1. Introduction
Adenomyosis is a common gynecological disorder; it
is a myometrial lesion characterized by the presence of ectopic
endometrial glands and stroma located deep within the surrounding
myometrium with adjacent myometrial hyperplasia and hypertrophy
(1). Symptoms are commonly reported
as menorrhagia, dysmenorrhea, pelvic pain, abnormal uterine
bleeding and bulk-related symptoms. Despite its common occurrence,
the etiology and pathogenesis of adenomyosis remains unclear.
Previous epidemiologic studies investigated risk factors for
adenomyosis. Risk factors include: age between 40 and 50 years,
early menarche, short menstrual cycles, a first birth at an early
age, multiparity, sharp curettage during early pregnancy, obesity
and tamoxifen use (1,2). Adenomyosis commonly coexists with
uterine leiomyomas (1).
Adenomyosis, adenomyoma and adenomyomatosis are
pseudotumors of several organs, including the uterus as well as the
bile ducts, gallbladder and Vater papilla (3,4).
Adenomyosis of the gallbladder is a benign, mostly asymptomatic
condition made up of 3.3% of patients who underwent cholecystectomy
(3). Histology confirmed the
presence of hyperplastic change in the gallbladder wall, overgrowth
of the mucosa, thickening of the muscular wall, and formation of
intramural diverticula or sinus tracts, also known as the formation
of Rokitansky-Aschoff sinuses (3,5).
Although the pathogenesis of uterine adenomyosis remains unclear,
the most frequent debate is whether the pathological alteration is
inflammation or adenoma (6).
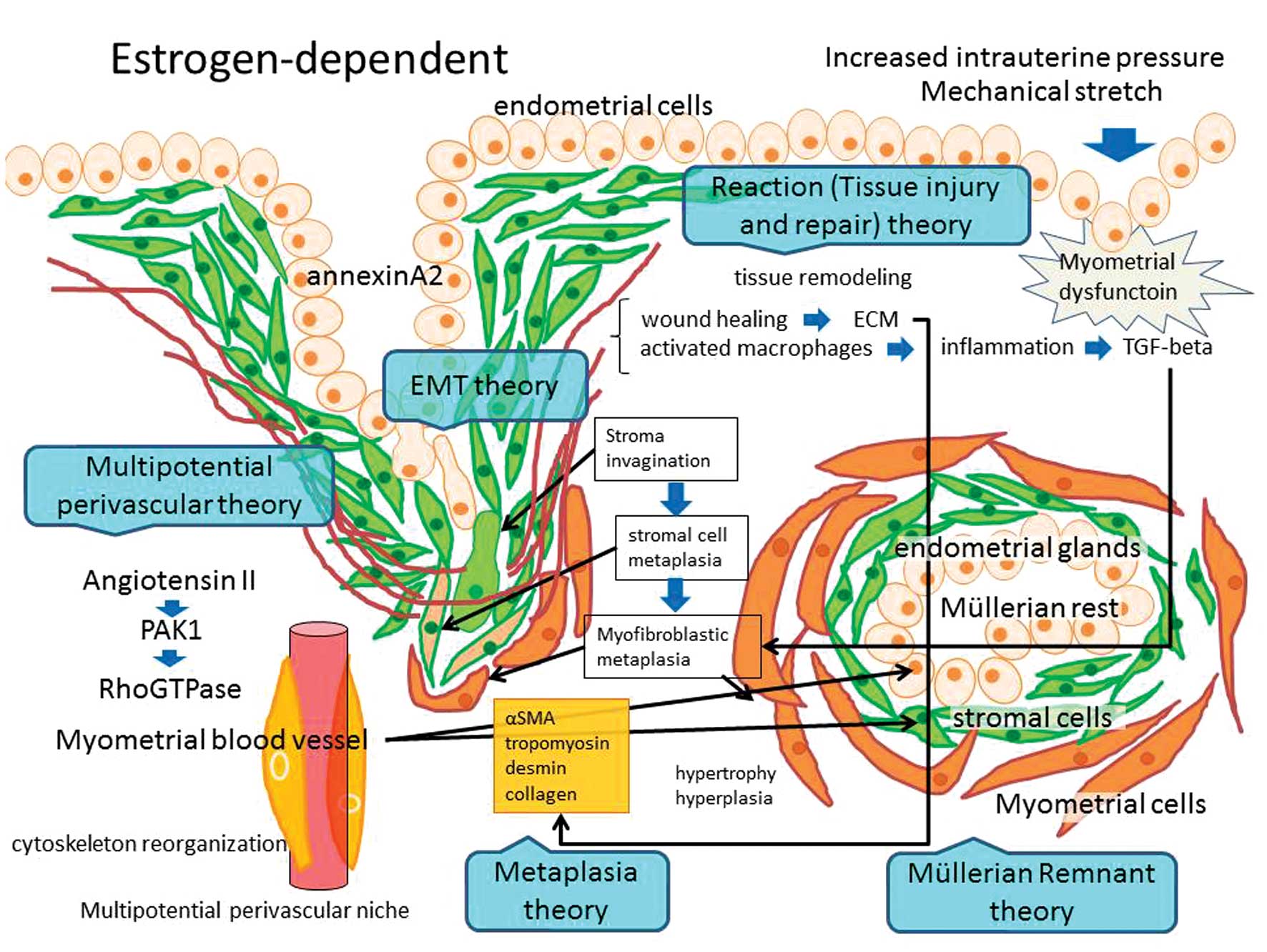
2. Pathogenesis of adenomyosis
Myometrial dysfunction
Adenomyosis can be diagnosed using magnetic
resonance imaging (MRI): irregular thickening of the junctional
zone is associated with adenomyosis. Human and experimental studies
suggest that adenomyosis occurs by invagination of the basal
endometrium into the inner layer of the uterine myometrium known as
junctional zone (1,7). Abnormal thickening of the
subendometrial myometrium includes basal endometrium and inner
myometrium. The inner layer of the myometrium may represent a
region of structural weakness and myometrial dysfunction of varying
severity susceptible to an invagination of the stromal cells. The
junctional zone was often found to be fissured (7). Myometrial smooth muscle dysfunction
also develops as a primary or steroid hormone-induced defect in
adenomyosis (8). Following
