Introduction
Trans-3,4′,5-trihydroxystilbene (resveratrol) is a
natural polyphenolic phytoalexin present in various plants, food
products, red wine, grape skin, berries, nuts, eucalyptus and
rheum. Its biological activities, including anti-inflammatory,
anti-oxidant, anti-cancer, anti-aging, anti-bacterial, anti-fungal
and anti-viral properties have been demonstrated (1). Resveratrol has been shown to prevent
angiogenesis and the migration of endothelial cells, and this
anti-angiogenic effect makes it a promising candidate for the
prevention of cancer progression (2,3).
Resveratrol has been demonstrated to inhibit proliferation and
induce apoptosis in gastric cancer cells. This provides the
therapeutic rationale for tumor chemotherapy using resveratrol
(4). Recent studies revealed that
resveratrol inhibited cell invasion in hepatoma cells in response
to hepatocyte growth factor in vitro as well as in hepatoma
and Lewis lung carcinoma in mice (5). In addition, resveratrol was reported
to suppress phorbol myristate acetate-induced cervical cancer cell
invasion (6). Resveratrol may
reduce proliferation, invasion, migration and apoptosis by
regulating nuclear factor (NF)-κB activities in various types of
cancer cells (7). Noteworthy,
resveratrol exerts biphasic effects where low concentrations are
estrogenic and high concentrations are anti-estrogenic (8). High concentrations of resveratrol have
anti-oxidant, pro-apoptotic, anti-growth, anti-angiogenic,
anti-invasive and anti-inflammatory effects. Therefore, resveratrol
is an important cancer-preventive agent (9). Another recent study found that
resveratrol exhibits anti-cancer properties in different tumor cell
types, including breast, prostate, stomach, colon, pancreatic and
thyroid cancers (10).
Tumor metastasis occurs through a complex series of
events including cell adhesion, migration, invasion, proliferation
and vessel formation (11).
Invasion and metastasis are fundamental properties of malignant
cancer cells. The degradation of the basement membrane and stromal
extracellular matrix (ECM), which exert biochemical and mechanical
barriers to cell movement, has been demonstrated as an important
biological process in the invasion and metastasis of cancer cells
(12). ECM degradation and
remodeling require the action of extracellular proteinases, among
which matrix metalloproteinases (MMPs) have been noted to play a
significant role. MMPs are a family of zinc-dependent proteinases
involved in the degradation of the ECM. The MMPs have been
implicated in the processes of tumor growth, invasion and
metastasis. Frequently, overexpression of MMPs has been detected in
malignant tumors and has been associated with an aggressive
malignant phenotype and poor prognosis in patients with cancer
(13). Among the MMPs, MMP-2
(72-kDa type IV collagenase) and MMP-9 (92-kDa type IV collagenase)
have been reported to play a major role in cancer metastasis. It
has been suggested that MMP-2 and MMP-9 are key mediators of tumor
metastasis, migration and invasion among these members (14). Therefore, the inhibition of MMP
expression and secretion is important to prevent cell adhesion,
migration, invasion and metastasis. Moreover, MMP-2 and MMP-9 may
be potential targets of anti-metastatic drugs for therapeutic
benefit in cancer (15). The
secretion and synthesis of MMPs may be promoted by a variety of
stimuli, including cytokines, during various pathological
processes, including tumor invasion, inflammation, atherosclerosis
and rheumatoid arthritis (16).
However, the mechanisms underlying the effect of resveratrol on
MMPs and the migratory abilities in HT1080 human fibrosarcoma cells
remain unclear.
Mitogen-activated protein kinase (MAPK) pathways are
evolutionarily conserved kinase modules that link extracellular
signals to the machinery that controls fundamental cellular
processes, such as proliferation, growth, differentiation,
migration, invasion and apoptosis in various cell types, including
cancer cells (17). Abnormalities
in MAPK signaling impinge on most, if not all of these processes
and play a critical role in the progression and development of
cancer (18). Recent studies have
demonstrated the role of the p38 MAPK signaling pathway in
mediating interleukin (IL)-28A-induced cell migration of UMUC-3
cells. The p38 pathway regulates IL-28A-induced cell migration
through MMP-9 expression by activating NF-κB and AP-1 binding
motifs (19). Salinomycin exhibited
significant growth inhibition and induction of apoptosis in the
human ovarian cancer cell line OV2008. Effects of salinomycin on
OV2008 cells have been associated with modulating the p38 MAPK
signaling pathway. A previous study found that salinomycin-induced
apoptosis in OV2008 cells may be associated with activating p38
MAPK and merits further investigation (20). Phosphatidylinositol 3-kinases (PI
3-kinases or PI-3Ks) are a family of enzymes involved in cellular
functions, such as cell growth, proliferation, survival,
differentiation, motility and intracellular trafficking. PI-3Ks are
involved in various types of cancers. Links between PI-3K activity
and several human maladies, including allergy, inflammation, heart
disease and cancer have been noted. Noteworthy, various members of
the PI-3K family have been linked to key cellular processes as well
as to several human diseases (21).
Recent studies have demonstrated that estrogen receptor β
growth-inhibitory effects are repressed through the activation of
MAPK and PI-3K signaling in mammary epithelial and breast cancer
cells (22). Another study
indicated that erufosine suppresses breast cancer in vitro
and in vivo via its activity on PI-3K, c-Raf and Akt
proteins. This study indicates that erufosine possesses high
anti-neoplastic activity not only in breast cancer cell lines in
vitro but also in rat mammary carcinoma in vivo. In
addition, this may indicate that the mechanism of action of
erufosine involves influence on both PI-3K/Akt and Ras/Raf/MAPK
signaling pathways (23).
Aromatic-turmerone was found to suppress the TPA-induced
upregulation of MMP-9 and COX-2 expression by blocking NF-κB,
PI-3K/Akt and ERK1/2 signaling in human breast cancer cells.
Aromatic-turmerone significantly inhibited TPA-induced invasion,
migration and colony formation in human breast cancer cells
(24). Therefore, in the present
study, we aimed to investigate the regulation by resveratrol of
both MMP-9 expression and cell migratory abilities, using HT1080
human fibrosarcoma cells as a model. We also investigated the
underlying signaling pathways involved in the molecular mechanism
of resveratrol on MMP-9 and cell migration in HT1080 cells. We
observed that resveratrol induces MMP-9 expression and cell
migration through the p38 kinase and PI-3K pathways in HT1080 human
fibrosarcoma cells.
Materials and methods
Materials
Resveratrol was purchased from Sigma-Aldrich (St.
Louis, MO, USA). RPMI-1640 medium and fetal bovine serum (FBS) were
purchased from Invitrogen (Burlington, ON, Canada). Streptomycin
and penicillin were obtained from Sigma-Aldrich. SB203580 and
LY294002 were purchased from Calbiochem (San Diego, CA, USA). MMP-9
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), pp38 (Cell
Signaling Technology, Danvers, MA, USA), p38 (Santa Cruz
Biotechnology, Inc.), pAkt (Cell Signaling Technology), Akt and
actin antibodies (both from Santa Cruz Biotechnology, Inc.) were
used for the experiment.
