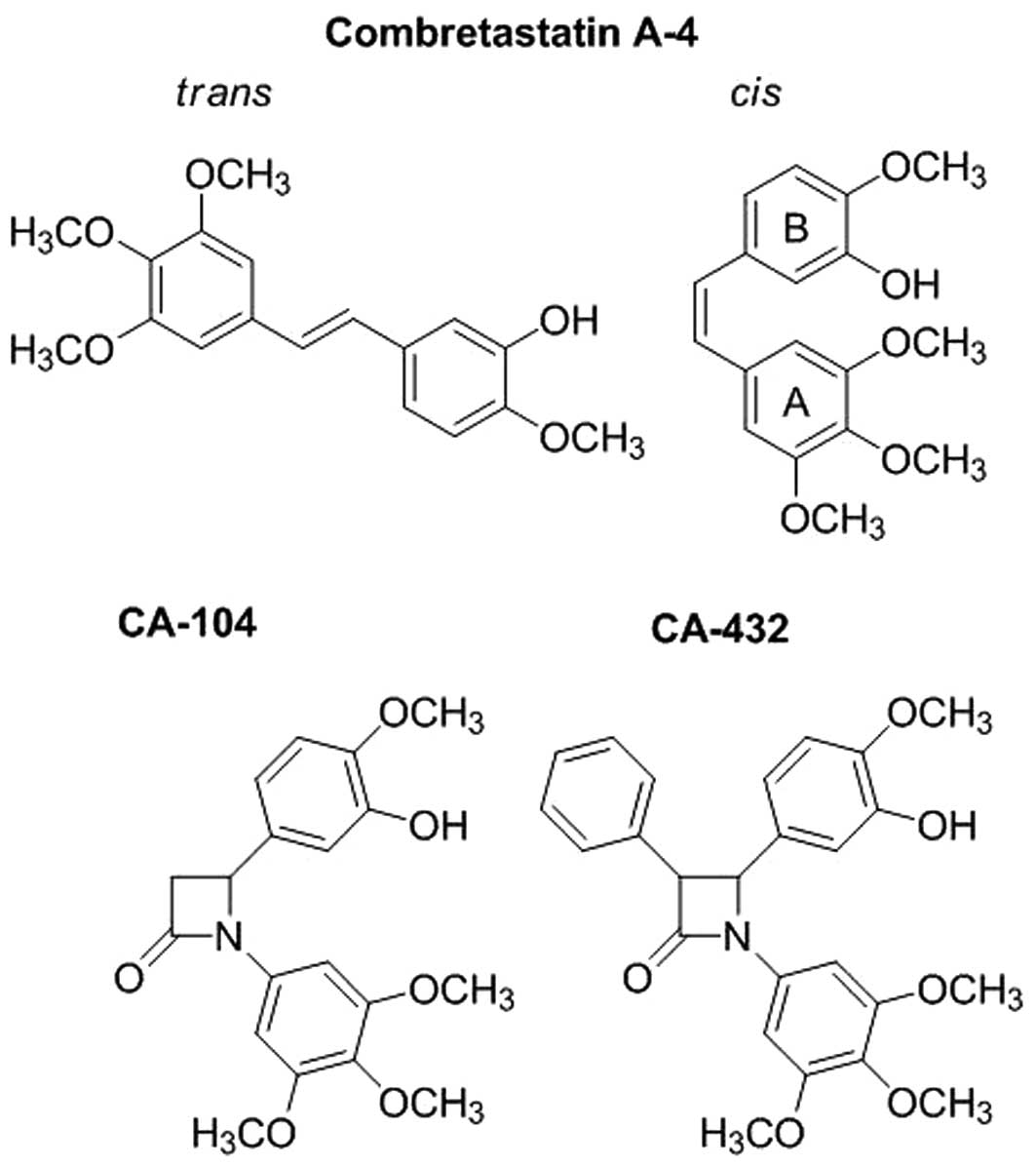
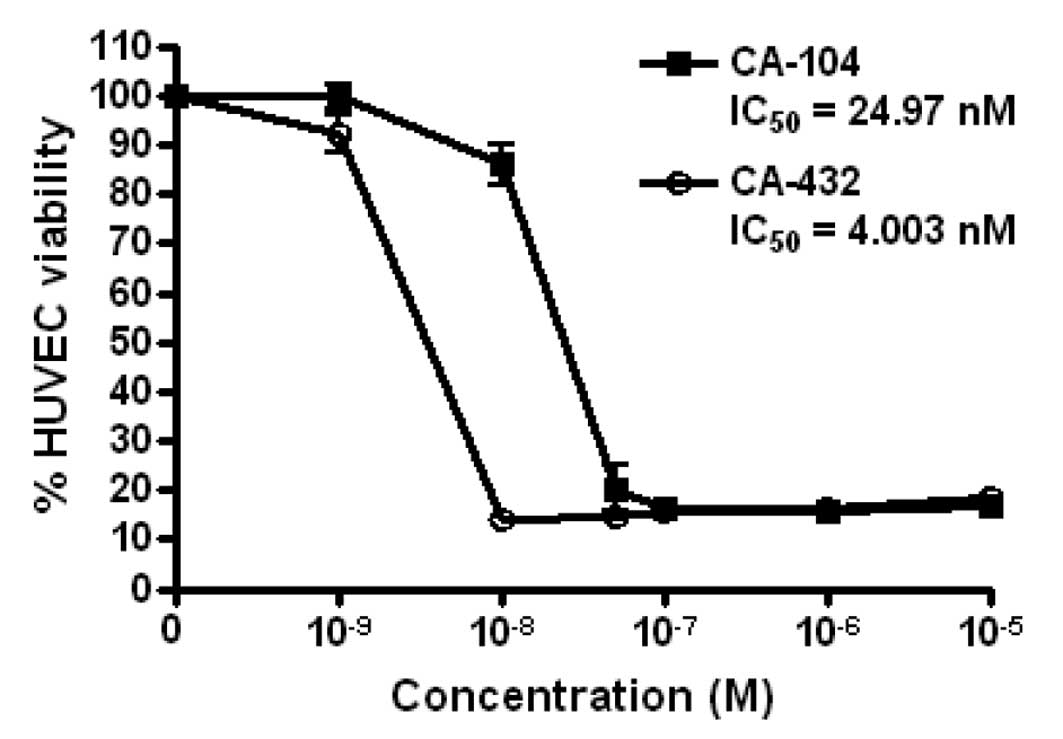
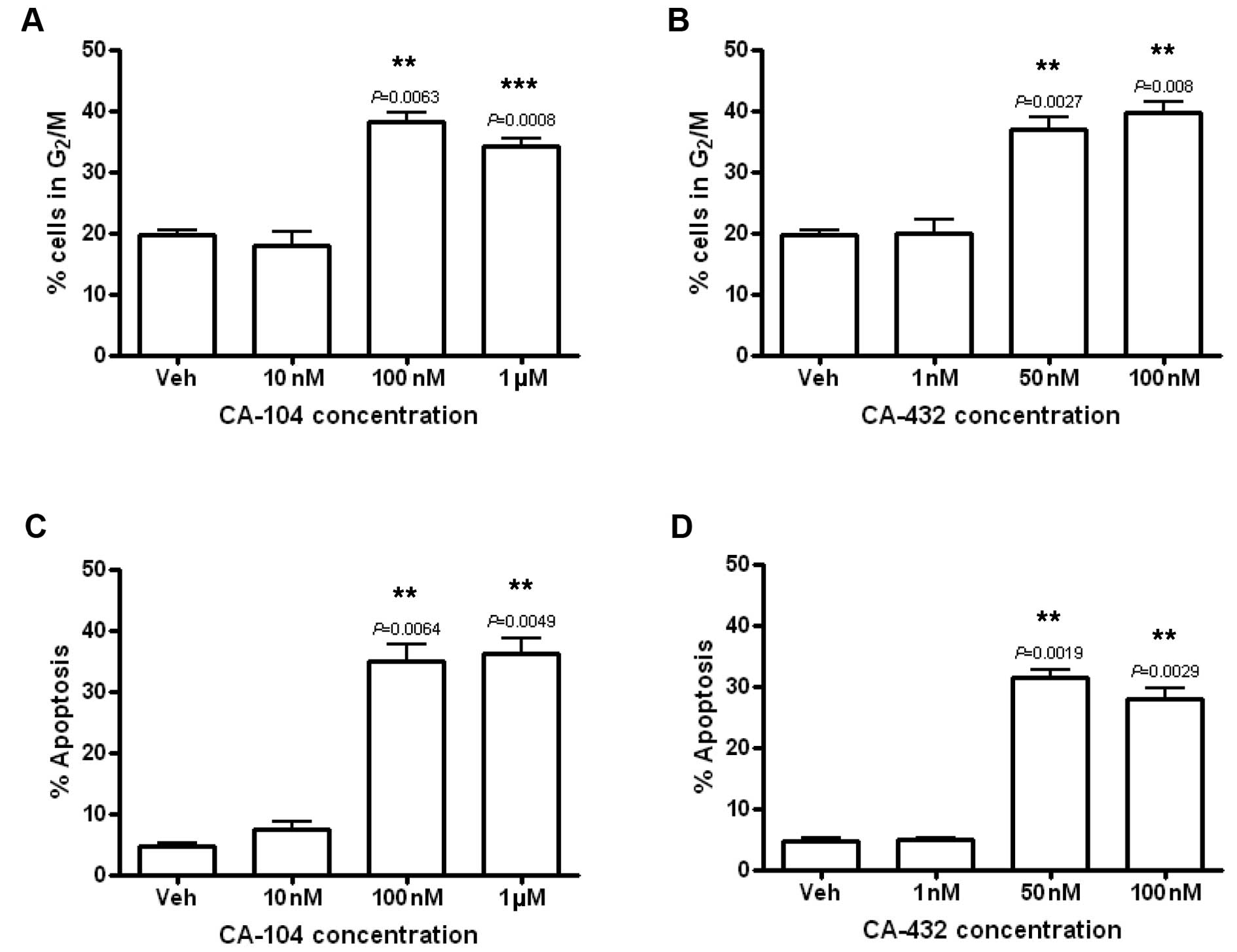
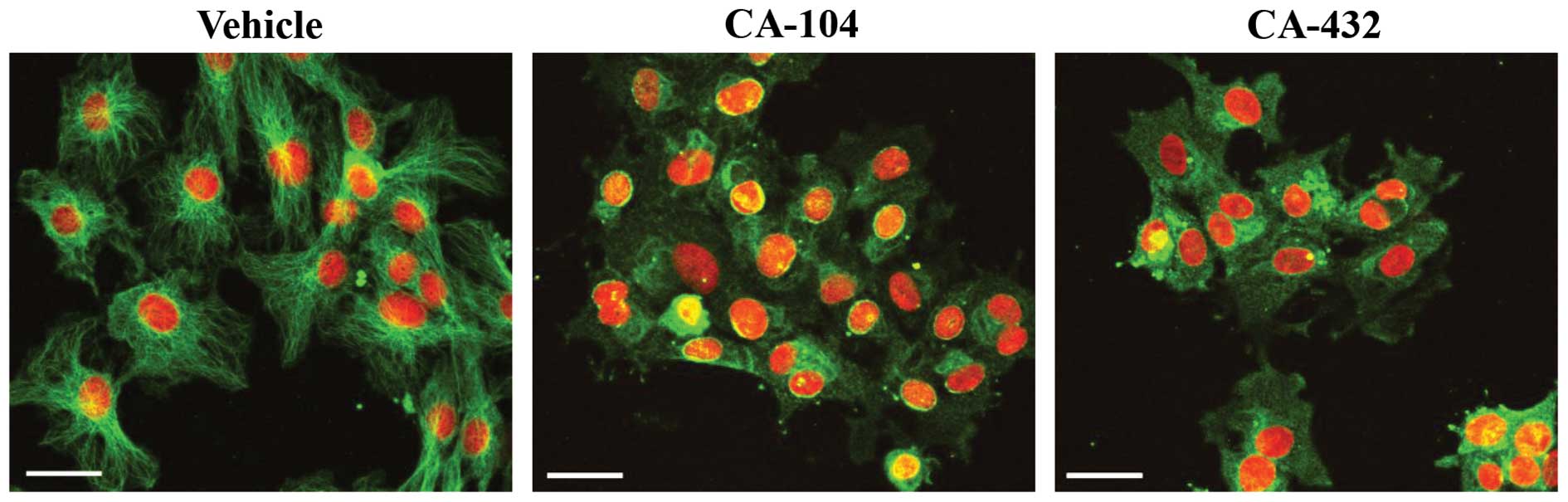
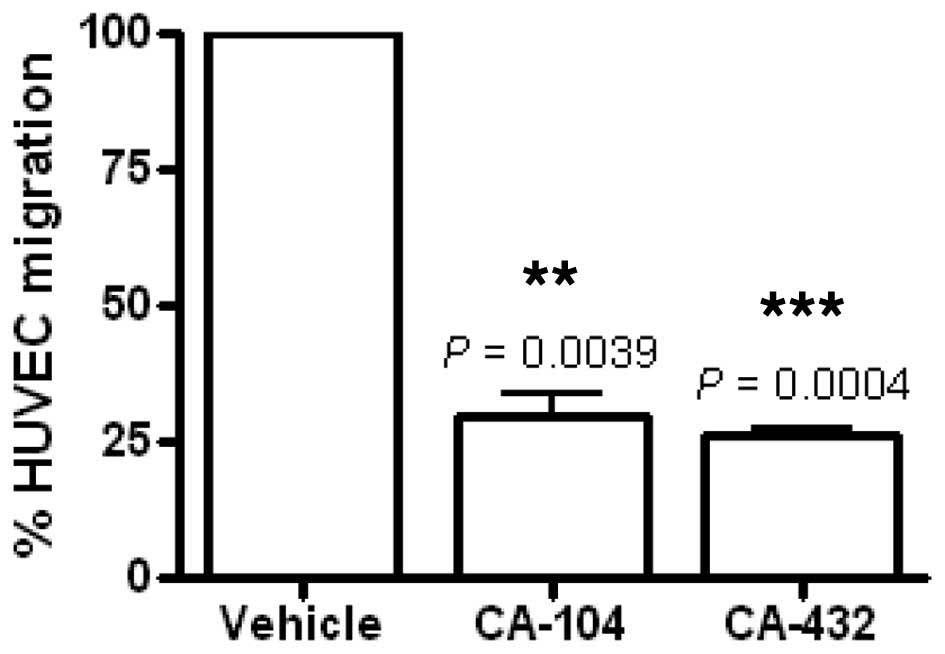
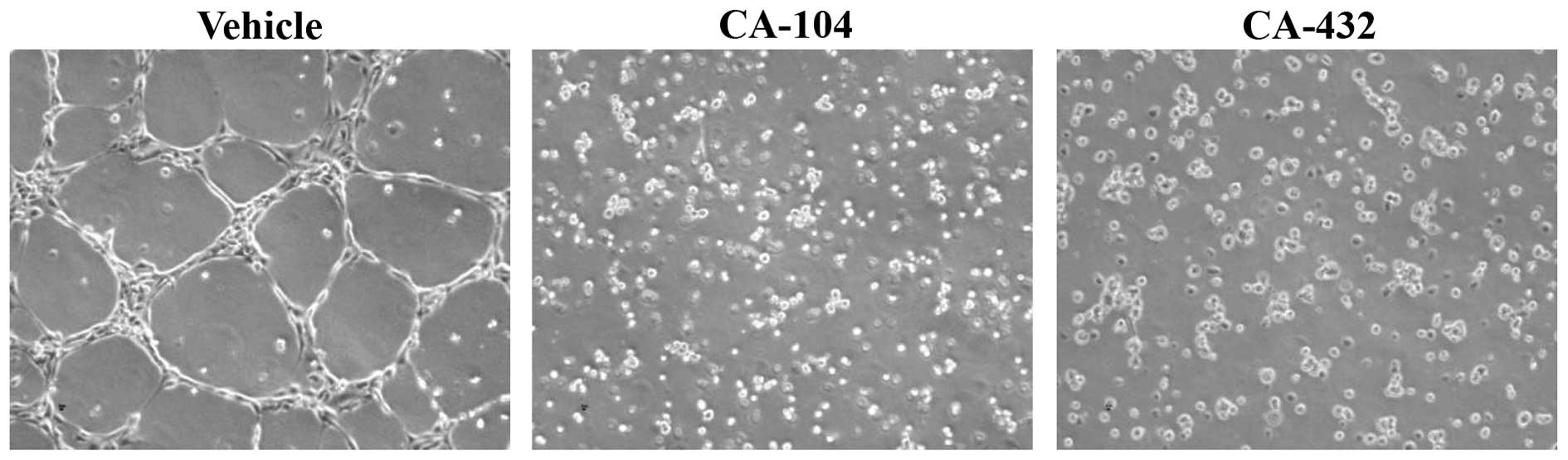
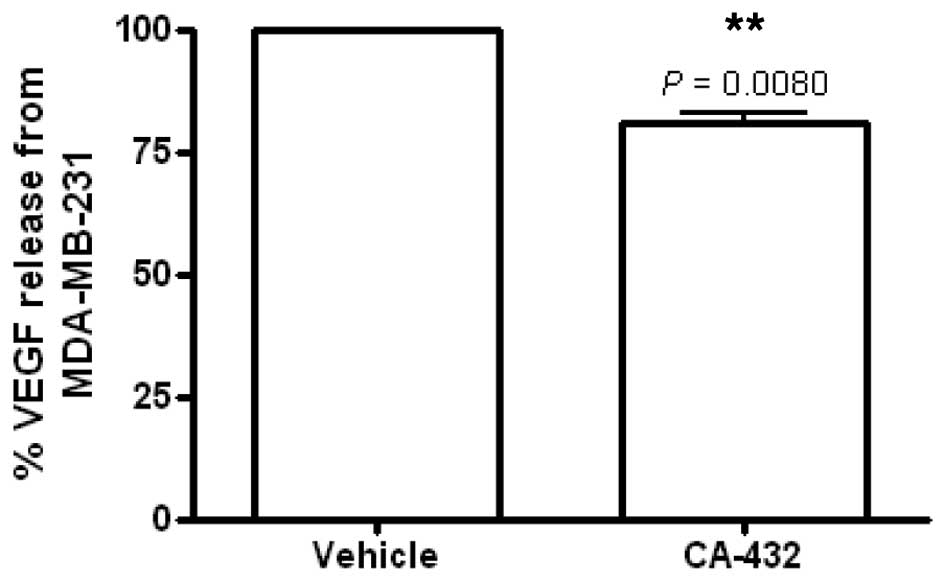
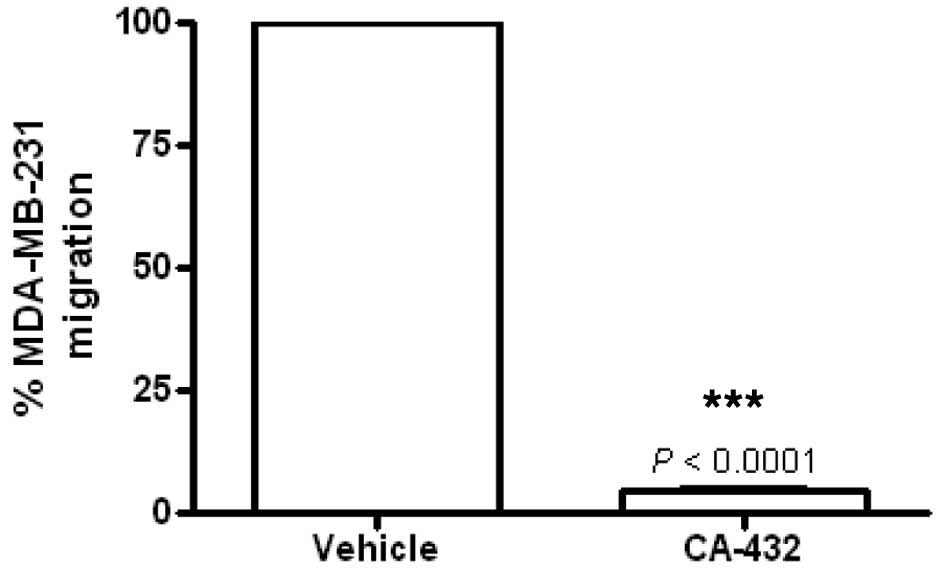
|
1
|
Pettit GR, Singh SB, Niven ML, Hamel E and
Schmidt JM: Isolation, structure, and synthesis of combretastatins
A-1 and B-1, potent new inhibitors of microtubule assembly, derived
from Combretum caffrum. J Nat Prod. 50:119–131. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin CM, Singh SB, Chu PS, Dempcy RO,
