Introduction
Of the skin cancers, melanoma is the leading cause
of death and the mortality rate is increasing (1–3). Thus,
for all ages, melanoma is the primary focus of early detection
campaigns. Sun UV has been recognized to cause skin cancer
(4–6). UV can cause DNA damage of skin cells
(4–6). DNA damage is involved in
neurodegeneration in age-related disease, cerebral ischemia, and
brain trauma including DNA damage (7–9). It
was reported that in anticancer therapy, irradiation and
DNA-damaging chemotherapeutic drugs play an important key role
based on their ability to induce DNA double-strand breaks leading
to cancer cell death (10–12). Thus, if agents can block DNA repair
proteins it may lead to increase in the sensitivity of DNA damaging
chemotherapeutic agents (13–16).
Triptolide (diterpenoid triepoxide; PG490) extracted
from Tripterygium wilfordii Hook F (TWHF) has been shown to
present anti-fertility function (17), anti-neoplastic activity such as
anti-leukemia (18–25), anti-human hepatocellular carcinoma
cells (25), colon cancer cells
(23,26,27)
and cervical cancer cells (28).
Furthermore, evidence has been shown that triptolide inhibited the
growth and metastasis of various solid tumors and has been
suggested capable of acting synergistically with conventional
chemotherapeutic drugs (29,30).
Substantial evidence has been demonstrated that
triptolide induced cytotoxic effects in many human cancer cell
lines but no available information exists to show
triptolide-induced DNA damage in human skin cancer cells.
Therefore, we investigated the effects of triptolide on DNA damage
associated DNA repair genes expression (mRNA) in A375.S2 human
malignant melanoma cells in vitro. Our findings demonstrated
that triptolide induced DNA damage and also inhibited the
expression of DNA repair genes in A375.S2 cells.
Materials and methods
Chemicals and reagents
Triptolide, dimethyl sulfoxide (DMSO), ethidium
bromide, propidium iodide (PI), Tris-HCl and Triton X-100 were
purchased from Sigma-Aldrich. RPMI-1640 medium, fetal bovine serum
(FBS), L-glutamine, penicillin-streptomycin and trypsin-EDTA were
purchased from Gibco®/Invitrogen (Grand Island, NY,
USA).
Cell culture and chemical treatment
The human malignant melanoma cell line (A375.S2) was
purchased from the Food Industry Research and Development Institute
(Hsinchu, Taiwan). Cells were cultured with minimum essential
medium (MEM) supplemented with 10% fetal bovine serum, 100 U/ml of
penicillin, 100 μg/ml of streptomycin, and 2 mmol/l of L-glutamine
in 75 cm2 tissue culture flasks and grown in a
humidified 5% CO2 and 95% air at 37°C (31,32).
Flow cytometric assay for percentage of
viable cells
Equal numbers of cells (2×105 cells/well)
were seeded in 12-well plates and allowed to attach overnight. The
cells were treated with 0.1% DMSO or triptolide (0, 15, 20, 25 and
30 nM) diluted in MEM with 5% FBS for 24 h. Cells from each
treatment were stained with PI (5 μg/ml) and were analyzed for
percentage of viable cells by using flow cytometry
(Becton-Dickinson, San Jose, CA, USA) and cell viability was
calculated as previously described (33,34).
Comet assay and DAPI staining for DNA
damage
A375.S2 cells at the density of 2×105
cells/well in 12-well plates were incubated with triptolide at
final concentrations of 0, 15, 20, 25 and 30 nM, vehicle (1 μl
DMSO) and 0.1% of H2O2 (positive control) for
24 h or the cells were treated with 20 nM triptolide for 0, 6, 12,
24 and 48 h in MEM medium grown at 37°C in 5% CO2 and
95% air. Cells were harvested for the measurement of DNA damage
using the Comet assay as described previously (33,35,36).
Comet tail length was calculated and quantified by using the TriTek
CometScore™ software image analysis system (TriTek Corp.,
Sumerduck, VA, USA) as described previously (33,35,36).
Harvested cells were stained by DAPI then examined and photographed
by using fluorescence microscopy as described elsewhere (33,35,37).
DNA gel electrophoresis for DNA
damage
A375.S2 cells at the density of 2×105
cells/well in 12-well plates were incubated with triptolide at
final concentrations of 0, 15, 20, 25 and 30 nM for 48 h in MEM
medium grown in 5% CO2 and 95% air at 37°C. Cells in
each well were individually isolated by using DNA isolation kit.
The isolated DNA (2 μg) from each treatment was examined for DNA
damage by using DNA electrophoresis which was carried out in 0.5%
agarose gel in Tris/acetate buffer at 15 V for 2 h. At the end of
electrophoresis the DNA was stained with ethidium bromide then
examined and photographed under a fluorescence microscope as
previously described (38–40).
Real-time PCR assay for examining the
expression of DNA repair genes
A375.S2 cells at the density of 1×106
cells/well in 6-well plates were incubated with or without 20 nM of
triptolide for 24 h in MEM medium grown at 37°C in 5%
CO2 and 95% air. The cells from each treatment were
collected and total RNA was individually extracted by using the
Qiagen RNeasy mini kit (Qiagen, Inc, Valencia, CA, USA) as
previously described (41–43). Isolated RNA samples were
individually reverse-transcribed for 30 min at 42°C with High
Capacity cDNA Reverse Transcription kit according to the standard
protocol of the supplier (Applied Biosystems, Carlsbad, CA, USA).
Quantitative PCR from each sample was conducted as follows: 2 min
at 50°C, 10 min at 95°C, and 40 cycles of 15 sec at 95°C, 1 min at
60°C using 1 μl of the cDNA reverse-transcribed as described above,
2X SYBR Green PCR Master Mix (Applied Biosystems) and 200 nM of
forward and reverse primers as shown in Table I, and previously described (41,43,44).