invagination of stromal cells, invasion of glandular cells,
abnormal growth and differentiation, these cells are subsequently
surrounded by hypertrophic and hyperplastic myometrium (9,10).
These data suggest that adenomyosis might be caused by defects in
the formation of the inner myometrial layer of the uterus (8).
Morphological changes are detected in the myometrial
architecture of uteri having adenomyosis (11). Microscopically, spindle cell
populations containing smooth muscle cells, frequent components of
adenomyotic lesions, are noted in direct contact to the adenomyotic
stroma (12). Expression of
α-smooth muscle actin (positive for smooth muscle cells and a
contractile phenotype called myofibroblasts) and desmin, an
intermediate filament (positive for skeletal, visceral and certain
vascular smooth muscle cells), were consistent with myometrial
hyperplasia and hypertrophy in adenomyotic myometrium (13). The vimentin expression (positive for
mesenchyme origin cells) differed and was lower in adenomyosis and
higher in eutopic endometrium. There was also a difference in the
expression of estrogen receptor (ER) and progesterone receptor (PR)
between the adenomyosis and non-adenomyosis groups such as
leiomyomas (14). ER-β expression
and the lack of PR expression are related to the development of
adenomyosis. Persistent estrogenic stimulation may have a
functional role in the characteristic of adenomyosis due to
myometrial smooth muscle cell hypertrophy and hyperplasia. It has
been established, for example, that the myometrial hyperplasia
during early gestation is induced by the estradiol-mediated PI3K
(phosphatidylinositol-3 kinase)/mTOR (mechanistic target of
rapamycin) signaling pathway (15).
Increased intrauterine pressure
Another possibility is increased intrauterine
pressure (1). Increase in the
growth of the uterus during pregnancy is due to smooth muscle cell
hypertrophy and hyperplasia (16).
Mechanical stretch is known to stimulate myometrial hyperplasia and
hypertrophy through alteration of intracellular calcium signaling.
Myometrial stretch and subsequent uterine contractile activity are
associated with the initiation of primary dysmenorrhea. A history
of severe dysmenorrhea has been associated with a subsequent
diagnosis of endometriosis (17).
Collectively, prolonged mechanical stretch and contraction can
modulate uterine myometrial growth and subsequent hypertrophy to
and hyperplasia of myometrial cells.
