Introduction
microRNAs (miRNAs) are a class of non-coding RNA
genes whose final product is a 22-nt functional RNA molecule. They
play important roles in the regulation of target genes by binding
to complementary regions of messenger transcripts to repress
translation or regulate degradation. This ultimately leads to
repression of protein translation and downregulation of protein
expression (1,2). Deregulation of miRNAs is emerging as
an important area of study in carcinogenesis since their regulatory
capabilities can drastically influence cell physiology (3). Current data indicate that every tumor
type analyzed by miRNA profiling shows differential expression of
miRNAs when compared to normal tissues, therefore, differential
expression of miRNAs may have diagnostic significance and
prognostic value (4).
Lung cancer is the leading cause of
cancer-associated mortality and is responsible for more deaths than
the next three most common tumors combined (breast, prostate and
colon) (5). Even for patients in
the earliest stage of the disease (stage IA), the 5-year survival
rate is only 73% after surgical resection (6). Non-small cell lung cancer (NSCLC)
accounts for ~87% of lung cancer cases (7). Recently, several studies have reported
that alterations in miRNA expression are directly involved in the
tumorigenesis of lung cancer and suggest that tissue miRNA
profiling can be used for diagnosis or predicting the prognosis of
lung cancer (8–10).
Our previous miRNA microarray analysis revealed that
miRNA-150, miR-18b-5p, miR-643 and miR-3940-5p were downregulated
in recurrent tumors when compared to primary tumors in NSCLC. To
confirm the possible role of these miRNAs in NSCLC, we investigated
the expression of miRNA-150, miR-18b-5p, miR-643 and miR-3940-5p in
normal, tumor-adjacent and tumor tissues in 90 NSCLC patients. We
also compared the expression levels with clinicopathological
features and examined the expression level in embryonic lung cells.
Our aim was to investigate the association of miRNA-150,
miR-18b-5p, miR-643 and miR-3940-5p expression levels with
clinicopathologic features and increase our understanding of the
miRNA signature in NSCLC. The findings may suggest a potential
diagnostic or prognostic strategy for targeting specific
miRNAs.
Materials and methods
Approval for the present study was obtained from the
ethics committees of the participating hospitals.
Patients and samples
Fresh frozen specimens from 90 cases of NSCLC used
in this study were obtained from Shanghai Pulmonary Hospital
between December 2010 and September 2011. The patients had not
received adjuvant chemotherapy before surgery. Three samples, which
included normal (N), tumor-adjacent normal (TN) and tumor (T)
tissue, were obtained from excision specimens for each lung cancer
patient. Tumor-adjacent samples were dissected 2 cm from the tumor
and included as little contamination of the cancerous tissue as
possible. Normal tissues consisted of the dissected pulmonary
tissue at a distance of least 5 cm from the tumor. Samples were
freshly resected after lung tissue excision and immediately frozen
in liquid nitrogen for subsequent total RNA extraction. Tumors were
classified according to the TNM staging for NSCLC (International
Association for the Study of Lung Cancer, IASLC, 2009). The
histological type, tumor size and pathologic differentiation were
precisely diagnosed through routine cytological and histological
examinations supplemented by histochemical and immunohistochemical
assays. Seventeen embryonic lung tissue cDNA samples were kindly
provided by Shanghai First Maternity and Infant Health
Hospital.
Total RNA extraction and real-time PCR
quantification of miRNAs
RNA was extracted from frozen tissue samples using a
miRNeasy Mini kit (Qiagen GmbH, Hilden, Germany) following the
manufacturer’s instructions. cDNA was reverse transcribed from
total RNA samples using the Qiagen miScript Reverse Transcription
kit (Qiagen), which can polyadenylate at the 3′ terminus of miRNAs
and reverse transcribe by a oligo-dT primer with a universal tag.
All RT reactions were carried out in a 10-μl volume, starting with
1000 ng of total RNA. The reaction mixture was initially heated to
37°C for 60 min and at 95°C for 5 min. PCR products were amplified
from cDNA samples using the Qiagen miScript SYBR-Green PCR kit with
specific miRNA primers and miScript Universal Primer. Specific
primer assays for each microRNA were obtained from Qiagen (order
no. 2591821): hs-miR-150 (lot no. 102594470); hs-miR-18b-5p (lot
no.1 02594478); hs-miR-643 (lot no. 102594477); hs-miR-3940-5p (lot
no. 102594472); and Hs-RUN6-2-1 (lot no. 102594473).
miRNA PCR quantification was performed using the ABI
PRISM 7500 Real-Time PCR system. The assay plates were initially
heated to 95°C for 15 min, followed by 40 cycles of 94°C for 15 sec
and 55°C for 30 sec. qPCR for each sample was performed in
duplicate. The −ΔΔCt method was used to analyze the relative
quantitative expression levels of miRNAs with snRNA U6 as an
internal control gene. TP53/p53 was amplified from 60 cases of
paired tumor and normal samples by SYBR-Green real-time PCR.
TP53/p53 forward primer was TCAACAAGATGTTTTGCCAACTG; reverse
primer, ATG TGCTGTGACTGCTTGTAGATG. cDNA was amplified using the
following steps: an initial 3-min denaturation at 95°C, followed by
40 cycles of 94°C for 5 sec, 61°C for 34 sec and a final melting
curve step.
Immunohistochemistry of p53, EGFR, Kras
and Ki-67
Immunohistochemistry was routinely performed to
determine p53, EGFR, Kras and Ki-67 in the formalin-fixed
paraffin-embedded surgical specimens. Anti-p53 (clone DO-7; Dako,
Glostrup, Denmark), anti-EGFR (clone EGFR.25; Gene Tech, Shanghai,
China), anti-Kras (clone 4D10; Gene Tech), and anti-Ki-67 (clone
MIB-1; Dako) monoclonal antibodies were used as primary Abs. A
Ventana NexES immunohistochemistry kit (Ventana Medical Systems)
was used for staining. Specimens were considered ‘positive’ when
>5% of tumor cells within the section exhibited positive nuclear
staining for Ki-67 (11), positive
nuclear staining for Kras, positive cytoplasmic or nuclear staining
for p53 and positive membranous staining for EGFR.
Immunohistochemical staining in surgical specimens was
independently assessed by two pathologists.
Statistical analysis
Data are expressed as medians (IQR interquartile
range). Statistical differences in miRNA expression between two
groups were calculated using the non-parametric Wilcoxon
Mann-Whitney test and a test for several independent samples used
the Kruskal-Wallis H test. A paired t-test was used for paired
samples. Pearson’s correlation was used to evaluate the correlation
coefficient of the two groups. A Chi-square test was used to
compare proportional data. The statistical analysis was performed
using SPSS 17.0 software (SPSS Inc., Chicago, IL). The level of
significance was defined as a P-value of <0.05.