Each assay was run on an Applied Biosystems 7300 Real-time PCR
system in triplicate. The expression fold-changes were performed by
using the comparative CT method.
 | Table IThe DNA sequence was evaluated using
the Primer Express software and each assay was run on an Applied
Biosystems 7300 Real-time PCR system. |
Table I
The DNA sequence was evaluated using
the Primer Express software and each assay was run on an Applied
Biosystems 7300 Real-time PCR system.
| Primer name | Sequences |
|---|
| Human BRCA1 | F:
CCAGGGAGTTGGTCTGAGTGA
R: ACTTCCGTAAGGCATCGTAACAC |
| Human DNA-PK | F:
CCAGCTCTCACGCTCTGATATG
R: CAAACGCATGCCCAAAGTC |
| Human MGMT | F:
CCTGGCTGAATGCCTATTTCC
R: TGTCTGGTGAACGACTCTTGCT |
| Human p53 | F:
GGGTTAGTTTACAATCAGCCACATT
R: GGGCCTTGAAGTTAGAGAAAATTCA |
| Human ATM | F:
TTTACCTAACTGTGAGCTGTCTCCAT
R: ACTTCCGTAAGGCATCGTAACAC |
| Human ATR | F:
GGGAATCACGACTCGCTGAA
R: CTAGTAGCATAGCTCGACCATGGA |
| Human GAPDH | F:
ACACCCACTCCTCCACCTTT
R: TAGCCAAATTCGTTGTCATACC |
Statistical analysis
All studies were performed in duplicate. Results are
presented as mean ± standard deviation. One-tailed Student’s t-test
was used to analyze the difference between control and triptolide
treated groups. Significance was defined as p<0.05.
Results
