Introduction
The combretastatins are a family of stilbenoids,
some of which possess potent anti-tumour and anti-vascular
properties. Originally isolated from the bark of the South African
bush willow tree Combretum caffrum, these naturally
occurring stilbenes consist of two phenyl rings, the A-ring and
B-ring, linked by an ethylene bridge (1). The most extensively studied member of
the family, combretastatin A-4 (CA-4) is well documented to
function as a microtubule targeting agent (2). Microtubules are dynamic filaments
composed of α- and β-tubulin heterodimers that are key components
of the mitotic spindle. Thus, agents that interfere with
microtubule dynamics perturb mitotic cell division (3). CA-4 is a tubulin depolymerising agent
that interacts with β-tubulin at or close to its colchicine binding
site leading to the destabilisation of microtubules and preventing
the formation of the mitotic spindle and hence results in mitotic
arrest and subsequently cell death of tumour cells (2,4,5). Its
water-soluble phosphate prodrug combretastatin A-4 phosphate
(CA-4P) or fosbretabulin is readily cleaved in vivo by
non-specific endogenous phosphatases to yield active CA-4 (6).
CA-4P induced rapid selective tumour vascular
shutdown and tumour regression in both subcutaneous and
orthotopical mouse tumour models at concentrations well below the
maximum tolerated dose (7,8). It also reduced tumour blood flow in
phase I clinical trials at well tolerated doses (9,10).
Consequently, CA-4P has recently completed numerous phase II
clinical trials including trials for the use of CA-4P in the
treatment of advanced anaplastic thyroid cancer (11), CA-4P in combination with paclitaxel
and/or carboplatin in the treatment of advanced solid tumours and
combinations of CA-4P, anti-angiogenic bevacizumab, carboplatin and
paclitaxel for chemotherapy naive non-small cell lung cancer
(12). In addition, phase II trials
for the treatment of neovascular age-related macular degeneration
and polypoidal choroidal vasculopathy have also been undertaken
(12). CA-4P in combination with
bevacizumab is currently recruiting for phase II trials for
reoccurring or persistent tumours of the ovarian epithelial,
fallopian tube or peritoneal cavity and is about to enter phase I
trials for the treatment of recurrent high grade gliomas (12).
While in vitro studies have demonstrated the
anti-proliferative and cytotoxic effects of CA-4 on endothelial
cells (7,13), the rapid vascular changes observed
in vivo occur too early to be attributed to endothelial cell
death. The rapid response of endothelial cells to CA-4 is thought
to involve disruption to interphase microtubules triggering rapid
remodelling of the actin cytoskeleton, assembly of actin stress
fibres, actinomyosin contractility, formation of focal adhesions
and disruption of cell-cell junctions (14).
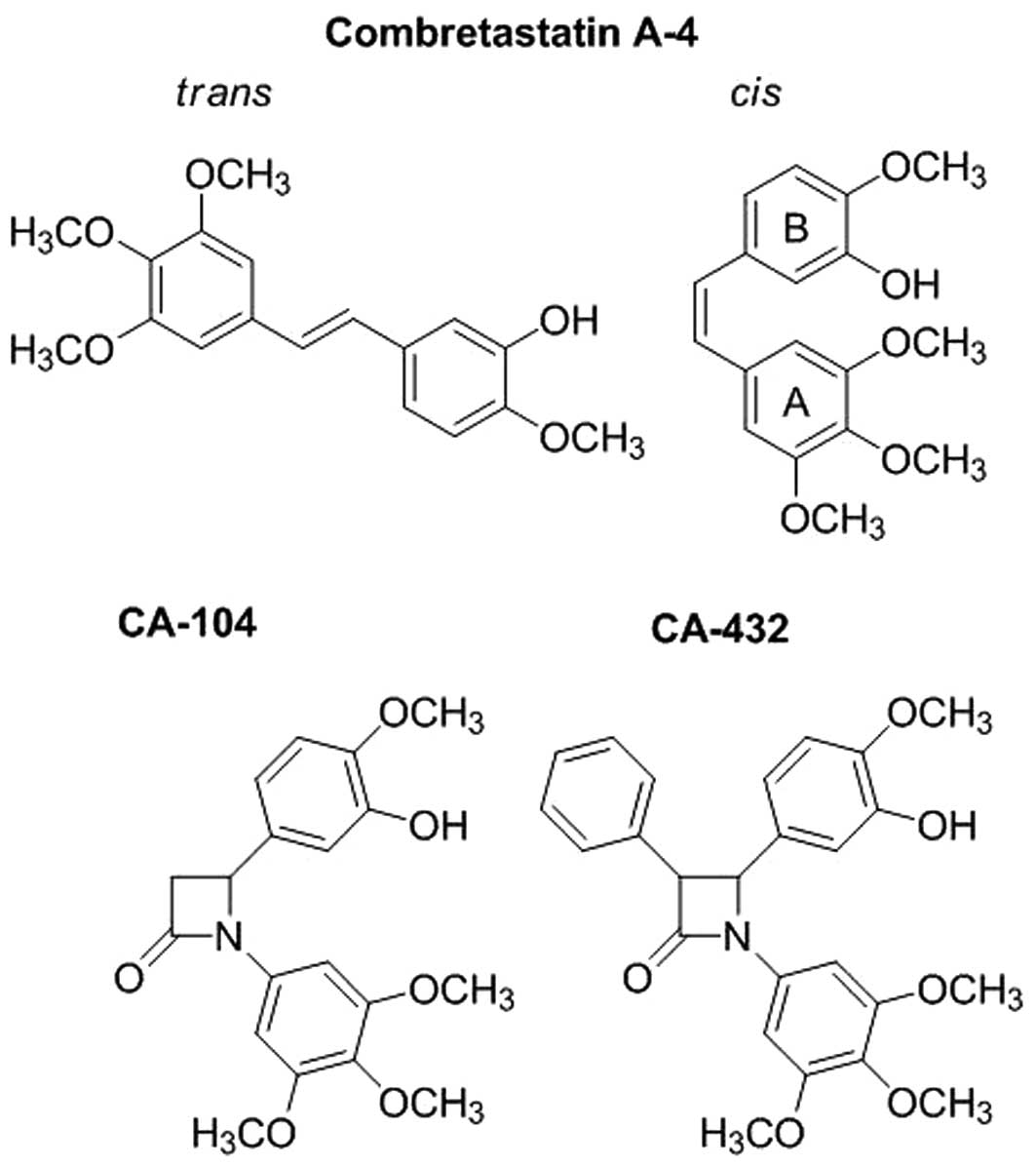
Both the success and the limitations of CA-4 lie in
its stilbene structure, illustrated in Fig. 1. Only the cis-configuration
of CA-4 is biologically active (15). The spatial arrangement between its
3,4,5-trimethyloxyphenyl A-ring and 3-hydroxy-4-methoxyphenyl
B-ring are crucial to its functionality and ability to interact
with tubulin (15–17). However, the cis-isomer is
intrinsically unstable and readily isomerises to the more
thermodynamically stable but inactive trans-configuration
(15). Cis-trans
isomerisation can be triggered by heat, light and protic media thus
lowering the therapeutic efficacy of the agent.
We recently synthesised a series of CA-4 analogues
that have been stabilised in their cis-configuration by the
replacement of the usual ethylene bridge of CA-4 with a
1,4-diaryl-2-azetidinone (β-lactam) ring (18,19).
The β-lactam ring provided a scaffold structure that retained a
similar spatial arrangement between the two phenyl rings as the
cis-conformation of CA-4. These compounds were either
unsubstituted at position C-3 of the β-lactam ring or substituted
with methyl groups (18) or aryl
rings (19). Molecular docking
studies indicated that representative compounds were capable of
interacting with tubulin with similar positioning to CA-4. Several
compounds inhibited tubulin polymerisation in vitro and
demonstrated potent anti-mitotic potential in a selection of tumour
cell lines derived of diverse origin including leukaemia, breast,
non-small cell lung, colon, CNS, melanoma, ovarian, cervical, renal
and prostate cancers (18–21).
Furthermore, low nanomolar concentrations of
representative compounds caused tubulin depolymerisation, resulting
in loss of cell viability mediated by G2M arrest and
apoptosis of breast carcinoma ER-positive MCF-7 and ER-negative
MDA-MB-231 cells whilst exerting no significant cytotoxicity to
normal murine breast epithelial cells (IC50 >10 mM)
(19). Significantly, lead compound
CA-432 also induced potent anti-tubulin, anti-proliferative and
anti-mitotic effects in human promyelocytic leukaemia HL60 cells
expressing multidrug-resistant transporters P-glycoprotein or
breast cancer resistance protein (BCRP) and in ovarian carcinoma
A2780 cells also expressing P-glycoprotein. Molecular docking
studies supported the notion that CA-432 was not a substrate for
P-glycoprotein. Furthermore, CA-432 induced apoptosis in ex
vivo samples from chronic myeloid leukaemia (CML) patients
including those displaying resistance to imatinib mesylate, the
frontline treatment for CML (20).