Schmidt JM, Pettit GR and Hamel E: Interactions of tubulin with
potent natural and synthetic analogs of the antimitotic agent
combretastatin: a structure-activity study. Mol Pharmacol.
34:200–208. 1988.PubMed/NCBI
|
|
3
|
Mollinedo F and Gajate C: Microtubules,
microtubule-interfering agents and apoptosis. Apoptosis. 8:413–450.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pettit GR, Singh SB, Hamel E, Lin CM,
Alberts DS and Garcia-Kendall D: Isolation and structure of the
strong cell growth and tubulin inhibitor combretastatin A-4.
Experientia. 45:209–211. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nabha SM, Wall NR, Mohammad RM, Pettit GR
and Al-Katib AM: Effects of combretastatin A-4 prodrug against a
panel of malignant human B-lymphoid cell lines. Anticancer Drugs.
11:385–392. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pettit GR, Temple C Jr, Narayanan VL,
Varma R, Simpson MJ, Boyd MR, Rener GA and Bansal N: Antineoplastic
agents 322. Synthesis of combretastatin A-4 prodrugs. Anticancer
Drug Des. 10:299–309. 1995.PubMed/NCBI
|
|
7
|
Dark GG, Hill SA, Prise VE, Tozer GM,
Pettit GR and Chaplin DJ: Combretastatin A-4, an agent that
displays potent and selective toxicity toward tumor vasculature.
Cancer Res. 57:1829–1834. 1997.PubMed/NCBI
|
|
8
|
Grosios K, Holwell SE, McGown AT, Pettit