Cell line and culture
The human fibrosarcoma cell line, HT1080, was
obtained from the American Type Culture Collection (Rockville, MD,
USA). HT1080 cells were maintained in RPMI-1640 medium containing
10% FBS, 50 μg/ml streptomycin and 50 U/ml penicillin. Cell
cultures were maintained at 37°C, in a humidified atmosphere of 5%
CO2 in an incubator. HT1080 cells were plated on culture
dishes at a density of 1.2×105 cells/dish, and cells at
70–80% confluency were used for treatment.
MTT assay
Cell growth inhibition was evaluated by MTT assay.
HT1080 cells were plated at a density of 1.2×105
cells/well on a 96-well plate and maintained overnight for
attachment. The next day, the medium was replaced. The cells were
treated with various concentrations of resveratrol and were allowed
to grow for 24 h. Four hours before the completion of incubation,
10 μl of MTT reagent I (methylthiazole tetrazolium, 10 mg/ml) was
added to each well. After incubation, 100 μl of MTT reagent II
(solubilization buffer, 10% SDS with 0.01 N HCl, DMSO) was added to
each well, and the cells were incubated overnight at 37°C and 5%
CO2. Finally, the color that developed after the
reaction was measured at 595 nm using an ELISA plate reader.
Flow cytometric analysis
Cells were then treated with various concentrations
of resveratrol for 24 h. HT1080 cells were untreated (control) or
treated with resveratrol in the absence or presence of SB203580 or
LY294002 for 24 h. Cells were harvested and washed twice with
phosphate-buffered saline (PBS). Cells were then centrifuged at
1,000 rpm for 10 min and were resuspended in 70% cold ethanol at
4°C overnight. The fixed cells were washed twice with cold PBS,
resuspended in a PBS containing RNase A (50 g/ml; Sigma-Aldrich) at
37°C for 30 min and stained with propidium iodide (50 μg/ml;
Molecular Probes, Eugene, OR, USA) for 30 min in the dark. Cell
populations undergoing cell cycle arrest were determined using a
flow cytometer (Partec, Munich, Germany).
Gelatin zymography
Gelatin zymography was performed in order to
determine the effect of resveratrol on the activity of MMPs. HT1080
cells were seeded on 35-mm tissue culture dishes and maintained
overnight for attachment. The next day, the medium was replaced
with RPMI containing 2% serum and cells were incubated overnight.
HT1080 cells were treated with resveratrol in the absence or
presence of inhibitors (SB203580 and LY294002) in RPMI containing
2% serum. The supernatants were collected to prepare samples with a
loading buffer. Proteins were resolved by 7.5% SDS-PAGE in the
presence of 0.1% gelatin. After SDS-PAGE, the gels were washed
three times with 2.5% Triton X-100 for 90 min and incubated with a
gelatin incubation buffer (5 mM CaCl2, 0.2 M NaCl and 50
mM Tris) for 24 h. The gels were then stained with Coomassie Blue
solution for 1 h and were exposed to an LAS-3000 imager (Fuji Film
Co., Tokyo, Japan). The experimental results were generated as
numerical values through the program ImageJ.
Western blot analysis
HT1080 cells grown in 35-mm tissue culture dishes
were treated with resveratrol in the absence or presence of
inhibitors (SB203580 and LY294002), harvested and washed with cold
PBS. Proteins were isolated via cold RIPA lysis buffer (50 mM
Tris-HCl pH 7.4, 150 mM NaCl, 1% Nonidet P-40 and 0.1% SDS),
supplemented with protease inhibitors [10 μg/ml aprotinin, 10 μg/ml
leupeptin, 10 μg/ml pepstatin and 1 mM 4-(2-aminoethyl)
benzenesulfonyl fluoride] and phosphatase inhibitors (1 mM sodium
orthovanadate, 1 mM NaF) and equal amounts of total cellular
proteins were resolved by SDS-PAGE. After SDS-PAGE, the proteins
were transferred to the nitrocellulose (NC) membranes (Whatman
Schleicher and Schuell, Dachen, Germany). The NC sheet was blocked
with 5% non-fat dry milk in Tris-buffered saline. Antibodies
against MMP-9, pp38, p38, pAkt, Akt and actin were used for probing
corresponding NC blots overnight at 4°C. NC membranes were then
washed with TBST (Tris-buffered saline/Tween-20) and incubated with
horseradish peroxidase-conjugated secondary antibody
(Sigma-Aldrich) for 2 h, followed by an exposure using an LAS-3000
imager. The experimental results were generated in numerical values
using the program Image J.
Wound healing assay
HT1080 cells were seeded into 35-mm tissue culture
dishes and were allowed to attach overnight to 70–80% confluency in
RPMI containing 2% serum. Cell monolayers were wounded by 1-mm
plastic tips and washed twice with PBS in order to remove cell
debris. Cells were treated with resveratrol in the absence or
presence of inhibitors (SB203580 and LY294002). HT1080 cells
migrated into the wound surface, and the number of migrating cells
was determined under an inverted microscope.
Data analysis and statistics
The results are expressed as the means ± SD. Values
were calculated from the specified number of determinations. The
significance of difference in the experimental and control groups
was assessed by one-way ANOVA. A value of P<0.05 was considered
to indicate a statistically significant difference.
