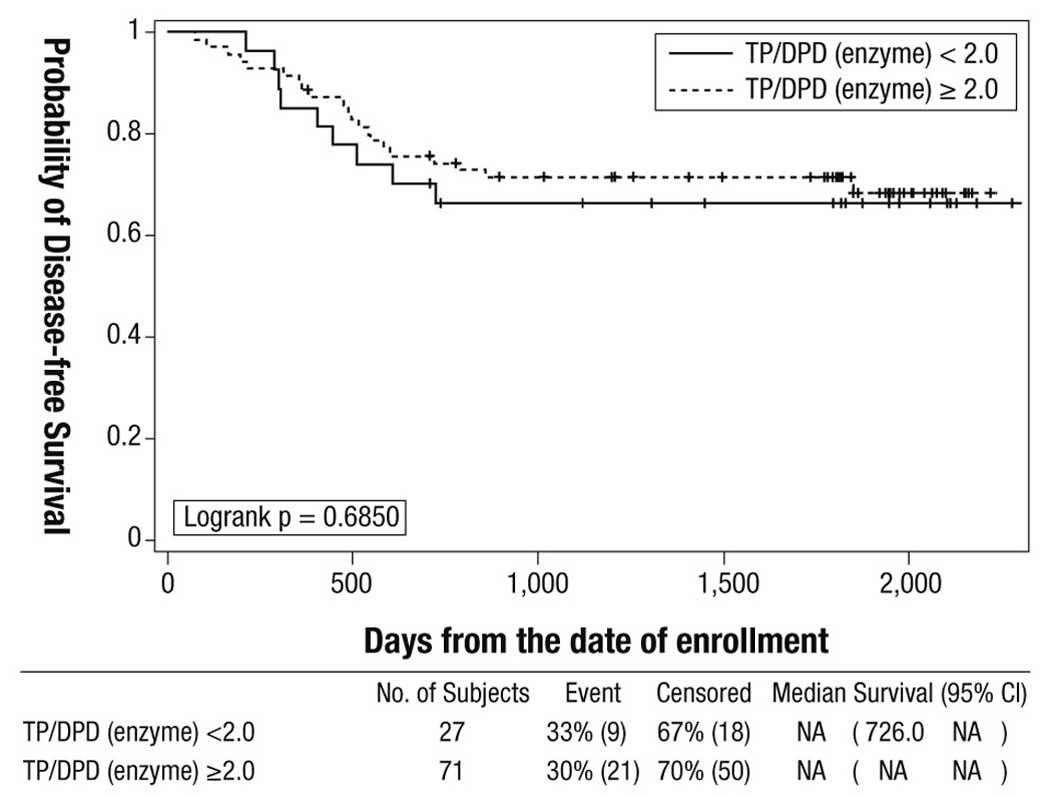
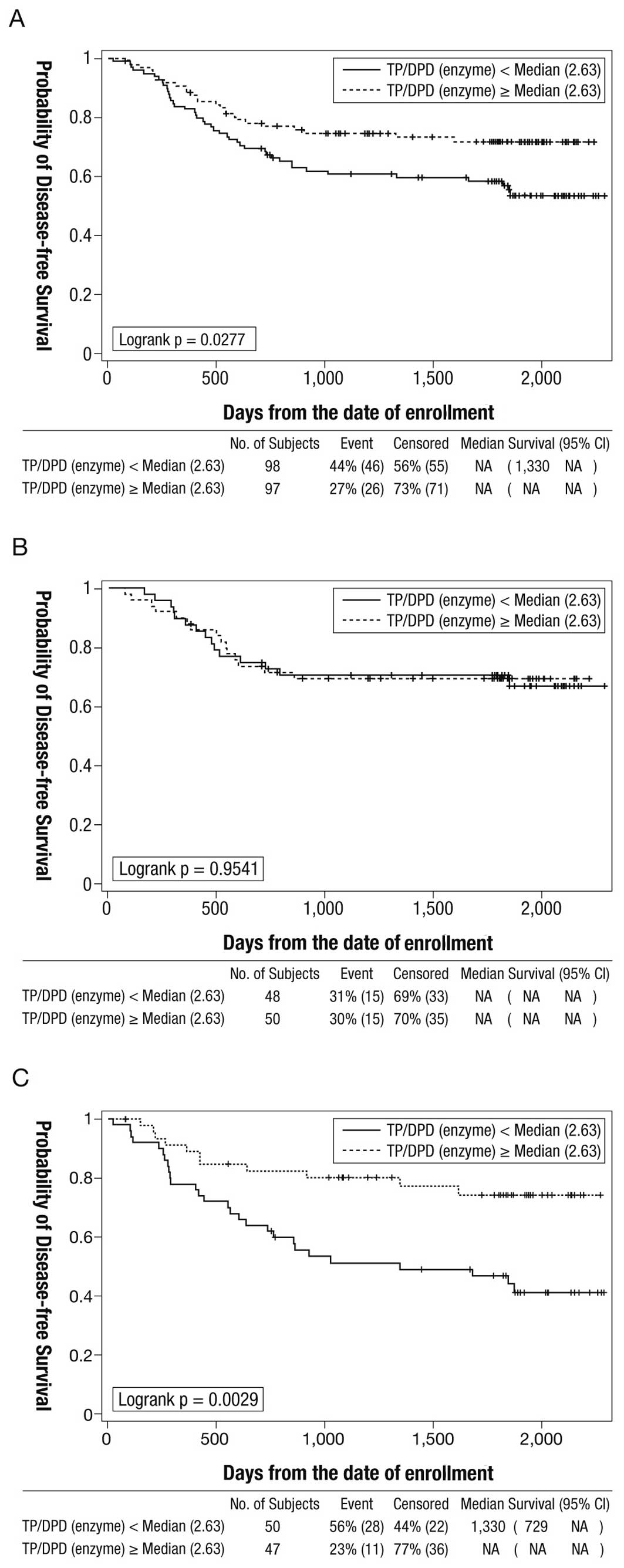
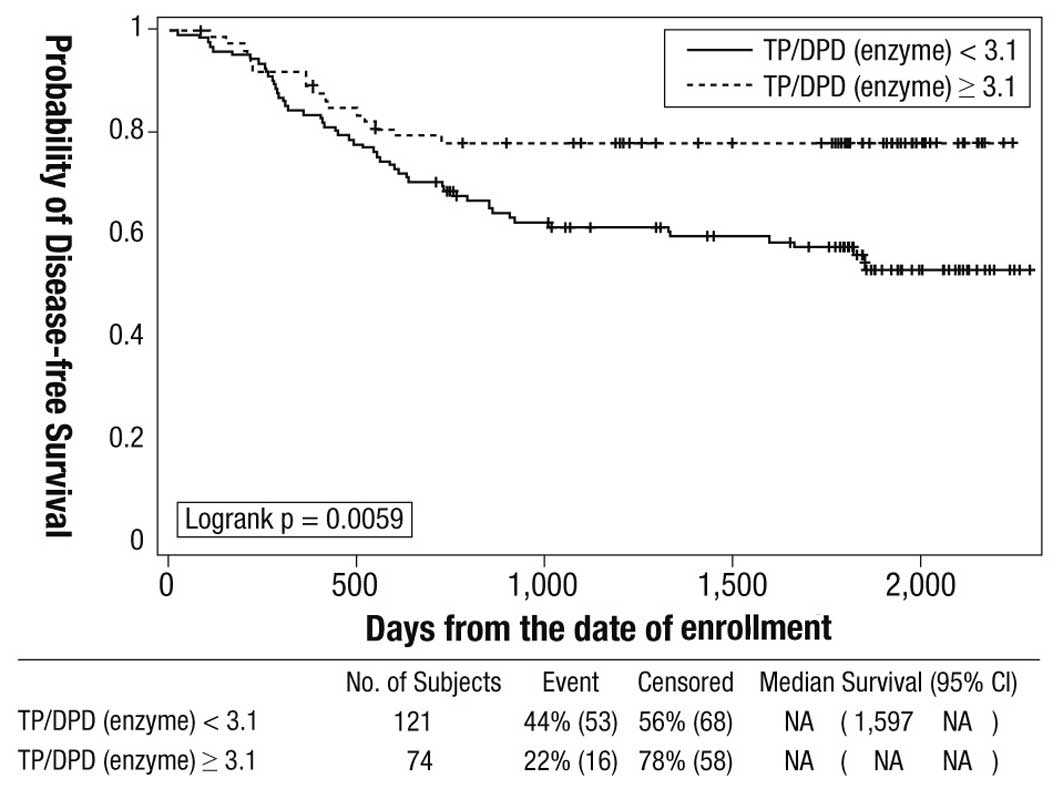
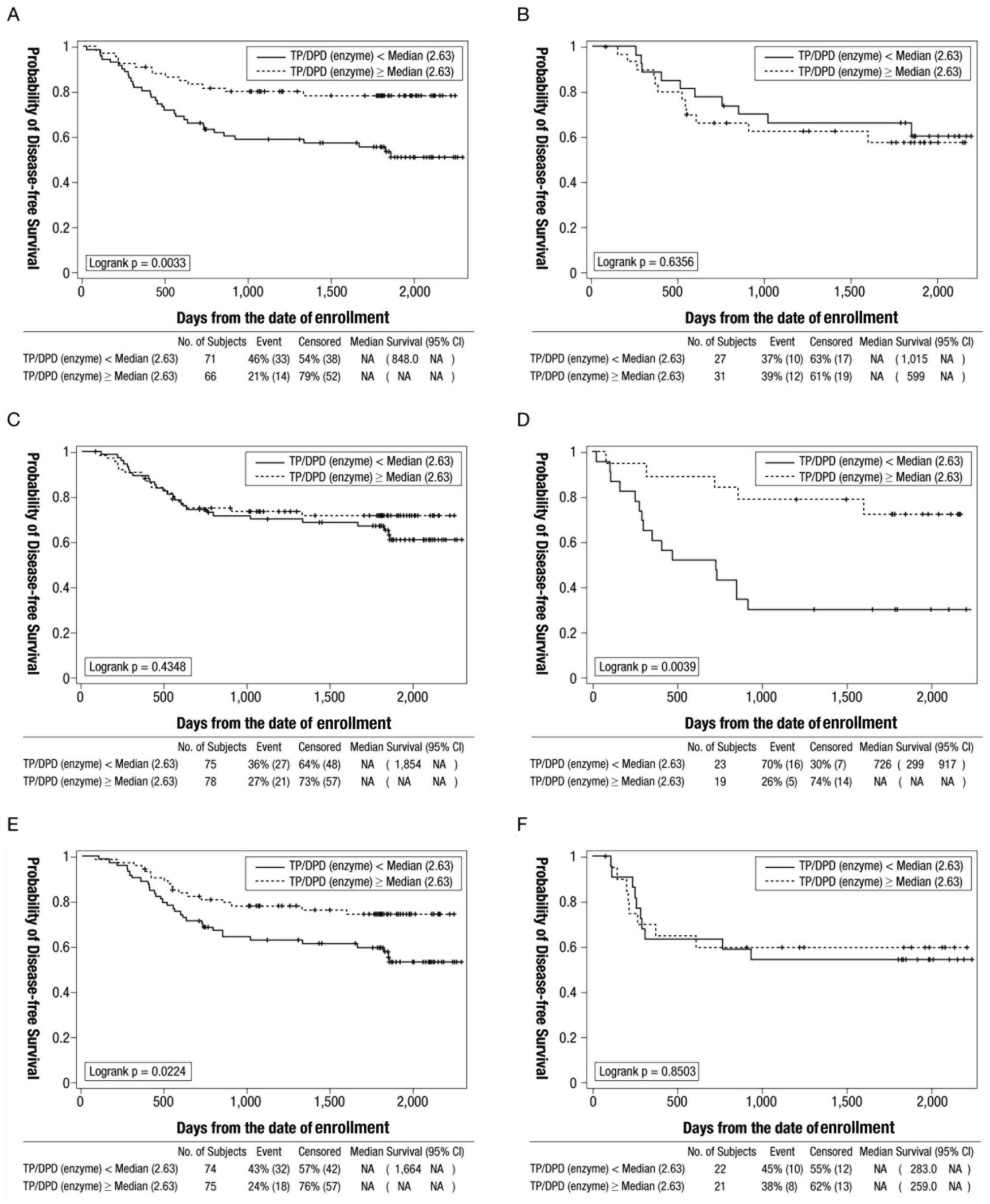
|
1
|
Center for Cancer Control and Information
Services, National Cancer Center, Japan. http://ganjoho.ncc.go.jp/public/statistics/index.html.
Accessed August 16, 2011
|
|
2
|
Efficacy of adjuvant fluorouracil and
folinic acid in colon cancer. International Multicentre Pooled
Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet.
