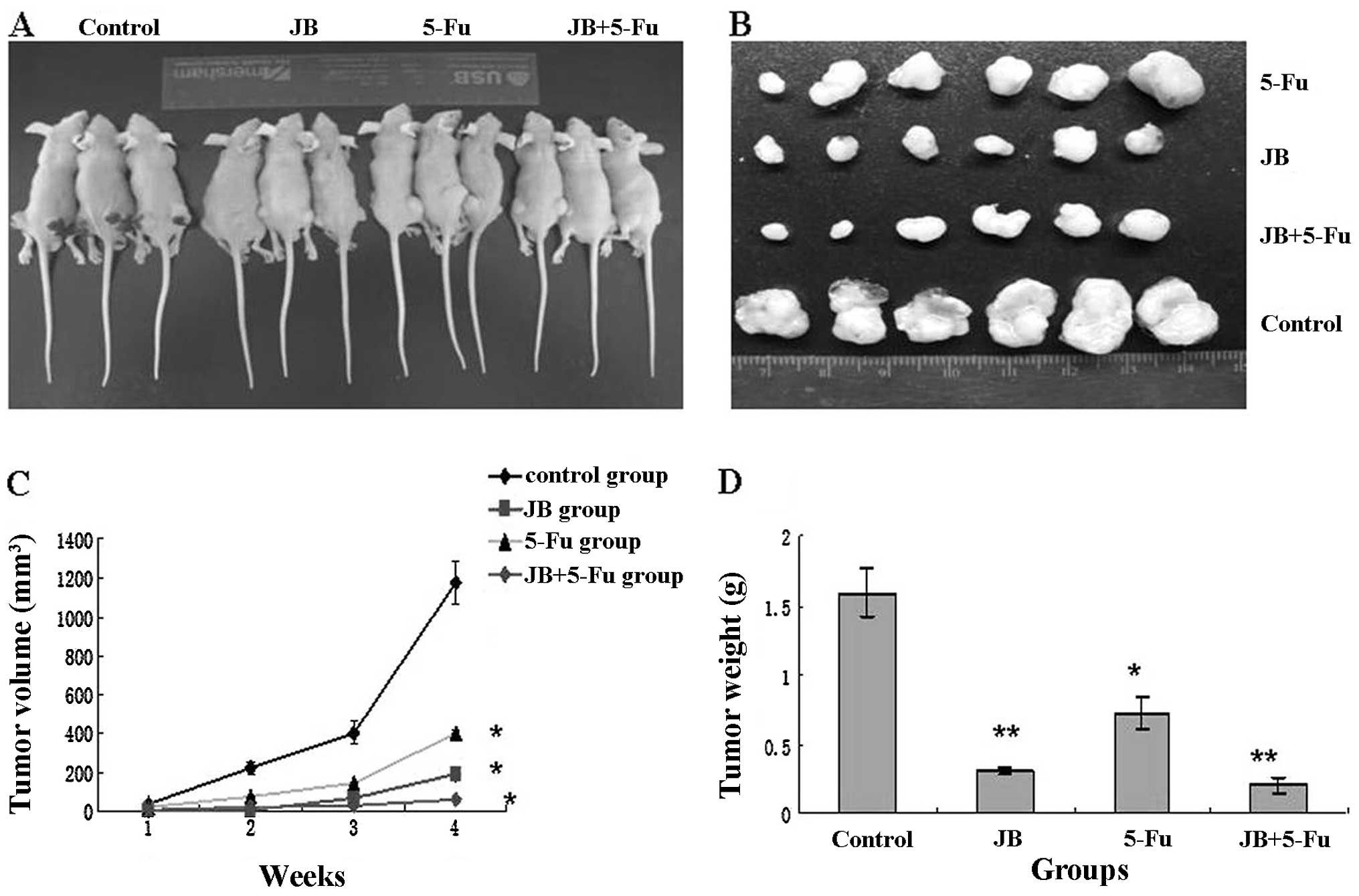
To confirm the antitumor effect of JB in
vivo, MCF-7 cells were transplanted into nude mice. There was
no statistical difference between the four groups at the start of
treatment (P>0.05), and they were randomly divided into four
groups: the negative control group, the JB group (40 mg/kg), the
5-Fu group (5 mg/kg), and the JB+5-Fu group. Tumor growth curves
were depicted (Fig. 4C) to compare
the differences in the antitumor efficacy during the course of the
experiments. At 28 days, tumor volume in the 5-Fu, the 5-Fu+JB and
the JB group were greatly reduced, while tumors in the control
group reached 1,207 mm3. The differences in the tumor
sizes of the four groups was significant (P<0.05); however, no
significant difference was observed between the JB and the JB+5-Fu
group (P>0.05). The results of tumor weight were consistent with
the results of tumor volume (P<0.05 vs. control group, P<0.01
vs. control group) (Fig. 4D).