Results
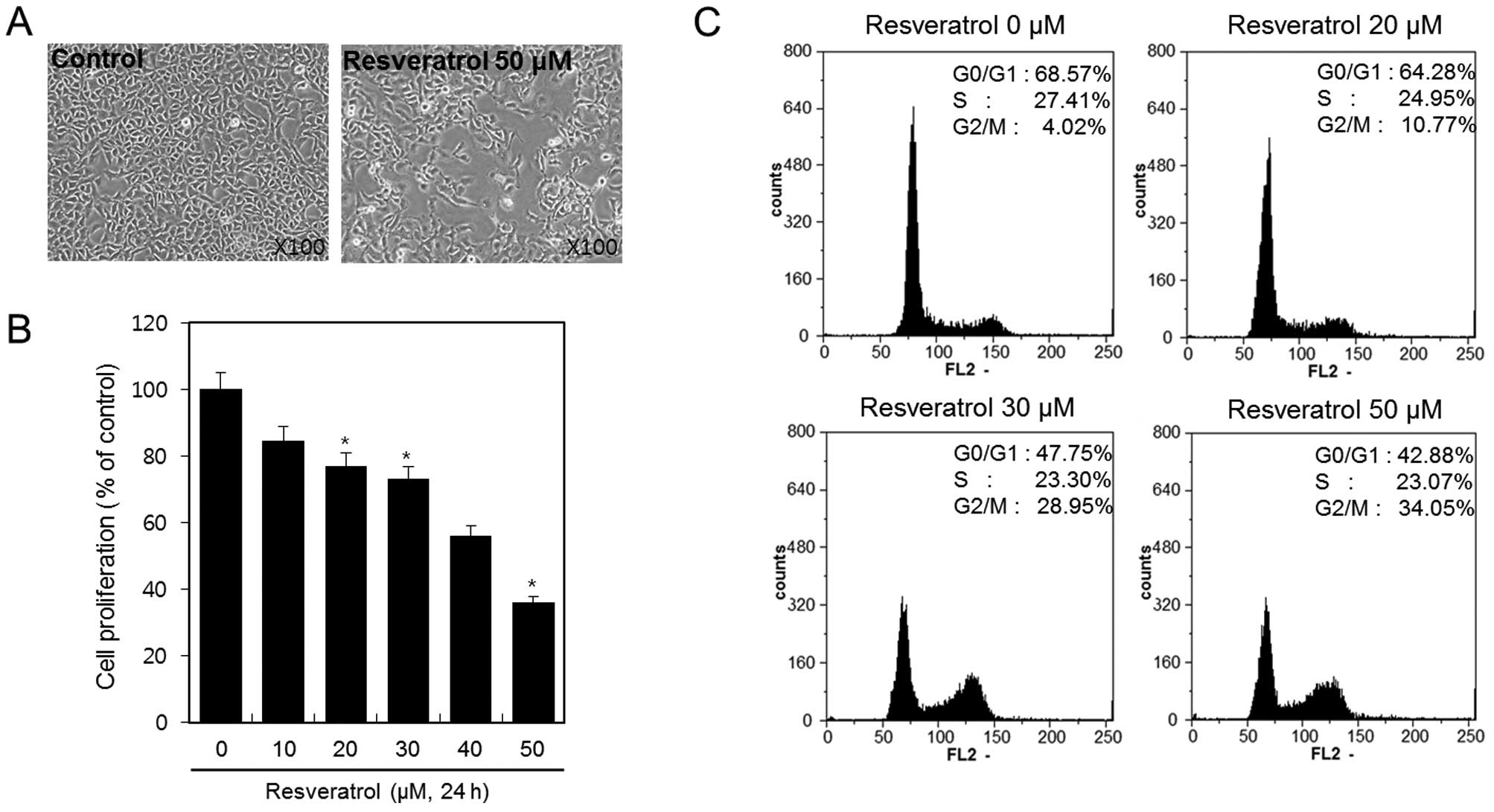
Resveratrol inhibits cell viability and
proliferation of HT1080 human fibrosarcoma cells
HT1080 cells were treated with or without 50 μM of
resveratrol for 24 h, and cellular morphology of the cells was
analyzed under a microscope. Resveratrol inhibited the growth of
HT1080 cells (Fig. 1A). To
determine the inhibitory effect of resveratrol on cell viability
and proliferation of the HT1080 cells more precisely, we treated
the cells with 10, 20, 30, 40 and 50 μM of resveratrol for 24 h and
determined the effect using MTT assay. The results demonstrated
that cell viability and proliferation decreased as the resveratrol
dose increased (Fig. 1B). With 50
μM of resveratrol, up to 50% inhibition of proliferation was
observed after a 24-h treatment. Specifically, resveratrol
inhibited cell viability and proliferation of HT1080 cells in a
dose-dependent manner. Using flow cytometric analysis, we
demonstrated the effect of resveratrol on cell cycle arrest in
HT1080 cells. HT1080 cells were treated with 20, 30 and 50 μM of
resveratrol for 24 h. We observed that resveratrol induced cell
cycle arrest at the G2 and M phases. The percentages of cells
arrested in the G2/M phase following treatment with resveratrol at
concentrations of 20, 30 and 50 μM were ~10.77, 28.95 and 34.05%,
respectively (Fig. 1C).
These results indicated that resveratrol inhibits
cell viability and proliferation in HT1080 human fibrosarcoma cells
via the induction of G2/M phase cell cycle arrest.
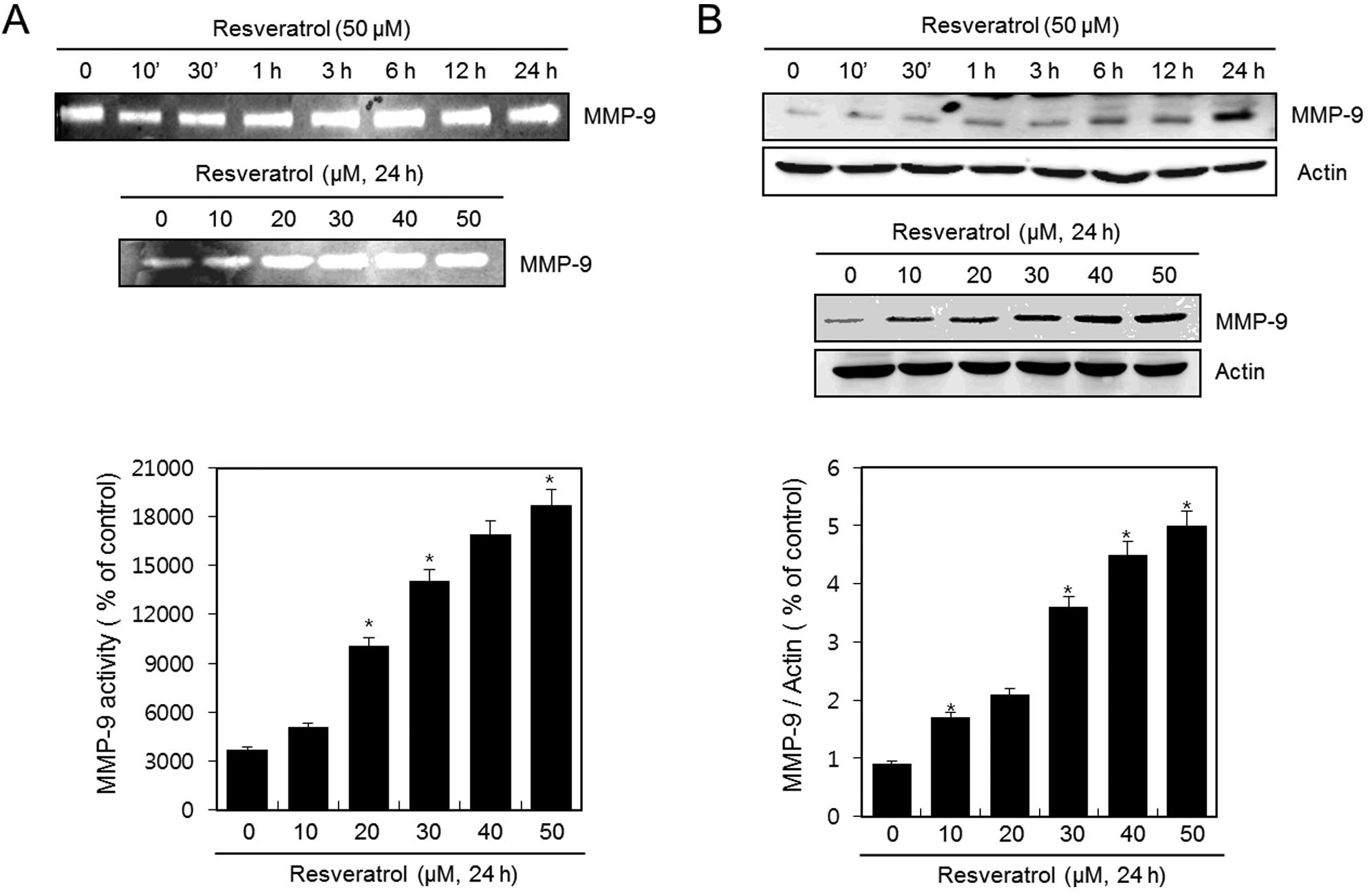
Resveratrol induces the activation and
expression of MMP-9 in HT1080 human fibrosarcoma cells
HT1080 cells were untreated (control) or treated
with 50 μM of resveratrol for the indicated time periods or with
various concentrations of resveratrol for 24 h. The activation of
MMP-9 was analyzed using gelatin zymography. Resveratrol
dramatically induced the activation of MMP-9 in a time- and
dose-dependent manner (Fig. 2A). In
addition, the expression of MMP-9 was detected using western blot
analysis. The expression of MMP-9 was increased after treatment
with resveratrol in a time-and dose-dependent manner (Fig. 2B). The experimental results were
obtained using ImageJ (Fig. 2A and
B).