Results
General information
Ninety NSCLC cases were enrolled and included in the
final analysis. Forty-one cases of NSCLC were localized on the left
side and 49 on the right side, which included, respectively, 21 and
20 cases localized on the upper and lower lobe on the left side and
27, 8 and 14 cases localized on the upper, middle and lower lobe on
the right side. Additionally, this group of patients included 72
(80.0%) males and 18 (20.0%) females, with a median age of 59 years
(range, 28–77, IQR 55–65). Seventy-eight patients (86.7%) were
<70 years of age and 12 (13.3%) were ≥70 years. Fifty-one
patients were never-smokers and 39 patients were current or
ever-smokers. Coexisting diseases, including hypertension, coronary
heart disease, diabetes, obstructive pneumonia, gastric ulcer and
varicose vein, were reported in 24 patients.
Ten patients underwent pneumonectomies, 66
lobectomies, and 6 bilobectomies. Lobectomy combined with extended
resection was performed in 8 cases (4 cases of sleeve resection, 1
case of angioplasty and 3 cases of tracheoplasty). Postoperative
complications developed in 14 cases and included prolonged air
leakage (>1 week in 8 cases), pulmonary infection (1 case),
empyema (1 case), atrial fibrillation, requiring intervention (1
case), chylothorax (2 cases, requiring further operation in 1 case)
and acute pulmonary embolism (1 case).
Pathology and immunohistochemistry
There were 41 (45.6%) squamous cell carcinoma, 32
(35.6%) adenocarcinoma, 16 (17.8%) adenosquamous carcinoma and 1
(1.1%) large-cell carcinoma cases in this group. Thirty-eight were
stage PI (7 Ia and 31 Ib), 18 PII (12 IIa and 6 IIb), 29 PIII (28
IIIa and 1 IIIb) and 5 PIV. The reasons for PIIIb was metastatic
isolate nodules in different lobes. The median tumor size was 3.5
cm (IQR 2.5–5 cm, range 1.2–9.5 cm). For tumor grading, well,
moderately and poorly differentiated carcinoma was noted in 16, 49
and 9 cases, respectively (16 missing cases were adenosquamous
carcinoma, for which the differentiation degree was not able to be
distinguished). A total of 1268 lymph nodes (1045 mediastinal and
223 regional LNs) were removed at an average of 14±6.9 nodes per
patient. One hundred and eighty-five N2 nodes in 34 cases proved
cancerous among the total LNs removed. The results of the IHC
analysis were obtained from 83 cases. Following IHC analysis,
positive expression of p53, EGFR, Kras and Ki-67 was observed in
45, 37, 41 and 63 specimens, respectively. Clinical characteristics
of the 90 patients are shown in Table
