Introduction
The age-adjusted incidence of colon cancer in Japan
in 2003 was second compared to stomach cancer in men (63.8/100,000
individuals) and breast cancer in women (35.9/100,000 individuals)
(1). However, it is estimated that
there will be 512,225 cases of colon cancer in Japan in 2020,
surpassing the number of breast cancer cases from 2010 onwards as
the most common type of cancer in women (1).
Adjuvant chemotherapy improves the overall survival
in patients with resected stage III colon cancer (2). In the late 1990's, intravenous
5-fluorouracil/leucovorin (5-FU/LV) was established as the standard
adjuvant treatment for patients with stage III colon cancer
(3–5). Since then several oral
fluoropyrimidines [i.e. capecitabine, uracil/tegafur (UFT) plus LV]
have been demonstrated as an effective alternative to 5-FU/LV in
the treatment of colon cancer (6,7).
Intratumoral expression of the metabolizing enzymes
thymidine phosphorylase (TP), dihydropyrimidine dehydrogenase
(DPD), thymidylate synthase (TS) and orotate
phosphoribosyltransferase (OPRT) are important for the clinical
activity of the drugs and may be predictive markers used to guide
decision-making regarding the treatment for individual patients.
For example, the TP/DPD ratio is significantly higher in cancer
cell lines with high sensitivity to doxifluridine (an intermediate
metabolite of capecitabine) compared to those with lower
sensitivity and the possibility of predicting the effectiveness of
doxifluridine from the TP/DPD ratio has been demonstrated (8,9). The
OPRT/DPD ratio may be a promising predictive marker for UFT (data
reported in UFT plus LV) (10).
We conducted a multicenter phase II study on 204
stage III colorectal cancer patients to identify potential
biomarkers predictive of outcome to adjuvant therapy with oral
fluoropyrimidines. Patients were treated orally for 12 months
(11) with doxifluridine or UFT,
which were the standard drug treatments in Japan in 2001 when the
study was initiated.
Patients and methods
Patients
Patients with histologically diagnosed stage III
(i.e. Dukes' C) colorectal cancer who had undergone curative
resection within the last 6 weeks prior to the start of the study
were enrolled. Other inclusion criteria were: age 20 to 75 years,
Eastern Cooperative Oncology Group (ECOG) performance status of
0–2, able to take oral medication, not treated with any prior
therapy other than surgical resection and adequate organ function.
Exclusion criteria were: cancer of the appendix or anal canal
derived from the anal glands, synchronous or metachronous cancers
or multiple invasive colon cancers (excluding intramucosal
cancer).
Patients were recruited from 13 institutions
belonging to the Japanese Society for Cancer of the Colon and
Rectum which achieved a consensus on the appropriate surgical
procedure and follow-up.
The study protocol was approved by the institutional
review boards of each participating institution. The study was in
accordance with the Ethical Guidelines for Clinical Studies of the
Health, Labor and Welfare Ministry in Japan and was conducted in
compliance with the Declaration of Helsinki. All patients provided
written informed consent.
Study design
Patients were randomly assigned (1:1 ratio) to
treatment groups. Tumor site (colon vs. rectosigmoid-upper rectum
vs. lower rectum), depth of invasion [(sm/mp/ss, a1) vs. (se,
a2/si, ai)], lymph node metastasis (n1 vs. n2/n3) and the study
site were selected as stratification factors.
Treatment
Patients received oral doxifluridine (800
mg/m2/day) in 3 divided doses or oral UFT (400
mg/m2/day) in 2 divided doses for 5 days with 2 rest
days and repeated weekly for 12 months. Patients were then followed
up until confirmation of recurrence. On the occurrence of adverse
events, dose reductions or temporary treatment interruptions were
performed as per protocol.
Tissue samples and analysis
Sixty milligrams (5 mm2) of tissue was
obtained from each resected tumor, from the marginal portion of the
primary lesion and not including necrotic tissue. The sample tissue
was divided in half, frozen in liquid nitrogen and stored at −80°C
or colder. For analysis of TS tandem repeat type, a 1 ml blood
sample was collected within 3 months after surgery and stored at
−80°C or colder.
Tissue specimens were sent to Nippon Roche Co., Ltd.
(now Chugai Pharmaceutical Co., Ltd.) Research Center for analysis.
TP and DPD protein levels were measured by ELISA (12,13)
and TP, DPD, TS and OPRT mRNA levels were measured by RT-PCR using
a LightCycler® (Roche Diagnostics KK, Tokyo, Japan).
Identification of TS tandem repeat type was conducted by PCR-RFLP
assay (14).
Evaluation
Physical examination, ultrasonography, chest X-rays
and tumor marker measurements were performed before the beginning
of the study treatment; every 4 months for the first year after
surgery and every 6 months from the second year onwards. Suspected
recurrence was confirmed via barium enema, CT or other appropriate
diagnostic imaging modality.
Safety was evaluated from reports of adverse events,
laboratory examination results and measurements of vital signs.
Adverse events were classified according to the Common Toxicity
Criteria of the National Cancer Institute (NCI-CTC, version 2). All
patients were followed up for a maximum of 5 years until death,
failure to follow up or completion of the study.
Study objectives
The primary objective was to examine the effect of
the intratumoral TP/DPD enzyme ratio (cut-off value 2.0) on
disease-free survival in the doxifluridine group. There were 3
secondary endpoints, which were examined on an exploratory basis:
i) Effects of TP and DPD protein levels in tumor samples and the
magnitude of the TP/DPD ratio on disease-free and overall survival
with oral fluoropyrimidine adjuvant therapy. ii) Effects of TP,
DPD, TS and OPRT mRNA levels in tumor samples and the magnitude of
the TP/DPD ratio on disease-free and overall survival with oral
fluoropyrimidine treatment. iii) Effects of the TS tandem repeat
type from blood samples on disease-free and overall survival with
oral fluoropyrimidine treatment.
Statistical analysis
Data were analyzed for the full analysis set,
comprising all patients who started protocol treatment and in which
TP and DPD protein levels were measured.