These results suggest that resveratrol induces both
the activation and expression of MMP-9 in HT1080 human fibrosarcoma
cells.
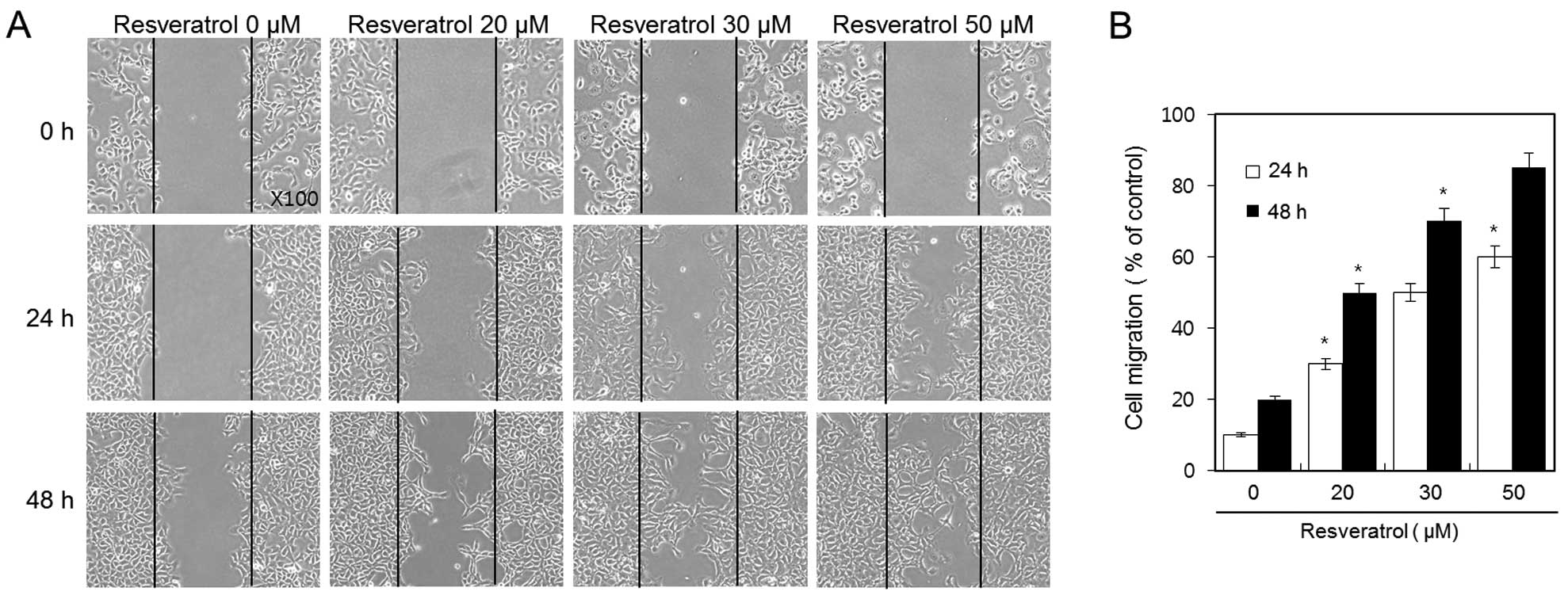
Resveratrol increases the cell migratory
ability of HT1080 human fibrosarcoma cells
We used a wound healing assay in order to evaluate
the effect of resveratrol on the cell migration of HT1080 cells.
Confluent cell monolayers were scraped and then treated with 20, 30
and 50 μM of resveratrol for 24 and 48 h. We discovered that
resveratrol dramatically increased cell migration of HT1080 cells
in a dose-and time-dependent manner (Fig. 3A). After 48 h, the wound was almost
covered due to the influx of highly migratory cells in the 50-μM
resveratrol group. In addition, a clear dose-response effect of
resveratrol was observed; the percentage of induction of the
migratory ability of 20, 30 and 50 μM of resveratrol was ~50, 70
and 85%, respectively (Fig.
3B).
These results indicate that resveratrol increases
the cell migratory ability of HT1080 fibrosarcoma cells in a dose-
and time-dependent manner.