I; tumor localization is shown in Table II.
 | Table IDemographic and clinical features of
the 90 NSCLC patients. |
Table I
Demographic and clinical features of
the 90 NSCLC patients.
| Characteristics | No. of patients
(%) |
|---|
| Age (years) |
| Median (IQR) | 59 (55–65) |
| ≥70 | 12 (13.3) |
| <70 | 78 (86.7) |
| Gender |
| Male | 72 (80) |
| Female | 18 (20) |
| Smoking history |
| Never-smokers | 51 (56.7) |
| Current or ever
smokers | 39 (43.3) |
| Cell type |
| Squamous cell
carcinoma | 41 (45.6) |
|
Adenocarcinoma | 32 (35.6) |
| Adenosquamous
carcinoma | 16 (17.8) |
| Others | 1 (1.1) |
|
Differentiation |
| Well | 16 (17.8) |
| Moderate | 49 (54.4) |
| Poor | 9 (10.0) |
| Missing data | 16 (17.8) |
| Tumor diameter
(cm) |
| <3 | 29 (32.2) |
| ≥3 | 61 (67.8) |
| Mediastinal lymph
nodes |
| Positive | 34 (37.8) |
| Negative | 56 (62.2) |
| Stage |
| Ia | 7 (7.8) |
| Ib | 31 (34.4) |
| IIa | 12 (13.3) |
| IIb | 6 (6.7) |
| IIIa | 28 (31.1) |
| IIIB | 1 (1.1) |
| IV | 5 (5.6) |
| IHC for p53, EGFR,
Krasa |
| p53(+) | 45 (54.2) |
| EGFR(+) | 37 (44.6) |
| Kras(+) | 41 (49.4) |
| Ki-67(+) | 63 (75.9) |
 | Table IIDistribution of tumor
localization. |
Table II
Distribution of tumor
localization.
| Localization | No. of patients
(%) |
|---|
| Right lung | 49 |
| Upper lobe | 27 (30) |
| Middle lobe | 8 (8.9) |
| Lower lobe | 14 (15.6) |
| Left lung | 41 |
| Upper lobe | 21 (23.3) |
| Lower lobe | 20 (22.2) |
miR-150 and miR-3940-5p expression was
reduced in tumor tissues and tumor-adjacent tissues compared to
normal tissues
The expression of miR-150, miR-18b-5p, miR-643,
miR-3940-5p and internal control snRNA U6 was detected by miRNA
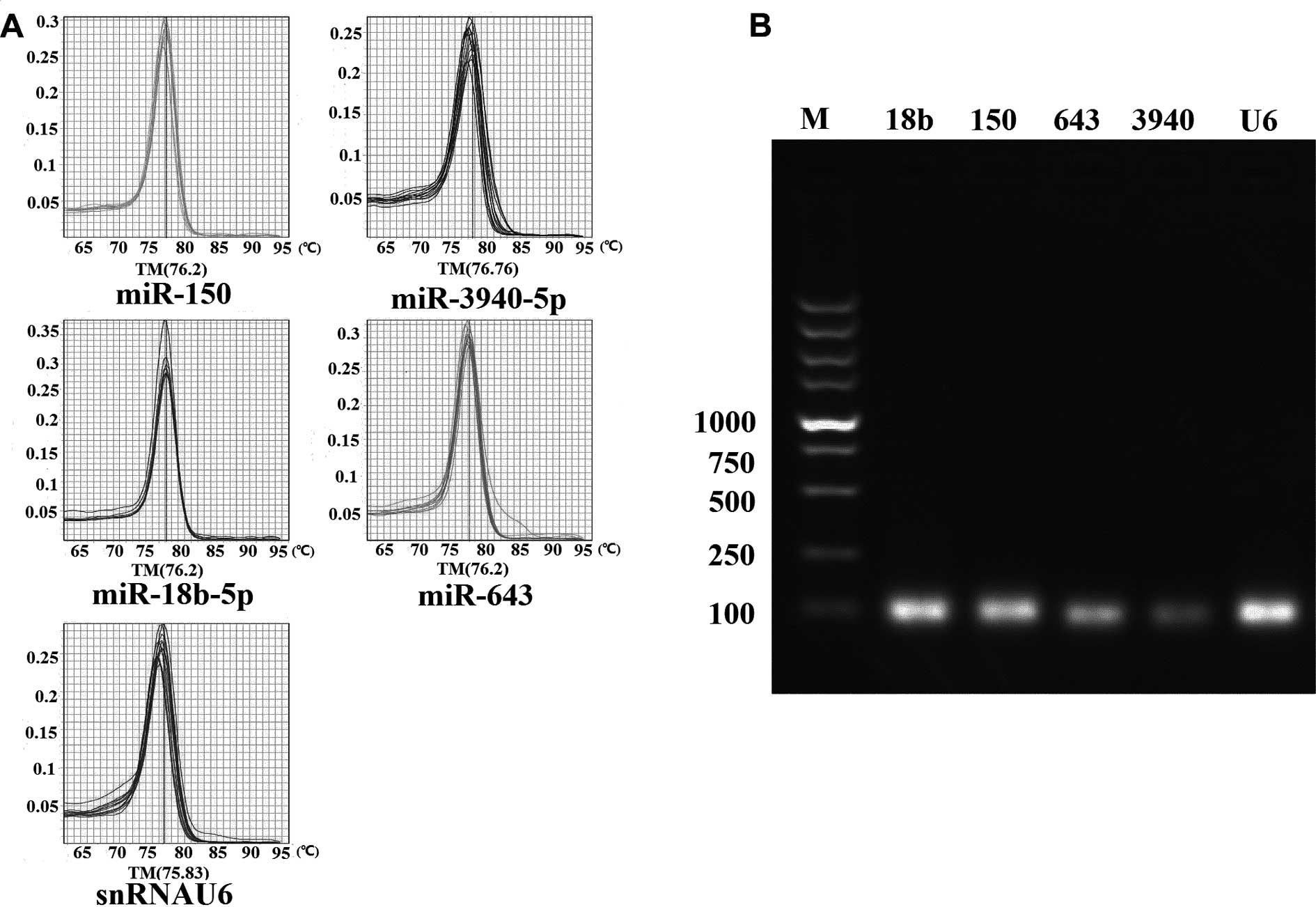
poly(A) tailing-based SYBR-Green real-time qPCR. The specificity of
each miRNA primer for amplification was confirmed by a melting
curve analysis at the end of the PCR procedure and subsequential
agarose gel electrophoresis of the PCR products (Fig. 1).
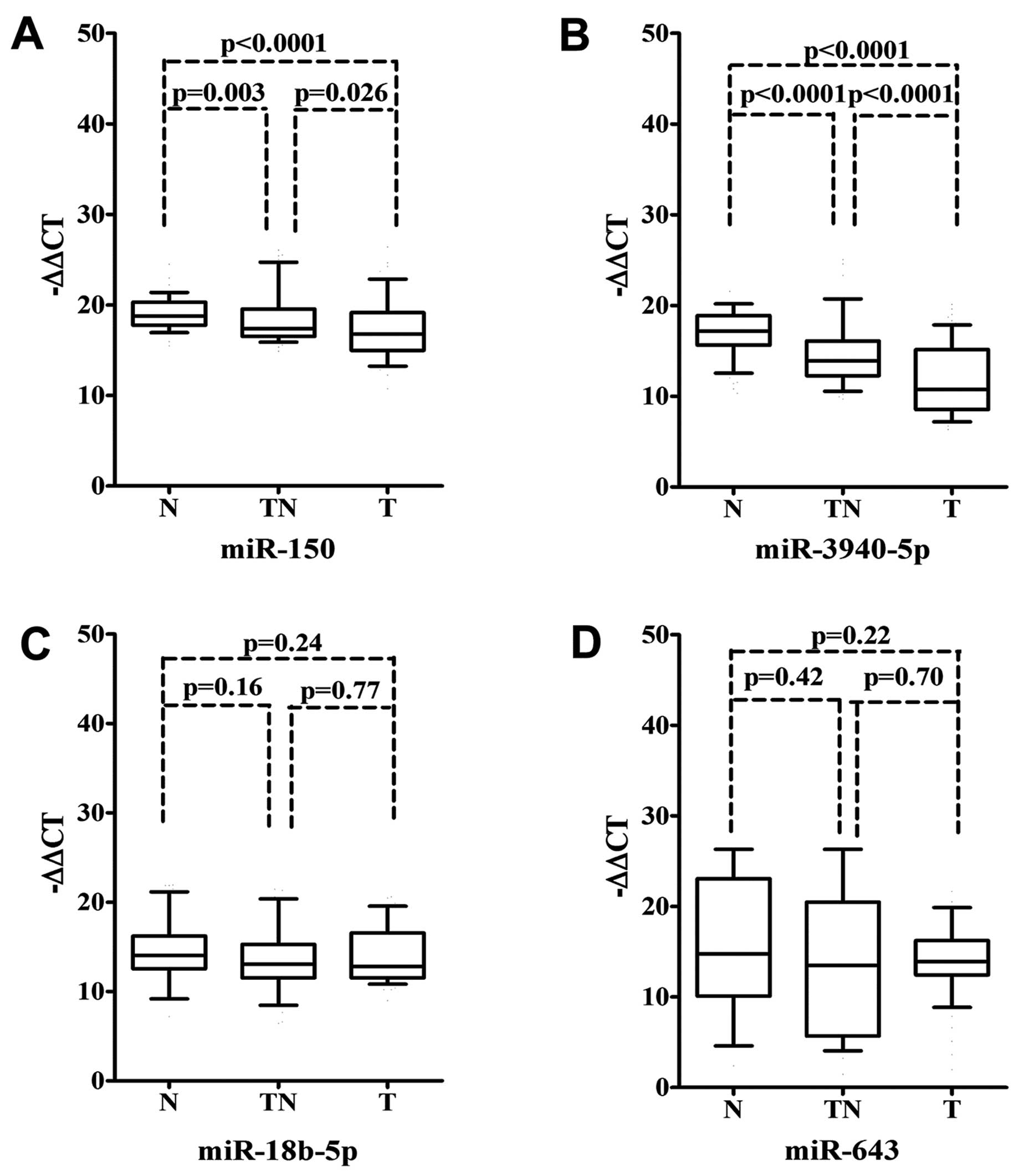
We examined the expression levels of miR-150,
miR-18b-5p, miR-643 and miR-3940-5p in the tumor, tumor-adjacent
and normal tissues of the 90 NSCLC cases. As a result, a marked
downregulation of miR-150 and miR-3940-5p expression was noted in
the tumor tissues when compared to the normal and tumor-adjacent
tissues (P<0.05). The expression of miR-150 and miR-3940-5p in
tumor-adjacent tissues was also lower than that in the the matched
normal tissues (P<0.05) (Fig. 2A and
B). As for miR-18b-5p and miR-643, no significant differences
were found among the groups (P>0.05) (Fig. 2C and D).