Time-to-event endpoints were analyzed using the
Kaplan-Meier method and 5-year survival rates were estimated with
95% confidence intervals (CI). Differences between patient cohorts
were examined with the log-rank test. Disease-free survival was
defined as the period from the date of enrollment to the date of
confirmation of either recurrence or death, whichever preceded.
Recurrence was defined as the occurrence of metachronous colon
cancer or secondary invasive cancer. Patients without recurrence or
death at the time of analysis were censored at the final
observation time. Overall survival was defined as the period from
the date of enrollment to the date of confirmation of death from
any cause. In the analysis of overall survival, patients who
survived were censored at the final observation time.
Proportional hazard models were used for the
analysis of disease-free and overall survival. We examined 3
different models: drug and the parameter (e.g., TP/DPD ratio), drug
and the parameter and the interactions between them and
stratification factors as covariates.
We estimated hazard ratios (HRs) and a two-sided
Wald P-value for each model. A P-value of ≤0.05 was used to
determine whether or not a factor had predictive value.
Further post-hoc exploratory analyses were
performed. A Cox regression analysis was performed to identify a
cut-off value for the intrartumoral TP/DPD enzyme ratio to
distinguish between patients with good and poor outcomes. The
effects of intratumoral TP/DPD enzyme ratio on disease-free
survival by tumor site, T and N category were analyzed.
The sample size was estimated assuming an HR of 2.2
for the doxifluridine group based on Nishimura et
al(9), who demonstrated that
disease-free survival was significantly longer in the patient
cohort with a TP/DPD ratio ≥2.0 with doxifluridine adjuvant
therapy. An estimated 240 patients in total were required, with 120
patients in the doxifluridine group. Assuming a 5-year disease-free
survival rate of 70% for patients with stage III (R0) colon cancer
and 60% for rectal cancer, a colon:rectal cancer ratio of 55:45 and
a 1-year enrollment period with a 5-year follow-up period, an α
error of 0.05 (one-sided) and detection power (1-β) of 63% were
ensured. Data were collected by EPS Co., Ltd. Statistical analyses
were conducted under the supervision of T.T., at the Center for
Medical Statistics.
Results
Two hundred and four patients were enrolled at 13
institutions from January 2002 to September 2003 and 102 patients
were assigned to the doxifluridine group and 102 patients to the
UFT group. One hundred and two patients in the doxifluridine group
and 99 patients in the UFT group received treatment as allocated,
respectively. The analysis set comprised 195 patients in which TP
and DPD protein levels were measured (doxifluridine, n=98; UFT,
n=97). There were no differences between the 2 groups in the
distribution of patient baseline characteristics (Table I).
 | Table IPatient baseline characteristics. |
Table I
Patient baseline characteristics.
| Doxifluridine
(n=102) | Uracil/tegafur
(n=102) |
|---|
|
|
|
|---|
| n (%) | n (%) |
|---|
| Age, years |
| Median (range) | 63 (26–75) | 62.5 (33–75) |
| Gender |
| Male | 55 (53.9) | 60 (58.8) |
| Female | 47 (46.1) | 42 (41.2) |
| Tumor location |
| Colon | 59 (57.8) | 57 (55.9) |
| Rectum | 43 (42.2) | 45 (44.1) |
| Histological
type |
| Well
differentiated | 35 (34.3) | 32 (31.4) |
| Moderately
differentiated | 62 (60.8) | 66 (64.7) |
| Poorly
differentiated | 4 (3.9) | 3 (2.9) |
| Mucinous | 1 (1.0) | 1 (1.0) |
| Depth of
invasiona |
| sm, mp | 13 (12.7) | 12 (11.8) |
| ss, a1 | 53 (52.0) | 57 (55.9) |
| se, a2 | 31 (30.4) | 32 (31.4) |
| si, ai | 5 (4.9) | 1 (1.0) |
| Metastasis to lymph
nodesb |
| n1(+) | 77 (75.5) | 78 (76.5) |
| n2(+) | 20 (19.6) | 22 (21.6) |
| n3(+) | 5 (4.9) | 2 (2.0) |
| Histological
stage |
| IIIa | 77 (75.5) | 78 (76.5) |
| IIIb | 25 (24.5) | 24 (23.5) |
| Lymphatic
invasion |
| ly0 | 11 (10.8) | 9 (8.8) |
| ly(+) | 91 (89.2) | 93 (91.2) |
| Venous
invasion |
| v0 | 18 (17.6) | 20 (19.6) |
| v(+) | 84 (82.4) | 82 (80.4) |
| Histological
curability |
| R0 | 102 (100.0) | 102 (100.0) |
Compliance and safety
Seventy-seven patients completed treatment and 25
patients discontinued intervention in the doxifluridine group
(death, n=1; relapsed/metastasis, n=7; adverse events related to
study drugs, n=10; declined treatment, n=6; other, n=1).
Sixty-seven patients completed treatment and 32 patients
discontinued intervention in the UFT group (relapsed/metastasis,
n=15; complications, n=1; adverse events related to study drugs,
n=13; declined treatment, n=2; other, n=1).
Treatment compliance was similar in both groups
(≥70% of treatment days completed for doxifluridine, 72.5%; UFT,
68.7%; ≥70% doses received for doxifluridine, 68.6%; UFT,
65.7%).