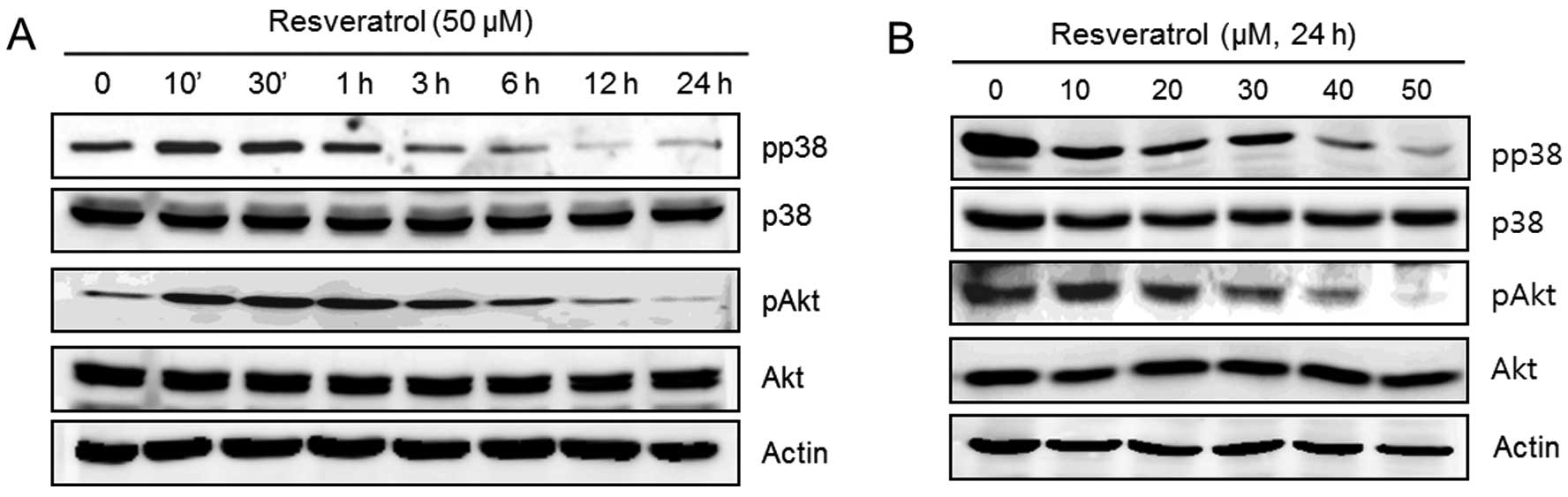
Resveratrol induces MMP-9 and cell
migratory ability via p38 and Akt kinases in human fibrosarcoma
HT1080 cells
The following experiments were performed in order to
verify that the upstream signaling pathways of MMP-9 and cell
migration were influenced by resveratrol. HT1080 cells were
untreated (control) or treated with 50 μM of resveratrol for the
indicated time periods or with the indicated concentrations of
resveratrol for 24 h. The expression of pp38, p38, pAkt, Akt and
actin was analyzed using western blot analysis. We observed that
resveratrol dramatically decreased the phosphorylation of p38 and
Akt in a time-dependent manner (Fig.
4A). In addition, the phosphorylation of p38 and Akt was
decreased after treatment with resveratrol in a dose-dependent
manner (Fig. 4B).
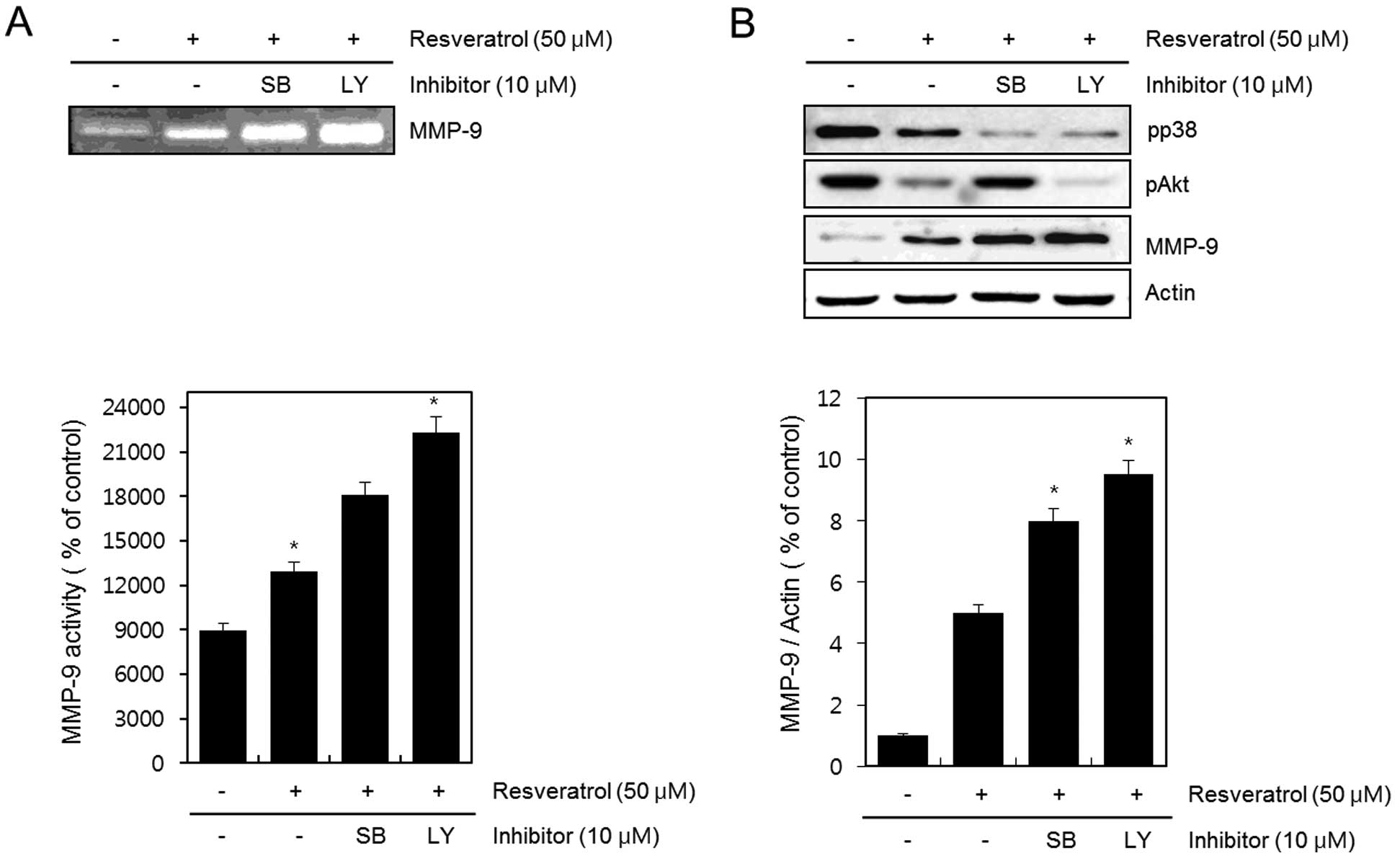
Next, cells were untreated (control) or treated with
resveratrol in the absence or presence of 10 μM SB203580 or 10 μM
LY294002 for 24 h. The activation of MMP-9 was detected using the
gelatin zymography assay (Fig. 5A).
The expression of pp38, pAkt, MMP-9 and actin was determined by
western blot analysis (Fig. 5B).
The inhibition of resveratrol-reduced p38 and Akt kinase with
SB203580 and LY294002 resulted in a more increased
resveratrol-induced activation and expression of MMP-9. The
experimental results were obtained using ImageJ (Fig. 5A and B).
The cells were untreated (control) or treated with
50 μM of resveratrol in the absence or presence of 10 μM SB203580
or 10 μM LY294002 for 24 h. We investigated whether cell
proliferation is involved with cell cycle arrest by flow cytometric
analysis. As a result, there was no difference in cell
proliferation following single treatment of resveratrol (50 μM)
when compared with the cells treated in combination with SB203580
or LY294002. Furthermore, treatment with SB203580 or LY294002
engendered an ~10–13% apoptosis (Fig.