JB induces tumor tissue apoptosis in
vivo
The transplanted tumor tissues were dispersed into
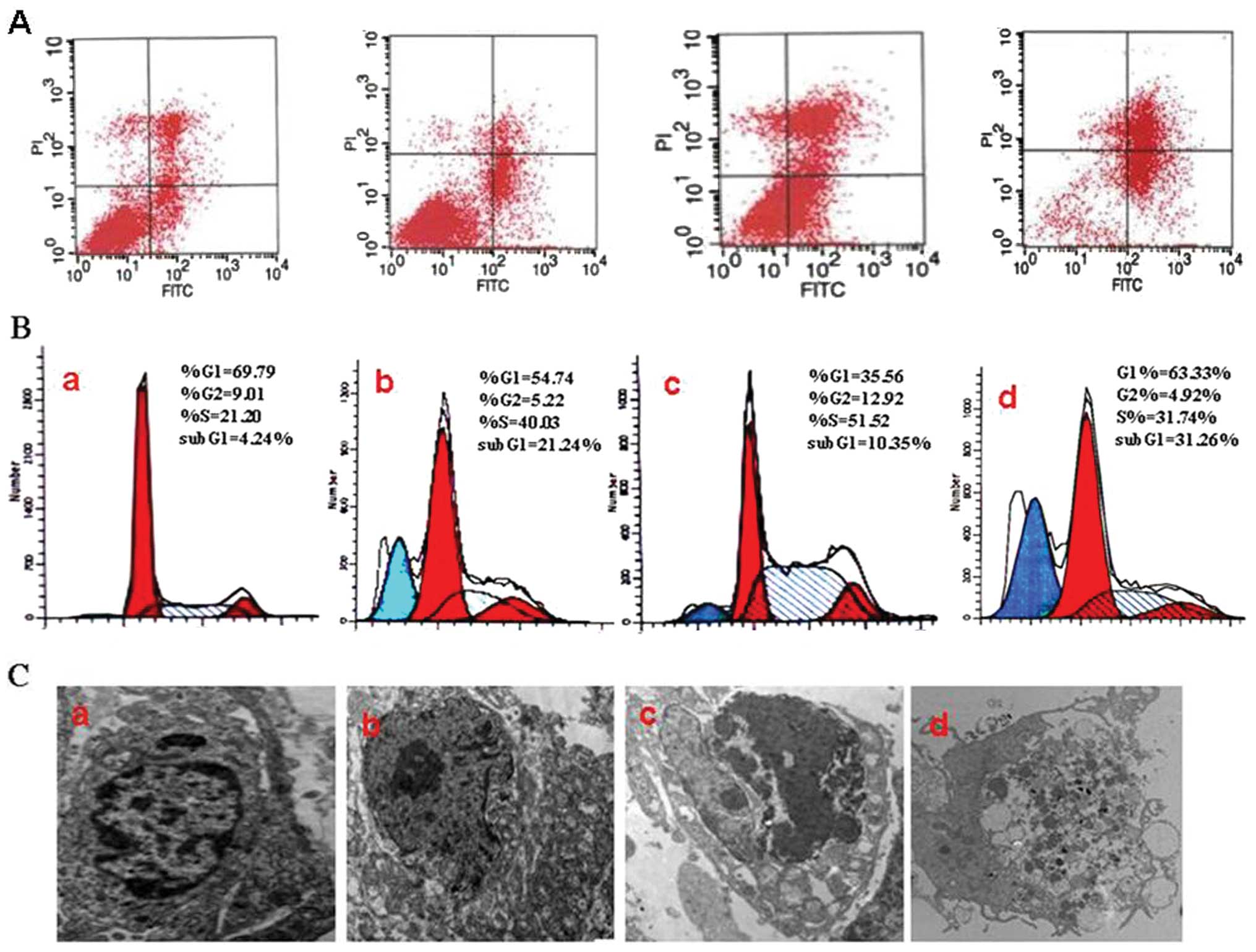
single cells for analysis by flow cytometry. Fig. 5B shows that tumor tissues from the
5-Fu, the JB and the JB+5-Fu group presented notable changes with
increased percentage of S cells and decreased percentage of G2/M
cells. The apoptotic rate in the JB+5-Fu group (31.26±3.15%) was
higher than that in the control group (4.24±1.25%). Annexin
V-FITC/PI dual labeling further showed that treatment with JB
induced a higher percentage of apoptosic cells in the JB 40