Adverse events were reported in 61 patients (59.8%)
in the doxifluridine group and 71 patients (71.7%) in the UFT group
and grade 3 events were reported in 7 patients in both groups (one
patient had grade 4 anorexia and stomach pains caused by adhesive
bowel obstruction) (Table II).
 | Table IIAdverse events graded according to
the Common Toxicity Criteria of the National Cancer Institute
(version 2). |
Table II
Adverse events graded according to
the Common Toxicity Criteria of the National Cancer Institute
(version 2).
| Doxifluridine
(n=102) | Uracil/tegafur
(n=99) |
|---|
|
|
|
|---|
| G1 | G2 | G3 | G4 | Total | G1 | G2 | G3 | G4 | Total |
|---|
| Nausea | 3 | 4 | | | 7 | 4 | 4 | 2 | | 10 |
| Vomiting | | | | | | | 2 | 1 | | 3 |
| Anorexia | 2 | 5 | 1 | | 8 | 13 | 5 | 1 | 1b | 20 |
| Diarrhea | 7 | 4 | 2 | | 13 | 4 | 5 | 1 | | 10 |
| Stomatitis | 5 | 1 | | | 6 | 2 | | | | 2 |
| Alopecia | 1 | | | | 1 | 2 | | | | 2 |
| Pigmentation
change | 3 | | | | 3 | 4 | | | | 4 |
|
Rash/desquamation | 1 | 4 | | | 5 | 4 | 7 | | | 11 |
| Fatiguea | 5 | 2 | 1 | | 8 | 8 | 4 | 1 | | 13 |
| Hematuria | 3 | | | | 3 | 2 | | | | 2 |
| Increased
creatinine | 3 | | | | 3 | 2 | | | | 2 |
| Decreased WBC | 7 | 3 | | | 10 | 4 | 7 | | | 11 |
| Decreased platelet
count | 9 | | | | 9 | 4 | | | | 4 |
| Decreased
hemoglobin | 7 | 6 | 1 | | 14 | 13 | 7 | | | 20 |
| Increased
bilirubin | 15 | 13 | | | 28 | 22 | 3 | | | 25 |
| Increased
GOT/GPT | 11 | 3 | 1 | | 15 | 22 | 5 | 2 | | 29 |
| Other | 4 | 2 | 2 | | 10c | 6 | 1 | 1 | 1b | 11d |
Adverse events assessed as grade ≥2 that resulted in
treatment discontinuation or dose reduction were reported in 56
patients in the UFT group and 55 patients in the doxifluridine
group (note: patients may have experienced ≥1 event).
Intratumoral markers
TP and DPD protein levels were measured in 195
patients and mRNA in 170 patients. Median TP and DPD protein levels
and TP, DPD, TS and OPRT mRNA levels showed no significant
differences between the 2 groups (Table III).
 | Table IIIIntratumoral markers. |
Table III
Intratumoral markers.
| Median (range) |
|---|
|
|
|---|
| Doxifluridine
(n=98) | Uracil/tegafur
(n=97) |
|---|
| Protein levels
(U/mg protein) |
| TP | 81.3
(13.2–242.5) | 80.3
(13.7–301.3) |
| DPD | 34.4
(4.9–124.2) | 32.8
(8.8–139.8) |
| TP/DPD | 2.663
(0.54–6.94) | 2.569
(0.75–9.57) |
|
| Doxifluridine
(n=85) | Uracil/tegafur
(n=85) |
|
| mRNA levels (copy
number)a |
| TP | 2.61
(0.21–21.49) | 2.93
(0.24–141.16) |
| DPD | 0.13
(0.02–1.92) | 0.18
(0.006–1.57) |
| TP/DPD | 19.47
(2.19–145.66) | 22.06
(1.32–558.16) |
| TS | 1.06
(0.23–14.86) | 1.17
(0.04–20.40) |
| OPRT | 0.82
(0.04–7.37) | 0.88
(0.182–6.18) |
Assessment of marker predictiveness
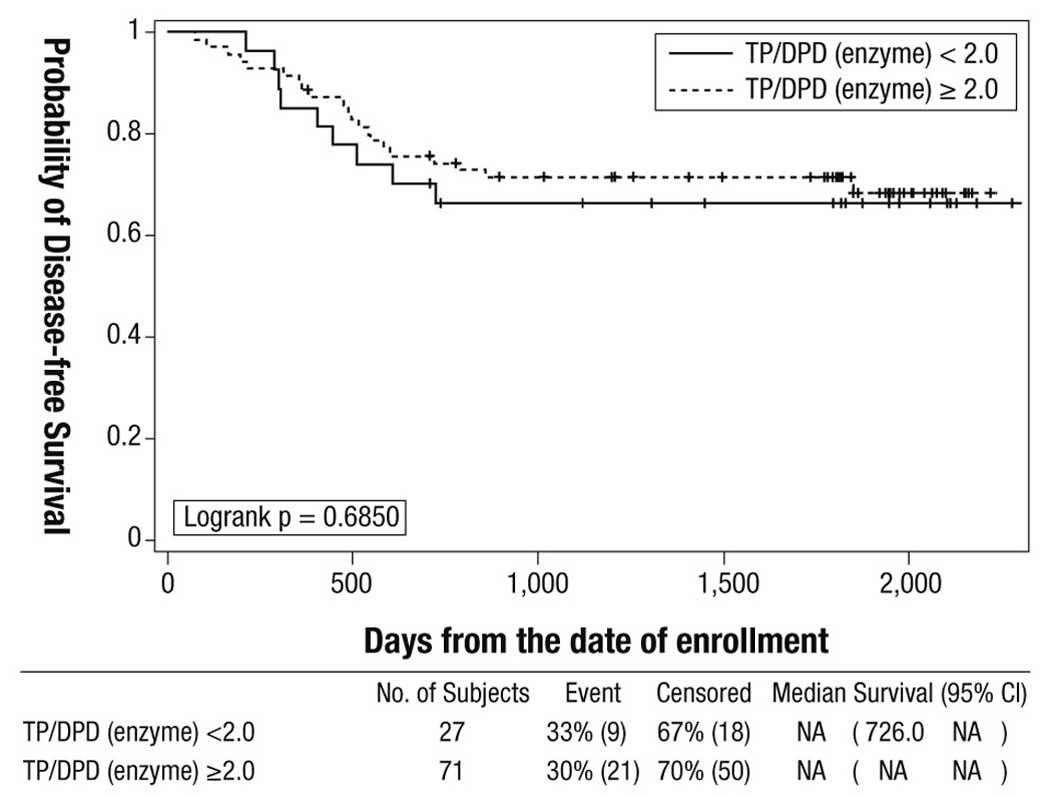
For the effect of intratumoral TP/DPD enzyme ratio
(cut-off value 2.0) on disease-free survival in the doxifluridine
group, the primary study endpoint, there was no statistically
significant difference between the 2 cohorts in the 5-year
disease-free survival rate: TP/DPD ratio ≥2.0, 71.4% (95% CI
59.3–80.5) vs. TP/DPD ratio <2.0, 66.5% (95% CI 45.4–80.9)
(log-rank P=0.6850) (Fig. 1).