miR-150 and miR-3940-5p expression is
reduced in embryonic lung tissue when compared to normal adult lung
tissue
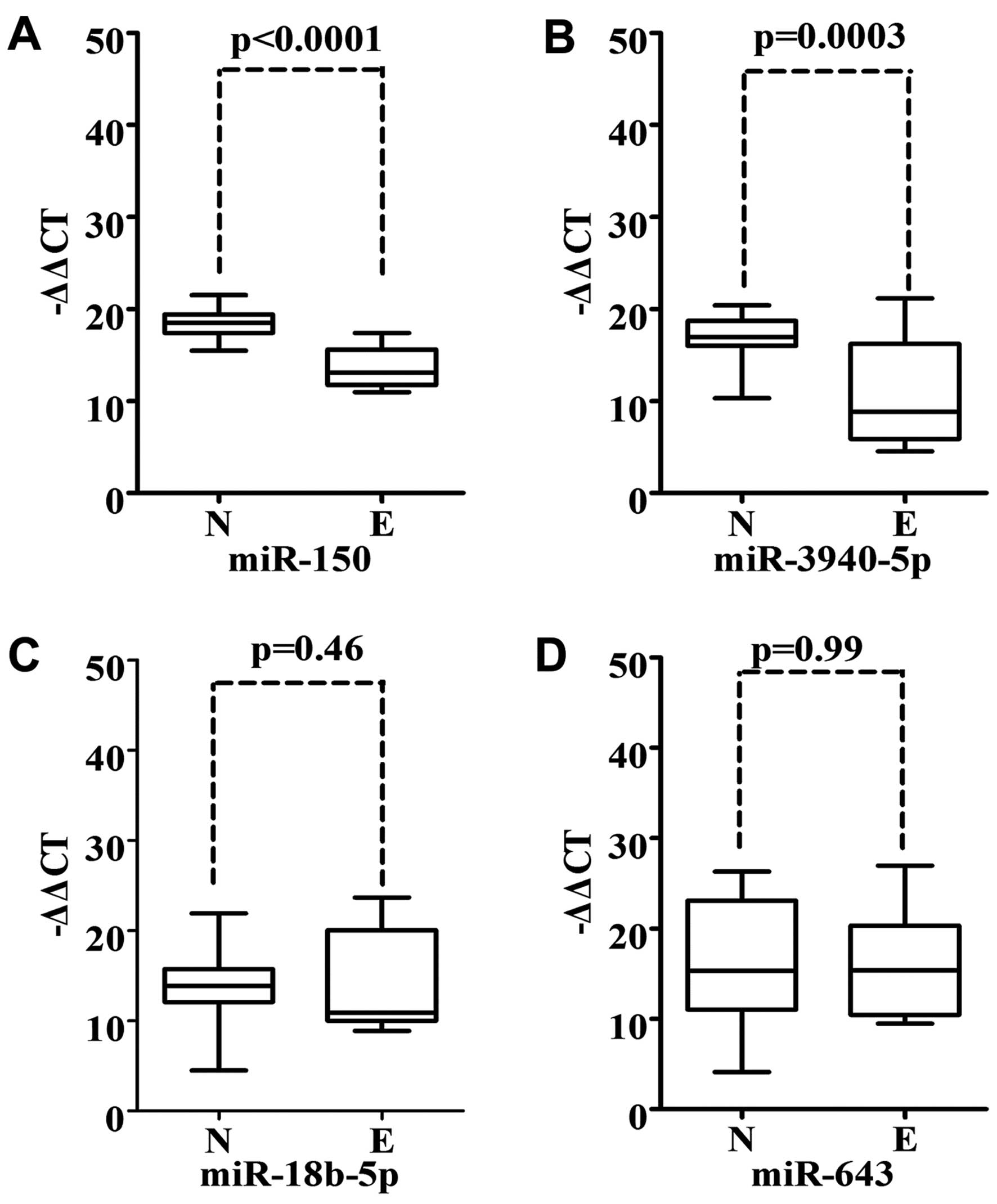
It has been reported that miRNAs are essential for
regulating cell differentiation and maintaining the pluripotent
state of stem cells (12).
Overlapping miRNA expression patterns may exist between tumor and
embryonic cells (13). Therefore,
we examined the expression of miR-150, miR-3940-5p, miR-18b-5p and
miR-643 in 17 cDNA samples of embryonic lung tissue. Notably, we
observed that the expression of miR-150 and miR-3940-5p was
significantly downregulated in embryonic lung tissue vs. normal
adult lung tissue (P<0.001) (Fig. 3A
and 3B). We did not note different expression levels of
miR-18b-5p and miR-643 in embryonic lung tissue when compared to
the normal adult lung tissue (Fig. 3C
and D).
Relationship between clinicopathological
features and miR-150 and miR-3940-5p expression levels
The expression levels of miR-150 and miR-3940-5p
were compared between different cohorts dependent on various
clinicopathological features (Table
III). There were no significant variations in miR-150 between
the subgroups regarding age, gender, tumor differentiation and
metastasis to mediastinal lymph nodes. However, a statistically
significant difference in miR-150 expression was observed in
regards to tumor size, smoking and stage. The expression level of
miR-150 was much lower in the subgroups of smokers and patients
with tumors of III–IV stage and a diameter ≥3 cm. A significant
difference in miR-3940-5p expression was not noted between
subgroups according to age, gender, tumor differentiation, smoking
status, histology, tumor stage, tumor size and metastasis to
mediastinal lymph nodes.
 | Table IIIRelationship between
clinicopathological features and miR-150 and miR-3940-5p
expression. |
Table III
Relationship between
clinicopathological features and miR-150 and miR-3940-5p
expression.