While our studies to date demonstrate the cytotoxic
effects of these cis-restricted β-lactam CA-4 analogues on a
variety of tumour cells derived from both the haematopoietic system
and from solid tumours, we have not investigated their effects on
endothelial cells. Tumour vascularisation is essential for tumour
growth and metastases. Therefore, the purpose of this study was to
examine the anti-vascular, anti-angiogenic and anti-metastatic
properties of these compounds through a series of in vitro
tests. We selected two lead analogues, CA-104 and CA-432, for this
purpose (Fig. 1). CA-104 was
unsubstituted at position C-3 of the β-lactam ring (18), while, CA-432 was substituted with a
4-(3-hydroxy-4-methoxyaryl ring) (19). We established that both compounds
displayed anti-endothelial properties in vitro. They induced
tubulin depolymerisation in primary human umbilical vein
endothelial cells (HUVECs). This effect was associated with a loss
in endothelial cell viability mediated by G2M arrest and
apoptosis. We also demonstrated both direct and indirect
anti-angiogenic events. Both compounds prevented migration and
in vitro capillary tubule formation by HUVECs whilst lead
compound, CA-432, reduced the release of VEGF from breast
adenocarcinoma MDA-MB-231 cells. Finally, we established that
CA-432 abrogated migration of these highly metastatic MDA-MB-231
cells. Of note, these anti-angiogenic and anti-metastatic events
preceded any cytotoxic effects attributed to the β-lactam
analogues.
These findings indicate a novel function for these
β-lactam CA-4 analogues. Our findings collectively demonstrated
that these rigid cis-restricted analogues exhibited
anti-tumour, anti-vascular, anti-angiogenic and anti-metastatic
properties with minimal toxicity to normal cells. Replacement of
the ethylene bridge with a β-lactam ring yielded compounds that
retained the in vitro functionality of CA-4 but with the
additional advantage of conformational stability.
Materials and methods
Unless otherwise stated, chemicals were obtained
from Sigma-Aldrich (Poole, Dorset, UK) and tissue culture vessels
were sourced from Greiner Bio-One GmbH (Frickenhausen,
Germany).
Cell culture
Pooled primary human umbilical vein endothelial
cells (HUVECs) and their associated reagents were all obtained from
Cascade Biologics (Invitrogen, Carlsbad, CA, USA). HUVECs were
maintained between passages 1–4 in Medium 200 supplemented with
LSGS (low serum growth factor supplement) and utilised for
experiments at passage 4. Human breast adenocarcinoma MDA-MB-231
cells (ATCC, Manassas, VA, USA) were maintained in Dulbecco’s
modified Eagle’s medium (DMEM) enhanced with GlutaMAX-I and
supplemented with 10% foetal bovine serum, 50 U/ml penicillin and
50 μg/ml streptomycin (all purchased from Gibco, Invitrogen). Cells
were maintained in a humidified incubator at 37°C in 5%
CO2 and were subcultured by trypsinisation upon reaching
70–80% confluency.
Reagents
Two representative cis-restricted β-lactam
combretastatin A-4 analogues were used in this study. Their
structures are illustrated in Fig.
1. Analogue
4-(3-hydroxy-4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one
(CA-104) was synthesised as previously described in Carr et
al(18) where this compound was
referred to as compound 12d. Analogue
4-(3-hydroxy-4-methoxyphenyl)-3-phenyl-1-(3,4,5-trimethoxyphenyl)azetidin-2-one
(CA-432) was synthesised as described in O’Boyle et
al(19) where it was referred
to as compound 35. The analogues were dissolved in ethanol and
stored in the dark at −20°C. Human recombinant VEGF165 (R&D
Systems Inc., Minneapolis, MN, USA) was reconstituted to 1 μg/ml in
0.1% (w/v) bovine serum albumin (BSA) and also stored at −20°C.
Cell viability assays
HUVECs (20,000 cells/well) or MDA-MB-231 cells
(12,000 cells/well) were grown on 96-well plates. The cells were
treated (24 h post-seeding) with a range of concentrations of the
analogues for up to 72 h. Cellular metabolic activity and hence
cell viability was monitored using AlamarBlue™ dye (BioSource,
Invitrogen) which was added to each well [final concentration of
10% (v/v)] and incubated at 37°C. The change from an oxidized
indigo blue non-fluorescent state to a fluorescent pink state in
the reduced environment of living cells was measured at an
excitation wavelength of 544 nm and an emission wavelength of 590
nm using a SpectraMax Gemini spectrofluorometric microtiter well
plate reader (Molecular Devices, Sunnyvale, CA, USA). The results
were expressed as the percentage of cell viability relative to the
vehicle-treated control cells (100%). Dose-response curves were
plotted, and IC50 values (the concentration of compound
resulting in 50% reduction in cell viability) were obtained using
Prism GraphPad 4.
Determination of DNA content
Following treatment, HUVECs were harvested by
centrifugation at 800 × g for 10 min. Cell pellets were resuspended
in 200 μl PBS and fixed by a drop-wise addition of 2 ml of ice-cold
70% (v/v) ethanol/PBS while gently vortexing. Following overnight
fixation at −20°C the cells were again centrifuged to remove the
ethanol and resuspended in PBS supplemented with 0.5 mg/ml RNase A
and 0.15 mg/ml propidium iodide. Cells were incubated in the dark
at 37°C for 30 min. The fluorescence emitted from the propidium
iodide was measured on a linear scale using a FACSCalibur flow
cytometer (Becton Dickinson, San Jose, CA, USA). Data collections
(10,000 events per sample) were gated to exclude cell debris and
cell aggregates. Fluorescence was proportional to the amount of DNA
present in each entity and therefore indicated the stage of the
cell cycle it was in. Cells in G0/G1 were
diploid (2N DNA content), cells in G2/M were tetraploid
(4N DNA content), cells in the S phase had DNA contents between 2N
and 4N, while apoptotic cells were hypoploid and contained <2N
DNA. All data were recorded and analysed using the CellQuest
software (Becton Dickinson).
Microtubule staining
HUVECs (60,000 cells/chamber) were cultured on BD
Falcon™ 4-chamber glass slides (BD Biosciences, Bedford, MA, USA)
for 24 h. Following treatment for 16 h, the cells were fixed in
100% methanol at −20°C and the microtubular network was detected by
indirect immunofluorescence as previously described (22). Briefly, the slides were sequentially
incubated in a blocking solution [5% (w/v) BSA/0.1% (v/v) Triton
X-100/PBS], monoclonal anti-α-tubulin antibodies (Merck
Biosciences, Nottingham, UK), fluorescein isothiocyanate
(FITC)-conjugated rabbit anti-mouse antibodies (DakoCytomation,
Glostrup, Denmark) and finally 0.2 μg/ml propidium iodide (to stain
DNA). An anti-quenching solution (2 μg/ml p-phenylenediamine
in 50:50 glycerol:PBS) was applied to the surface of the slides and
coverslips were mounted. The organisation of the microtubule
network (green) and the cellular DNA (red) was visualised under a
x60 oil-immersion lens using an Olympus IX81 fluorescent microscope
(Olympus Corporation, Tokyo, Japan).
Endothelial cell migration
Costar® 8 μM-pore Transwell inserts
(Corning Incorporated, Corning, NY, USA) were coated overnight at
4°C with 10 μg/ml human fibronectin. HUVECs (10,000 cells in 100 μl
medium) were seeded onto the transwell inserts, placed in 24-well
plates containing 0.6 ml medium and incubated for 1 h. HUVEC
migration was stimulated by addition of 10 ng/ml VEGF to the lower
well. Vehicle or the indicated analogues were also added to the
lower wells. After a period of 6 h the upper surfaces of the
inserts were swabbed to remove non-migrated cells. Filters were
incubated overnight in a solution containing 0.5% toluidine blue O
and 0.5% sodium tetraborate to stain the migrated cells. Following
solubilisation of the cells using 0.2% (w/v) SDS in 20 mM Tris-HCl,
pH 7.7, the absorbance was determined at 650 nm in a
spectrophotometer (Molecular Devices).
In vitro tubule formation
HUVECs (1.5×106 cells/well), were
incubated on BD Biocoat™ Matrigel™-coated 6-well plates (BD
Biosciences) for 6 h in the presence of vehicle or the indicated
compounds. The ability of the HUVECs to spontaneously form
capillary-like tubules on the Matrigel (basement membrane matrix
preparation) was photographed under a Nikon Eclipse TE300 phase
contrast microscope (Nikon Instruments Inc., Melville, NY, USA) at
a magnification of ×100.