Results for the secondary endpoints were as follows:
i) The effect of the magnitude of intratumoral TP/DPD enzyme ratio
on disease-free survival with oral fluoropyrimidine treatment was
only significant (HR=2.76, P=0.00469) when analyzed in the model
including the drug and the parameter and the interactions between
them. Proportional hazards analysis showed that the effects of TP
and DPD protein levels on disease-free survival and the effects of
the parameters (i.e. TP, DPD protein levels and TP/DPD enzyme
ratio) on overall survival were not significant in the models (data
not shown).
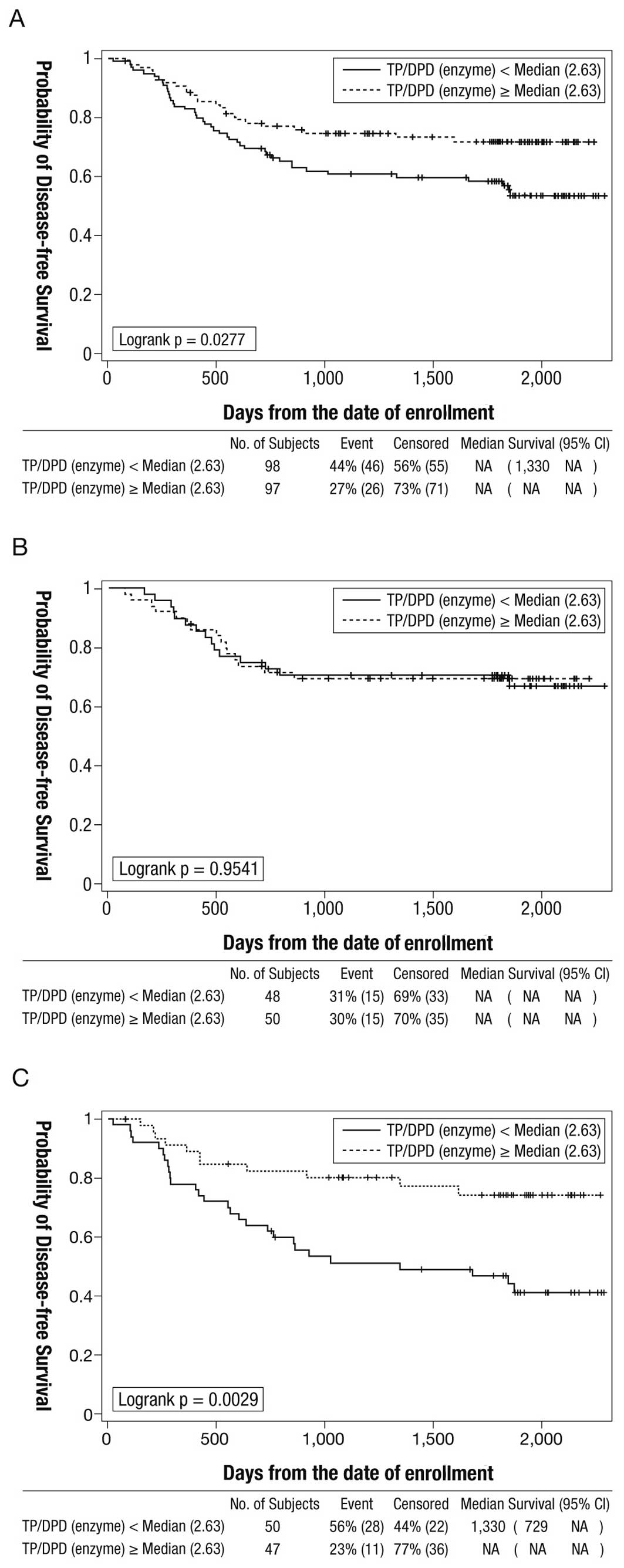
The 5-year disease-free survival rate was
statistically significantly higher in the cohort with a higher
intratumoral TP/DPD enzyme ratio (median ≥2.63) compared to the
cohort with a lower TP/DPD ratio (<2.63): 71.9% (95% CI
61.4–80.0) vs. 57.0% (95% CI 46.3–66.3) (log-rank P=0.0277)
(Fig. 2A). The effects of the
magnitude of the TP/DPD ratio on disease-free survival in each of
the doxifluridine and UFT groups are shown in Fig. 2. In the UFT group, the 5-year
disease-free survival rate was statistically significantly higher
in the cohort with the higher TP/DPD ratio (median ≥2.63) than in
the cohort with a lower TP/DPD ratio (median <2.63): 74.4% (95%
CI 58.2–85.1) vs. 44.1% (95% CI 29.7–57.6) (log-rank P=0.0029)
(Fig. 2C). In the doxifluridine
group, the effect of the magnitude of the TP/DPD ratio on
disease-free survival was not statistically significant (log-rank
P=0.9541) (Fig. 2B).
ii) Proportional hazard analysis demonstrated that
the effects of the parameters (i.e. intratumoral TP, DPD, TS and
OPRT mRNA levels and TP/DPD mRNA ratio) on disease-free and overall
survival were not significant in any of the models (data not
shown).
iii) Proportional hazards analysis showed that the
effects of the parameters (i.e. TS tandem repeat type 2R/2R, 2R/3R
or 3R/3R) on disease-free and overall survival were not significant
in the models (data not shown).