mg/mg+5-Fu 5 mg/mg (31.26±6.24%) than in the control group
(4.24±2.19%) (P<0.05) (Fig. 5A).
Fig. 5C shows cellular morphology
by TEM. The JB group showed that the nuclear DNA of apoptotic cells
displayed nucleus pyknosis and distortion, chromatin condensation,
margination, and fracture.
Effect on the PI3K/Akt/mTOR signaling
pathway in tumor tissue
The expression of cyclinD1, cyclinE, mTOR, p-PI3K
and p-Akt in the the tumor tissues of the JB group was
significantly lower than that in the control group (P<0.05)
(Fig. 6). This is in contrast to
the upregulation of PTEN and p-eIF4E compared to that in the
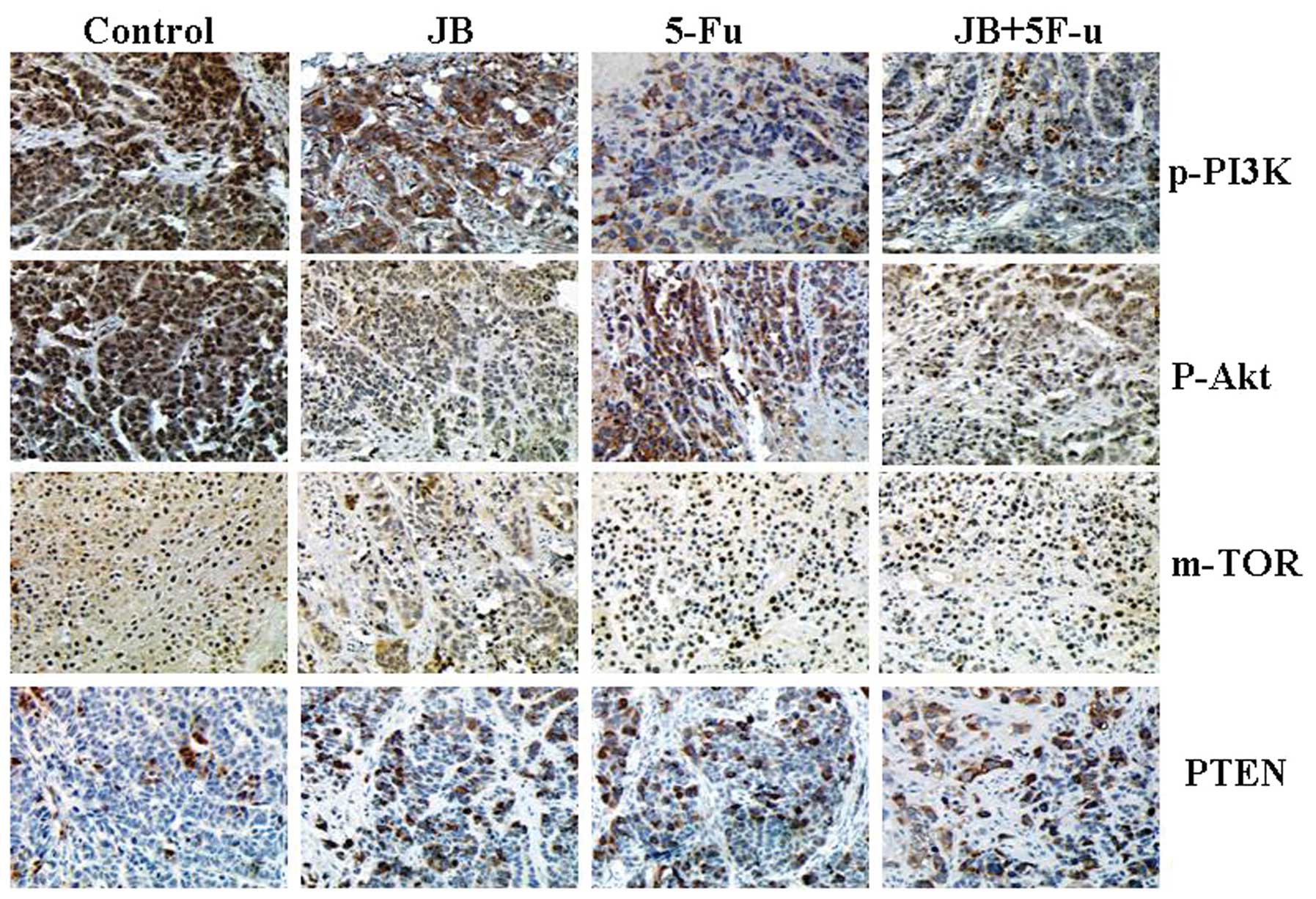
control group (P<0.05). Immunohistochemical staining of p-PI3K,
p-Akt, PTEN, mTOR showed the expression of proteins in tumor
tissues. By staining the PTEN of the JB group, its staining
intensity became gradually stronger than in the control group
(P<0.05), indicating high cell proliferation activity. Staining
intensity of p-PI3K, mTOR and p-Akt was significantly weaker in
nude mice treated with JB compared to the control group (P<0.01)
(Fig. 7). These results reveal that
JB was able to downregulate the expression of the p-PI3K, mTOR and
p-Akt proteins whereas it upregulated the expression of the PTEN
protein.