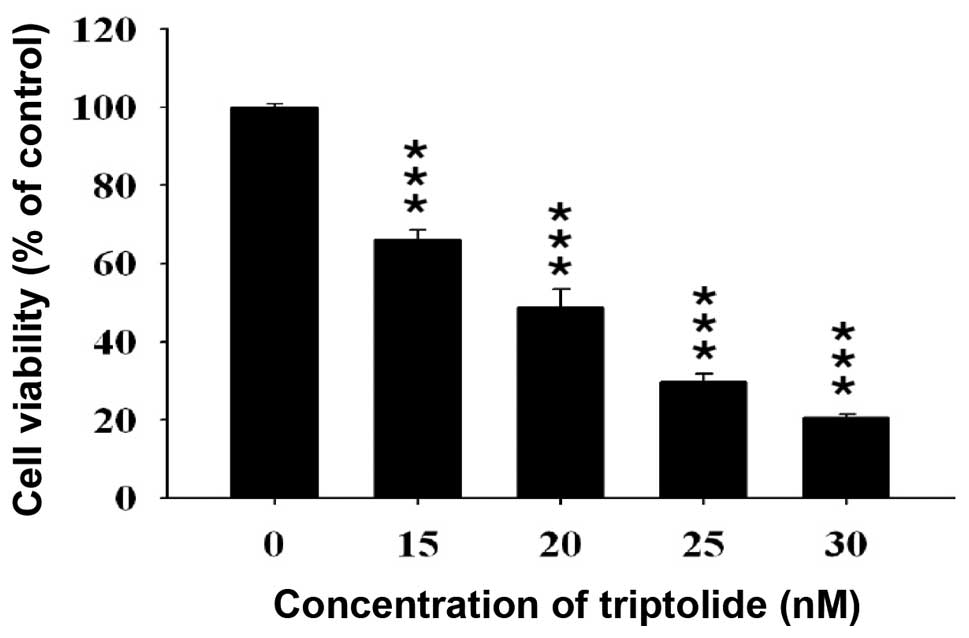
Effect of triptolide on the percentage of
viable A375.S2 cells
A375.S2 cells were incubated with 15, 20, 25 and 30
nM of triptolide for 24 h. At the end of incubation, all samples
were collected for determining the percentage of viable cells and
the results are presented in Fig.
1, which indicated that triptolide decreased the percentage of
viable cells at the concentration of 15–30 nM.
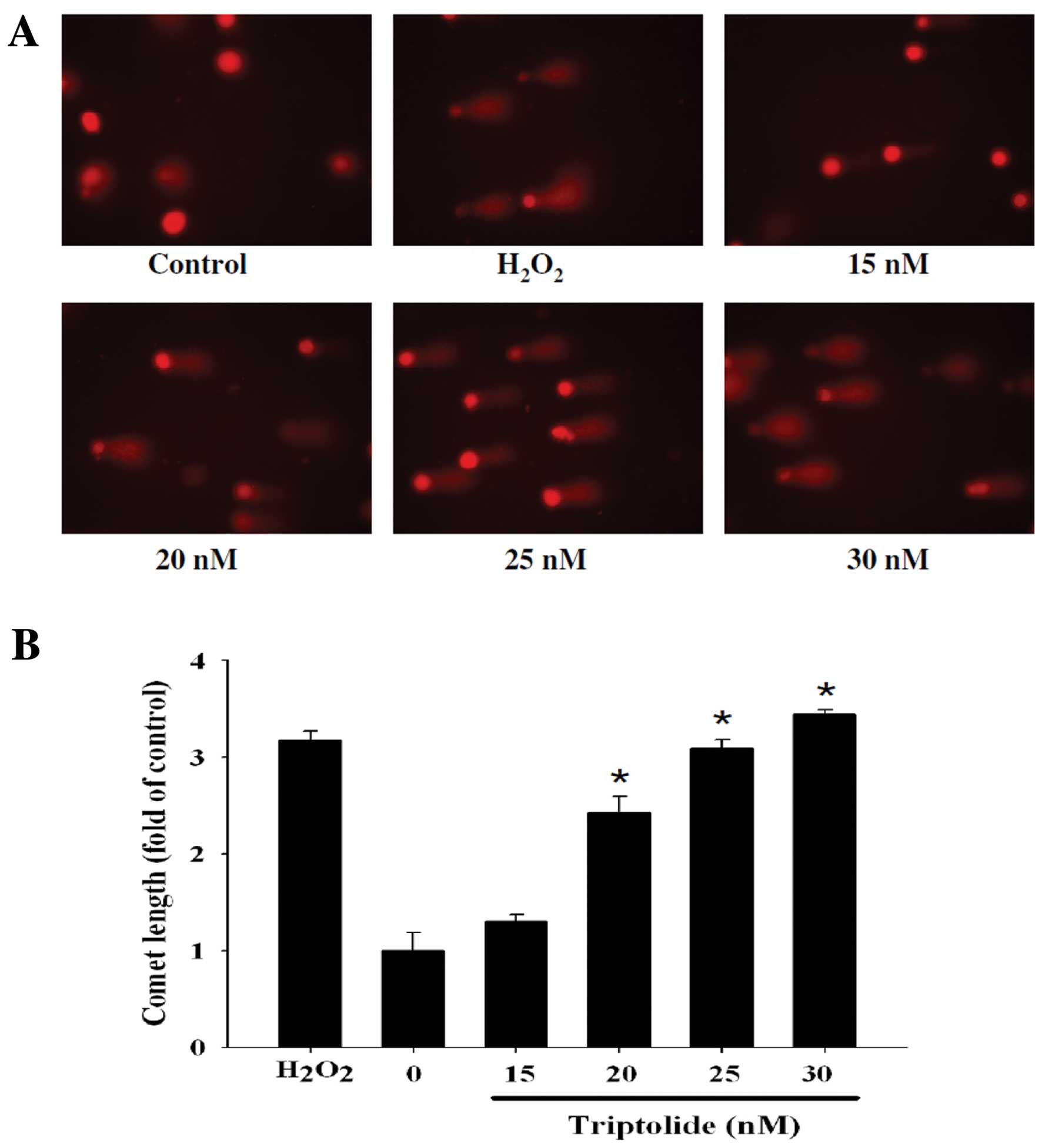
Effects of triptolide on DNA in A375.S2
cells examined by Comet assay and DAPI staining
To confirm whether triptolide can induce DNA damage
in A375.S2 cells, after cells were treated with triptolide DNA
damage was examined by Comet assay and the results are presented in
Fig. 2. Triptolide induced DNA
damage in A375.S2 cells and these effects were dose-dependent
(Fig. 2B) and time-dependent
(Fig. 2D). The higher concentration
of triptolide led to a longer DNA migration smear (Comet tail).
H2O2 is known to be a highly reactive oxygen
species, in the present studies, 0.1% H2O2
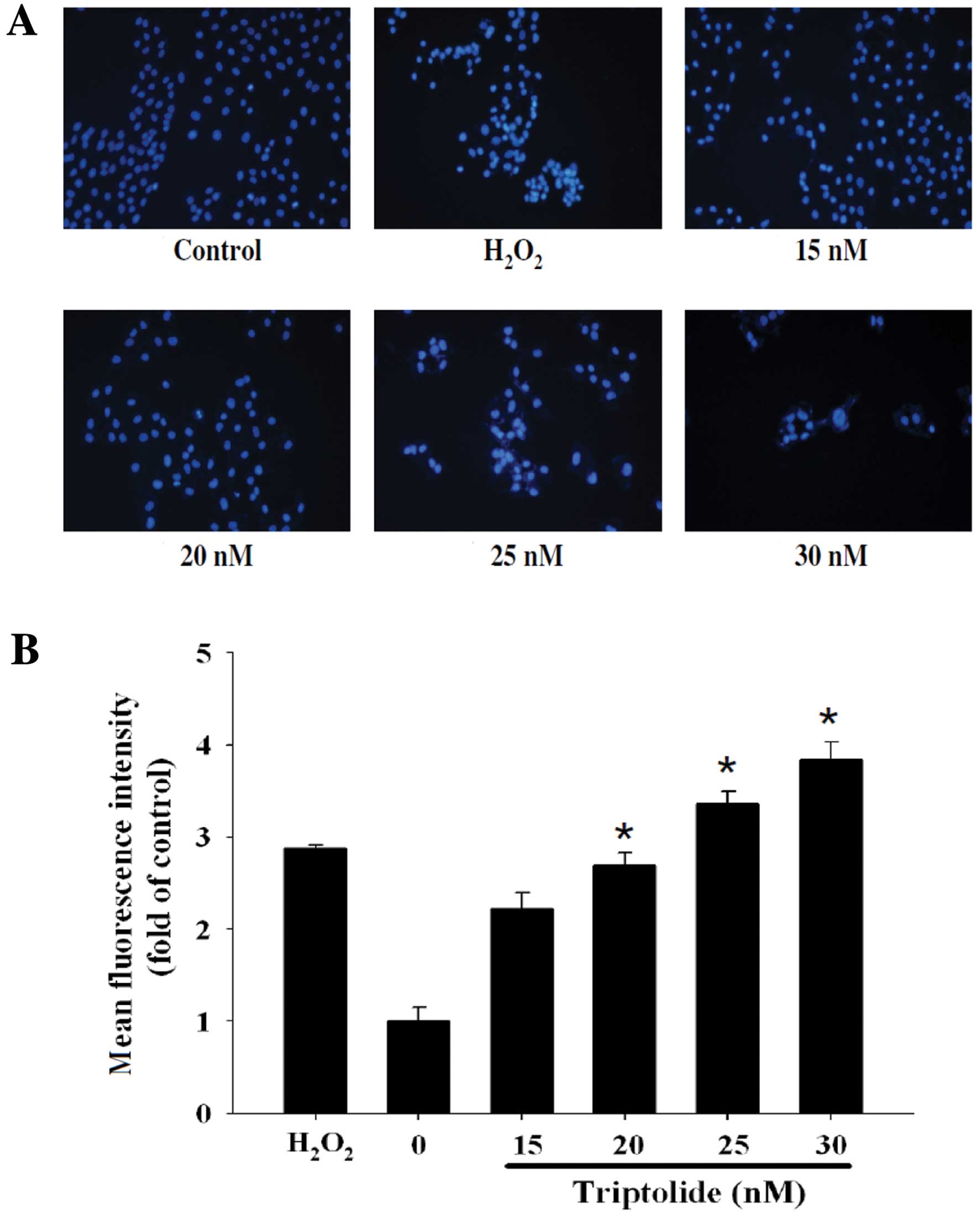
induced Comet tails. Fig. 3 shows
DNA damage by DAPI stain and the effects based on the mean
fluorescence intensity (Fig. 3A)
are dose-dependent (Fig. 3B).