Exploratory analyses
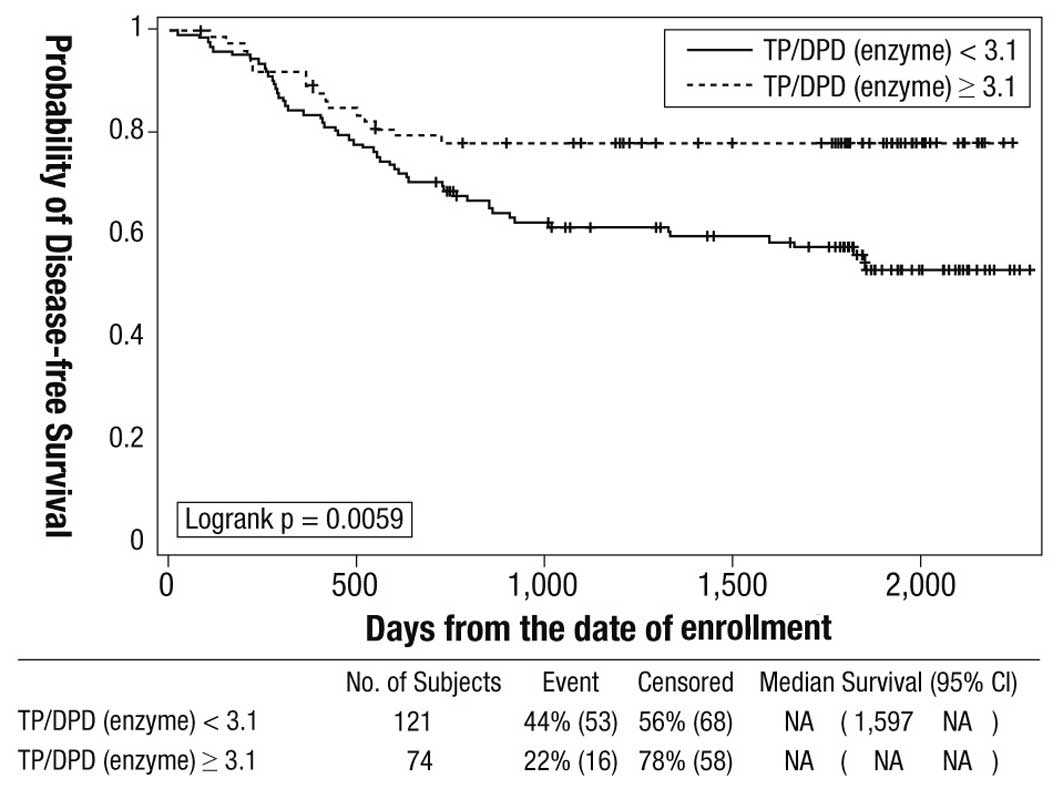
An exploratory Cox regression analysis identified a
cut-off value of 3.1 for the intratumoral TP/DPD enzyme ratio to
distinguish between patients with good and poor outcomes with oral
fluoropyrimidine treatment. The 5-year disease-free survival rates
in cohorts with TP/DPD ratios ≥3.1 and <3.1 were 77.7 and 56.1%,
respectively (log-rank P=0.0059) (Fig.
3). Multiple regression analysis using Cox's method identified
T4 (P=0.0068; HR=2.225; 95% CI 1.247–3.969) and intratumoral TP/DPD
enzyme ratio ≥3.1 (P=0.0035; HR=0.394; 95% CI 0.211–0.735) as
independent variables for prognosis. The P-values for other
variables examined in the model (i.e. N category, tumor site, drug,
TP/DPD mRNA ratio) were not statistically significant (data not
shown).
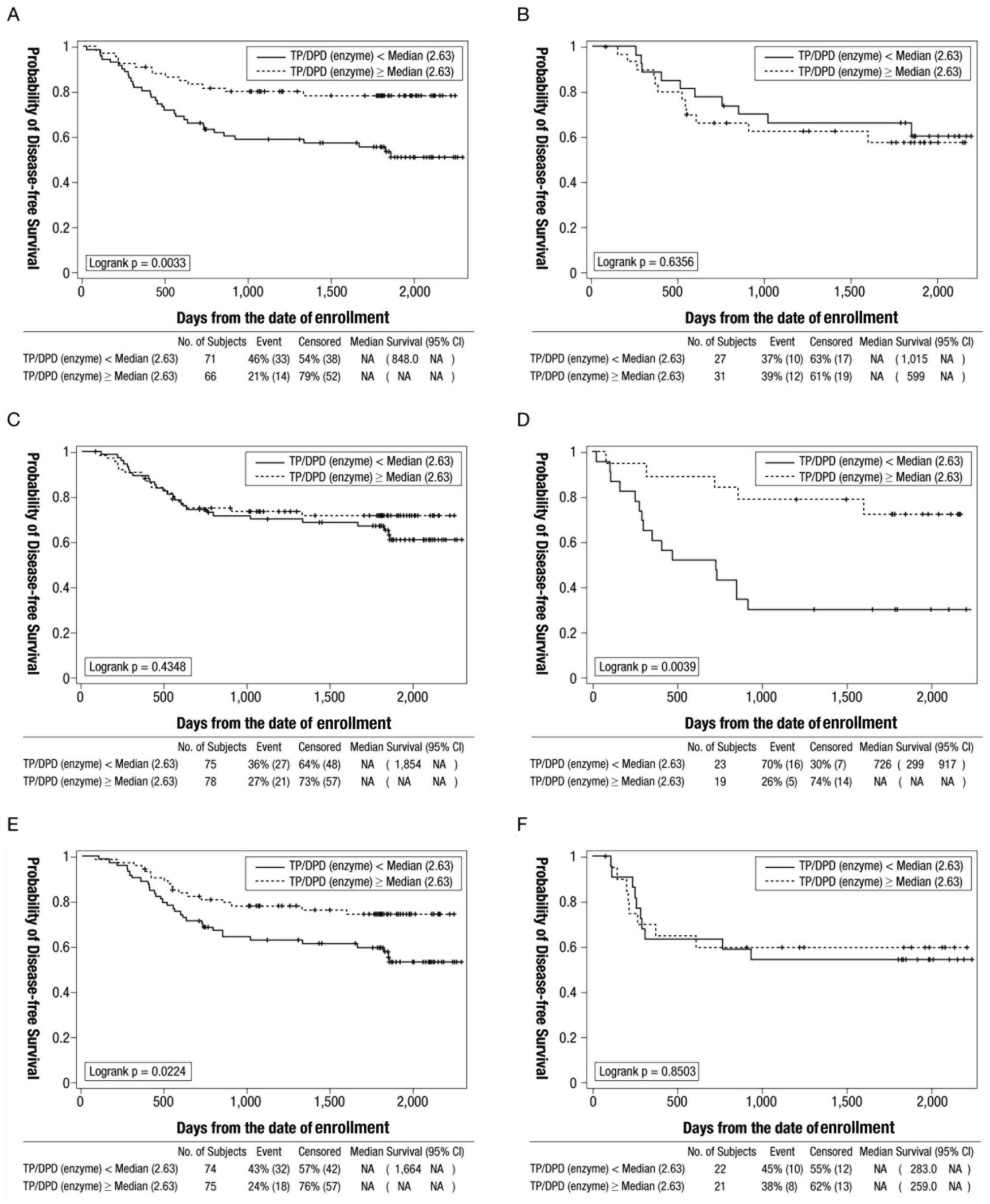
Additionally, exploratory analyses to examine the
effect of the intratumoral TP/DPD enzyme ratio on disease-free
survival by tumor site, T stage and N stage in patients treated
with oral fluoropyrimidines were conducted (Fig. 4). Analysis by tumor site displayed
that the TP/DPD ratio was significantly associated with the
disease-free survival (log-rank P=0.0033) in patients with colon
cancer. Analysis by T category displayed an association between
TP/DPD ratio and disease-free survival (log-rank P=0.0039) in
patients with T4, but not T1-3. Analysis by N category displayed an
association between TP/DPD ratio and disease-free survival
(log-rank P=0.0224) in patients with N1, but without N2
disease.
Discussion
In this study, we examined several biomarkers for
their possible predictive value in relation to the outcome
following adjuvant chemotherapy with oral fluoropyrimidines in
patients with stage III colorectal cancer in order to identify
subgroups of patients who may benefit from this intervention. Our
findings on the predictive value of biomarkers in tumor cells and
blood samples were unexpected.
The TP/DPD ratio is significantly higher in cancer
cell lines with high sensitivity for doxifluridine and capecitabine
compared to those with lower sensitivity (8). In addition, synergistic effects were
observed when doxifluridine was used in combination with
chemotherapeutic agents or radiotherapy in vivo, since tumor
TP levels are increased by these treatments (15). This synergistic antitumor activity,
which results from TP upregulation, is specific for doxifluridine,
not for UFT or 5-FU (15). These
data suggest that TP may be a potential factor for determining the
outcome following doxifluridine treatment. There was a significant
association between the magnitude of intratumoral TP/DPD enzyme
ratio and disease-free survival (HR=2.76; P=0.00469) when the
interaction between the drug and this parameter was analyzed. The
5-year disease-free survival rate in the group with a high TP/DPD
ratio (median ≥2.63) was statistically significantly higher
compared to the group with a low TP/DPD ratio (median <2.63;