Detection of VEGF release
MDA-MB-231 cells (60,000 cells/cm2) were
grown for 24 h, then washed in PBS and incubated in a low serum
environment [DMEM supplemented with 1% (v/v) FBS] in the presence
or absence of lead analogue CA-432 for 6 h. The conditioned medium
was then centrifuged at 400 × g and the supernatants were collected
while the cells were lysed in sample buffer [62.5 mM Tris (pH 6.8),
2% (w/v) SDS, 10% (v/v) glycerol], sonicated briefly and denatured
by boiling for 3 min. The concentration of VEGF in the supernatants
was determined by ELISA using a Quantikine Human VEGF Immunoassay
kit (R&D Systems Inc.) in accordance with manufacturer’s
handbook. The colour intensity (proportional to the concentration
of VEGF) was determined by measuring the absorbance in a SpectraMax
340PC spectrophotometer (Molecular Devices) at wavelength 450 nm
with correction at 540 nm. The readings were normalised to account
for the total protein content of the cells which was determined
from an aliquot of cell lysate using a BCA protein assay kit
(Pierce, Thermo Fisher Scientific Inc., Rockford, IL, USA) in
accordance with the manufacturer’s instructions. Normalised
VEGF-release was then expressed as a percentage of release from
vehicle-treated control cells (100%).
Tumour cell migration
MDA-MB-231 cells were seeded at a density of 25,000
cells/well onto 24-well Falcon migration inserts (8-μm pore size)
(BD Biosciences) in serum-free medium. Inserts were placed into
Falcon companion plates containing DMEM supplemented with 20% FBS
in the presence or absence of CA-432. Following 6 h of incubation,
the upper surfaces of the inserts were swabbed to remove
non-migrated cells. Migrated cells on the underside of the membrane
were fixed in methanol, stained with Mayer’s hematoxylin,
dehydrated in methanol and mounted on a glass slide. The cells in 5
fields at ×10 magnification were counted.
Statistical analysis
Results were presented as mean ± SEM. Statistical
analysis of the experimental data was performed using the computer
program Prism GraphPad 4. P-values were determined using a paired
two-tailed Student’s t-test. A value of P<0.05 was considered to
be statistically significant.
Results
cis-restricted β-lactam combretastatin
A-4 analogues, CA-104 and CA-432, reduce endothelial cell
viability
To determine the effect of cis-restricted
β-lactam CA-4 analogues on endothelial cell viability, we tested a
range of concentrations of the representative analogues, CA-104 and
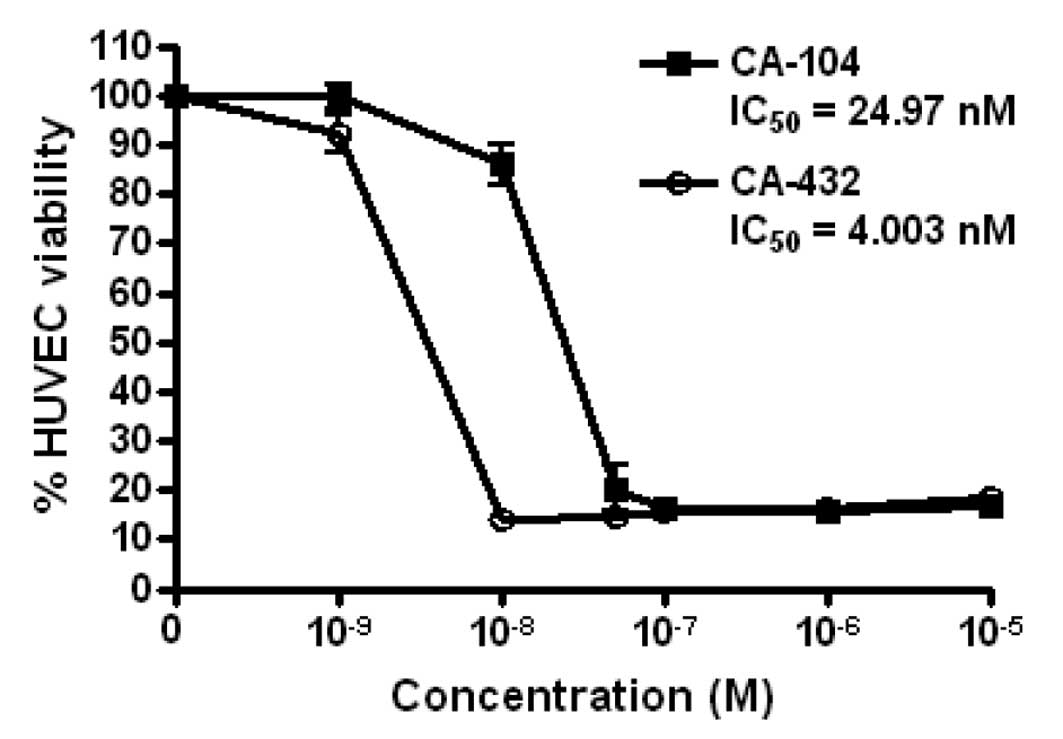
CA-432, on primary HUVECs for 72 h. We established that both agents
led to a reduction in the metabolic activity and hence viability of
HUVECs, with respective IC50 values for CA-104 and
CA-432 of 24.97 and 4.00 nM (Fig.
2). From these IC50 values, it was deduced that
analogue CA-432 was a more potent inhibitor of endothelial cell
viability than CA-104. The concentrations of CA-104 and CA-432 used
for the rest of this study were chosen to reflect the values
obtained from this cell viability assay.
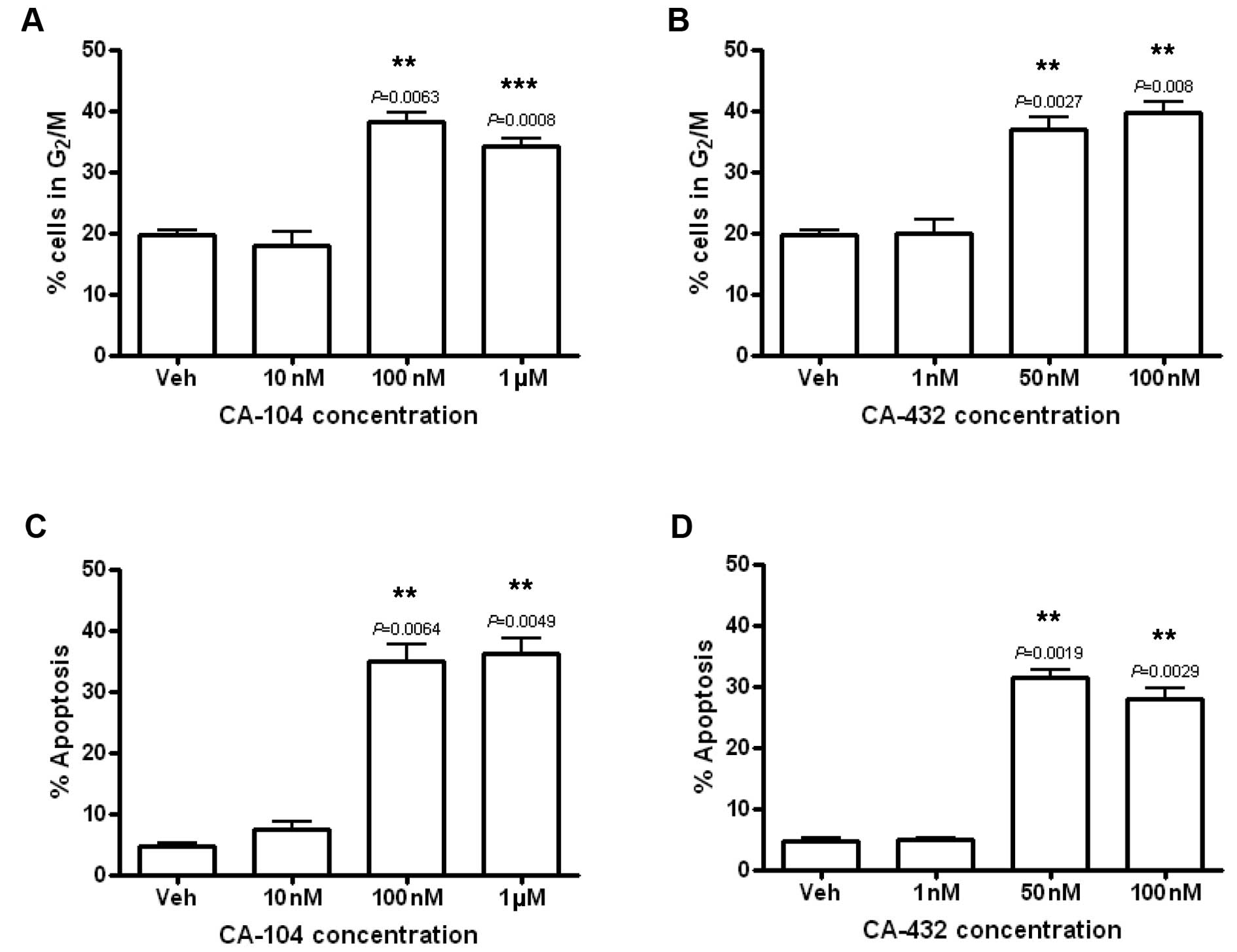
CA-104 and CA-432 induce G2/M
arrest and apoptosis in endothelial cells
Cell cycle profiles were examined to define the
mechanisms underlying the reduction in endothelial cell viability
following exposure to CA-104 and CA-432. HUVECs were treated with
vehicle [0.5% (v/v) ethanol], CA-104 (0.01–1 μM) or CA-432 (1–100
nM) for 24 h. Subsequently, their DNA was fluorescently stained
with propidium iodide and analysed by flow cytometry. Examination
of their DNA profiles indicated that both CA-104 and CA-432 induced
a dose-dependent increase in the percentage of HUVECs with 4N DNA
content compared to vehicle-treated cells. Approximately 20% of
HUVECs treated with the vehicle alone displayed tetraploid DNA
contents compared to 38.1±1.5% (P=0.0063) or 37.0±1.8% (P=0.0027)
of the HUVECs treated with CA-104 (100 nM) or CA-432 (50 nM)
respectively (Fig. 3A and B). These
statistically significant increases in 4N DNA content indicated
that the compounds induced arrest in the G2/M phase of
the cell cycle.