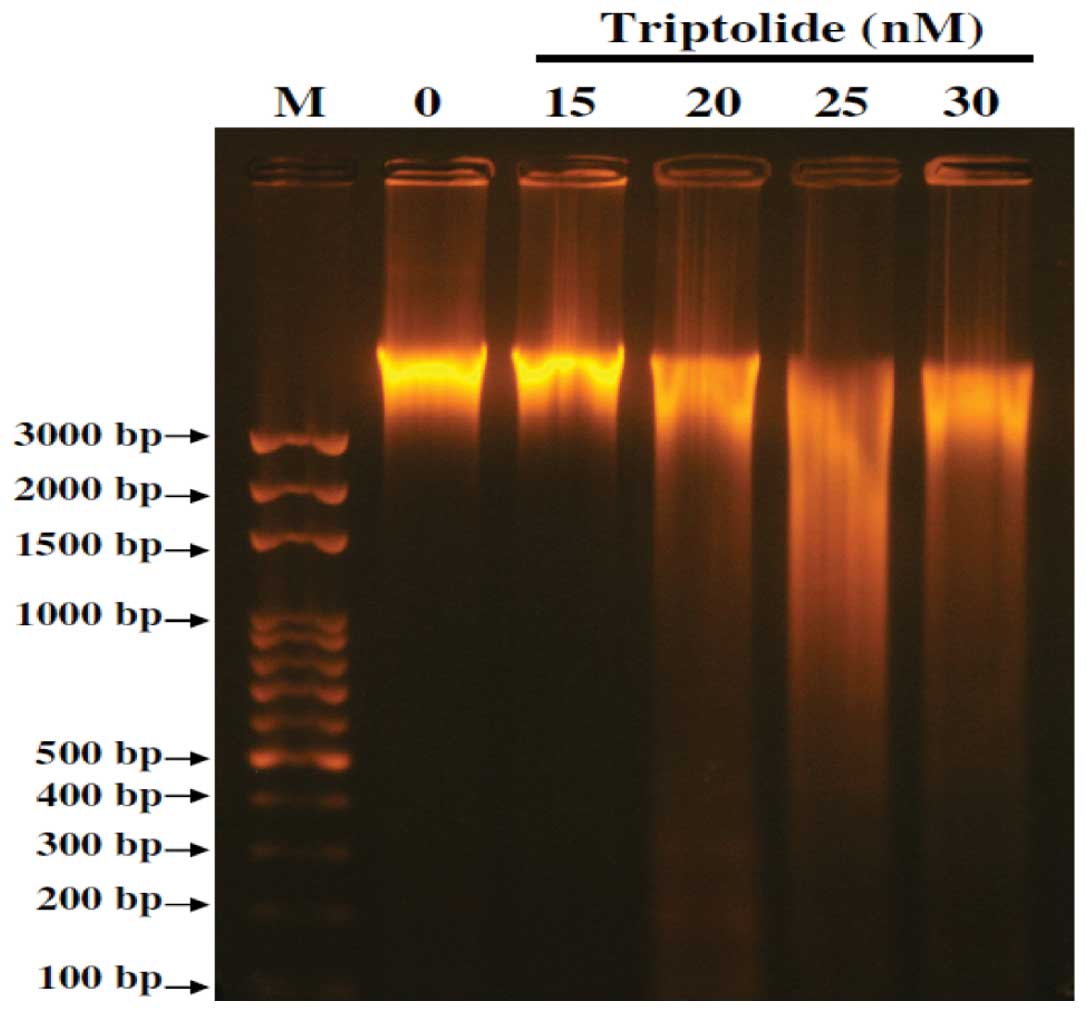
Effects of triptolide on DNA in A375.S2
cells examined by DNA gel electrophoresis
To confirm whether or not triptolide can induced DNA
damage in A375.S2 cells, DNA gel electrophoresis was used and
results are shown in Fig. 4. The
results show that triptolide induced DNA damage and fragments in
A375.S2 cells (Fig. 4). The higher
dose of triptolide (30 nM) led to more DNA damage and fragments
than that of low dose (15 nM) incubation in A375.S2 cells.
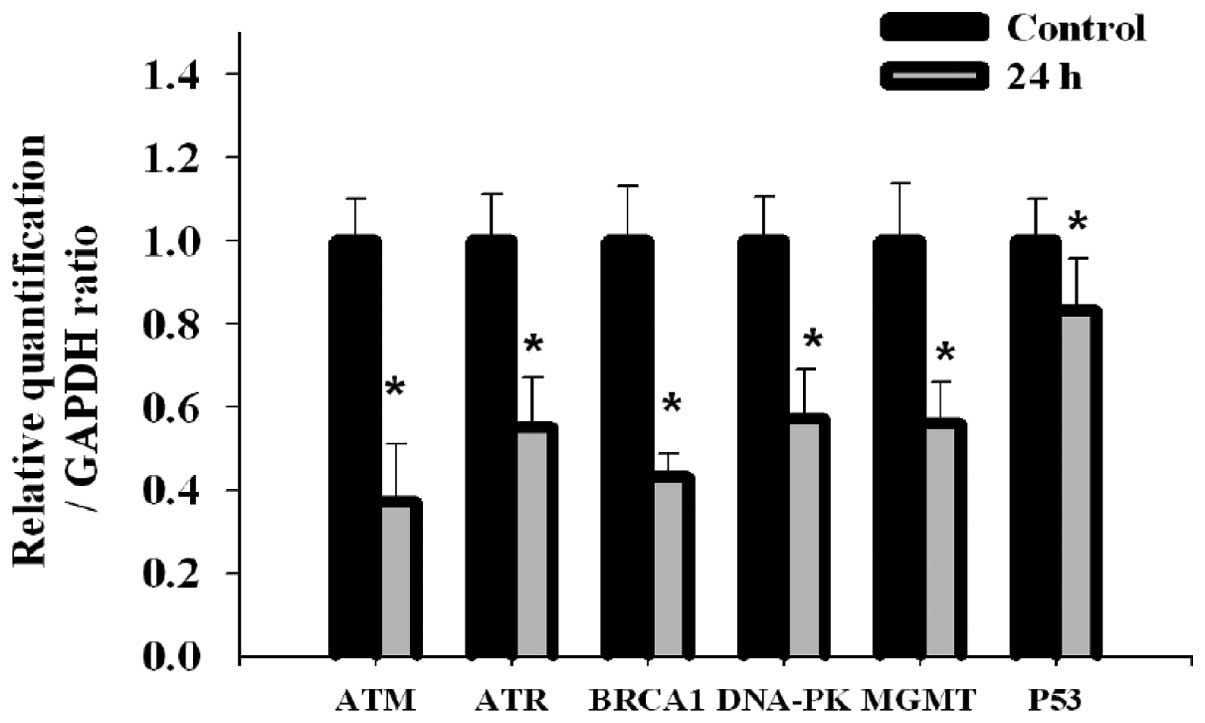
Effects of triptolide on DNA damage and
of repair gene expression in A375.S2 cells measured by real-time
PCR
Figs. 2 and 3 results show that triptolide induced DNA
damage and fragments in A375.S2 cells. We investigated whether or
not triptolide affects the gene expression of DNA damage and repair
in A375.S2 cells. We used DNA agarose gel electrophoresis for
examining the products from real-time PCR and results are shown in
Fig. 5. The results indicated that
all examined gene expression including ATM, ATR, BRCA-1, DNA-PK,
MGMT and p53 mRNA were decreased in 24 h treatment with triptolide.