3. Histogenesis of adenomyosis
Several key steps are required to establish an
adenomyosis: epithelial-mesenchymal transition (EMT), survival and
growth of the ectopic tissue within the myometrium, myometrial
hypertrophy and hyperplasia, and induction of an immunosuppressive
microenvironment. The theory of stromal invagination into the inner
layer of the myometrium with gland invasion, in conjunction with
microenvironmental factors to stimulate smooth muscle cell growth,
is the most widely accepted hypothesis. It is plausible that
myometrial hypertrophy and hyperplasia are considered as metaplasia
of stromal cells. It can also be explained by the embryonic
remnants/rests theory or as response/reaction to the presence of
ectopic glands. Although the exact etiology of adenomyosis is
unknown, several hypotheses about its origin exist, as described
below.
Metaplasia theory
Although the functional contributions of smooth
muscle cells to adenomyosis development are poorly understood,
Mechsner et al reported that epithelial and stromal cells in
endometriosis develop from persistent coelomic epithelial cells by
metaplasia (18). In pelvic
endometriosis, eutopic endometrial cells arriving through
retrograde transplantation at ectopic sites could induce the
surrounding tissue to undergo smooth muscle metaplasia. Similar to
endometriosis, adenomyosis-associated smooth muscle cells may be of
metaplastic origin. In skin, fibroblast-to-myofibroblast transition
is a key event during hypertrophic and keloid scar formation.
Myofibroblastic metaplasia with formation of an abnormal myometrial
hypertrophy may contribute to an impaired uterine function.
Immunohistochemical study in adenomyosis tissue
revealed expression of specific uterine marker molecules, including
the essential components characterizing uterine myometrial cells
such as oxytocin receptor (OTR), vasopressin receptors (VPR), ER
and PR (12). OTR, ER and PR are
also expressed in peritoneal endometrial smooth muscle cells
(11). The endometrial stromal
cells have an elongated fibroblast-like appearance with
immunopositivity for α-smooth muscle actin, indicating a pure
smooth muscle phenotype. This theory is the myofibroblastic
differentiation from fibroblasts or stromal cells, suggesting
smooth muscle metaplasia out of stromal cells.
Müllerian remnants theory
Another possibility is that the de novo
development of adenomyosis occurs from Müllerian rests in a uterine
myometrial location (1).
Adenomyosis may stem from the capacity of the secondary Müllerian
system to differentiate into both endometrial glands and stroma and
surrounding smooth muscle cells. The deeply infiltrating
retroperitoneal nodule is considered to be an adenomyosis whose
pathogenesis is related to metaplasia of Müllerian remnants.
Tissue remodeling theory
On the other hand, van Kaam et al reported
that the presence of adenomyotic nodules in deeply infiltrating
endometriosis lesions is accounted for by a reaction of the local
environment to the presence of ectopic endometrium (19). Adenomyosis is caused by trauma,
known as tissue injury and repair (20). The response to any implant is wound
healing comprised of inflammation and tissue remodeling. The wound
healing process involves more extensive tissue remodeling through
production of extracellular matrix (ECM) components, remodeling
enzymes, cellular adhesion molecules, growth factors, cytokines and
chemokine genes. Activated macrophages generate various cytokines
including transforming growth factor (TGF)-β, which promotes tissue
remodeling and subsequently causes fibroblasts to differentiate
into myofibroblasts. Stromal cells were characterized as
myofibroblasts due to their expression of α-smooth muscle actin,
tropomyosin, desmin and collagens (21). Myofibroblasts play an important role
in the development of adenomyosis by their expression of ECM
proteins. These data suggest that myometrial hypertrophy is a
response/reaction of the ectopic endometrial cells to the
surrounding tissue, which shares characteristics with physiological
and pathological mechanisms of wound healing (tissue injury and
repair) (1,19,20).
The human uterine endometrium undergoes scarless
repair. Chronic persistent normoperistalsis leads to the same
extent of microtraumatization (20). This mechanism overlaps those
controlling other histopathological occurrences of tissue
remodeling.
Epithelial-mesenchymal transition
theory
Adenomyosis is an estrogen-dependent disease. High
estrogen concentrations in ectopic endometrium may be necessary for
the maintenance of adenomyosis (1).
The estrogen dependency is often accompanied by the appearance of
EMT features, which is a crucial step for the acquisition of
invasive properties of endometrial epithelial cells during
adenomyosis progression (22).
Estrogen enhances endometrial tissue growth, metastasis and
angiogenesis in an adenomyosis model via annexin A2 (ANXA2)-induced
EMT (23). These data implicate the
crucial role of estrogen-induced EMT in the development of
adenomyosis (22).