345:939–944. 1995. View Article : Google Scholar
|
|
3
|
Haller DG, Catalano PJ, Macdonald JS, et
al: Phase III study of fluorouracil, leucovorin, and levamisole in
high-risk stage II and III colon cancer: final report of Intergroup
0089. J Clin Oncol. 23:8671–8678. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Comparison of fluorouracil with additional
levamisole, higher-dose folinic acid, or both, as adjuvant
chemotherapy for colorectal cancer: a randomised trial. QUASAR
Collaborative Group. Lancet. 355:1588–1596. 2000. View Article : Google Scholar
|
|
5
|
André T, Quinaux E, Louvet C, et al: Phase
III study comparing a semimonthly with a monthly regimen of
fluorouracil and leucovorin as adjuvant treatment for stage II and
III colon cancer patients: final results of GERCOR C96.1. J Clin
Oncol. 25:3732–3738. 2007.PubMed/NCBI
|
|
6
|
Twelves C, Wong A, Nowacki MP, et al:
Capecitabine as adjuvant treatment for stage III colon cancer. N
Engl J Med. 352:2696–2704. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lembersky BC, Wieand HS, Petrelli NJ, et
al: Oral uracil and tegafur plus leucovorin compared with
intravenous fluorouracil and leucovorin in stage II and III
carcinoma of the colon: results from National Surgical Adjuvant
Breast and Bowel Project Protocol C-06. J Clin Oncol. 24:2059–2064.
2006. View Article : Google Scholar
|
|
8
|
Ishikawa T, Sekiguchi F, Fukase Y, et al:
Positive correlation between the efficacy of capecitabine and
doxyfluridine and the ratio of thymidine phosphorylase to
dihydropyrimidine dehydrogenase activities in tumors in human
cancer xenografts. Cancer Res. 58:685–690. 1998.
|
|
9
|
Nishimura G, Terada I, Kobayashi T, et al:
Thymidine phosphorylase and dihydropyrimidine dehydrogenase levels
in primary colorectal cancer show a relationship to clinical
effects of 5′-deoxy-5-fluorouridine as adjuvant chemotherapy. Oncol
Rep. 9:479–482. 2002.PubMed/NCBI
|
|
10
|
Ichikawa W, Uetake H, Shirota Y, et al:
Both gene expression for orotate phosphoribosyltransferase and its
ratio to dihydropyrimidine dehydrogenase influence outcome
following fluoropyrimidine-based chemotherapy for metastatic
colorectal cancer. Br J Cancer. 89:1486–1492. 2003. View Article : Google Scholar
|
|
11
|
Sakamoto J, Ohashi Y, Hamada C, et al:
Efficacy of oral adjuvant therapy after resection of colorectal
cancer: 5-year results from three randomized trials. J Clin Oncol.
22:484–492. 2004.PubMed/NCBI
|
|
12
|
Nishida M, Hino A, Mori K, et al:
Preparation of anti-human thymidine phosphorylase monoclonal
antibodies useful for detecting the enzyme levels in tumor tissues.
Biol Pharm Bull. 19:1407–1411. 1996. View Article : Google Scholar
|
|
13
|
Mori K, Hasegawa M, Nishida M, et al:
Expression levels of thymidine phosphorylase and dihydropyrimidine
dehydrogenase in various human tumor tissues. Int J Oncol.
17:33–38. 2000.PubMed/NCBI
|
|
14
|
Kawakami K and Watanabe G: Identification
and functional analysis of single nucleotide polymorphism in the
tandem repeat sequence of thymidylate synthase gene. Cancer Res.
63:6004–6007. 2003.PubMed/NCBI
|
|
15
|
Sawada N, Ishikawa T, Fukase Y, et al:
Induction of thymidine phosphorylase activity and enhancement of
capecitabine efficacy by taxol/taxotere in human cancer xenografts.
Clin Cancer Res. 4:1013–1019. 1998.PubMed/NCBI
|
|
16
|
Gieschke R, Burger HU, Reigner B, et al:
Population pharmacokinetics and concentration-effect relationships
of capecitabine metabolites in colorectal cancer patients. Br J
Clin Pharmacol. 55:252–263. 2003. View Article : Google Scholar
|
|
17
|
Patterson AV, Zhang H, Moghaddam A, et al:
Increased sensitivity to the prodrug 5′-deoxy-5-fluorouridine and
modulation of 5-fluoro-2′-deoxyuridine sensitivity in MCF-7 cells
transfected with thymidine phosphorylase. Br J Cancer. 72:669–675.
1995.
|
|
18
|
Evrard A, Cuq P, Ciccolini J, et al:
Increased cytotoxicity and bystander effect of 5-fluorouracil and
5-deoxy-5-fluorouridine in human colorectal cancer cells
transfected with thymidine phosphorylase. Br J Cancer.