ATM and BRCA-1 gene were more sensitive than the other genes (ATR,
DNA-PK, MGMT and p53). P53 was the least sensitive compared to the
other genes.
Discussion
Numerous experiments have shown that triptolide
induces cell death via induction of apoptosis in human cancer cell
lines (26,45,46),
but no available information exists to demonstrate triptolide
induced DNA damage and affected DNA repair gene expression in human
skin cancer cells. We found that A375.S2 cells treated with various
concentrations of triptolide led to decreased percentage of viable
cells (Fig. 1) and it also induced
DNA damage (Figs. 2 and 3) and inhibited gene expression of DNA
repair genes (Fig. 5) in A375.S2
cells. These findings are based on the observations from i) flow
cytometric assay showing the decrease of percentage of viable cells
(Fig. 1); ii) Comet assay and DAPI
staining, the longer comet tail means higher DNA damage (Fig. 2); the light of fluorescence means
higher DNA condensation (Fig. 3);
iii) DNA fragments in DNA gel electrophoresis indicate high dose of
triptolide treatment led to high DNA damage and fragments (Fig. 4) and iv) RT-PCR showed that
triptolide inhibited the gene expression (mRNA) of DNA associated
repair genes (Fig. 5).
It is well documented that Comet assay is a highly
sensitive technique for DNA damage examination (47,48)
and trend-break formation during the process of excision repair of
DNA in cells (49,50). Herein, our results showed
triptolide-induced DNA damage, which was examined by Comet assay
and DAPI staining. The DNA damage of A375.S2 cells from triptolide
treatment was also confirmed by DNA gel electophoresis (Fig. 4).
It was reported that agent-induced DNA damage can be
reduced in cells via the DNA repair system through eliminating DNA
lesions (49,50). Thus, we further investigated whether
or not triptolide can affect the DNA repair gene expression in
A375.S2 cells and results indicated that triptolide inhibit the
expression of mRNA such as ataxia telangiectasia mutated (ATM),
ataxia-telangiectasia (ATR), breast cancer gene 1 (BRCA-1), p53,
DNA-dependent protein kinase (DNA-PK) and
O6-methylguanine DNA methyltransferase (MGMT) in the
examined A375.S2 cells. The results in Fig. 5 indicate that p53 gene has the
lowest sensitivity to triptolide when compared to the other
examined genes.
It was reported that DNA damage responses of cells
could lead to p53 activation and activated p53 regulates the cell
cycle arrest, DNA repair and apoptosis (51,52).
The role of p53 in skin cancer cell response to triptolide-induced
DNA damage and repair is unclear. Our results show that triptolide
inhibited p53 gene expression in A375.S2 cells. In response to DNA
damage, DNA damage checkpoints associate with cell cycle for
maintaining genomic integrity (53–55).
It was reported that both ATM and ATR are master checkpoint kinases
which can be activated by double-stranded DNA breaks (52,56).
Our results also show that triptolide inhibited the ATM and ATR
gene expression in A375.S2 cells.
DNA-PK plays an important role in DNA damage repair
(52) and the deficiency in DNA-PK
activity of human glioblastoma cells can lead to a slow, error
prone repair process causing increased formation of chromosome
aberrations (52). BRCA1 plays and
important roles in DNA damage and repair response, homologous
recombination, cell cycle regulation, protein ubiquitination and
apoptosis (57,58) and loss of BRCA1 causes a defective
DNA repair response and G2/M cell cycle checkpoint in
breast cancer cells (57,59). MGMT reduces cytotoxicity of
therapeutic or environmental alkylating agents (60,61).
Our results showed that triptolide inhibited the gene expression
(mRNA) of DNA-PK, MGMT and BRCA-1.