| | miR-150 | miR-3940-5p |
|---|
| |
|
|
|---|
| No. of samples | −ΔΔCt | P-value | −ΔΔCt | P-value |
|---|
| Age (years) | 90 | | | | |
| ≥70 | 12 | 17.2
(14.7–20.5) | 0.44 | 17.2
(14.7–20.5) | |
| <70 | 78 | 16.5
(15.9–19.1) | | 16.5
(15.9–19.1) | 0.38 |
| Gender | 90 | | | | |
| Male | 72 | 16.77
(14.91–19.06) | | 10.46
(8.29–14.98) | |
| Female | 18 | 16.85
(15.44–20.11) | 0.68 | 12.25
(8.28–17.07) | 0.53 |
| Smoking habit | 90 | | | | |
| Never-smokers | 51 | 17.69
(16.35–20.29) | | 9.80
(8.06–15.09) | |
| Current or ever
smokers | 39 | 16.05
(14.56–18.70) | 0.03c | 12.21
(9.26–15.24) | 0.18 |
| Histological
subtype | 89a | | | | |
| Squamous cell | 41 | 16.46
(14.85–20.17) | 0.07 | 10.75
(8.19–15.26) | 0.72 |
|
Adenocarcinoma | 32 | 18.07
(15.46–21.84) | | 12.61
(8.60–16.82) | |
| Adenosquamous | 16 | 16.11
(14.45–17.13) | | 9.75
(8.18–12.03) | |
| Stage | 90 | | | | |
| I+II | 56 | 17.41
(15.42–20.29) | 0.04c | 10.39
(8.98–15.21) | 0.34 |
| III–IV | 34 | 16.08
(14.67–18.10) | | 10.34
(7.81–14.83) | |
|
Differentiation | 74b | | | | |
| Well | 16 | 17.94
(16.53–19.43) | 0.36 | 11.52
(8.60–15.85) | 0.44 |
| Moderate | 49 | 16.68
(14.67–20.31) | | 12.41
(8.67–15.28) | |
| Poor | 9 | 15.88
(14.38–20.65) | | 8.84
(7.18–15.72) | |
| Tumor diameter
(cm) | 90 | | | | |
| <3 | 29 | 18.07
(16.30–21.07) | 0.007c | 10.17
(8.07–14.94) | 0.20 |
| ≥3 | 61 | 16.35
(14.50–18.63) | | 12.61
(8.81–15.63) | |
| MLN | 90 | | | | |
| Metastasis
(+) | 34 | 16.32
(14.78–18.24) | 0.25 | 11.04
(8.84–15.26) | 0.47 |
| No metastasis
(−) | 56 | 17.05
(14.95–20.29) | | 10.06
(8.42–15.19) | |
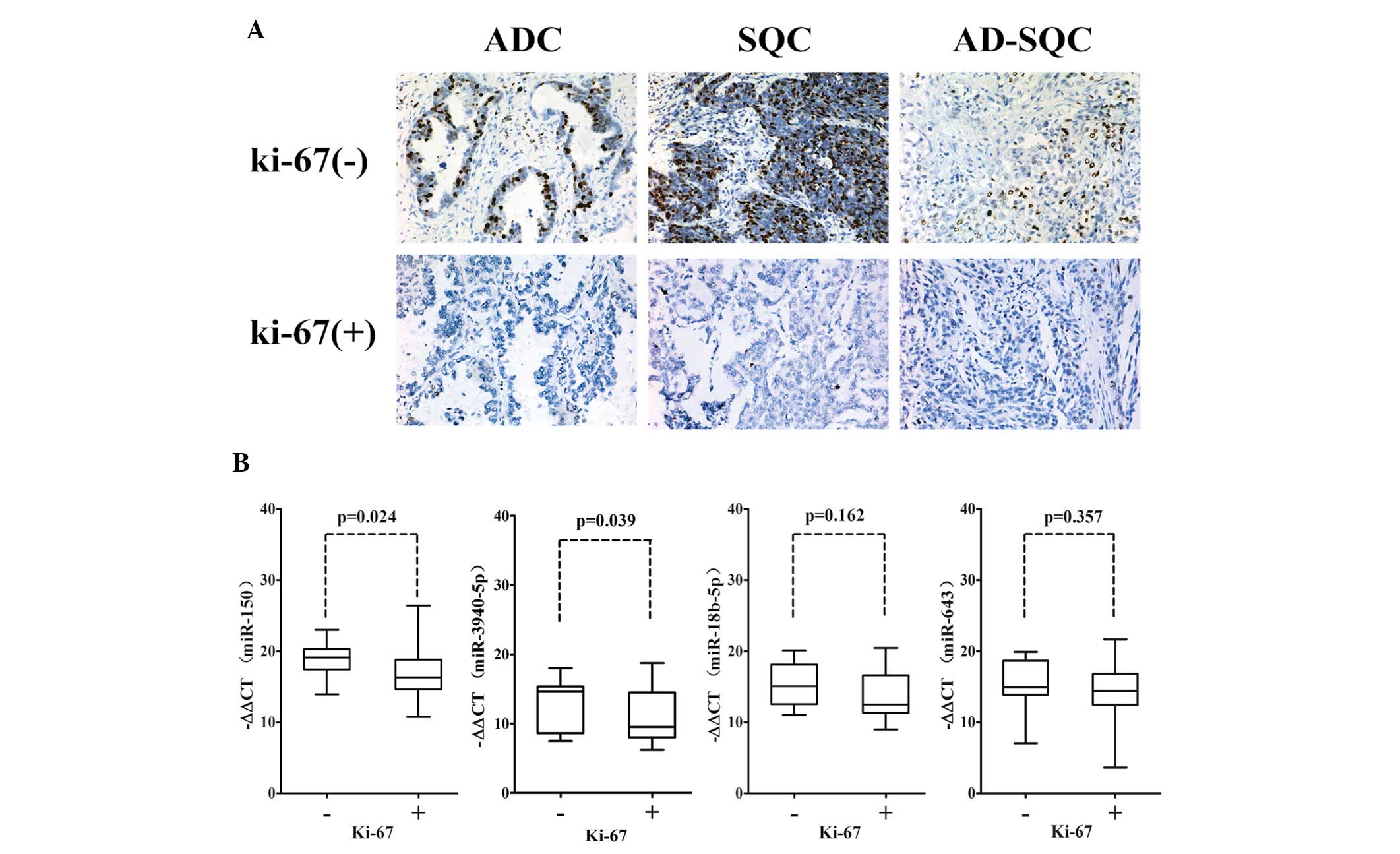
Low expression of miR-150 and miR-3940-5p
is associated with the Ki-67 proliferation index in NSCLC
The percentage of Ki-67-positive tumor cells in a
tumor is an excellent marker of tumor proliferation, and is
correlated with prognosis for survival and tumor recurrence. After
designating patients with a Ki-67 labeling index >5% as the
positive group, we found that miR-150 and miR-3940-5p expression
levels were specifically downregulated in the Ki-67-positive NSCLC
group, while miR-18b-5p and miR-643 expression levels were not
altered significantly between the Ki-67-positive and -negative
NSCLC groups (Fig. 4). This finding
implies that miR-150 and miR-3940-5p expression may be associated
with tumor growth and proliferation in NSCLC.