Multipotential perivascular theory
The phenomenon of vascular involvement in
adenomyosis is relatively common. Angiogenesis is an important
factor in the development of adenomyosis (7,24).
Vascular endothelial growth factor (VEGF), fibroblast growth factor
(FGF)-1, FGF-2, thrombospondin 1 (TSP-1) and platelet-derived
growth factor (PDGF) are potent angiogenic factors. The FGF2 754C/G
polymorphism is considered to be associated with a risk for
developing adenomyosis in Chinese women (25). Pathophysiological vascular
remodeling leads to vascular smooth muscle cell hypertrophy,
proliferation, or migration (26).
Angiotensin II is a well-known participant of vascular remodeling
and activates a downstream target PAK1 (serine/threonine
p21-activating kinase). Pak1 is a critical effector that links
RhoGTPases to cytoskeleton reorganization and regulates cell
motility and morphology. Expression of PAK1 is increased in
adenomyosis, suggesting that PAK1 is involved in
adenomyosis-associated vascular remodeling (27).
Meenakshi and McCluggage raise the possibility that
mesenchymal stem-like cells reside in a perivascular niche and
adenomyosis may derive from myometrial blood vessels, perhaps
multipotential perivascular cells (28). Sieiński found that proliferation of
the adenomyotic stroma originates from a perivascular stromal
proliferation, indicating that a multipotential stem cell
population within adenomyosis appears to be associated with
perivascular cells surrounding the blood vessels (29). These studies suggest that the
possible existence of multipotential perivascular stromal and
myometrial cells is an important factor in the development of
adenomyosis.
Mast cell activation theory
Mast cells are present within the endometrium and
are also observed in close association with uterine smooth muscle
cells (30). Activation and release
of mast cell-derived mediators occur in endometriosis (31). Mast cells could contribute to the
differentiation and development of the myometrium, possibly through
the production of nerve growth factor (NGF), preadipocyte factor-1
(Pref-1), and insulin-like growth factor-2 (8,32).
NGF plays a critical role in the production of pain
and can be used as an indicator for the severity of adenomyosis.
Pref-1 may have a role in maintaining cells in an undifferentiated
state. These data support the hypothesis that the mast cell is an
important factor in the maintenance of adenomyosis (7,24).
Others
DNA microarray and proteomics analysis identified
that specific genes are differentially expressed in adenomyosis and
matched eutopic endometrium. Abnormalities of inflammatory
response, cytokine/chemokine expression, protease activation,
autophagy, immunosuppressive microenvironment and epigenetic
regulation may be present in adenomyosis. Numerous genes, including
CXCL10, calbindin D-28K, prostaglandin-related factors [such as
prostaglandin E receptor 3 (subtype EP3) and prostaglandin
synthase], COX-2 and cancer- and cell death-related genes are
differentially expressed in adenomyosis (10,33).
CXCL10 modulates adhesion molecule expression through stimulation
of monocytes and natural killer and T-cell migration. Patients with
adenomyosis had HLA-G expression in eutopic and ectopic endometrial
cells (34). This could explain the
ability of cells to escape host immunosurveillance and to survive
without being eliminated by the immune system. Calbindin, a
cytosolic calcium binding protein, is expressed in female
reproductive tissues (35). Uterine
calbindin may be involved in controlling myometrial activity
related with intracellular calcium level. The expression of
interleukin-10 (IL-10) was elevated in the eutopic endometrium of
women with adenomyosis (36). IL-10
downregulates the expression of Th1 cytokines and MHC class II
antigens. The matrix metalloproteinase (MMP)-2 -1306C/T
polymorphism may be associated with the risk of adenomyosis
(37). COX-2 induces migration,
invasion and anti-apoptotic capabilities of adenomyosis-derived
mesenchymal stem cells (38).
Beclin 1 has a central role in autophagy, a process of programmed
cell survival, which is increased during periods of cell stress
(39). Beclin 1 expression was
decreased in eutopic endometria of women with adenomyosis (40). Histone deacetylases (HDACs) are
involved in adenomyosis, suggesting that it may be an epigenetic
disease (41). These data suggest
that genetic and epigenetic abnormalities contribute to the
pathogenesis of adenomyosis.
Animal experiments
The findings and theories on the pathogenesis of
adenomyosis have been obtained by research using animal models. A
variety of animals, including non-human primates, horses, dogs,
cats, rabbits and laboratory rodents, can be used as models for
developing spontaneous adenomyosis (24,42).