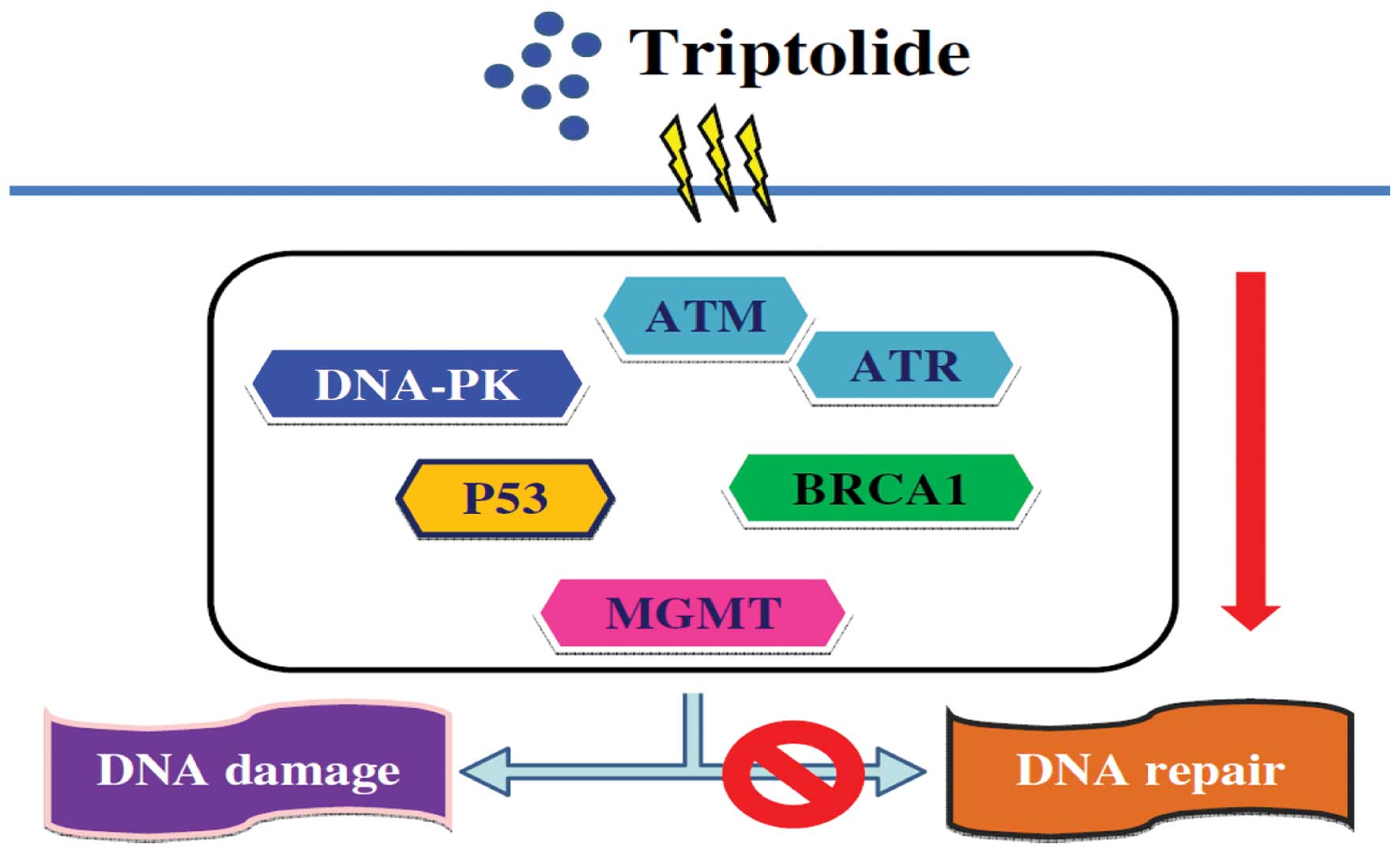
In conclusion, A375.S2 cells were exposed to various
concentrations of triptolide and DNA damage occurred. Moreover, the
proposed flow chart for triptolide effect on DNA in A375.S2 human
malignant melanoma cells is summarized in Fig. 6. Triptolide induces DNA damage in a
dose response followed by inhibition of DNA repair-associated gene
expression including ATM, ATR, BRCA-1, p53, DNA-PK and MGMT, then
leading to DNA damage (Fig. 6).
Acknowledgements
This study was supported by the grant CMU-100-ASIA-4
from China Medical University.
References
|
1
|
Pang J, Assaad D, Breen D, et al:
Extramammary Paget disease: review of patients seen in a
non-melanoma skin cancer clinic. Curr Oncol. 17:43–45. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martinez JC and Otley CC: The management
of melanoma and nonmelanoma skin cancer: a review for the primary
care physician. Mayo Clin Proc. 76:1253–1265. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rigel DS: Epidemiology of melanoma. Semin
Cutan Med Surg. 29:204–209. 2010. View Article : Google Scholar
|
|
4
|
Berwick M: How do solar UV irradiance and
smoking impact the diagnosis of second cancers after diagnosis of
melanoma?: No answer yet. Dermatoendocrinology. 4:18–19. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pfeifer GP and Besaratinia A: UV
wavelength-dependent DNA damage and human non-melanoma and melanoma
skin cancer. Photochem Photobiol Sci. 11:90–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aceituno-Madera P, Buendia-Eisman A, Olmo
FJ, Jimenez-Moleon JJ and Serrano-Ortega S: Melanoma, altitude, and
UV-B radiation. Actas Dermosifiliogr. 102:199–205. 2011.(In
Spanish).
|
|
7
|
Mathew A, Lindsley TA, Sheridan A, et al:
Degraded mitochondrial DNA is a newly identified subtype of the
damage associated molecular pattern (DAMP) family and possible
trigger of neurodegeneration. J Alzheimers Dis. 30:617–627.
2012.
|
|
8
|
Baltanas FC, Casafont I, Weruaga E, Alonso
JR, Berciano MT and Lafarga M: Nucleolar disruption and cajal body
disassembly are nuclear hallmarks of DNA damage-induced
neurodegeneration in purkinje cells. Brain Pathol. 21:374–388.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barzilai A: DNA damage, neuronal and glial
cell death and neurodegeneration. Apoptosis. 15:1371–1381. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Engelmann D and Putzer BM: Translating DNA
damage into cancer cell death-A roadmap for E2F1 apoptotic
signalling and opportunities for new drug combinations to overcome
chemoresistance. Drug Resist Updat. 13:119–131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ye Y, Xiao Y, Wang W, et al: Inhibition of
expression of the chromatin remodeling gene, SNF2L, selectively
leads to DNA damage, growth inhibition, and cancer cell death. Mol
Cancer Res. 7:1984–1999. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suganuma M, Kawabe T, Hori H, Funabiki T
and Okamoto T: Sensitization of cancer cells to DNA damage-induced
cell death by specific cell cycle G2 checkpoint abrogation. Cancer
Res. 59:5887–5891. 1999.PubMed/NCBI
|
|
13
|
Potter AJ and Rabinovitch PS: The cell
cycle phases of DNA damage and repair initiated by topoisomerase
II-targeting chemotherapeutic drugs. Mutat Res. 572:27–44. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Friedmann B, Caplin M, Hartley JA and
Hochhauser D: Modulation of DNA repair in vitro after treatment
with chemotherapeutic agents by the epidermal growth factor
receptor inhibitor gefitinib (ZD1839). Clin Cancer Res.