GR and Bibby MC: In vivo and in vitro evaluation of combretastatin
A-4 and its sodium phosphate prodrug. Br J Cancer. 81:1318–1327.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rustin GJ, Galbraith SM, Anderson H,
Stratford M, Folkes LK, Sena L, Gumbrell L and Price PM: Phase I
clinical trial of weekly combretastatin A4 phosphate: clinical and
pharmacokinetic results. J Clin Oncol. 21:2815–2822. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nathan P, Zweifel M, Padhani AR, Koh DM,
Ng M, Collins DJ, Harris A, Carden C, Smythe J, Fisher N, Taylor
NJ, Stirling JJ, Lu SP, Leach MO, Rustin GJ and Judson I: Phase I
trial of combretastatin A4 phosphate (CA4P) in combination with
bevacizumab in patients with advanced cancer. Clin Cancer Res.
18:3428–3439. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mooney CJ, Nagaiah G, Fu P, Wasman JK,
Cooney MM, Savvides PS, Bokar JA, Dowlati A, Wang D, Agarwala SS,
Flick SM, Hartman PH, Ortiz JD, Lavertu PN and Remick SC: A phase
II trial of fosbretabulin in advanced anaplastic thyroid carcinoma
and correlation of baseline serum-soluble intracellular adhesion
molecule-1 with outcome. Thyroid. 19:233–240. 2009. View Article : Google Scholar
|
|
12
|
A service of the U.S. National Institutes
of Health. www.clinicaltrials.govurisimplewww.clinicaltrials.gov.