6A). Confluent cell monolayers were scraped and then used as
control, or treated with 50 μM resveratrol in the absence or
presence of 10 μM SB203580 or 10 μM LY294002 for 24 and 48 h
(Fig. 6B). Cell migratory ability
was detected using a wound healing assay. The inhibition of p38 and
Akt kinases with SB203580 and LY294002 resulted in increased
resveratrol-induced cell migration of HT1080 cells. The percentages
of induction in cell migration following treatment with 50 μM of
resveratrol, when compared with treatment with SB203580 and
LY294002, was ~76 and 79% at 24 h, respectively and ~79 and 82% at
48 h, respectively (Fig. 6C).
These results suggest that resveratrol induces the
activation and expression of MMP-9 and cell migration via p38
kinase and PI-3K in HT1080 human fibrosarcoma cells. Moreover, the
results indicate that resveratrol increases cancer metastasis in
HT1080 human fibrosarcoma cells.
Discussion
Invasion is an important step in the growth and
metastasis of tumor cells, Metastasis of cancer cells is a complex
multistep process involving cell invasion, adhesion, invasion and
motility (25). The initial step of
tumor cell invasion is characterized by the breakdown of the
basement membrane, a process dependent on type IV
collagen-degrading enzymes (26).
Tumor metastasis and invasion are the main causes of morbidity and
mortality worldwide. MMPs, a family of zinc-dependent
endopeptidases, have the ability to degrade extracellular matrix
(ECM) components. Therefore, MMPs play a fundamental role in tumor
invasion and tissue remodeling (27). MMPs facilitate tumor progression and
metastasis (28). The secretion and
activation of MMP-2 and MMP-9 have been associated with a high
metastatic potential in several human carcinomas including breast,
colon, lung and pancreatic carcinomas (29). The induction of MMP-2 and MMP-9
involves multiple signaling cascades, particularly the MAPK pathway
(30–32). It has been reported that the
activation of the MAPK and PI-3K pathways plays a critical role in
the modulation of MMP-9 (33). The
activation of ERK1/2 and p38 has been found in other systems to be
the mechanism for promoting the production of MMPs, which are
important for cell proliferation and invasion. It has also been
reported that the PI-3K/Akt and nuclear factor-κB (NF-κB) pathways
are involved in the regulation of MMP-9 (34). It has been suggested that inhibition
of p-ERK1/2 and p-JNK leads to a reduction in tumor cells (35). Recent studies have demonstrated that
flavonoid baicalein suppresses adhesion, migration and invasion of
MDA-MB-231 human breast cancer cells. Baicalein mediates invasive
properties of MDA-MB-231 cells by suppressing the activation and
expression of MMP-2 and MMP-9, which are important factors for
breast cancer cells, including MDA-MB-231. The MAPK/MMP signaling
pathway in MDA-MB-231 cells contributes to the underlying mechanism
of an invasive potential, which suggests that the MAPK signaling
pathway is a potential therapeutic target in highly invasive breast
cancer cells (36). Dieckol from
Ecklonia cava regulates the invasion of human fibrosarcoma
cells and modulates MMP-2 and MMP-9 expression via the NF-κB
pathway. It acts as an inhibitor of MMP-2 and MMP-9 expression
levels by the downregulation of the NF-κB pathway without
significant influence on AP-1 and MAPK pathways. Furthermore, the
modulation of MMP-2 and MMP-9 expression levels may be a reason for
the suppression of the invasiveness of the HT1080 cell line
(37). Previous studies indicate
the inhibitory effects of wogonin on the invasion of human breast
carcinoma cells by downregulating the expression and activity of
MMP-9. This study illustrated that wogonin may inhibit tumor
invasion and metastasis in vitro via decreasing both the
endogenous and PMA-induced MMP-9 expression and activation.
Furthermore, it demonstrated that the downregulation of MMP-9 is
potentially associated with the inhibition of PKC translocation and
ERK1/2 protein phosphorylation (38).
Resveratrol has been demonstrated to possess
anti-cancer, anti-aging, anti-inflammatory and neuroprotective
activities. Resveratrol has been shown to inhibit migration and
invasion of human breast cancer cells (39). Resveratrol was previously reported
to protect cells from oxidative DNA damage caused by hydrogen
peroxide and peroxynitrite (40).
Recent studies have demonstrated that resveratrol inhibits human
lung adenocarcinoma cell metastasis by suppressing the heme
oxygenase 1-mediated (HO-1) NF-κB pathway and subsequently,
downregulating the expression of MMPs. This study revealed that
resveratrol inhibited the expression of MMP-2 and MMP-9 and reduced
human lung adenocarcinoma cell metastasis by suppressing HO-1.
Moreover, HO-1 inhibition or silencing induced the downregulation
of MMP-2 and MMP-9, which was due to the inhibition of the
NF-κB-dependent signaling pathway. These findings suggest that
resveratrol may act as a therapeutic agent in the inhibition of
cancer progression and provide a novel mechanistic insight into the
potential effects of resveratrol on the inhibition of tumor
invasion and metastasis (41). In a
recent study, resveratrol via anti-oxidant activity inhibited MMP
via modulation of SIRT1 in human fibrosarcoma cells. The results
suggest that the activation of SIRT1 in the presence of resveratrol
inhibits the expression of MMP-9 in human fibrosarcoma cells
through the suppression of ROS and AP-1 as well as NF-κB activation
by the enhanced activity of SIRT1 (42). Resveratrol was found to inhibit
tumor cell adhesion to endothelial cells by blocking ICAM-1
expression. Resveratrol blocks ICAM-1 expression and cell adhesion
between HT1080 and ECV304 cells, which suggests that the
downregulation of ICAM-1 by resveratrol is involved in reducing
cell adhesion (43). Moreover,
resveratrol has been demonstrated to modulate MED28 (magicin/EG-1)
expression and inhibit epidermal growth factor (EGF)-induced
migration in MDA-MB-231 human breast cancer cells. The role of
MED28 in cell migration reinforces the importance of this
multifaceted protein and raises the possibility of clinical
application in a combination therapy of resveratrol and MED28
suppression in breast cancer cells (44). Another recent study revealed the
effects of resveratrol on cyclooxygenase-1 and -2, NF-κB, MMP-9 and
sirtuin 1 mRNA expression in the hearts of streptozotocin-induced
diabetic rats (45). It reported
the anti-invasive effect of resveratrol and related methoxy
analogues on human hepatocarcinoma cells. Resveratrol may be used
to further examine their signal transduction pathways on MMP-9
suppression and TIMP-2 induction for the prevention of hepatoma
invasion or metastasis (46).
Resveratrol was found to induce gastric cancer cell apoptosis via
ROS, independently of sirtuin 1 (47). In addition, it has been reported
that resveratrol arrests the cell cycle and induces apoptosis in
human hepatocellular carcinoma cells (48).