10:6476–6486. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kerklaan PR, Bouter S, van Elburg PE and
Mohn GR: Evaluation of the DNA repair host-mediated assay. II
Presence of genotoxic factors in various organs of mice treated
with chemotherapeutic agents. Mutat Res. 164:19–29. 1986.PubMed/NCBI
|
|
16
|
Norin AJ and Goldschmidt EP: Effect of
mutagens, chemotherapeutic agents and defects in DNA repair genes
on recombination in F′ partial diploid Escherichia coli.
Mutat Res. 59:15–26. 1979.PubMed/NCBI
|
|
17
|
Hikim AP, Lue YH, Wang C, Reutrakul V,
Sangsuwan R and Swerdloff RS: Posttesticular antifertility action
of triptolide in the male rat: Evidence for severe impairment of
cauda epididymal sperm ultrastructure. J Androl. 21:431–437.
2000.
|
|
18
|
Zhou GS, Hu Z, Fang HT, et al: Biologic
activity of triptolide in t(8;21) acute myeloid leukemia cells.
Leuk Res. 35:214–218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mak DH, Schober WD, Chen W, et al:
Triptolide induces cell death independent of cellular responses to
imatinib in blast crisis chronic myelogenous leukemia cells
including quiescent CD34+ primitive progenitor cells.
Mol Cancer Ther. 8:2509–2516. 2009. View Article : Google Scholar
|
|
20
|
Shi X, Jin Y, Cheng C, et al: Triptolide
inhibits Bcr-Abl transcription and induces apoptosis in
STI571-resistant chronic myelogenous leukemia cells harboring T315I
mutation. Clin Cancer Res. 15:1686–1697. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pigneux A, Mahon FX, Uhalde M, et al:
Triptolide cooperates with chemotherapy to induce apoptosis in
acute myeloid leukemia cells. Exp Hematol. 36:1648–1659. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yao GH, Luan JF, Ye D, et al: Effects of
triptolide on proliferation and apoptosis of Jurkat cell line in
acute T lymphocytic leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
16:506–509. 2008.(In Chinese).
|
|
23
|
Tong X, Zheng S, Jin J, Zhu L, Lou Y and
Yao H: Triptolide inhibits cyclooxygenase-2 and inducible nitric
oxide synthase expression in human colon cancer and leukemia cells.
Acta Biochim Biophys Sin. 39:89–95. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lou YJ and Jin J: Triptolide
down-regulates bcr-abl expression and induces apoptosis in chronic
myelogenous leukemia cells. Leuk Lymphoma. 45:373–376. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chan EW, Cheng SC, Sin FW and Xie Y:
Triptolide induced cytotoxic effects on human promyelocytic
leukemia, T cell lymphoma and human hepatocellular carcinoma cell
lines. Toxicol Lett. 122:81–87. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu J, Shen M, Yue Z, et al: Triptolide
inhibits colon-rectal cancer cells proliferation by induction of G1
phase arrest through upregulation of p21. Phytomedicine.
19:756–762. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Y, Song F, Wu WK, et al: Triptolide
inhibits colon cancer cell proliferation and induces cleavage and
translocation of 14-3-3 epsilon. Cell Biochem Funct. 30:271–278.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim MJ, Lee TH, Kim SH, Choi YJ, Heo J and
Kim YH: Triptolide inactivates Akt and induces caspase-dependent
death in cervical cancer cells via the mitochondrial pathway. Int J
Oncol. 37:1177–1185. 2010.PubMed/NCBI
|
|
29
|
Zhang C, Cui GH, Liu F, Wu QL and Chen Y:
Inhibitory effect of triptolide on lymph node metastasis in
patients with non-Hodgkin lymphoma by regulating SDF-1/CXCR4 axis
in vitro. Acta Pharmacol Sin. 27:1438–1446. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang S, Chen J, Guo Z, et al: Triptolide
inhibits the growth and metastasis of solid tumors. Mol Cancer
Ther. 2:65–72. 2003.PubMed/NCBI
|
|
31
|
Hsiao YP, Yu CS, Yu CC, et al: Triggering
apoptotic death of human malignant melanoma a375. S2 cells by
bufalin: involvement of caspase cascade-dependent and independent
mitochondrial signaling pathways. Evid Based Complement Alternat
Med. 2012:5912412012. View Article : Google Scholar
|
|
32
|
Lo C, Lai TY, Yang JS, et al: Gallic acid
inhibits the migration and invasion of A375. S2 human melanoma
cells through the inhibition of matrix metalloproteinase-2 and Ras.
Melanoma Res. 21:267–273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu CS, Huang AC, Yang JS, et al: Safrole
induces G0/G1 phase arrest via inhibition of cyclin E and provokes
apoptosis through endoplasmic reticulum stress and
mitochondrion-dependent pathways in human leukemia HL-60 cells.
Anticancer Res. 32:1671–1679. 2012.
|
|
34
|
Liu KC, Ho HC, Huang AC, et al: Gallic
acid provokes DNA damage and suppresses DNA repair gene expression
in human prostate cancer PC-3 cells. Environ Toxicol. Sept
2–2011.(Epub ahead of print).
|
|
35
|
Ni CH, Yu CS, Lu HF, et al:
Chrysophanol-induced cell death (necrosis) in human lung cancer
A549 cells is mediated through increasing reactive oxygen species
and decreasing the level of mitochondrial membrane potential.
Environ Toxicol. Jul 30–2012.(Epub ahead of print).
|
|
36
|
Yu CC, Ko FY, Yu CS, et al: Norcantharidin
triggers cell death and DNA damage through S-phase arrest and
ROS-modulated apoptotic pathways in TSGH 8301 human urinary bladder
carcinoma cells. Int J Oncol. 41:1050–1060. 2012.
|
|
37
|
Ni CH, Chen PY, Lu HF, et al:
Chrysophanol-induced necrotic-like cell death through an impaired
mitochondrial ATP synthesis in Hep3B human liver cancer cells. Arch
Pharm Res. 35:887–895. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tsai SC, Yang JS, Peng SF, et al: Bufalin
increases sensitivity to AKT/mTOR-induced autophagic cell death in
SK-HEP-1 human hepatocellular carcinoma cells. Int J Oncol.