Accessed September 14, 2012
|
|
13
|
Galbraith SM, Chaplin DJ, Lee F, Stratford
MR, Locke RJ, Vojnovic B and Tozer GM: Effects of combretastatin A4
phosphate on endothelial cell morphology in vitro and
relationship to tumour vascular targeting activity in vivo.
Anticancer Res. 21:93–102. 2001.PubMed/NCBI
|
|
14
|
Kanthou C and Tozer GM: The tumor vascular
targeting agent combretastatin A-4-phosphate induces reorganization
of the actin cytoskeleton and early membrane blebbing in human
endothelial cells. Blood. 99:2060–2069. 2002. View Article : Google Scholar
|
|
15
|
Pettit GR, Rhodes MR, Herald DL, Hamel E,
Schmidt JM and Pettit RK: Antineoplastic agents. 445 Synthesis and
evaluation of structural modifications of (Z)- and
(E)-combretastatin A-41. J Med Chem. 48:4087–4099. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Woods JA, Hadfield JA, Pettit GR, Fox BW
and McGown AT: The interaction with tubulin of a series of
stilbenes based on combretastatin A-4. Br J Cancer. 71:705–711.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McGown AT and Fox BW: Structural and
biochemical comparison of the anti-mitotic agents colchicine,
combretastatin A4 and amphethinile. Anticancer Drug Des. 3:249–254.
1989.PubMed/NCBI
|
|
18
|
Carr M, Greene LM, Knox AJ, Lloyd DG,
Zisterer DM and Meegan MJ: Lead identification of conformationally
restricted β-lactam type combretastatin analogues: synthesis,
antiproliferative activity and tubulin targeting effects. Eur J Med
Chem. 45:5752–5766. 2010.
|
|
19
|
O’Boyle NM, Carr M, Greene LM, Bergin O,
Nathwani SM, McCabe T, Lloyd DG, Zisterer DM and Meegan MJ:
Synthesis and evaluation of azetidinone analogues of combretastatin
A-4 as tubulin targeting agents. J Med Chem. 53:8569–8584.
2010.PubMed/NCBI
|
|
20
|
Greene LM, Nathwani SM, Bright SA, Fayne
D, Croke A, Gagliardi M, McElligott AM, O’Connor L, Carr M, Keely
NO, O’Boyle NM, Carroll P, Sarkadi B, Conneally E, Lloyd DG, Lawler
M, Meegan MJ and Zisterer DM: The vascular targeting agent
combretastatin-A4 and a novel cis-restricted {beta}-lactam
analogue, CA-432, induce apoptosis in human chronic myeloid
leukemia cells and ex vivo patient samples including those
displaying multidrug resistance. J Pharmacol Exp Ther. 335:302–313.
2010.PubMed/NCBI
|
|
21
|
Greene LM, Carr M, Keeley NO, Lawler M,
Meegan MJ and Zisterer DM: BubR1 is required for the mitotic block
induced by combretastatin-A4 and a novel cis-restricted
β-lactam analogue in human cancer cells. Int J Mol Med. 27:715–23.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nathwani SM, Butler S, Meegan MJ, Campiani
G, Lawler M, Williams DC and Zisterer DM: Dual targeting of tumour
cells and host endothelial cells by novel microtubule-targeting
agents, pyrrolo-1,5-benzoxazepines. Cancer Chemother Pharmacol.
65:289–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Auerbach R, Lewis R, Shinners B, Kubai L
and Akhtar N: Angiogenesis assays: a critical overview. Clin Chem.
49:32–40. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kubota Y, Kleinman HK, Martin GR and
Lawley TJ: Role of laminin and basement membrane in the
morphological differentiation of human endothelial cells into
capillary-like structures. J Cell Biol. 107:1589–1598. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hoeben A, Landuyt B, Highley MS, Wildiers
H, Van Oosterom AT and De Bruijn EA: Vascular endothelial growth
factor and angiogenesis. Pharmacol Rev. 56:549–580. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fidler IJ: The pathogenesis of cancer
metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003.
|
|
27
|
Hughes L, Malone C, Chumsri S, Burger AM
and McDonnell S: Characterisation of breast cancer cell lines and
establishment of a novel isogenic subclone to study migration,
invasion and tumourigenicity. Clin Exp Metastasis. 25:549–557.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hotchkiss KA, Ashton AW, Mahmood R,
Russell RG, Sparano JA and Schwartz EL: Inhibition of endothelial
cell function in vitro and angiogenesis in vivo by docetaxel
(Taxotere): association with impaired repositioning of the
microtubule organizing center. Mol Cancer Ther. 1:1191–1200.