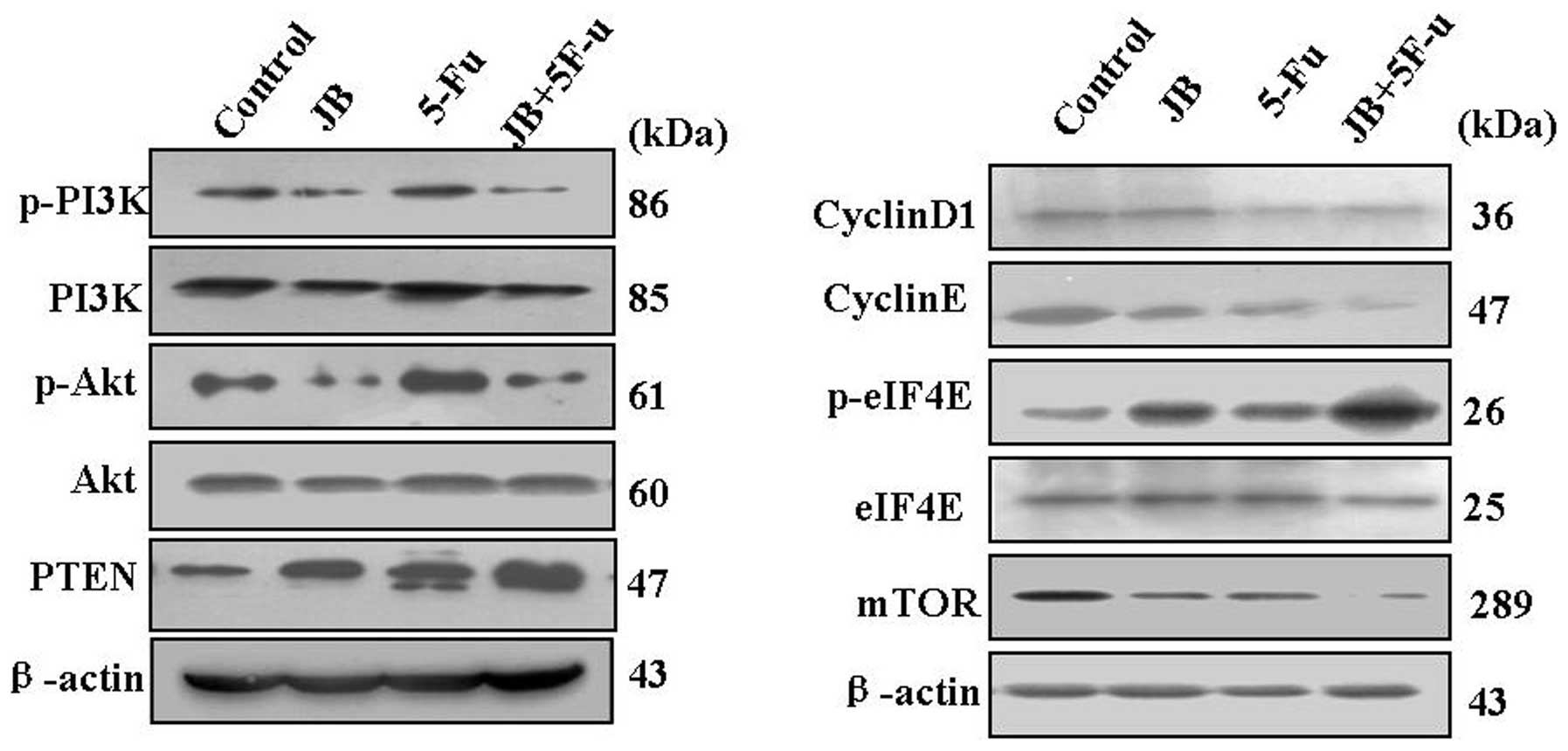 | Figure 6Effect of JB on the PI3K/Akt/mTOR
signal pathway in tumor tissues. Proteins were extracted, then
cyclinD1, cyclinE, p-PI3K, p-Akt, PTEN, mTOR and p-eIF4E
expressions were determined by western blot analysis. The results
showed downregulation of cyclinD1, cyclinE, mTOR, p-PI3K and p-Akt,
and upregulation of PTEN and p-eIF4E. Densitometric values of
protein bands were normalized to those of β-actin. The expression
of PI3K, Akt and eIF4E had no significance in any of the
groups. |
Discussion
JB was employed in this study to investigate its
mechanism to induce apoptosis in vitro and in vivo.
The results demonstrated that jolkinolide B (JB) inhibited the
PI3K/Akt/mTOR signaling pathway leading to apoptotic cell death.
The dried roots of Euphorbia fischeriana Steud have a long
history of being used as folk medicine, and antitumorigenic
constituents, such as the diterpenoid compound 17-acetoxyjokinolide
B and JB, have been identified (5).