41:1431–1442. 2012.PubMed/NCBI
|
|
39
|
Chen HY, Lu HF, Yang JS, et al: The novel
quinolone CHM-1 induces DNA damage and inhibits DNA repair gene
expressions in a human osterogenic sarcoma cell line. Anticancer
Res. 30:4187–4192. 2010.PubMed/NCBI
|
|
40
|
Lin YT, Yang JS, Lin SY, et al: Diallyl
disulfide (DADS) induces apoptosis in human cervical cancer Ca Ski
cells via reactive oxygen species and Ca2+-dependent
mitochondria-dependent pathway. Anticancer Res. 28:2791–2799.
2008.PubMed/NCBI
|
|
41
|
Chen YY, Chiang SY, Lin JG, et al: Emodin,
aloe-emodin and rhein induced DNA damage and inhibited DNA repair
gene expression in SCC-4 human tongue cancer cells. Anticancer Res.
30:945–951. 2010.PubMed/NCBI
|
|
42
|
Ho YT, Lu CC, Yang JS, et al: Berberine
induced apoptosis via promoting the expression of caspase-8, -9 and
-3, apoptosis-inducing factor and endonuclease G in SCC-4 human
tongue squamous carcinoma cancer cells. Anticancer Res.
29:4063–4070. 2009.
|
|
43
|
Lu HF, Yang JS, Lai KC, et al:
Curcumin-induced DNA damage and inhibited DNA repair genes
expressions in mouse-rat hybrid retina ganglion cells (N18).
Neurochem Res. 34:1491–1497. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ji BC, Yu CC, Yang ST, et al: Induction of
DNA damage by deguelin is mediated through reducing DNA repair
genes in human non-small cell lung cancer NCI-H460 cells. Oncol
Rep. 27:959–964. 2012.PubMed/NCBI
|
|
45
|
Wu PP, Liu KC, Huang WW, et al: Triptolide
induces apoptosis in human adrenal cancer NCI-H295 cells through a
mitochondrial-dependent pathway. Oncol Rep. 25:551–557.
2011.PubMed/NCBI
|
|
46
|
Huang W, He T, Chai C, et al: Triptolide
inhibits the proliferation of prostate cancer cells and
down-regulates SUMO-specific protease 1 expression. PLoS One.
7:e376932012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Petriccione M and Ciniglia C: Comet assay
to assess the genotoxicity of Persian walnut (Juglans regia
L.) husks with statistical evaluation. Bull Environ Contam Toxicol.
89:166–171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kwasniewska J, Grabowska M, Kwasniewski M
and Kolano B: Comet-FISH with rDNA probes for the analysis of
mutagen-induced DNA damage in plant cells. Environ Mol Mutagen.
53:369–375. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Goutham HV, Mumbrekar KD, Vadhiraja BM, et
al: DNA double-strand break analysis by γ-H2AX foci: A useful
method for determining the overreactors to radiation-induced acute
reactions among head-and-neck cancer patients. Int J Radiat Oncol
Biol Phys. Jul 24–2012.(Epub ahead of print).
|
|
50
|
Savina NV, Smal MP, Kuzhir TD,
Ershova-Pavlova AA and Goncharova RI: DNA-damage response
associated with occupational exposure, age and chronic inflammation
in workers in the automotive industry. Mutat Res. 748:21–28. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pennington KP, Walsh T, Lee M, et al:
BRCA1, TP53, and CHEK2 germline mutations in uterine serous
carcinoma. Cancer. Jul 18–2012.(Epub ahead of print).
|
|
52
|
Serrano MA, Li Z, Dangeti M, et al:
DNA-PK, ATM and ATR collaboratively regulate p53-RPA interaction to
facilitate homologous recombination DNA repair. Oncogene. Jul
16–2012.(Epub ahead of print).
|
|
53
|
Fortini P, Ferretti C, Pascucci B, et al:
DNA damage response by single-strand breaks in terminally
differentiated muscle cells and the control of muscle integrity.
Cell Death Differ. Jun 15–2012.(Epub ahead of print).
|
|
54
|
Jia YG, Yang YM, Zuo B, Guo LD and Lou JY:
DNA damage response in ovarian clear cell adenocarcinoma. Sichuan
Da Xue Xue Bao Yi Xue Ban. 43:331–334. 2012.(In Chinese).
|
|
55
|
Wong VC, Cash HL, Morse JL, Lu S and
Zhitkovich A: S-phase sensing of DNA-protein crosslinks triggers
TopBP1-independent ATR activation and p53-mediated cell death by
formaldehyde. Cell Cycle. 11:2526–2537. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Boltz KA, Leehy K, Song X, Nelson AD and
Shippen DE: ATR cooperates with CTC1 and STN1 to maintain telomeres
and genome integrity in Arabidopsis. Mol Biol Cell. 23:1558–1568.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tammaro C, Raponi M, Wilson DI and Baralle
D: BRCA1 exon 11 alternative splicing, multiple functions and the
association with cancer. Biochem Soc Trans. 40:768–772. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Pessetto ZY, Yan Y, Bessho T and Natarajan
A: Inhibition of BRCT(BRCA1)-phosphoprotein interaction enhances
the cytotoxic effect of olaparib in breast cancer cells: a proof of
concept study for synthetic lethal therapeutic option. Breast
Cancer Res Treat. 134:511–517. 2012. View Article : Google Scholar
|
|
59
|
Yarden RI, Metsuyanim S, Pickholtz I,
Shabbeer S, Tellio H and Papa MZ: BRCA1-dependent Chk1
phosphorylation triggers partial chromatin disassociation of
phosphorylated Chk1 and facilitates S-phase cell cycle arrest. Int
J Biochem Cell Biol. 44:1761–1769. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Silber JR, Bobola MS, Blank A and
Chamberlain MC: O(6)-Methylguanine-DNA methyltransferase in glioma
therapy: Promise and problems. Biochim Biophys Acta. 1826:71–82.
2012.PubMed/NCBI
|
|
61
|
Fumagalli C, Pruneri G, Possanzini P, et
al: Methylation of O6-methylguanine-DNA methyltransferase (MGMT)
promoter gene in triple-negative breast cancer patients. Breast
Cancer Res Treat. 134:131–137. 2012. View Article : Google Scholar : PubMed/NCBI
|