Correlation between expression levels of
miR-150, miR-3940-5p and tumor-related genes Kras, EGFR, p53
As shown in Table
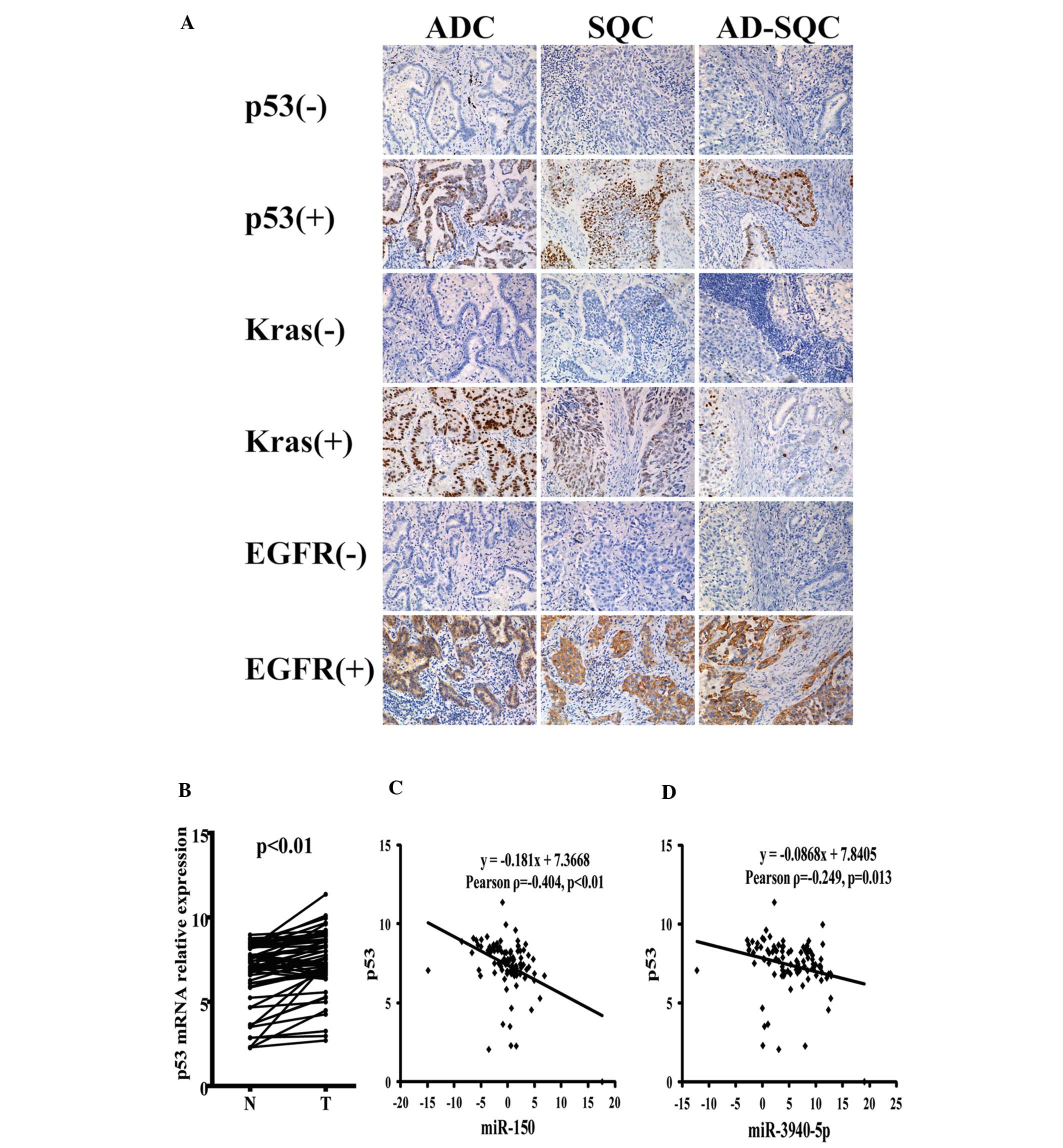
IV and Fig. 5A, we analyzed the
miR-150 and miR-3940-5p expression levels in 83 cases of NSCLC in
subgroups categorized according to positive or negative IHC
staining for tumor-related genes p53, Kras and EGFR. The levels of
miR-150 and miR-3940-5p expression were significantly downregulated
in the p53 IHC-positive NSCLC, but not in the Kras- and
EGFR-positive NSCLC cases. To confirm the possible p53 association
with miR-150 and miR-3940-5p, we also examined the p53 mRNA
expression in 60 cases of paired NSCLC samples by quantitative
real-time PCR. p53 mRNA was highly expressed in the tumor samples
of NSCLC (Fig. 5B). Additionally,
the p53 mRNA expression level was negatively correlated with
miR-150 and miR-3940-5p (Fig. 5C)
expression in the normal and tumor tissues of NSCLC.
 | Table IVmiR-150 and miR-3940-5p expression in
p53, EGFR, Kras IHC-positive and -negative NSCLC cases. |
Table IV
miR-150 and miR-3940-5p expression in
p53, EGFR, Kras IHC-positive and -negative NSCLC cases.
| | miR-150 | miR-3940-5p |
|---|
| |
|
|
|---|
| Protein
expression | No of samples | −ΔΔCt | P-value | −ΔΔCt | P-value |
|---|
| p53 | 83 | | | | |
| (+) | 45 | 16.50
(15.36–18.19) | 0.04a | 10.01
(8.19–15.01) | 0.04a |
| (−) | 38 | 18.19
(15.46–21.88) | | 13.73
(9.48–15.95) | |
| EGFR | 83 | | | | |
| (+) | 37 | 16.45
(14.58–20.29) | 0.34 | 12.21
(9.01–16.02) | 0.49 |
| (−) | 46 | 17.70
(15.50–19.13) | | 10.35
(8.71–15.10) | |
| Kras | 83 | | | | |
| (+) | 41 | 16.77
(14.88–19.11) | 0.25 | 10.04
(8.07–15.20) | 0.12 |
| (−) | 42 | 17.25
(15.89–20.27) | | 12.21
(9.40–15.58) | |
Discussion
The present study investigated the expression
pattern of miR-150 and miR-3940-5p in lung cancers. Specifically,
this investigation yielded the following findings. i) A significant
decrease in the expression levels of miR-150 and miR-3940-5p were
noted in the tumor tissues when compared to normal tissues and
tumor-adjacent tissues vs. normal tissues. ii) Downregulated
expression of miR-150 and miR-3940-5p was found in embryonic lung
tissue. iii) miR-150 expression was associated with smoking in
NSCLC patients; decreased miR-150 expression in tumor tissues was
found in a cohort of smokers. iv) miR-150 was preferentially
downregulated in a subgroup of NSCLC patients with a tumor diameter
≥3 cm. v) miR-150 was downregulated in stages III and IV compared
to stages I and II tumors. vi) miR-150 and miR-3940-5p were most
aberrantly decreased in nuclear proliferating antigen
Ki-67-positive NSCLC. vii) miR-150 and miR-3940-5p were expressed
to a lesser degree in p53 IHC-positive NSCLC. viii) Expression
levels of miR-150 and miR-3940-5p were negatively correlated with
p53 mRNA in NSCLC. There was no significant difference in the
expression level of miR-150 in regards to age, tumor histology and
differentiation (Table III).