2002.PubMed/NCBI
|
|
29
|
Vacca A, Iurlaro M, Ribatti D, Minischetti
M, Nico B, Ria R, Pellegrino A and Dammacco F: Antiangiogenesis is
produced by nontoxic doses of vinblastine. Blood. 94:4143–4155.
1999.PubMed/NCBI
|
|
30
|
Tozer GM, Kanthou C, Parkins CS and Hill
SA: The biology of the combretastatins as tumour vascular targeting
agents. Int J Exp Pathol. 83:21–38. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pasquier E, Honore S and Braguer D:
Microtubule-targeting agents in angiogenesis: where do we stand?
Drug Resist Updat. 9:74–86. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kerbel RS: Inhibition of tumour
angiogenesis as a strategy to circumvent acquired resistance to
anti-cancer therapeutic agents. Bioessays. 13:31–36. 1991.
View Article : Google Scholar
|
|
33
|
Kamat AA, Kim TJ, Landen CN Jr, Lu C, Han
LY, Lin YG, Merritt WM, Thaker PH, Gershenson DM, Bischoff FZ,
Heymach JV, Jaffe RB, Coleman RL and Sood AK: Metronomic
chemotherapy enhances the efficacy of antivascular therapy in
ovarian cancer. Cancer Res. 67:281–288. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ohsumi K, Hatanaka T, Fujita K, Nakagawa
R, Fukuda Y, Nihei Y, Suga Y, Morinaga Y, Akiyama Y and Tsuji T:
Syntheses and antitumor activity of cis-restricted
combretastatins: 5-membered heterocyclic analogues. Bioorg Med Chem
Lett. 8:3153–3158. 1998.PubMed/NCBI
|
|
35
|
Wang L, Woods KW, Li Q, Barr KJ, McCroskey
RW, Hannick SM, Gherke L, Credo RB, Hui YH, Marsh K, Warner R, Lee
JY, Zielinski-Mozng N, Frost D, Rosenberg SH and Sham HL: Potent,
orally active heterocycle-based combretastatin A-4 analogues:
synthesis, structure-activity relationship, pharmacokinetics, and
in vivo antitumor activity evaluation. J Med Chem. 45:1697–1711.
2002. View Article : Google Scholar
|
|
36
|
Barrett I, Carr M, O’Boyle N, Greene LM,
Knox AJ, Lloyd DG, Zisterer DM and Meegan MJ: Lead identification
of conformationally restricted benzoxepin type combretastatin
analogs: synthesis, antiproliferative activity, and tubulin
effects. J Enzyme Inhib Med Chem. 25:180–194. 2010. View Article : Google Scholar
|
|
37
|
Burns CJ, Fantino E, Powell AK, Shnyder
SD, Cooper PA, Nelson S, Christophi C, Malcontenti-Wilson C,
Dubljevic V, Harte MF, Joffe M, Phillips ID, Segal D, Wilks AF and
Smith GD: The microtubule depolymerizing agent CYT997 causes
extensive ablation of tumor vasculature in vivo. J Pharmacol Exp
Ther. 339:799–806. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Böhle AS, Leuschner I, Kalthoff H and
Henne-Bruns D: Combretastatin A-4 prodrug: a potent inhibitor of
malignant hemangioendothelioma cell proliferation. Int J Cancer.
87:838–843. 2000.PubMed/NCBI
|
|
39
|
Wang J, Lou P, Lesniewski R and Henkin J:
Paclitaxel at ultra low concentrations inhibits angiogenesis
without affecting cellular microtubule assembly. Anticancer Drugs.
14:13–19. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Merchan JR, Jayaram DR, Supko JG, He X,
Bubley GJ and Sukhatme VP: Increased endothelial uptake of
paclitaxel as a potential mechanism for its antiangiogenic effects:
potentiation by Cox-2 inhibition. Int J Cancer. 113:490–498. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pasquier E, Honore S, Pourroy B, Jordan
MA, Lehmann M, Briand C and Braguer D: Antiangiogenic
concentrations of paclitaxel induce an increase in microtubule
dynamics in endothelial cells but not in cancer cells. Cancer Res.