On the other hand, an animal model of endometriosis appears limited
to non-human primates, since rodents do not develop spontaneous
endometriosis (24). These two
phenotypic variants suggest different etiology and pathophysiology
of adenomyosis and endometriosis (24,43).
In animal models, adenomyosis was characterized by benign invasion
of endometrial glands and stroma into the myometrium, growth of
these cells and subsequent hypertrophy and hyperplasia of
myometrial smooth muscle cells. CD-1 mice spontaneously develop
uterine adenomyosis, with a disease prevalence of ~80% by 12 months
of age (24). Other animal
experiments also showed that tamoxifen-induced disruption and
disorganization of the inner myometrium play a role in the
development of adenomyosis (44).
Abnormal development of the inner circular muscle layer, exhibiting
discontinuity in the inner myometrial layer, marked thinning, and
lack of orientation and bundling of the muscle cells, may be
involved in the development of premature uterine adenomyosis.
Increased incidence and severity was evident in mice dosed with
tamoxifen (45). Upregulated genes
include nerve growth factor (NGF), cathepsin B, transforming growth
factor-β induced (Tqfbi) and collagens (Colla1, Colla2) (45). NGF is associated with the severity
of adenomyosis and plays a critical role in producing pain, neural
plasticity and release of inflammatory factors (46). In equine models, ECM proteins,
including collagen IV, laminin and fibronectin deposition outside
the basement membrane, might be involved in the development of
endometriosis (21). These findings
will be helpful to provide a molecular basis for understanding the
mechanism underlying steroid hormone-induced tissue remodeling and
the development of adenomyosis (45).
4. Malignant transformation of
adenomyosis
It is believed that endometriosis can undergo
malignant transformation into endometrioid and clear cell ovarian
carcinoma. We reviewed whether adenomyosis serves as a precursor of
neoplastic disease. Sampson’s criteria were proposed for a
diagnosis of malignant transformation of adenomyosis (47). Kumar and Anderson emphasized the
necessity of the transitions between the benign and malignant
glandular structures (48,49). Colman and Rosenthal identified
criteria that endometrial stromal cells supporting a diagnosis of
adenomyosis must be present (50).
Herein we reviewed the cases of carcinoma arising in
adenomyosis.
The pathological criteria used for case
identification are: i) evidence of pre-existing adenomyosis at the
site of the malignant lesion; ii) presence of glandular cells
and/or endometrial stromal cells supporting a diagnosis of
adenomyosis; iii) evidence of transitions between the benign and
malignant glandular structures; iv) carcinoma must be absent from
invasion or metastasis from another source; and v) carcinoma must
be absent from the eutopic endometrium.
A frequent association of adenomyosis with other
hormone-dependent uterine lesions, including leiomyoma, endometrial
hyperplasia and endometrial carcinoma has been described in the
literature (1,51). Adenomyosis is commonly seen in
hysterectomy specimens for endometrial adenocarcinoma (1,51).
Malignant changes in adenomyosis were present in 6.8% of patients
with endometrial cancer (52). A
majority of cases with adenocarcinoma arising in adenomyosis were
associated with the adjacent endometrial adenocarcinomas (53). Adenocarcinomas developing within
adenomyosis often originate from endometrial carcinomas which arise
from the eutopic endometrium, then invade into pre-existing
adenomyosis (54). In this review,
we selected cases whose endometrium was completely examined and
tumor-free.
Although malignant transformation of adenomyosis is
a rare event, there are several case reports of adenocarcinomas
developing within adenomyosis. Rolly published the first report in
1897 (55). In our review, to date,
44 cases of malignant tumors arising from adenomyosis have been
documented (48,51,54–70).
Most of the patients were postmenopausal. Malignant
transformation of adenomyosis in premenopausal women with normal
endometrium is extremely rare (54). Abnormal vaginal bleeding, vulvar
itching, slight fever and weight loss were clinical presentations.
These uteri showed no evidence of endometrial malignancy in the
endometrial cavity. One patient had received tamoxifen for
treatment of her breast cancer over the past five years (59). There were transitions between
endometrial epithelium of adenomyosis, borderline malignancy
(noninvasive), and invasive carcinoma. The histological subtypes of
the tumors were endometrioid and, to a lesser degree, serous, clear
cell and poorly differentiated adenocarcinoma. Serous endometrial
intraepithelial carcinoma (serous EIC) arising in adenomyosis is
rare (56,59).