G2/M arrest was accompanied by
significant increases in the number of entities presenting with
hypodiploid (<2N) quantities of DNA as indicated by a sub
G0/G1 peak on the DNA profiles. While only
approximately 5% of vehicle-treated HUVECs were found to be
hypodiploid, treatment with CA-104 (100 nM) or CA-432 (50 nM)
resulted in an increase in the amount of hypodiploid cells to
35.0±2.6% (P=0.0064) or 31.4±1.3% (P=0.0019) respectively (Fig. 3C and D). These statistically
significantly increases in hypodiploid cells represented an
increase in the levels of apoptosis. Cell cycle analysis
illustrated that both CA-104 and CA-432 inhibited proliferation and
survival of endothelial cells through the induction of
G2/M arrest and apoptosis.
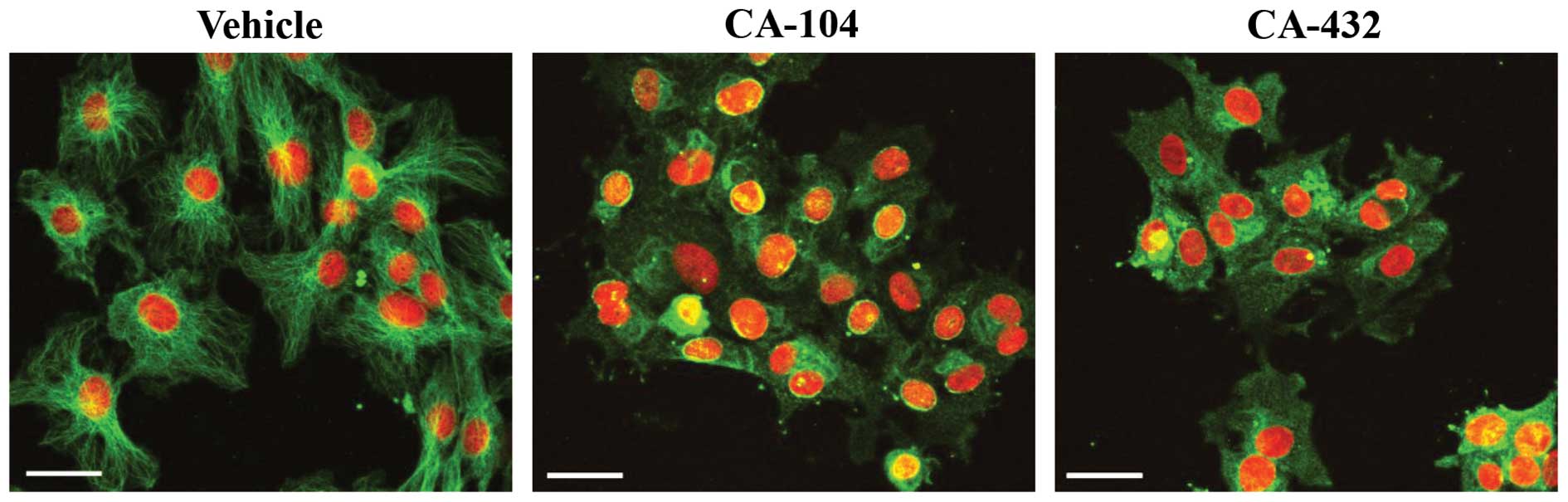
CA-104 and CA-432 cause destabilisation
of the microtubule network in endothelial cells
We next established the effect of CA-104 and CA-432
on the gross morphology of the microtubular network in endothelial
cells. HUVECs, grown on glass 4-chamber slides, were treated for 16
h with vehicle [0.5% (v/v) ethanol], CA-104 (100 nM) or CA-432 (50
nM). Immunofluorescent staining was used to detect morphological
changes in the tubulin cytoskeleton such as alterations in
organisation and arrangement. In normal cells, the microtubule
network is organised into cytoplasmic tubulin filaments radiating
from a central point to the periphery. HUVECs treated with vehicle
alone (0.5% ethanol) displayed this typical tubulin morphology
(Fig. 4). Treatment of cells with
tubulin polymerising agents (for example, paclitaxel) results in a
highly concentrated accumulation of filaments into dense peripheral
bundles indicative of microtubule stabilisation. In contrast,
exposure of cells to tubulin depolymerising agents (such as
vincristine) results in diffuse tubule staining with no definition
of structure caused by microtubule disassembly. These typical
morphological changes associated with tubulin depolymerising agents
were observed when HUVECs were treated with either CA-104 or CA-432
(Fig. 4).
CA-104 and CA-432 reduce endothelial cell
migration
Having established that cis-restricted
β-lactam combretastatin A-4 derivatives altered endothelial cell
function, we then examined their implications in angiogenic
processes in vitro. Firstly, their effect on HUVEC migration
was evaluated using a modified Transwell migration assay. This
chemotactic model representative of tumour-induced endothelial cell
migration (23) consisted of an
upper and a lower chamber separated by a membrane. Migration of
HUVECs from the upper to the lower chamber was stimulated by the
addition of VEGF to the lower chamber. The effect of CA-104 (100
nM) and CA-432 (50 nM) on this migration was determined by their
addition along with VEGF into the lower chamber. Migration was
expressed as a percentage of migration by vehicle-treated cells
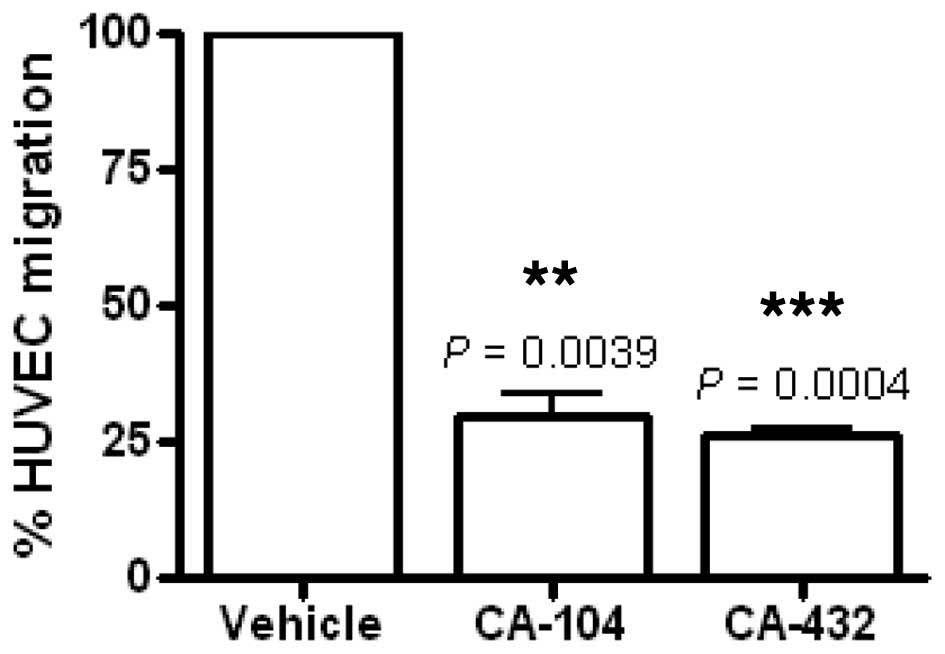
(100%). Incubation for 6 h with CA-104 or CA-432 significantly
inhibited VEGF-stimulated HUVEC migration by 70.3±4.4% (P=0.0039)
and 74.0±1.5% (P=0.0004) respectively (Fig. 5). This effect preceded endothelial
cell toxicity since no loss of HUVEC viability was evident
following a 6-h treatment with CA-104 or CA-432 (data not
shown).
CA-104 and CA-432 inhibit endothelial
cell differentiation
To further investigate the anti-angiogenic potential
of CA-104 and CA-432, an endothelial tube formation assay was
performed. The spontaneous formation of capillary-like structures
by endothelial cells, when incubated on an extracellular basement
membrane matrix preparation known as Matrigel, is a standard in
vitro angiogenesis test (24).