However, the effect of resveratrol on MMP-9 and the
migratory ability in HT1080 human fibrosarcoma cells remains
unclear. Therefore, in the present study we investigated the effect
of resveratrol on MMP-9 and cell migration in HT1080 cells. In the
present study, we discovered that resveratrol at various
concentrations inhibited the HT1080 cell viability using MTT assay
and FACS analysis (Fig. 1).
However, resveratrol dramatically increased the activation and
expression of MMP-9 in a dose- and time-dependent manner as
determined by gelatin zymography assay and western blot analysis
(Fig. 2). We found that resveratrol
induced the migratory ability of HT1080 cells as determined by a
wound healing assay. Resveratrol induced the migration of HT1080
cells in a dose- and time-dependent manner (Fig. 3). Phosphorylation of p38 and Akt
kinases was inhibited by resveratrol in the HT1080 cells in a dose-
and time-dependent manner (Fig. 4).
To confirm the importance of the p38 and Akt kinases in the
regulatory pathway of resveratrol-induced MMP-9 and cell migration,
we treated cells with SB203580 and LY294002. The inhibition of p38
and Akt kinases with SB203580 and LY294002 further increased
resveratrol-induced MMP-9 activation and expression (Fig. 5). In addition, the suppression of
p38 and Akt kinases with SB203580 and LY294002 further increased
the resveratrol-induced migratory ability of HT1080 cells (Fig. 6).
In conclusion, our data indicated that resveratrol
inhibited cell proliferation. However, resveratrol also increased
the activity and expression of MMP-9 and the migratory ability in
HT1080 cells. In addition, resveratrol decreased the
phosphorylation of p38 and Akt. Inhibition of p38 and Akt kinases
further enhanced the resveratrol-induced MMP-9 activity and
expression and cell migration. Our results suggest that resveratrol
regulates MMP-9 and cell migratory ability through the p38 kinase
and PI-3K pathways in human fibrosarcoma HT1080 cells. Furthermore,
resveratrol enhances cancer metastasis in HT1080 human fibrosarcoma
cells.
Acknowledgements
This study was supported by a grant from the
National Research Foundation of Korea (NRF) and funded by the
Korean Government (MEST) (2011–0027473 and 2012-0004359).
References
|
1
|
Brisdelli F, D’Andrea G and Bozzi A:
Resveratrol: a natural polyphenol with multiple chemopreventive
properties. Curr Drug Metab. 10:530–546. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cao Y, Cao R and Brakenhielm E:
Antiangiogenic mechanisms of diet-derived polyphenols. J Nutr
Biochem. 13:380–390. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brakenhielm E, Cao R and Cao Y:
Suppression of angiogenesis, tumor growth, and wound healing by
resveratrol, a natural compound in red wine and grapes. FASEB J.
15:1798–1800. 2001.PubMed/NCBI
|
|
4
|
Aquilano K, Baldelli S, Rotilio G and
Ciriolo MR: Trans-Resveratrol inhibits
H2O2-induced adenocarcinoma gastric cell
proliferation via inactivation of MEK1/2-ERK1/2-c-jun signalling
axis. Biochem Pharmacol. 77:337–347. 2009.PubMed/NCBI
|
|
5
|
Kozuki Y, Miura Y and Yagasaki K:
Resveratrol suppresses hepatoma cell invasion independently of its
anti-proliferative action. Cancer Lett. 167:151–156. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Woo JH, Lim JH, Kim YH, et al: Resveratrol
inhibits phorbol myristate acetate-induced matrix
metalloproteinase-9 expression by inhibiting JNK and PKC delta
signal transduction. Oncogene. 23:1845–1853. 2004. View Article : Google Scholar
|
|
7
|
Sun C, Hu Y, Liu X, et al: Resveratrol
downregulates the constitutional activation of nuclear
factor-kappaB in multiple myeloma cells, leading to suppression of
proliferation and invasion, arrest of cell cycle, and induction of
apoptosis. Cancer Genet Cytogenet. 165:9–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Klinge CM, Blankenship KA, Risinger KE, et
al: Resveratrol and estradiol rapidly activate MAPK signaling
through estrogen receptors alpha and beta in endothelial cells. J
Biol Chem. 280:7460–7468. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aziz MH, Kumar R and Ahmad N: Cancer
chemoprevention by resveratrol: in vitro and in vivo
studies and the underlying mechanisms (Review). Int J Oncol.
23:17–28. 2003.PubMed/NCBI
|
|
10
|
Tang FY, Chiang EP and Sun YC: Resveratrol
inhibits heregulin-beta1-mediated matrix metalloproteinase-9
expression and cell invasion in human breast cancer cells. J Nutr
Biochem. 19:287–294. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yilmaz M, Christofori G and Lehembre F:
Distinct mechanisms of tumor invasion and metastasis. Trends Mol
Med. 13:535–541. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Woessner JF Jr: Matrix metalloproteinases
and their inhibitors in connective tissue remodeling. FASEB J.
5:2145–2154. 1991.PubMed/NCBI
|
|
13
|
Hidalgo M and Eckhardt SG: Development of
matrix metalloproteinase inhibitors in cancer therapy. J Natl
Cancer Inst. 93:178–193. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roach DM, Fitridge RA, Laws PE, Millard
SH, Varelias A and Cowled PA: Up-regulation of MMP-2 and MMP-9
leads to degradation of type IV collagen during skeletal muscle
reperfusion injury; protection by the MMP inhibitor, doxycycline.
Eur J Vasc Endovasc Surg. 23:260–269. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bachmeier BE, Iancu CM, Jochum M and
Nerlich AG: Matrix metalloproteinases in cancer: comparison of
known and novel aspects of their inhibition as a therapeutic
approach. Expert Rev Anticancer Ther. 5:149–163. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee SO, Jeong YJ, Im HG, Kim CH, Chang YC
and Lee IS: Silibinin suppresses PMA-induced MMP-9 expression by
blocking the AP-1 activation via MAPK signaling pathways in MCF-7
human breast carcinoma cells. Biochem Biophys Res Commun.
354:165–171. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bradham C and McClay DR: p38 MAPK in
development and cancer. Cell Cycle. 5:824–828. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee SJ, Kim WJ and Moon SK: Role of the
p38 MAPK signaling pathway in mediating interleukin-28A-induced
migration of UMUC-3 cells. Int J Mol Med. 30:945–952.
2012.PubMed/NCBI
|
|
20
|
Zhang B, Wang X, Cai F, Chen W, Loesch U,
Bitzer J and Zhong XY: Effects of salinomycin on human ovarian
cancer cell line OV2008 are associated with modulating p38 MAPK.
Tumour Biol. July 7–2012.(Epub ahead of print).
|
|
21
|
Foster FM, Traer CJ, Abraham SM and Fry
MJ: The phosphoinositide (PI) 3-kinase family. J Cell Sci.