65:2433–2440. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pourroy B, Honoré S, Pasquier E,
Bourgarel-Rey V, Kruczynsk A, Briand C and Braguer D:
Antiangiogenic concentrations of vinflunine increase the interphase
microtubule dynamics and decrease the motility of endothelial
cells. Cancer Res. 66:3256–3263. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kanthou C, Greco O, Stratford A, Cook I,
Knight R, Benzakour O and Tozer G: The tubulin-binding agent
combretastatin A-4-phosphate arrests endothelial cells in mitosis
and induces mitotic cell death. Am J Pathol. 165:1401–1411. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Iyer S, Chaplin DJ, Rosenthal DS, Boulares
AH, Li LY and Smulson ME: Induction of apoptosis in proliferating
human endothelial cells by tumour-specific antiangiogenesis agent
combretastatin A-4. Cancer Res. 58:4510–4514. 1998.PubMed/NCBI
|
|
45
|
Nabha SM, Mohammad RM, Dandashi MH,
Coupaye-Gerard B, Aboukameel A, Pettit GR and Al-Katib AM:
Combretastatin-A4 prodrug induces mitotic catastrophe in chronic
lymphocytic leukemia cell line independent of caspase activation
and poly(ADP-ribose) polymerase cleavage. Clin Cancer Res.
8:2735–2741. 2002.PubMed/NCBI
|
|
46
|
Ding X, Zhang Z, Li S and Wang A:
Combretastatin A4 phosphate induces programmed cell death in
vascular endothelial cells. Oncol Res. 19:303–309. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Greene LM, O’Boyle NM, Nolan DP, Meegan MJ
and Zisterer DM: The vascular targeting agent combretastatin-A4
directly induces autophagy in adenocarcinoma-derived colon cancer
cells. Biochem Pharmacol. 84:612–624. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
O’Boyle NM, Carr M, Greene LM, Keely NO,
Knox AJ, McCabe T, Lloyd DG, Zisterer DM and Meegan MJ: Synthesis,
biochemical and molecular modelling studies of antiproliferative
azetidinones causing microtubule disruption and mitotic
catastrophe. Eur J Med Chem. 46:4595–4607. 2011.
|
|
49
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29:15–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Conn G, Soderman DD, Schaeffer MT, Wile M,
Hatcher VB and Thomas KA: Purification of a glycoprotein vascular
endothelial cell mitogen from a rat glioma-derived cell line. Proc
Natl Acad Sci USA. 87:1323–1327. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Belotti D, Vergani V, Drudis T, Borsotti
P, Pitelli MR, Viale G, Giavazzi R and Taraboletti G: The
microtubule-affecting drug paclitaxel has antiangiogenic activity.
Clin Cancer Res. 2:1843–1849. 1996.PubMed/NCBI
|
|
52
|
Avramis IA, Kwock R and Avramis VI:
Taxotere and vincristine inhibit the secretion of the angiogenesis
inducing vascular endothelial growth factor (VEGF) by wild-type and
drug-resistant human leukemia T-cell lines. Anticancer Res.
21:2281–2286. 2001.
|
|
53
|
Nör JE, Christensen J, Liu J, Peters M,
Mooney DJ, Strieter RM and Polverini PJ: Up-regulation of Bcl-2 in
microvascular endothelial cells enhances intratumoral angiogenesis
and accelerates tumor growth. Cancer Res. 61:2183–2188.
2001.PubMed/NCBI
|
|
54
|
Tran J, Master Z, Yu JL, Rak J, Dumont DJ
and Kerbel RS: A role for survivin in chemoresistance of
endothelial cells mediated by VEGF. Proc Natl Acad Sci USA.
99:4349–4354. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li F, Ambrosini G, Chu EY, Plescia J,
Tognin S, Marchisio PC and Altieri DC: Control of apoptosis and
mitotic spindle checkpoint by survivin. Nature. 396:580–584. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Giodini A, Kallio MJ, Wall NR, Gorbsky GJ,
Tognin S, Marchisio PC, Symons M and Altieri DC: Regulation of
microtubule stability and mitotic progression by survivin. Cancer
Res. 62:2462–2467. 2002.PubMed/NCBI
|
|
57
|
Tozer GM, Prise VE, Wilson J, Cemazar M,
Shan S, Dewhirst MW, Barber PR, Vojnovic B and Chaplin DJ:
Mechanisms associated with tumor vascular shut-down induced by
combretastatin A-4 phosphate: intravital microscopy and measurement
of vascular permeability. Cancer Res. 61:6413–6422. 2001.PubMed/NCBI
|
|
58
|
Tozer GM, Kanthou C and Baguley BC:
Disrupting tumour blood vessels. Nat Rev Cancer. 5:423–435. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Vincent L, Kermani P, Young LM, Cheng J,
Zhang F, Shido K, Lam G, Bompais-Vincent H, Zhu Z, Hicklin DJ,
Bohlen P, Chaplin DJ, May C and Rafii S: Combretastatin A4
phosphate induces rapid regression of tumor neovessels and growth
through interference with vascular endothelial-cadherin signaling.
J Clin Invest. 115:2992–3006. 2005. View Article : Google Scholar
|
|
60
|
Tozer GM, Kanthou C, Lewis G, Prise VE,
Vojnovic B and Hill SA: Tumour vascular disrupting agents:
combating treatment resistance. Br J Radiol. 81:S12–S20. 2008.
View Article : Google Scholar : PubMed/NCBI
|