These findings demonstrate that miR-150 and miR-3940-5p may play a
tumor-suppressing role in lung carcinogenesis.
The present study showed that nuclear positive Ki-67
of NSCLC is associated with miR-150 and miR-3940-5p
down-regulation. Ki-67 is commonly used as a marker to evaluate
proliferation of tumor cells. It is strictly associated with cell
proliferation and is present during all active phases of the cell
cycle, but is absent in resting cells (14). Findings convincingly support that
Ki-67 overexpression in resected NSCLC is associated with a poor
prognosis (15–17). Downregulation of miR-150 and
miR-3940-5p in Ki-67-positive specimens suggests that miR-150 and
miR-3940-5p may be involved in tumor cell proliferation and
contribute to poor prognosis of NSCLC.
miR-150 has been reported as a
hematopoietic-specific miRNA in malignant lymphoma and is
significantly downregulated in tumor cells relative to healthy
cells (18). In solid tumor tissue,
miR-150 expression levels were reduced when compared to paired
non-cancerous tissue in colorectal cancer, indicating that low
miR-150 expression is associated with shorter survival and a worse
response to adjuvant chemotherapy (19). Our results demonstrated that miR-150
expression is also reduced in another solid tumor - NSCLC.
Additionally, we found that miR-150 expression was strongly
associated with tumor stage and size as significantly low
expression in advanced-stage and large-size tumors was noted.
Moreover, we obtained significant findings concerning the effect of
tobacco smoking on miR-150 expression; the expression of miR-150
was much lower in the tumors of smokers than that in non-smokers.
It has been observed that smoking status may have differential
impacts on miRNA expression for mutational patterns of EGFR in
NSCLC (20), and downregulation of
let-7 associated with tobacco smoking was also observed in rats
(21). Modification of miR-150
expression in our research suggests that smoking status has a
negative effect on the potential tumor-suppressor miR-150 in NSCLC.
However, we did not conduct a hierarchical analysis on the effect
of cigarettes because of the small number of cases. Other unknown
factors may have contributed to our results; therefore, larger
samples or functional tests are necessary to explicate our
results.
miR-3940-5p is located at chr-19 p13.3, and the
5p-mature-sequence is GUGGGUUGGGGCGGGCUCUG; this is a novel miRNA
and was first reported in human embryonic stem cells (candidate-18)
(22). Next-generation sequencing
identified a large number of new miRNAs, including miR-3940-5p.
These new miRNAs are generally less evolutionarily conserved than
known miRNAs, with a large percentage unique to humans or primates;
the importance of non-conserved miRNAs has not been fully
appreciated (23). To date, there
is no report on the expression level and regulation of miR-3940-5p
in any cell line or solid tumor. The results of our study found
that miR-3940-5p was downregulated in NSCLC and embryonic lung
tissue and may have the potential function as a tumor suppressor in
the carcinogenesis of lung cancer.
Our findings also demonstrated that miR-150 and
miR-3940-5p expression was much lower in p53 IHC-positive NSCLC.
p53 protein is expressed at low levels in normal cells and at high
levels in a variety of transformed cell lines, where it is believed
to contribute to transformation and malignancy (24). Nuclear p53 protein accumulation, in
most cases, is due to mutations within the gene and a strong
association was noted between TP53 mutations and nuclear p53
protein accumulation (25).
Mutations in this gene and accumulation of the abnormal p53 protein
are frequently associated with malignancy and tumor progression;
accumulation of the mutant p53 protein has been associated with a
poor prognosis in patients with breast, gastric and colorectal
carcinomas (26). It is possible
that miR-150 and miR-3940-5p directly or indirectly regulate the
p53 expression or degradation. Regulation of p53 activity, by
different miRNAs, has been verified in several pathways. For
example, miR-34a acts as a negative regulator of p53 through
deacetylating p53 (27); miR-29
family members upregulate p53 protein levels and induce
p53-mediated apoptosis through direct downregulation of the
regulatory subunit of PI3K-p85 α (28,29).
miR-122 was found to modulate p53 through the inhibition of cyclin
G1 (30). Prediction of miR-150
target gene by TargetScan 6.0 demonstrated that a conserved 8mer
binding site of miR-150 exists at the 3′UTR of TP53/p53. Thus, as a
potential tumor suppressor, hsa-miR-150 may be involved in the
alteration of p53 gene expression. Few studies have been conducted
on the expression and function of hsa-miR-3940-5p in carcinoma. Our
results suggest that hsa-miR-3940-5p may also be involved in the
regulation of p53 expression, directly or indirectly, but further
elucidation of the underlying mechanism of decreased miR-3940-5p
expression in p53-positive cases is needed.
We also examined the downregulation of miR-150 and
miR-3940-5p in embryonic lung tissue compared to a normal adult
sample. miRNAs play critical roles in the maintenance of stemness
in ES cells as well as in the differentiation to multiple cell
lineages (12). In previous
reports, oncogenic miRNAs were upregulated in hES cells, which
suggests a possible function in the blockade of cell
differentiation (31,32). Overexpression of let-7b led to
reduced neural stem cell proliferation and increased neural
differentiation (33), and
inhibition of the let-7 family promotes de-differentiation of
somatic cells to induce pluripotent stem (iPS) cells (34). It has been reported that the
downregulation of let-7 is essential for self-renewal and
maintenance of the undifferentiated state of cancer stem cells
(35). Downregulation of miR-150
and miR-3940-5p in embryonic lung tissue implies that they can
interfere with the differentiation of stem or carcinoma stem
cells.
We found that miR-150 and miR-3940-5p were
significantly differentially expressed between tumor,
tumor-adjacent and normal tissues, and miR-150 was associated with
tumor stage and size. This is evidenced by the significantly low
expression in advanced-stage and large-size tumors. Notably,
downregulated miR-150 was significantly associated with smoking
status in NSCLC, which implies that miR-150 and miR-3940-5p are
involved in the pathogenesis of NSCLCS. miR-150 and miR-3940-5p
were also significantly downregulated in embryonic lung tissues
compared to a normal sample, which suggests they may be involved in
the differentiation of stem cells of normal or cancer cells. We
also found that miR-150 and miR-3940-5p were specifically
downregulated in nuclear cell proliferation antigen Ki-67-positive
NSCLC, which suggests that miR-150 and miR-3940-5p may be involved
in tumor cell proliferation and may contribute to poor prognosis.
miR-150 and miR-3940-5p were significantly downregulated in p53
IHC-positive cases, but not in Kras- and EGFR-positive cases. We
confirmed that miR-150 and miR-3940-5p expression was negatively
correlated with p53 mRNA by real-time PCR in NSCLC. These findings
suggest that miR-150 and miR-3940-5p interfere in NSCLC
tumorigenesis and progression, and in the direct or indirect
pathways of miR-150 and miR-3940-5p to affect p53 expression. These
findings warrant further research.