80:1726–1733. 1999. View Article : Google Scholar
|
|
19
|
Toi M, Rahman MA, Bando H and Chow LW:
Thymidine phosphorylase (platelet-derived endothelial-cell growth
factor) in cancer biology and treatment. Lancet Oncol. 6:158–166.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eda H, Fujimoto K, Watanabe S, et al:
Cytokines induce thymidine phosphorylase expression in tumor cells
and make them more susceptible to 5′-deoxy-5-fluorouridine. Cancer
Chemother Pharmacol. 32:333–338. 1993.PubMed/NCBI
|
|
21
|
Miyadera K, Sumizawa T, Haraguchi M, et
al: Role of thymidine phosphorylase activity in the angiogenic
effect of platelet-derived endothelial cell growth factor/thymidine
phosphorylase. Cancer Res. 55:1687–1690. 1995.PubMed/NCBI
|
|
22
|
Lenz HJ, Hayashi K, Salonga D, et al: p53
point mutations and thymidylate synthase messenger RNA levels in
disseminated colorectal cancer: an analysis of response and
survival. Clin Cancer Res. 4:1243–1250. 1998.PubMed/NCBI
|
|
23
|
Paradiso A, Simone G, Petroni S, et al:
Thymidilate synthase and p53 primary tumour expression as
predictive factors for advanced colorectal cancer patients. Br J
Cancer. 82:560–567. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sinicrope FA, Rego RL, Halling KC, et al:
Thymidylate synthase expression in colon carcinomas with
microsatellite instability. Clin Cancer Res. 12:2738–2744. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ochiai T, Nishimura K, Noguchi H, et al:
Prognostic impact of orotate phosphoribosyl transferase among
5-fluorouracil metabolic enzymes in resectable colorectal cancers
treated by oral 5-fluorouracil-based adjuvant chemotherapy. Int J
Cancer. 118:3084–3088. 2006. View Article : Google Scholar
|
|
26
|
Salonga D, Danenberg KD, Johnson M, et al:
Colorectal tumors responding to 5-fluorouracil have low gene
expression levels of dihydropyrimidine dehydrogenase, thymidylate
synthase, and thymidine phosphorylase. Clin Cancer Res.
6:1322–1327. 2000.
|
|
27
|
Lassmann S, Hennig M, Rosenberg R, et al:
Thymidine phosphorylase, dihydropyrimidine dehydrogenase and
thymidylate synthase mRNA expression in primary colorectal tumors -
correlation to tumor histopathology and clinical follow-up. Int J
Colorectal Dis. 21:238–247. 2006. View Article : Google Scholar
|
|
28
|
Ciaparrone M, Quirino M, Schinzari G, et
al: Predictive role of thymidylate synthase, dihydropyrimidine
dehydrogenase and thymidine phosphorylase expression in colorectal
cancer patients receiving adjuvant 5-fluorouracil. Oncology.
70:366–377. 2006. View Article : Google Scholar
|
|
29
|
Park DJ, Stoehlmacher J, Zhang W, et al:
Thymidylate synthase gene polymorphism predicts response to
capecitabine in advanced colorectal cancer. Int J Colorectal Dis.
17:46–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park CM, Lee WY, Chun HK, et al:
Relationship of polymorphism of the tandem repeat sequence in the
thymidylate synthase gene and the survival of stage III colorectal
cancer patients receiving adjuvant 5-flurouracil-based
chemotherapy. J Surg Oncol. 101:22–27. 2010. View Article : Google Scholar
|
|
31
|
Goto H, Kohno K, Sone S, et al: Interferon
gamma-dependent induction of thymidine
phosphorylase/platelet-derived endothelial growth factor through
gamma-activated sequence-like element in human macrophages. Cancer
Res. 61:469–473. 2001.
|
|
32
|
Takebayashi Y, Akiyama S, Akiba S, et al:
Clinicopathologic and prognostic significance of an angiogenic
factor, thymidine phosphorylase, in human colorectal carcinoma. J
Natl Cancer Inst. 88:1110–1117. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tokunaga Y, Hosogi H, Hoppou T, et al:
Prognostic value of thymidine phosphorylase/platelet-derived
endothelial cell growth factor in advanced colorectal cancer after
surgery: evaluation with a new monoclonal antibody. Surgery.
131:541–547. 2002. View Article : Google Scholar
|