This process requires cell-matrix interaction, inter-cellular
communication as well as cell motility and differentiation. HUVECs
were seeded onto Matrigel in the presence of vehicle (0.5%
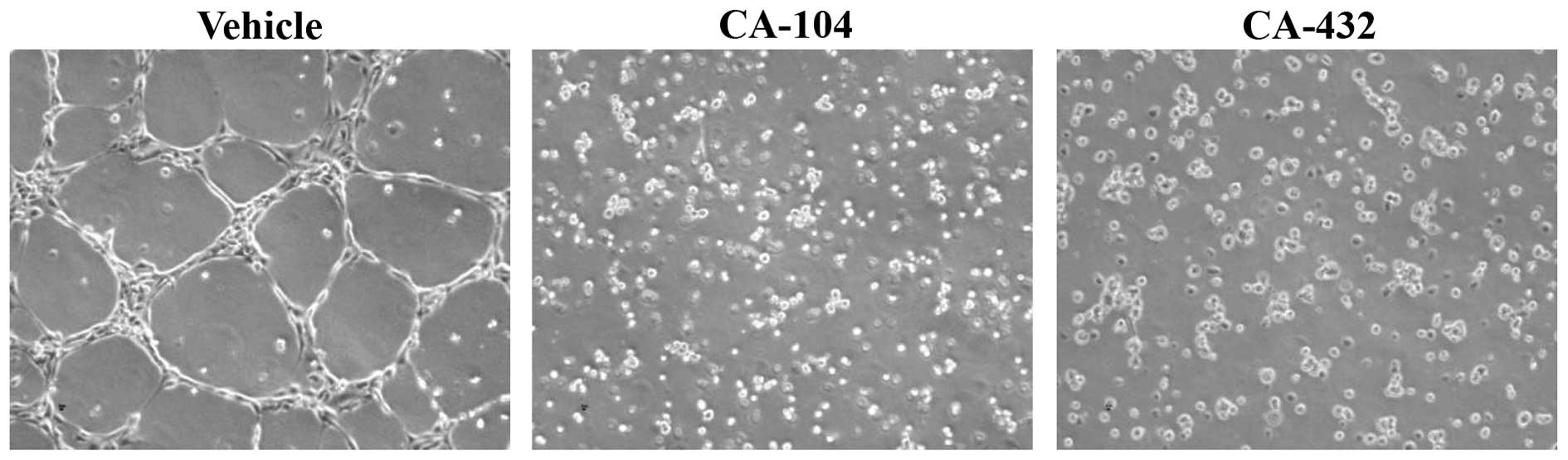
ethanol), CA-104 (100 nM) or CA-432 (50 nM) for 6 h. The alignment
of the cells on the 3D-Matrigel was assessed using a phase contrast
microscope (Fig. 6).
Vehicle-treated cells underwent alignment into capillary-like
structures while treatment with either CA-104 or CA-432 reduced
tubule formation. Again, as observed during the endothelial cell
migration assay, inhibition of in vitro tubule formation
preceded any cytotoxic effects attributed to CA-104 or CA-432.
CA-432 reduces VEGF release from breast
carcinoma cells
The release of VEGF from tumour cells plays a key
role in the stimulation of angiogenesis and promotion of
endothelial cell survival (25).
Therefore, we next investigated the effect of lead CA-4 analogue
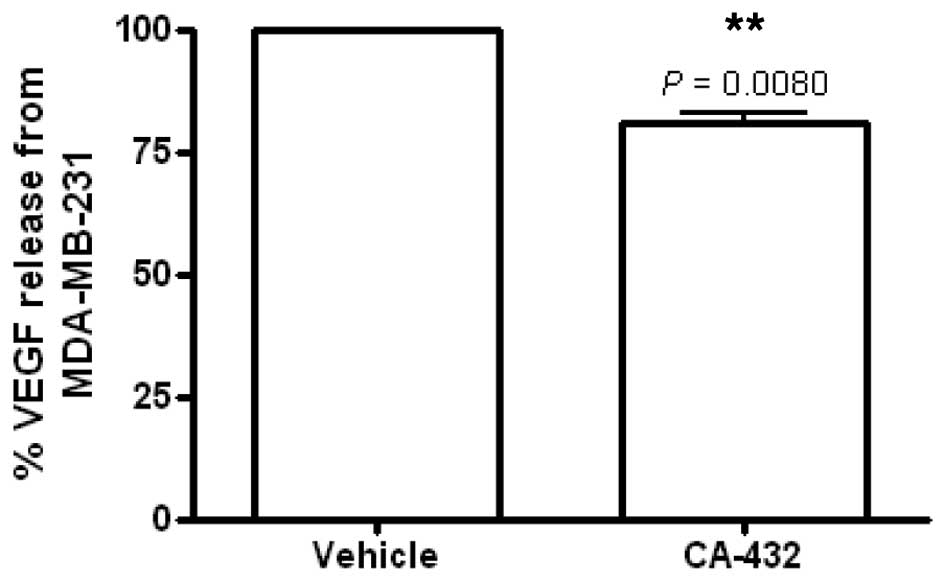
CA-432 (50 nM) on the release of VEGF from human breast
adenocarcinoma MDA-MB-231 cells. VEGF release was stimulated by
incubating the cells in a low serum environment. We found that the
CA-432 (50 nM) reduced the release of VEGF from MDA-MB-231 cells to
80.9±1.7% (P=0.0080) of that released from the vehicle-treated
control cells (Fig. 7). This event
preceded any cytotoxic effects due to CA-432, since at 6 h
post-treatment, no loss in MDA-MB-231 cell viability was detected
(data not shown). This finding suggested an indirect
anti-angiogenic function for CA-432 through targeting of tumour
cells.
CA-432 prevents migration of breast
carcinoma MDA-MB-231 cells
The migration of tumour cells from the primary site
is a critical step during tumour metastasis (26). We previously demonstrated that
MDA-MB-231 cells display good migratory capabilities (27). Therefore, the migration of
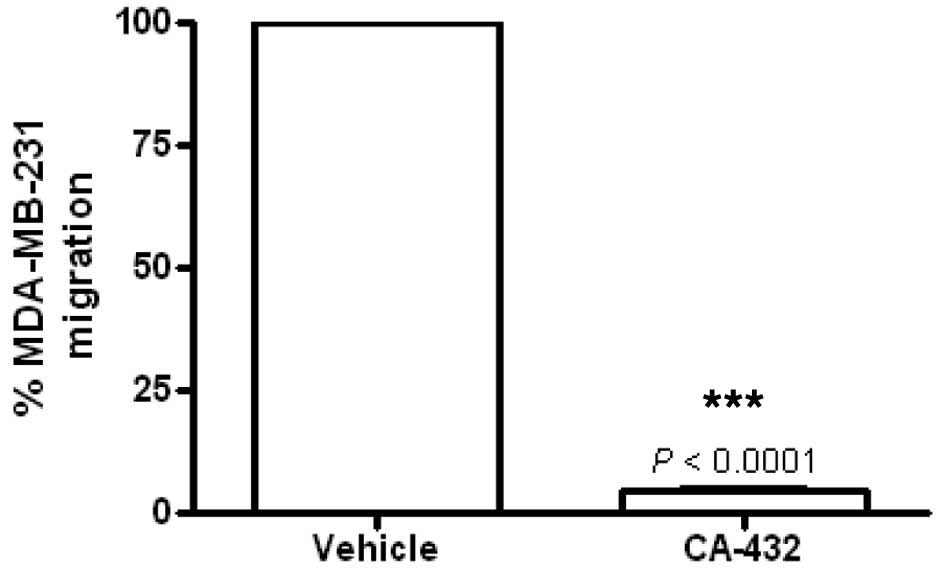
MDA-MB-231 cells across Transwell filters in the presence of
vehicle [0.5% (v/v) ethanol] or CA-432 (50 nM) was compared.
Migration was expressed as a percentage of the migration of
vehicle-treated cells (100%). Incubation for 6 h with CA-432
prevented MDA-MB-231 cell migration by 95.7±0.7% (P<0.0001)
(Fig. 8). This event preceded any
cytotoxic events, since following a 6 h treatment with CA-432, no
loss in MDA-MB-231 cell viability was detected (data not
shown).
Discussion
Both tumour vascularisation and the migration of
tumour cells are key events during the growth and metastatic
progression of tumours. Therefore, agents that disrupt these events
could potentially prove useful as anti-cancer therapies.
Anti-vascular therapies can be divided into two main strategic
subtypes: vascular-disrupting therapies that target the existing
tumour vasculature and anti-angiogenic therapies that prevent the
de novo synthesis of tumour blood vessels. CA-4 and its
water soluble prodrug CA-4P fall into the first category (7) while microtubule-targeting agents such
as the tubulin-stabilising taxanes (28) and the tubulin-destabilising vinca
alkaloids (29) form the latter
group. However, between these two subtypes there are overlaps, as
depending on the dosing strategies, anti-angiogenic agents can also
induce vascular disruption and the converse is also true (30). Therefore, the in vitro assays
used to detect both strategies are similar. Anti-vascular therapies
maximise their effects on the tumour vasculature while minimising
damage to the systemic vasculature system by exploiting the
fundamental differences between the normal mature vasculature and
the immature tumour vasculature which tends to be disorganised,
leaky and poorly associated with perivascular cells (31).