However, the molecular mechanism of JB remains largely unknown. In
the present study, the mechanism of apoptosis was examined by the
proliferation of MCF-7 cells and the tumor growth curve, cell
cycle, cell apoptosis, cell morphology and animal experiments. We
found that the Akt-dependent signaling pathway could be involved in
the effect of JB on nude mice.
Cell cycle was determined by flow cytometry in
vivo and in vitro. The results were consistent. MCF-7
cells were arrested in the cell cycle at S phase in vitro
and in vivo. However, the tumor tissue had cellular debris
and necrotic tissue making the animal experimentation
difficult.
Transmission electron microscopy (TEM) showed that
JB markedly induced apoptosis in vivo and in vitro.
The control group cells grew significantly. The JB and the 5-Fu
group cells kept their membrane integrity and cytoplasm condensed.
Cells became pyknotic and small, nuclear chromatin was dense with
lumps of different sizes, located mainly in the nuclear membrane.
Nuclear DNA of apoptotic cells displayed ladder bands
characteristic of internucleosomal DNA fragmentation and
apoptosis.
The PI3K/Akt signaling pathway has been reported to
play an essential role in various cellular processes including
apoptosis, survival, proliferation and differentiation (6,7).
Activated PI3K initiates the activation of the downstream Akt
kinase. Moreover, phosphorylated Akt could facilitate cell survival
and proliferation and regulate various signaling pathways (8–10). It
has been shown that the activation of PI3K and Akt could inhibit
autophagy. Yuan et al(12)
confirmed that there is a close relationship between the excessive
expression and activation of Akt kinase and the malignant
biological behavior of ovarian cancer (11,12).
The PI3K/Akt signaling pathway has been closely related to the
occurrence of human malignant tumors, such as endometrial cancer
and non-small cell lung cancer (13,14).
JB treatment of MDA-MB-231 cells decreased the expression of the
PI3K p85 subunit and the phosphorylated Akt in a
concentration-dependent manner (4).
Disregulation of the PI3K/AKT pathway leads to unchecked cellular
growth and proliferation.
Due to the complexity of this signaling cascade,
especially as applied to the regulation of the mammalian target of
rapamycin (mTOR), the activation of PI3K/Akt may be through the
TSC1/2 complex.
The eIF-4E suppresses molecules with protein 1
(4E-BP1) and ribosomal protein P70S6K (16–18).
4E-BP1 phosphorylation is reduced after treatment with EIF-4E
(19). PI3K/Akt signaling pathways
may adjust inhibition the PTEN (20). PTEN can make PI-3, 4, 5-P3 to
phosphoric acid and thus inhibits Akt activation, restraining the
cyclin E-CDK2 complex, making the cell block in the G1 phase
(21). Tumor-suppressor gene
mutations or lack of PTEN will lead to PI-3, 4, 5-P3
phosphorylation and Akt activation.
In conclusion, results of the MTT assay, flow
cytometry and TEM analysis showed that JB was able to inhibit the
growth of MCF-7 cells and induce apoptosis. JB significantly
decreased tumor volume and weight in nude mice. In addition, tumor
tissue treatment with JB was able to induce downregulation of
cyclinD1, cyclinE, mTOR, p-PI3K and p-Akt, and upregulation of PTEN
and p-eIF4E. JB-induced apoptosis of MCF-7 cells occurs through the
inhibition of the PI3K/Akt/mTOR signaling pathway. This appears to
be a key mechanism behind the JB-induced suppression of the breast
cancer cell growth in animal models. Therefore, JB may serve as a
novel promising compound in cancer therapy.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (No. 30973902), the Province Natural
Science Foundation of Hei Long Jiang (No. D200929) and the Science
and Technology Bureau mandatory subject of Qiqihar (SF-08001).
References
|
1
|
Wang YB and Huang R: Diterpenoids from the
roots of Euphorbia fischeriana. J Nat Prod. 69:967–970.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu WK and Ho JC: Jolkinolide B induces
neuroendocrine differentiation of human prostate LNCaP cancer cell
line. Biochem Pharmacol. 63:951–957. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luo H and Wang A: Induction of apoptosis
in K562 cells by jolkinolide B. Can J Physiol Pharmacol.