log-rank P=0.0277).
However, the effect of the intratumoral TP/DPD
enzyme ratio (cut-off value both 2.0 and median of 2.63) on
disease-free survival was not statistically significant in the
doxifluridine group (log-rank P=0.6850, 0.9541, respectively). TP
levels measured using tumor samples were found to be more important
for doxifluridine (and capecitabine) compared to using normal or
blood samples, since only fluoropyrimidine converted by TP in the
tumor has a direct effect (16,17).
For measurement, tissues which included both the tumor and stroma
cells were used, since fluoropyrimidine converted in cells with
high TP expression near the tumor may also have a tumor response
(18). Unexpected results may be
caused by TP levels not being stable before and after treatment
initiation, since TP is induced by a number of cytokines (19,20).
TP levels were measured from resected primary lesion tumors and
outcomes were analyzed 5 years after treatment initiation, which
may explain the different outcome from the in vivo study
(8).
The effect of the intratumoral TP/DPD enzyme ratio
(cut-off median of 2.63) on disease-free survival was statistically
significant in the UFT group (log-rank P=0.0029). UFT confers its
effect by maintaining high fluoropyrimidine levels in the blood and
not in the tumor. However, since TP levels in the tumor catalyze
the conversion between fluoropyrimidine and
2′-deoxy-5-fluorouridine (FUdR) [precursor of
5-fluoro-2′-deoxyuridine-5′-monophosphate (FdUMP) which has an
antitumor effect] and high TP levels produce deoxy
ribose-1-phosphate (dR1P) from thymidine (dR1P promotes the
conversion between fluoropyrimidine and FUdR) (21), high TP expression may be meaningful
for UFT.
The association between TS, OPRT and the sensitivity
to fluoropyrimidine drugs has been observed in previous research
(10,22–25).
It has also been reported that combined analysis, such as analysis
with TS and TP, DPD, with OPRT and DPD may predict efficacy or
outcome of treatment with fluoropyrimidines more precisely
(10,26–28).
In the present study we did not demonstrate an association between
any factors other than the TP/DPD enzyme ratio and the efficacy of
fluoropyrimidine drugs.
For TS tandem repeat type, it was previously
observed that the level of expression of the TS protein is higher
and that fluoropyrimidine drugs are less efficacious in patients
with the 3R/3R allele compared to those with 2R/2R or 2R/3R alleles
(29). Previous research reported
that TS tandem polymorphisms are potentially predictive biomarkers,
not only of response, but also for the occurrence of adverse
events. Conversely, other research has claimed that TS-tandem
polymorphisms are not associated with the efficacy of
fluoropyrimidine drugs (30). The
results of our study were negative for the possibility that
TS-tandem type is potentially predictive of an outcome following
oral fluoropyrimidine adjuvant therapy.
Our study has several important design features.