Endothelial cells are desirable targets for cancer
therapies due to accessibility and genetic stability and unlike
tumour cells, they do not readily acquire drug resistance (32). Hence, anti-vascular drugs can cause
tumour regression even in drug-resistant tumours. For example,
anti-tumour activity was reported with docetaxel in a xenograft
tumour that had been established by inoculating mice with
docetaxel-resistant cancer cells, an effect that was attributed to
the anti-angiogenic actions of docetaxel (33). After tumour vascularisation, several
key events are critical for progression of a tumour to a metastatic
phenotype. These events include migration of tumour cells from the
primary site into the blood/lymph system followed by their invasion
into a different site to begin the process of angiogenesis and the
growth of a secondary tumour (26).
Therefore, agents which target either the tumour vasculature or any
of the steps that occur during tumour metastasis are potentially
useful anticancer therapies, with agents that target both events
the most desirable.
Naturally occurring stilbene, CA-4, is a potent
inhibitor of tubulin polymerisation that displays potent
anti-mitotic activities in both cancer and endothelial cells
(2,4,5,7). In
vivo, its water-soluble prodrug, CA-4P, induces a rapid and
selective shutdown of tumour blood flow and subsequently tumour
regression (7,8) and thus the compound is currently
undergoing clinical trials as a vascular-disrupting agent. The
therapeutic efficacy of CA-4 is limited by its intrinsic
instability rendering it readily isomerisable to its inactive
trans-conformation (15–17).
Therefore, non-isomerisable CA-4 analogues have been developed to
provide more stable alternatives to CA-4. Approaches to prevent
isomerisation have included the replacement of the
cis-double bond in CA-4 with heterocyclic rings such as
tetrazole, imidazole and benzoxepin (34–36).
We previously developed and patented a series of
CA-4 analogues that are conformationally cis-restricted.
Replacement of the ethylene bridge of CA-4 with a β-lactam
(2-azetidinone) ring provided a rigid scaffold that prevented
cis-trans isomerisation and maintained a similar spatial
arrangement between the two phenol rings as the
cis-conformation of CA-4 (18). We have already established that some
of these compounds displayed potent anti-proliferative activity in
several cancer cell lines and ex vivo patient samples,
including those displaying multidrug resistance (18–21).
However, the anti-vascular and anti-metastatic properties of these
cis-restricted analogues remained to be elucidated. The
purpose of this study was to perform a series of in vitro
experiments to investigate the anti-vascular effects of the
compounds directly on primary HUVECs and indirectly on the release
of pro-angiogenic VEGF from tumour cells. Finally, we assessed the
effect of the analogues on one of the critical events involved in
metastasis, namely, tumour cell migration.
We selected two of the lead cis-restricted
CA-4 analogues from our previous studies as representative
compounds, CA-104 and its derivative CA-432, which contained an
aryl ring substituent at position C-3 of the β-lactam ring
(Fig. 1). We determined that both
analogues potently inhibited HUVEC proliferation with
IC50 values of 24.9 nM and 4 nM for CA-104 and CA-432,
respectively. These values were either similar or lower than those
we previously observed in tumour cells such as breast carcinoma
MCF-7 and MDA-MB-231 cells, promyelocytic leukaemia HL60 cells,
chronic myeloid leukaemia K562 cells and ovarian carcinoma A2780
cells, where IC50 values ranged between 17 and 60 nM for
CA-104 and 7.5 and 28 nM for CA-432 (18–20).
The magnitude of response obtained with CA-432 was similar to those
reported for CA-4 and CA-4P which also demonstrated
anti-proliferative effects on endothelial cells at lower
concentrations than tumour cells (7,37). As
we previously reported that exposure of normal breast epithelial
cells to CA-432 induced only a minimal amount of cytotoxicity with
an IC50 value of greater than 10 mM (19), it is interesting that the compounds
had such a potent effect on non-cancerous endothelial cells. This
phenomenon has been reported with CA-4P (38) and numerous other
microtubule-targeting agents (22,39)
and is postulated to be attributable to a variety of mechanisms
including enhanced uptake mechanisms in endothelial cells (40) or differences in endothelial cell
tubulin composition, its post-translational modifications or its
microtubule-associated proteins (41,42).
The loss in endothelial cell viability following
exposure to CA-104 and CA-432 was mediated by significant levels of
G2M arrest and apoptosis which was accompanied by
depolymerisation of the microtubular networks in HUVECs. Tubulin
depolymerisation and G2M arrest are typical responses
observed in tumour cells after exposure to the β-lactam analogues
(19,20) and also parallels the effects
observed with CA-4/CA-4P in endothelial cells (43). Studies suggest that the type of cell
death culminating from CA-4(P)-induced G2M arrest varies
depending on the conditions and the type of cell. Modes of
cytotoxicity reported include apoptosis (21,44),
mitotic catastrophe (45) and
autophagy (46,47). Recently we found that CA-432 induced
mitotic catastrophe in breast carcinoma cells (48) and autophagy in
adenocarcinoma-derived colon cancer cells (47). Mitotic arrest prevents the supply of
endothelial cells required for angiogenesis and cytotoxicity leads
to the destruction of existing tumour blood vessels, indicating a
novel anti-vascular function for the β-lactam CA-4 derivatives.
Apart from the proliferation and survival of
endothelial cells, angiogenesis requires several other critical
events including the migration of endothelial cells into the
extracellular matrix and their differentiation into new capillary
networks (31,49). In addition, stimulation of these
angiogenic events requires pro-angiogenic or vascular-survival
signals such as the release of VEGF by tumour cells (50). Interference in these processes has
been reported in endothelial and tumour cells treated with MTAs
such as paclitaxel, docetaxel, vinblastine and vincristine
(28,29,51,52).
We established that β-lactam CA-4 analogues were capable of
directly interfering with angiogenic events since they completely
abrogated VEGF-stimulated HUVEC migration and their spontaneous
differentiation into capillary-like structures when grown on
Matrigel, both standard tests for angiogenesis in vitro.
Furthermore, the derivatives may also indirectly influence
angiogenesis, as CA-432 significantly reduced the release of VEGF
from metastatic breast carcinoma MDA-MB-231 cells by almost 20%.
This is an interesting effect as in addition to its angiogenic
activity, VEGF can protect endothelial cells from apoptosis by
stimulating the activation of survival pathways such as
phosphoinositol-3-kinase (PI3 kinase) and upregulation of
anti-apoptotic Bcl-2 and in particular survivin which is an
important microtubule-binding apoptosis inhibitor involved in
mitotic spindle regulation (53–56).
These anti-angiogenic findings provided additional
support to the anti-vascular profile of these CA-4 derivatives and
it is interesting to note that these anti-angiogenic responses
occurred at time points that preceded the onset of cytoxicity
indicating that the anti-vascular phenotype of these compounds
cannot solely be attributed to endothelial cell death. This effect
was also observed with CA-4P which can induce complete vascular
shutdown within 20 min of drug exposure in vivo(57). As drug-induced effects on
endothelial cell proliferation or cytotoxicity occur too slowly to
account for this rapid response, it has been postulated that
morphological and functional changes in endothelial cells are more
likely to cause tumour vascular collapse (30,58).
Such changes may include rounding-up of cells due to disruption of
interphase microtubules leading to rapid remodelling of the actin
cytoskeleton, assembly of actin stress fibres, actinomyosin
contractility, formation of focal adhesions, disruption of
cell-cell junctions, including those involving N- and VE-cadherin
and an increase in monolayer permeability of macromolecules
(14,59,60).
It would therefore, be interesting in the future to investigate
some of these mechanisms to extend our study of the β-lactam CA-4
analogues. It is important to note that our initial observations
which illustrated inhibition of endothelial cell proliferation and
cytotoxicity are still important therapeutically, since these
mechanisms can play a role in the prevention of tumour re-growth in
chronic dosing schedules.
Finally, we investigated the effect of CA-432 on one
of the key events that occurs during metastasis, tumour cell
migration. We chose breast adenocarcinoma MDA-MB-231 cells as a
model since we previously found that these cells displayed good
migratory potential (27). We
determined that CA-432 abrogated MDA-MB-231 migration from a low to
a high serum environment. We previously showed that CA-432 has an
IC50 value of 28.8±0.02 nM in these cells (19), however, similarly to endothelial
cell migration, interference with tumour cell migration preceded
cytotoxicity.
In summary, these findings demonstrated novel
anti-vascular and anti-metastatic functions for our
cis-restricted β-lactam combretastatin A-4 analogues, CA-104
and CA-432. Collectively, this report along with our previous
studies indicate that these β-lactam CA-4 analogues induced
anti-tumour, anti-vascular and anti-metastatic events with minimal
toxicity to normal quiescent cells in vitro. These events
are analogous with the functions of CA-4 in vitro.