84:959–965. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin Y, Cui H, Xu H, Yue L, Xu H, Jiang L
and Liu J: Jolkinolide B induces apoptosis in MDA-MB-231 cells
through inhibition of the PI3K/Akt signaling pathway. Oncol Rep.
27:1976–1980. 2012.PubMed/NCBI
|
|
5
|
Wang HB and Chu WJ: Diterpenoids from the
roots of Euphorbia fischeriana. J Asian Nat Prod Res.
12:1038–1043. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Osaki M and Oshimura M: PI3K-Akt pathway:
its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Franke TF: PI3K/Akt: getting it right
matters. Oncogene. 27:6473–6488. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Geering B and Cutillas PR: Regulation of
class IA PI3Ks: is there a role for monomeric PI3K subunits.
Biochem Soc Trans. 35:199–203. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sussman M: ‘AKT’ing lessons for stem
cells: regulation of cardiac myocyte and progenitor cell
proliferation. Trends Cardiovasc Med. 17:235–240. 2007.
|
|
10
|
Tsurutani J and Steinberg SM: Prognostic
significance of clinical factors and Akt activation in patients
with bronchioloalveolar carcinoma. Lung Cancer. 55:115–121. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu YT and Tan HL: Activation of the
PI3K-Akt-mTOR signaling pathway promotes necrotic cell death via
suppression of autophagy. Autophagy. 5:824–834. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan ZQ and Sun M: Frequent activation of
AKT2 and induction of apoptosis by inhibition of
phosphoinositide-3-OH kinase/Akt pathway in human ovarian cancer.
Oncogene. 19:2324–2330. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Achiwa Y and Haseqawa K: Regulation of
phosphatidylinositol 3-kinase-Akt and the mitogen-activated protein
kinase pathways by ursolic acid in human endometrial cancer cells.
Biosci Biotechnol Biochem. 71:31–37. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang JM and He QY: Phosphorylated Akt
overexpression and loss of PTEN expression in non-small cell lung
cancer confers poor prognosis. Lung Cancer. 51:181–191. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Coffey JC and Wang JH: Phosphoinositide 3
kinase accelerates postoperative tumor growth by inhibiting
apoptosis and enhancing resistance to chemotherapy-induced
apoptosis. Novel role for an old enemy. J Biol Chem.
280:20968–20977. 2005. View Article : Google Scholar
|
|
16
|
Huang S and Houghton PJ: Targeting mTOR
signaling for cancer therapy. Curr Opin Pharmacol. 3:371–377. 2003.
View Article : Google Scholar
|
|
17
|
Zhou X and Tan M: Activation of the
Akt/mammalian target of Rapamycin/4E-BP1 pathway by ErbB2
overexpression predicts tumor progression in breast cancers. Clin
Cancer Res. 10:6779–6788. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Asnaghi L, Bruno P, Priulla M, et al:
mTOR: a protein kinase switching between life and death. Pharmacol
Res. 50:545–549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Coleman LJ and Peter MB: Combined analysis
of eIF4E and 4E-binding protein expression predicts breast cancer
survival and estimates eIF4E activity. Br J Cancer. 100:1393–1399.
2009. View Article : Google Scholar
|
|
20
|
Bonifazi P and D’Angelo C: Intranasally
delivered siRNA targeting PI3K/Akt/mTOR inflammatory pathways
protects from aspergillosis. Mucosal Immunol. 3:193–206. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Madlener S and Saiko P: Multifactorial
anticancer effects of digalloyl-resveratrol encompass apoptosis,
cell-cycle arrest, and inhibition of lymph endothelial gap
formation in vitro. Br J Cancer. 102:1361–1370. 2010. View Article : Google Scholar : PubMed/NCBI
|