First, since this study targeted stage III cancer, the number of
residual cancer cells persisting after standard curative resection
may have had a major effect on postoperative survival. For this
reason, the skill of the surgeon is a major factor. Since the
institutions participating in our study belonged to the Japanese
Society for Cancer of the Colon and Rectum, we hypothesize that
surgeon-related factors were minimized. Other factors which may
have influenced our findings were potential differences in the
timing of the collection and handling of tissue specimens and the
differences between individuals in medication compliance.
Participating sites were rigorously drilled in the methodology for
the collection of specimens. Institutions which returned specimens
with considerable degradation during the study were provided with
on-site guidance and the requirement for prompt processing was
reinforced. Medication compliance was assessed every quarter by the
investigator by directly interviewing each patient. Patients were
asked for detailed reasons in the event of interruption or
discontinuation of the medication. We hypothesize that biases in
this study were minimal as a result of these measures.
Our study has some limitations. One of the most
serious issues is that there was no control arm (i.e. surgery-alone
group). TP is identical to platelet-derived endothelial cell growth
factor (PD-ECGF) (31) and patients
with high levels of tumoral TP expression have a poor prognosis
(32,33). This may be important, since patients
in the high-TP group may be especially responsive to doxifluridine
(9), although there were no
significant differences compared with UFT in our study. This may
indicate that TP is a prognostic, as well as a predictive, marker.
A surgery-alone group may have helped to clarify this issue and
explain our unexpected results. In conclusion, the magnitude of the
intratumoral TP/DPD enzyme ratio may predict outcomes in patients
with stage III colorectal cancer who are treated with adjuvant
chemotherapy with oral fluoropyrimidines. The intratumoral TP/DPD
enzyme ratio may therefore allow the individualization of
postoperative oral fluoropyrimidine adjuvant therapy in stage III
colorectal cancer. Further exploration and verification of the
magnitude of the intratumoral TP/DPD enzyme ratio as a biomarker is
required.
Acknowledgements
The authors thank all the investigators who
participated in this trial: Kazuo Hase, National Defense Medical
College Hospital, Tokorozawa; Naohiro Tomita, Kansai Rosai Hospital
(now transferred to Hyogo College of Medicine), Amagasaki; Makoto
Akaike, Kanagawa Cancer Center, Yokohama; Akihiko Murata, Hirosaki
University School of Medicine and Hospital, Hirosaki; Kazuo
Shirouz, Kurume University School of Medicine, Kurume and Yozi
Nishimura, Saitama Cancer Center, Ina-cho, Saitama, Japan. We also
wish to thank Chugai Pharmaceutical Co., Ltd Research Center,
Kamakura, for providing tumor tissue assay and analysis and EPS
Co., Ltd, Tokyo, for clinical data collection. Funding support was
provided by the Japan-China Medical Association.
References
|
1
|
Center for Cancer Control and Information
Services, National Cancer Center, Japan. http://ganjoho.ncc.go.jp/public/statistics/index.html.
Accessed August 16, 2011
|
|
2
|
Efficacy of adjuvant fluorouracil and
folinic acid in colon cancer. International Multicentre Pooled
Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet.
345:939–944. 1995. View Article : Google Scholar
|
|
3
|
Haller DG, Catalano PJ, Macdonald JS, et
al: Phase III study of fluorouracil, leucovorin, and levamisole in
high-risk stage II and III colon cancer: final report of Intergroup
0089. J Clin Oncol. 23:8671–8678. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Comparison of fluorouracil with additional
levamisole, higher-dose folinic acid, or both, as adjuvant
chemotherapy for colorectal cancer: a randomised trial. QUASAR
Collaborative Group. Lancet. 355:1588–1596. 2000. View Article : Google Scholar
|
|
5
|
André T, Quinaux E, Louvet C, et al: Phase
III study comparing a semimonthly with a monthly regimen of
fluorouracil and leucovorin as adjuvant treatment for stage II and
III colon cancer patients: final results of GERCOR C96.1. J Clin
Oncol. 25:3732–3738. 2007.PubMed/NCBI
|
|
6
|
Twelves C, Wong A, Nowacki MP, et al:
Capecitabine as adjuvant treatment for stage III colon cancer. N
Engl J Med. 352:2696–2704. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lembersky BC, Wieand HS, Petrelli NJ, et
al: Oral uracil and tegafur plus leucovorin compared with
intravenous fluorouracil and leucovorin in stage II and III
carcinoma of the colon: results from National Surgical Adjuvant
Breast and Bowel Project Protocol C-06. J Clin Oncol. 24:2059–2064.
2006. View Article : Google Scholar
|
|
8
|
Ishikawa T, Sekiguchi F, Fukase Y, et al:
Positive correlation between the efficacy of capecitabine and
doxyfluridine and the ratio of thymidine phosphorylase to
dihydropyrimidine dehydrogenase activities in tumors in human
cancer xenografts. Cancer Res. 58:685–690. 1998.
|
|
9
|
Nishimura G, Terada I, Kobayashi T, et al:
Thymidine phosphorylase and dihydropyrimidine dehydrogenase levels
in primary colorectal cancer show a relationship to clinical
effects of 5′-deoxy-5-fluorouridine as adjuvant chemotherapy. Oncol
Rep. 9:479–482. 2002.PubMed/NCBI
|
|
10
|
Ichikawa W, Uetake H, Shirota Y, et al:
Both gene expression for orotate phosphoribosyltransferase and its
ratio to dihydropyrimidine dehydrogenase influence outcome
following fluoropyrimidine-based chemotherapy for metastatic
colorectal cancer. Br J Cancer. 89:1486–1492. 2003. View Article : Google Scholar
|
|
11
|
Sakamoto J, Ohashi Y, Hamada C, et al:
Efficacy of oral adjuvant therapy after resection of colorectal
cancer: 5-year results from three randomized trials. J Clin Oncol.
22:484–492. 2004.PubMed/NCBI
|
|
12
|
Nishida M, Hino A, Mori K, et al:
Preparation of anti-human thymidine phosphorylase monoclonal
antibodies useful for detecting the enzyme levels in tumor tissues.