Therefore, these analogues should now be considered for further
in vivo investigation of their anti-tumour and anti-vascular
capabilities to further evaluate their potential as useful
alternatives to the intrinsically unstable CA-4.
Acknowledgements
This study was kindly funded by the Health Research
Board Ireland (Grant RP/2007/42). We acknowledge the assistance of
the technical staff of the School of Biochemistry and Immunology,
Biomedical Sciences Institute, Trinity College Dublin, Ireland, in
particular, Dr Orla Hanrahan and Dr Gavin McManus of the Microscopy
and Imaging Facility and Mr. Barry Moran of the Flow Cytometry
Facility.
References
|
1
|
Pettit GR, Singh SB, Niven ML, Hamel E and
Schmidt JM: Isolation, structure, and synthesis of combretastatins
A-1 and B-1, potent new inhibitors of microtubule assembly, derived
from Combretum caffrum. J Nat Prod. 50:119–131. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin CM, Singh SB, Chu PS, Dempcy RO,
Schmidt JM, Pettit GR and Hamel E: Interactions of tubulin with
potent natural and synthetic analogs of the antimitotic agent
combretastatin: a structure-activity study. Mol Pharmacol.
34:200–208. 1988.PubMed/NCBI
|
|
3
|
Mollinedo F and Gajate C: Microtubules,
microtubule-interfering agents and apoptosis. Apoptosis. 8:413–450.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pettit GR, Singh SB, Hamel E, Lin CM,
Alberts DS and Garcia-Kendall D: Isolation and structure of the
strong cell growth and tubulin inhibitor combretastatin A-4.
Experientia. 45:209–211. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nabha SM, Wall NR, Mohammad RM, Pettit GR
and Al-Katib AM: Effects of combretastatin A-4 prodrug against a
panel of malignant human B-lymphoid cell lines. Anticancer Drugs.
11:385–392. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pettit GR, Temple C Jr, Narayanan VL,
Varma R, Simpson MJ, Boyd MR, Rener GA and Bansal N: Antineoplastic
agents 322. Synthesis of combretastatin A-4 prodrugs. Anticancer
Drug Des. 10:299–309. 1995.PubMed/NCBI
|
|
7
|
Dark GG, Hill SA, Prise VE, Tozer GM,
Pettit GR and Chaplin DJ: Combretastatin A-4, an agent that
displays potent and selective toxicity toward tumor vasculature.
Cancer Res. 57:1829–1834. 1997.PubMed/NCBI
|
|
8
|
Grosios K, Holwell SE, McGown AT, Pettit
GR and Bibby MC: In vivo and in vitro evaluation of combretastatin
A-4 and its sodium phosphate prodrug. Br J Cancer. 81:1318–1327.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rustin GJ, Galbraith SM, Anderson H,
Stratford M, Folkes LK, Sena L, Gumbrell L and Price PM: Phase I
clinical trial of weekly combretastatin A4 phosphate: clinical and
pharmacokinetic results. J Clin Oncol. 21:2815–2822. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nathan P, Zweifel M, Padhani AR, Koh DM,
Ng M, Collins DJ, Harris A, Carden C, Smythe J, Fisher N, Taylor
NJ, Stirling JJ, Lu SP, Leach MO, Rustin GJ and Judson I: Phase I
trial of combretastatin A4 phosphate (CA4P) in combination with
bevacizumab in patients with advanced cancer. Clin Cancer Res.
18:3428–3439. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mooney CJ, Nagaiah G, Fu P, Wasman JK,
Cooney MM, Savvides PS, Bokar JA, Dowlati A, Wang D, Agarwala SS,
Flick SM, Hartman PH, Ortiz JD, Lavertu PN and Remick SC: A phase
II trial of fosbretabulin in advanced anaplastic thyroid carcinoma
and correlation of baseline serum-soluble intracellular adhesion
molecule-1 with outcome. Thyroid. 19:233–240. 2009. View Article : Google Scholar
|
|
12
|
A service of the U.S. National Institutes
of Health. www.clinicaltrials.govurisimplewww.clinicaltrials.gov.
Accessed September 14, 2012
|
|
13
|
Galbraith SM, Chaplin DJ, Lee F, Stratford
MR, Locke RJ, Vojnovic B and Tozer GM: Effects of combretastatin A4
phosphate on endothelial cell morphology in vitro and
relationship to tumour vascular targeting activity in vivo.
Anticancer Res. 21:93–102. 2001.PubMed/NCBI
|
|
14
|
Kanthou C and Tozer GM: The tumor vascular
targeting agent combretastatin A-4-phosphate induces reorganization
of the actin cytoskeleton and early membrane blebbing in human
endothelial cells. Blood. 99:2060–2069. 2002. View Article : Google Scholar
|
|
15
|
Pettit GR, Rhodes MR, Herald DL, Hamel E,
Schmidt JM and Pettit RK: Antineoplastic agents. 445 Synthesis and
evaluation of structural modifications of (Z)- and
(E)-combretastatin A-41. J Med Chem. 48:4087–4099. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Woods JA, Hadfield JA, Pettit GR, Fox BW
and McGown AT: The interaction with tubulin of a series of
stilbenes based on combretastatin A-4. Br J Cancer. 71:705–711.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McGown AT and Fox BW: Structural and
biochemical comparison of the anti-mitotic agents colchicine,
combretastatin A4 and amphethinile. Anticancer Drug Des. 3:249–254.
1989.PubMed/NCBI
|
|
18
|
Carr M, Greene LM, Knox AJ, Lloyd DG,
Zisterer DM and Meegan MJ: Lead identification of conformationally
restricted β-lactam type combretastatin analogues: synthesis,
antiproliferative activity and tubulin targeting effects. Eur J Med
Chem. 45:5752–5766. 2010.
|
|
19
|
O’Boyle NM, Carr M, Greene LM, Bergin O,
Nathwani SM, McCabe T, Lloyd DG, Zisterer DM and Meegan MJ:
Synthesis and evaluation of azetidinone analogues of combretastatin
A-4 as tubulin targeting agents. J Med Chem. 53:8569–8584.
2010.PubMed/NCBI
|
|
20
|
Greene LM, Nathwani SM, Bright SA, Fayne
D, Croke A, Gagliardi M, McElligott AM, O’Connor L, Carr M, Keely
NO, O’Boyle NM, Carroll P, Sarkadi B, Conneally E, Lloyd DG, Lawler
M, Meegan MJ and Zisterer DM: The vascular targeting agent
combretastatin-A4 and a novel cis-restricted {beta}-lactam
analogue, CA-432, induce apoptosis in human chronic myeloid
leukemia cells and ex vivo patient samples including those
displaying multidrug resistance. J Pharmacol Exp Ther. 335:302–313.
2010.PubMed/NCBI
|
|
21
|
Greene LM, Carr M, Keeley NO, Lawler M,
Meegan MJ and Zisterer DM: BubR1 is required for the mitotic block
induced by combretastatin-A4 and a novel cis-restricted
β-lactam analogue in human cancer cells. Int J Mol Med. 27:715–23.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nathwani SM, Butler S, Meegan MJ, Campiani
G, Lawler M, Williams DC and Zisterer DM: Dual targeting of tumour
cells and host endothelial cells by novel microtubule-targeting
agents, pyrrolo-1,5-benzoxazepines. Cancer Chemother Pharmacol.
65:289–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Auerbach R, Lewis R, Shinners B, Kubai L
and Akhtar N: Angiogenesis assays: a critical overview. Clin Chem.
49:32–40. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kubota Y, Kleinman HK, Martin GR and
Lawley TJ: Role of laminin and basement membrane in the
morphological differentiation of human endothelial cells into
capillary-like structures. J Cell Biol. 107:1589–1598. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hoeben A, Landuyt B, Highley MS, Wildiers
H, Van Oosterom AT and De Bruijn EA: Vascular endothelial growth
factor and angiogenesis. Pharmacol Rev. 56:549–580. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fidler IJ: The pathogenesis of cancer
metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003.
|
|
27
|
Hughes L, Malone C, Chumsri S, Burger AM
and McDonnell S: Characterisation of breast cancer cell lines and
establishment of a novel isogenic subclone to study migration,
invasion and tumourigenicity. Clin Exp Metastasis. 25:549–557.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hotchkiss KA, Ashton AW, Mahmood R,
Russell RG, Sparano JA and Schwartz EL: Inhibition of endothelial
cell function in vitro and angiogenesis in vivo by docetaxel
(Taxotere): association with impaired repositioning of the
microtubule organizing center. Mol Cancer Ther. 1:1191–1200.
2002.PubMed/NCBI
|
|
29
|
Vacca A, Iurlaro M, Ribatti D, Minischetti
M, Nico B, Ria R, Pellegrino A and Dammacco F: Antiangiogenesis is
produced by nontoxic doses of vinblastine. Blood. 94:4143–4155.