116:3037–3040. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cotrim CZ, Fabris V, Doria ML, et al:
Estrogen receptor beta growth-inhibitory effects are repressed
through activation of MAPK and PI3K signalling in mammary
epithelial and breast cancer cells. Oncogene. July 2–2012.(Epub
ahead of print).
|
|
23
|
Dineva IK, Zaharieva MM, Konstantinov SM,
Eibl H and Berger MR: Erufosine suppresses breast cancer in vitro
and in vivo for its activity on PI3K, c-Raf and Akt proteins. J
Cancer Res Clin Oncol. June 30–2012.(Epub ahead of print).
|
|
24
|
Park SY, Kim YH, Kim YH and Lee SJ:
Aromatic-turmerone attenuates invasion and expression of MMP-9 and
COX-2 through inhibition of NF-κB activation in TPA-induced breast
cancer cells. J Cell Biochem. June 27–2012.(Epub ahead of
print).
|
|
25
|
Nam KS and Shon YH: Suppression of
metastasis of human breast cancer cells by chitosan
oligosaccharides. J Microbiol Biotechnol. 19:629–633.
2009.PubMed/NCBI
|
|
26
|
Lu N, Ling Y, Gao Y, et al: Endostar
suppresses invasion through downregulating the expression of matrix
metalloproteinase-2/9 in MDA-MB-435 human breast cancer cells. Exp
Biol Med (Maywood). 233:1013–1020. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sato H, Takino T, Okada Y, Cao J,
Shinagawa A, Yamamoto E and Seiki M: A matrix metalloproteinase
expressed on the surface of invasive tumour cells. Nature.
370:61–65. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Khokha R: Suppression of the tumorigenic
and metastatic abilities of murine B16-F10 melanoma cells in vivo
by the overexpression of the tissue inhibitor of the
metalloproteinases-1. J Natl Cancer Inst. 86:299–304. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rosenblum G, Meroueh SO, Kleifeld O, et
al: Structural basis for potent slow binding inhibition of human
matrix metalloproteinase-2 (MMP-2). J Biol Chem. 278:27009–27015.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim ES, Sohn YW and Moon A:
TGF-beta-induced transcriptional activation of MMP-2 is mediated by
activating transcription factor (ATF)2 in human breast epithelial
cells. Cancer Lett. 252:147–156. 2007. View Article : Google Scholar
|
|
31
|
Cohen M, Meisser A, Haenggeli L and
Bischof P: Involvement of MAPK pathway in TNF-alpha-induced MMP-9
expression in human trophoblastic cells. Mol Hum Reprod.
12:225–232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kajanne R, Miettinen P, Mehlem A, et al:
EGF-R regulates MMP function in fibroblasts through MAPK and AP-1
pathways. J Cell Physiol. 212:489–497. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Westermarck J, Holmström T, Ahonen M, et
al: Enhancement of fibroblast collagenase-1 (MMP-1) gene expression
by tumor promoter okadaic acid is mediated by stress-activated
protein kinases Jun N-terminal kinase and p38. Matrix Biol.
17:547–557. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cho HJ, Kang JH, Kwak JY, et al:
Ascofuranone suppresses PMA-mediated matrix metalloproteinase-9
gene activation through the Ras/Raf/MEK/ERK- and Ap1-dependent
mechanisms. Carcinogenesis. 28:1104–1110. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hong IK, Kim YM, Jeoung DI, Kim KC and Lee
H: Tetraspanin CD9 induces MMP-2 expression by activating p38 MAPK,
JNK and c-Jun pathways in human melanoma cells. Exp Mol Med.
37:230–239. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang L, Ling Y, Chen Y, et al: Flavonoid
baicalein suppresses adhesion, migration and invasion of MDA-MB-231
human breast cancer cells. Cancer Lett. 297:42–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang C, Li Y, Qian ZJ, Lee SH, Li YX and
Kim SK: Dieckol from Ecklonia cava regulates invasion of
human fibrosarcoma cells and modulates MMP-2 and MMP-9 expression
via NF-κB pathway. Evid Based Complement Alternat Med.
2011:1404622011.PubMed/NCBI
|
|
38
|
Chen P, Lu N, Ling Y, et al: Inhibitory
effects of wogonin on the invasion of human breast carcinoma cells
by downregulating the expression and activity of matrix
metalloproteinase-9. Toxicology. 282:122–128. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tang FY, Su YC, Chen NC, Hsieh HS and Chen
KS: Resveratrol inhibits migration and invasion of human
breast-cancer cells. Mol Nutr Food Res. 52:683–691. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Johnson MK and Loo G: Effects of
epigallocatechin gallate and quercetin on oxidative damage to
cellular DNA. Mutat Res. 459:211–218. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu PL, Tsai JR, Charles AL, et al:
Resveratrol inhibits human lung adenocarcinoma cell metastasis by
suppressing heme oxygenase 1-mediated nuclear factor-kappaB pathway
and subsequently downregulating expression of matrix
metalloproteinases. Mol Nutr Food Res. 54(Suppl 2): S196–S204.
2010. View Article : Google Scholar
|
|
42
|
Lee SJ and Kim MM: Resveratrol with
antioxidant activity inhibits matrix metalloproteinase via
modulation of SIRT1 in human fibrosarcoma cells. Life Sci.
88:465–472. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Park JS, Kim KM, Kim MH, et al:
Resveratrol inhibits tumor cell adhesion to endothelial cells by
blocking ICAM-1 expression. Anticancer Res. 29:355–362.
2009.PubMed/NCBI
|
|
44
|
Lee MF, Pan MH, Chiou YS, Cheng AC and
Huang H: Resveratrol modulates MED28 (Magicin/EG-1) expression and
inhibits epidermal growth factor (EGF)-induced migration in
MDA-MB-231 human breast cancer cells. J Agric Food Chem.
59:11853–11861. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yar AS, Menevse S and Alp E: The effects
of resveratrol on cyclooxygenase-1 and -2, nuclear factor kappa
beta, matrix metalloproteinase-9, and sirtuin 1 mRNA expression in
hearts of streptozotocin-induced diabetic rats. Genet Mol Res.
10:2962–2975. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Weng CJ, Wu CF, Huang HW, Wu CH, Ho CT and
Yen GC: Evaluation of anti-invasion effect of resveratrol and
related methoxy analogues on human hepatocarcinoma cells. J Agric
Food Chem. 58:2886–2894. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liao PC, Ng LT, Lin LT, Richardson CD,
Wang GH and Lin CC: Resveratrol arrests cell cycle and induces
apoptosis in human hepatocellular carcinoma Huh-7 cells. J Med
Food. 13:1415–1423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang Z, Li W, Meng X and Jia B:
Resveratrol induces gastric cancer cell apoptosis via reactive
oxygen species, but independent of sirtuin1. Clin Exp Pharmacol
Physiol. 39:227–232. 2012. View Article : Google Scholar : PubMed/NCBI
|