Biol Pharm Bull. 19:1407–1411. 1996. View Article : Google Scholar
|
|
13
|
Mori K, Hasegawa M, Nishida M, et al:
Expression levels of thymidine phosphorylase and dihydropyrimidine
dehydrogenase in various human tumor tissues. Int J Oncol.
17:33–38. 2000.PubMed/NCBI
|
|
14
|
Kawakami K and Watanabe G: Identification
and functional analysis of single nucleotide polymorphism in the
tandem repeat sequence of thymidylate synthase gene. Cancer Res.
63:6004–6007. 2003.PubMed/NCBI
|
|
15
|
Sawada N, Ishikawa T, Fukase Y, et al:
Induction of thymidine phosphorylase activity and enhancement of
capecitabine efficacy by taxol/taxotere in human cancer xenografts.
Clin Cancer Res. 4:1013–1019. 1998.PubMed/NCBI
|
|
16
|
Gieschke R, Burger HU, Reigner B, et al:
Population pharmacokinetics and concentration-effect relationships
of capecitabine metabolites in colorectal cancer patients. Br J
Clin Pharmacol. 55:252–263. 2003. View Article : Google Scholar
|
|
17
|
Patterson AV, Zhang H, Moghaddam A, et al:
Increased sensitivity to the prodrug 5′-deoxy-5-fluorouridine and
modulation of 5-fluoro-2′-deoxyuridine sensitivity in MCF-7 cells
transfected with thymidine phosphorylase. Br J Cancer. 72:669–675.
1995.
|
|
18
|
Evrard A, Cuq P, Ciccolini J, et al:
Increased cytotoxicity and bystander effect of 5-fluorouracil and
5-deoxy-5-fluorouridine in human colorectal cancer cells
transfected with thymidine phosphorylase. Br J Cancer.
80:1726–1733. 1999. View Article : Google Scholar
|
|
19
|
Toi M, Rahman MA, Bando H and Chow LW:
Thymidine phosphorylase (platelet-derived endothelial-cell growth
factor) in cancer biology and treatment. Lancet Oncol. 6:158–166.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eda H, Fujimoto K, Watanabe S, et al:
Cytokines induce thymidine phosphorylase expression in tumor cells
and make them more susceptible to 5′-deoxy-5-fluorouridine. Cancer
Chemother Pharmacol. 32:333–338. 1993.PubMed/NCBI
|
|
21
|
Miyadera K, Sumizawa T, Haraguchi M, et
al: Role of thymidine phosphorylase activity in the angiogenic
effect of platelet-derived endothelial cell growth factor/thymidine
phosphorylase. Cancer Res. 55:1687–1690. 1995.PubMed/NCBI
|
|
22
|
Lenz HJ, Hayashi K, Salonga D, et al: p53
point mutations and thymidylate synthase messenger RNA levels in
disseminated colorectal cancer: an analysis of response and
survival. Clin Cancer Res. 4:1243–1250. 1998.PubMed/NCBI
|
|
23
|
Paradiso A, Simone G, Petroni S, et al:
Thymidilate synthase and p53 primary tumour expression as
predictive factors for advanced colorectal cancer patients. Br J
Cancer. 82:560–567. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sinicrope FA, Rego RL, Halling KC, et al:
Thymidylate synthase expression in colon carcinomas with
microsatellite instability. Clin Cancer Res. 12:2738–2744. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ochiai T, Nishimura K, Noguchi H, et al:
Prognostic impact of orotate phosphoribosyl transferase among
5-fluorouracil metabolic enzymes in resectable colorectal cancers
treated by oral 5-fluorouracil-based adjuvant chemotherapy. Int J
Cancer. 118:3084–3088. 2006. View Article : Google Scholar
|
|
26
|
Salonga D, Danenberg KD, Johnson M, et al:
Colorectal tumors responding to 5-fluorouracil have low gene
expression levels of dihydropyrimidine dehydrogenase, thymidylate
synthase, and thymidine phosphorylase. Clin Cancer Res.
6:1322–1327. 2000.
|
|
27
|
Lassmann S, Hennig M, Rosenberg R, et al:
Thymidine phosphorylase, dihydropyrimidine dehydrogenase and
thymidylate synthase mRNA expression in primary colorectal tumors -
correlation to tumor histopathology and clinical follow-up. Int J
Colorectal Dis. 21:238–247. 2006. View Article : Google Scholar
|
|
28
|
Ciaparrone M, Quirino M, Schinzari G, et
al: Predictive role of thymidylate synthase, dihydropyrimidine
dehydrogenase and thymidine phosphorylase expression in colorectal
cancer patients receiving adjuvant 5-fluorouracil. Oncology.
70:366–377. 2006. View Article : Google Scholar
|
|
29
|
Park DJ, Stoehlmacher J, Zhang W, et al:
Thymidylate synthase gene polymorphism predicts response to
capecitabine in advanced colorectal cancer. Int J Colorectal Dis.
17:46–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park CM, Lee WY, Chun HK, et al:
Relationship of polymorphism of the tandem repeat sequence in the
thymidylate synthase gene and the survival of stage III colorectal
cancer patients receiving adjuvant 5-flurouracil-based
chemotherapy. J Surg Oncol. 101:22–27. 2010. View Article : Google Scholar
|
|
31
|
Goto H, Kohno K, Sone S, et al: Interferon
gamma-dependent induction of thymidine
phosphorylase/platelet-derived endothelial growth factor through
gamma-activated sequence-like element in human macrophages. Cancer
Res. 61:469–473. 2001.
|
|
32
|
Takebayashi Y, Akiyama S, Akiba S, et al:
Clinicopathologic and prognostic significance of an angiogenic
factor, thymidine phosphorylase, in human colorectal carcinoma. J
Natl Cancer Inst. 88:1110–1117. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tokunaga Y, Hosogi H, Hoppou T, et al:
Prognostic value of thymidine phosphorylase/platelet-derived
endothelial cell growth factor in advanced colorectal cancer after
surgery: evaluation with a new monoclonal antibody. Surgery.
131:541–547. 2002. View Article : Google Scholar
|