Acknowledgements
This study was supported by the Science and
Technology Commission of Shanghai Municipality (10411956100),
(12ZR1426100) and Wu Jieping Funding (320.6720.1003). We are
extremely grateful to Mr. Liang Tang and Miss Xiaojun Yang for
their experimental expertise and Dr Gang Chen for providing the
resection specimens.
References
|
1
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eder M and Scherr M: MicroRNA and lung
cancer. N Engl J Med. 352:2446–2448. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scott GK, Goga A, Bhaumik D, Berger CE,
Sullivan CS and Benz CC: Coordinate suppression of ERBB2 and ERBB3
by enforced expression of micro-RNA miR-125a or miR-125b. J Biol
Chem. 282:1479–1486. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar
|
|
6
|
Goldstraw P, Crowley J, Chansky K, et al:
The IASLC Lung Cancer Staging Project: proposals for the revision
of the TNM stage groupings in the forthcoming (seventh) edition of
the TNM Classification of Malignant Tumours. J Thorac Oncol.
2:706–714. 2007. View Article : Google Scholar
|
|
7
|
Govindan R, Page N, Morgensztern D, et al:
Changing epidemiology of small-cell lung cancer in the United
States over the last 30 years: analysis of the surveillance,
epidemiologic, and end results database. J Clin Oncol.
24:4539–4544. 2006.PubMed/NCBI
|
|
8
|
Takamizawa J, Konishi H, Yanagisawa K, et
al: Reduced expression of the let-7 microRNAs in human lung cancers
in association with shortened postoperative survival. Cancer Res.
64:3753–3756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu SL, Chen HY, Chang GC, et al: MicroRNA
signature predicts survival and relapse in lung cancer. Cancer
Cell. 13:48–57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raponi M, Dossey L, Jatkoe T, et al:
MicroRNA classifiers for predicting prognosis of squamous cell lung
cancer. Cancer Res. 69:5776–5783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Soomro IN, Holmes J and Whimster WF:
Predicting prognosis in lung cancer: use of proliferation marker,
Ki67 monoclonal antibody. J Pak Med Assoc. 48:66–69.
1998.PubMed/NCBI
|
|
12
|
Tiscornia G and Izpisua Belmonte JC:
MicroRNAs in embryonic stem cell function and fate. Genes Dev.
24:2732–2741. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Monzo M, Navarro A, Bandres E, et al:
Overlapping expression of microRNAs in human embryonic colon and
colorectal cancer. Cell Res. 18:823–833. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scholzen T and Gerdes J: The Ki-67
protein: from the known and the unknown. J Cell Physiol.
182:311–322. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takahashi S, Kamata Y, Tamo W, et al:
Relationship between postoperative recurrence and expression of
cyclin E, p27, and Ki-67 in non-small cell lung cancer without
lymph node metastases. Int J Clin Oncol. 7:349–355. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martin B, Paesmans M, Mascaux C, et al:
Ki-67 expression and patients survival in lung cancer: systematic
review of the literature with meta-analysis. Br J Cancer.
91:2018–2025. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Woo T, Okudela K, Yazawa T, et al:
Prognostic value of KRAS mutations and Ki-67 expression in stage I
lung adenocarcinomas. Lung Cancer. 65:355–362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Watanabe A, Tagawa H, Yamashita J, et al:
The role of microRNA-150 as a tumor suppressor in malignant
lymphoma. Leukemia. 25:1324–1334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma Y, Zhang P, Wang F, et al: miR-150 as a
potential biomarker associated with prognosis and therapeutic
outcome in colorectal cancer. Gut. 61:1447–1453. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Toyooka S, Matsuo K, Shigematsu H, et al:
The impact of sex and smoking status on the mutational spectrum of
epidermal growth factor receptor gene in non-small cell lung
cancer. Clin Cancer Res. 13:5763–5768. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Izzotti A, Calin GA, Arrigo P, Steele VE,
Croce CM and De Flora S: Downregulation of microRNA expression in
the lungs of rats exposed to cigarette smoke. FASEB J. 23:806–812.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liao JY, Ma LM, Guo YH, et al: Deep
sequencing of human nuclear and cytoplasmic small RNAs reveals an
unexpectedly complex subcellular distribution of miRNAs and tRNA 3′
trailers. PLoS One. 5:e105632010.PubMed/NCBI
|
|
23
|
Persson H, Kvist A, Rego N, et al:
Identification of new microRNAs in paired normal and tumor breast
tissue suggests a dual role for the ERBB2/Her2 gene. Cancer Res.
71:78–86. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Starzynska T, Bromley M, Ghosh A and Stern
PL: Prognostic significance of p53 overexpression in gastric and
colorectal carcinoma. Br J Cancer. 66:558–562. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Andersen TI, Holm R, Nesland JM, Heimdal
KR, Ottestad L and Borresen AL: Prognostic significance of TP53
alterations in breast carcinoma. Br J Cancer. 68:540–548. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chava S, Mohan V, Shetty PJ, et al:
Immunohistochemical evaluation of p53, FHIT, and IGF2 gene
expression in esophageal cancer. Dis Esophagus. 25:81–87. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamakuchi M, Ferlito M and Lowenstein CJ:
miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci
USA. 105:13421–13426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park SY, Lee JH, Ha M, Nam JW and Kim VN:
miR-29 miRNAs activate p53 by targeting p85 alpha and CDC42. Nat
Struct Mol Biol. 16:23–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou BP, Liao Y, Xia W, Zou Y, Spohn B and
Hung MC: HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2
phosphorylation. Nat Cell Biol. 3:973–982. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fornari F, Gramantieri L, Giovannini C, et
al: MiR-122/cyclin G1 interaction modulates p53 activity and
affects doxorubicin sensitivity of human hepatocarcinoma cells.
Cancer Res. 69:5761–5767. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Laurent LC, Chen J, Ulitsky I, et al:
Comprehensive microRNA profiling reveals a unique human embryonic
stem cell signature dominated by a single seed sequence. Stem
Cells. 26:1506–1516. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ren J, Jin P, Wang E, Marincola FM and
Stroncek DF: MicroRNA and gene expression patterns in the
differentiation of human embryonic stem cells. J Transl Med.
7:202009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao C, Sun G, Li S, et al: MicroRNA
let-7b regulates neural stem cell proliferation and differentiation
by targeting nuclear receptor TLX signaling. Proc Natl Acad Sci
USA. 107:1876–1881. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Melton C, Judson RL and Blelloch R:
Opposing microRNA families regulate self-renewal in mouse embryonic
stem cells. Nature. 463:621–626. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu F, Yao H, Zhu P, et al: let-7 regulates
self renewal and tumorigenicity of breast cancer cells. Cell.
131:1109–1123. 2007. View Article : Google Scholar : PubMed/NCBI
|