1999.PubMed/NCBI
|
|
30
|
Tozer GM, Kanthou C, Parkins CS and Hill
SA: The biology of the combretastatins as tumour vascular targeting
agents. Int J Exp Pathol. 83:21–38. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pasquier E, Honore S and Braguer D:
Microtubule-targeting agents in angiogenesis: where do we stand?
Drug Resist Updat. 9:74–86. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kerbel RS: Inhibition of tumour
angiogenesis as a strategy to circumvent acquired resistance to
anti-cancer therapeutic agents. Bioessays. 13:31–36. 1991.
View Article : Google Scholar
|
|
33
|
Kamat AA, Kim TJ, Landen CN Jr, Lu C, Han
LY, Lin YG, Merritt WM, Thaker PH, Gershenson DM, Bischoff FZ,
Heymach JV, Jaffe RB, Coleman RL and Sood AK: Metronomic
chemotherapy enhances the efficacy of antivascular therapy in
ovarian cancer. Cancer Res. 67:281–288. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ohsumi K, Hatanaka T, Fujita K, Nakagawa
R, Fukuda Y, Nihei Y, Suga Y, Morinaga Y, Akiyama Y and Tsuji T:
Syntheses and antitumor activity of cis-restricted
combretastatins: 5-membered heterocyclic analogues. Bioorg Med Chem
Lett. 8:3153–3158. 1998.PubMed/NCBI
|
|
35
|
Wang L, Woods KW, Li Q, Barr KJ, McCroskey
RW, Hannick SM, Gherke L, Credo RB, Hui YH, Marsh K, Warner R, Lee
JY, Zielinski-Mozng N, Frost D, Rosenberg SH and Sham HL: Potent,
orally active heterocycle-based combretastatin A-4 analogues:
synthesis, structure-activity relationship, pharmacokinetics, and
in vivo antitumor activity evaluation. J Med Chem. 45:1697–1711.
2002. View Article : Google Scholar
|
|
36
|
Barrett I, Carr M, O’Boyle N, Greene LM,
Knox AJ, Lloyd DG, Zisterer DM and Meegan MJ: Lead identification
of conformationally restricted benzoxepin type combretastatin
analogs: synthesis, antiproliferative activity, and tubulin
effects. J Enzyme Inhib Med Chem. 25:180–194. 2010. View Article : Google Scholar
|
|
37
|
Burns CJ, Fantino E, Powell AK, Shnyder
SD, Cooper PA, Nelson S, Christophi C, Malcontenti-Wilson C,
Dubljevic V, Harte MF, Joffe M, Phillips ID, Segal D, Wilks AF and
Smith GD: The microtubule depolymerizing agent CYT997 causes
extensive ablation of tumor vasculature in vivo. J Pharmacol Exp
Ther. 339:799–806. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Böhle AS, Leuschner I, Kalthoff H and
Henne-Bruns D: Combretastatin A-4 prodrug: a potent inhibitor of
malignant hemangioendothelioma cell proliferation. Int J Cancer.
87:838–843. 2000.PubMed/NCBI
|
|
39
|
Wang J, Lou P, Lesniewski R and Henkin J:
Paclitaxel at ultra low concentrations inhibits angiogenesis
without affecting cellular microtubule assembly. Anticancer Drugs.
14:13–19. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Merchan JR, Jayaram DR, Supko JG, He X,
Bubley GJ and Sukhatme VP: Increased endothelial uptake of
paclitaxel as a potential mechanism for its antiangiogenic effects:
potentiation by Cox-2 inhibition. Int J Cancer. 113:490–498. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pasquier E, Honore S, Pourroy B, Jordan
MA, Lehmann M, Briand C and Braguer D: Antiangiogenic
concentrations of paclitaxel induce an increase in microtubule
dynamics in endothelial cells but not in cancer cells. Cancer Res.
65:2433–2440. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pourroy B, Honoré S, Pasquier E,
Bourgarel-Rey V, Kruczynsk A, Briand C and Braguer D:
Antiangiogenic concentrations of vinflunine increase the interphase
microtubule dynamics and decrease the motility of endothelial
cells. Cancer Res. 66:3256–3263. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kanthou C, Greco O, Stratford A, Cook I,
Knight R, Benzakour O and Tozer G: The tubulin-binding agent
combretastatin A-4-phosphate arrests endothelial cells in mitosis
and induces mitotic cell death. Am J Pathol. 165:1401–1411. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Iyer S, Chaplin DJ, Rosenthal DS, Boulares
AH, Li LY and Smulson ME: Induction of apoptosis in proliferating
human endothelial cells by tumour-specific antiangiogenesis agent
combretastatin A-4. Cancer Res. 58:4510–4514. 1998.PubMed/NCBI
|
|
45
|
Nabha SM, Mohammad RM, Dandashi MH,
Coupaye-Gerard B, Aboukameel A, Pettit GR and Al-Katib AM:
Combretastatin-A4 prodrug induces mitotic catastrophe in chronic
lymphocytic leukemia cell line independent of caspase activation
and poly(ADP-ribose) polymerase cleavage. Clin Cancer Res.
8:2735–2741. 2002.PubMed/NCBI
|
|
46
|
Ding X, Zhang Z, Li S and Wang A:
Combretastatin A4 phosphate induces programmed cell death in
vascular endothelial cells. Oncol Res. 19:303–309. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Greene LM, O’Boyle NM, Nolan DP, Meegan MJ
and Zisterer DM: The vascular targeting agent combretastatin-A4
directly induces autophagy in adenocarcinoma-derived colon cancer
cells. Biochem Pharmacol. 84:612–624. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
O’Boyle NM, Carr M, Greene LM, Keely NO,
Knox AJ, McCabe T, Lloyd DG, Zisterer DM and Meegan MJ: Synthesis,
biochemical and molecular modelling studies of antiproliferative
azetidinones causing microtubule disruption and mitotic
catastrophe. Eur J Med Chem. 46:4595–4607. 2011.
|
|
49
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29:15–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Conn G, Soderman DD, Schaeffer MT, Wile M,
Hatcher VB and Thomas KA: Purification of a glycoprotein vascular
endothelial cell mitogen from a rat glioma-derived cell line. Proc
Natl Acad Sci USA. 87:1323–1327. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Belotti D, Vergani V, Drudis T, Borsotti
P, Pitelli MR, Viale G, Giavazzi R and Taraboletti G: The
microtubule-affecting drug paclitaxel has antiangiogenic activity.
Clin Cancer Res. 2:1843–1849. 1996.PubMed/NCBI
|
|
52
|
Avramis IA, Kwock R and Avramis VI:
Taxotere and vincristine inhibit the secretion of the angiogenesis
inducing vascular endothelial growth factor (VEGF) by wild-type and
drug-resistant human leukemia T-cell lines. Anticancer Res.
21:2281–2286. 2001.
|
|
53
|
Nör JE, Christensen J, Liu J, Peters M,
Mooney DJ, Strieter RM and Polverini PJ: Up-regulation of Bcl-2 in
microvascular endothelial cells enhances intratumoral angiogenesis
and accelerates tumor growth. Cancer Res. 61:2183–2188.
2001.PubMed/NCBI
|
|
54
|
Tran J, Master Z, Yu JL, Rak J, Dumont DJ
and Kerbel RS: A role for survivin in chemoresistance of
endothelial cells mediated by VEGF. Proc Natl Acad Sci USA.
99:4349–4354. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li F, Ambrosini G, Chu EY, Plescia J,
Tognin S, Marchisio PC and Altieri DC: Control of apoptosis and
mitotic spindle checkpoint by survivin. Nature. 396:580–584. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Giodini A, Kallio MJ, Wall NR, Gorbsky GJ,
Tognin S, Marchisio PC, Symons M and Altieri DC: Regulation of
microtubule stability and mitotic progression by survivin. Cancer
Res. 62:2462–2467. 2002.PubMed/NCBI
|
|
57
|
Tozer GM, Prise VE, Wilson J, Cemazar M,
Shan S, Dewhirst MW, Barber PR, Vojnovic B and Chaplin DJ:
Mechanisms associated with tumor vascular shut-down induced by
combretastatin A-4 phosphate: intravital microscopy and measurement
of vascular permeability. Cancer Res. 61:6413–6422. 2001.PubMed/NCBI
|
|
58
|
Tozer GM, Kanthou C and Baguley BC:
Disrupting tumour blood vessels. Nat Rev Cancer. 5:423–435. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Vincent L, Kermani P, Young LM, Cheng J,
Zhang F, Shido K, Lam G, Bompais-Vincent H, Zhu Z, Hicklin DJ,
Bohlen P, Chaplin DJ, May C and Rafii S: Combretastatin A4
phosphate induces rapid regression of tumor neovessels and growth
through interference with vascular endothelial-cadherin signaling.
J Clin Invest. 115:2992–3006. 2005. View Article : Google Scholar
|
|
60
|
Tozer GM, Kanthou C, Lewis G, Prise VE,
Vojnovic B and Hill SA: Tumour vascular disrupting agents:
combating treatment resistance. Br J Radiol. 81:S12–S20. 2008.
View Article : Google Scholar : PubMed/NCBI
|