Two types of endometrial carcinoma, type I or type
II, have been delineated on the basis of clinicopathological and
genetic studies. In most cases with malignant transformation of
adenomyosis, different stages of atypical or hyperplastic changes
were simultaneously identified (52). This observation highlights a similar
pathway of carcinogenesis in adenomyosis as is known in
estrogen-responsive endometrial cancer type I (52). In general, malignant transformation
of adenomyosis was positive for ER, PR, cyclooxygenase-2 (COX-2),
CA125 and focally weak-positive for aromatase. Hormonal receptor
expression is associated with low grade and early-stage tumors.
Aromatase activity is upregulated by increased levels of the enzyme
COX-2.
By contrast, advanced adenocarcinomas in adenomyosis
were reported as negative for ER and PR (58). Ohta et al reported a case of
a clear cell adenocarcinoma arising from adenomyosis, with
overexpression of p53 and laminin-5 γ2 chain (62). The poorly differentiated
adenocarcinoma cells stained positively for p53, but did not
express either ER or PR, suggesting that this adenocarcinoma was a
type II carcinoma with biologically aggressive behavior (48). This has led to the proposal that
some cases with malignant transformation of adenomyosis develop
de novo.
5. Discussion
This review aimed at summarizing and emphasizing the
pathogenesis of adenomyosis and its malignant transformation.
Several pathological theories of adenomyosis have been proposed: i)
metaplasia, ii) Müllerian rests, iii) reaction (tissue injury and
repair) theory, as well as other hypotheses (Fig. 1). It is likely that intrinsic
factors within the myometrium are key for the development of
adenomyosis. Early signs of the development of adenomyosis might be
the disruption of smooth muscle cells of the inner layer of the
myometrium. Associated with these changes, stromal connective
tissue penetrates into the myometrium followed by uterine gland
invasion and growth. Adenomyosis-associated smooth muscle cells
respond to changes in environmental factors and regulate smooth
muscle function. Smooth muscle thin filaments are dynamic and
remodel the actin cytoskeleton. Smooth muscle cell aggregation,
hypertrophy and hyperplasia in premature uterine adenomyosis is
considered as smooth muscle metaplasia with various grades of
differentiation.
Recent progress in the understanding of the
molecular environment surrounding adenomyosis demonstrated that
growth and regression are determined by the rate of cell
proliferation, differentiation, apoptosis, angiogenesis, protease
production, and ECM deposition. These changes might determine the
patterns of gene expression appropriate for transformation of
stromal cells into functional smooth muscle cells. Ovarian steroids
are key regulators of a number of these processes. Activation of
several of these pathways and their downstream targeted genes are
involved in regulating a wide variety of processes that regulate
ovarian steroid synthesis, immune response, metabolism, matrix
accumulation, angiogenesis and local inflammatory response
(10). Although the full
implications of the differentially expressed genes are not yet
completely understood, adenomyosis samples clustered closely with
endometrium samples (10). It may
provide evidence for a possible metaplastic origin of endometrial
stromal cells.
We reviewed cases of adenocarcinoma arising from
adenomyotic foci in the uterus. Adenomyosis shares some aspects of
malignancy such as increased growth, angiogenesis and invasion. The
development of malignant disease is a rare occurrence. The
occurrence is attributable to malignant transformation of an
existing adenomyosis. Transition from endometrial epithelium of
adenomyosis to the pre-malignant single-layered tumor cells, and
finally to carcinoma lesions of various degrees was recognized.
These processes depend on the accumulation of genetic and
epigenetic alterations in a multistep process. However, the
identity of molecule(s) that initiate myometrial cellular
transformation and subsequently regulate their growth remains
unknown. The pathological and molecular plausibility is not well
substantiated.
There have been few data reporting genetic changes,
mutational analysis and the inactivation of specific tumor
suppressor genes in adenomyosis. Goumenou et al reported for
the first time that loss of heterozygosity (LOH) occurs in
adenomyosis (71). DNA mismatch
repair genes (hMSH2, hMLH1), p16Ink4 (CDKN2A,
cyclin-dependent kinase inhibitor 2A) and GALT
(galactose-1-phosphate uridylyltransferase) genes were associated
with adenomyosis (71). Stromal
bcl-2 expression in adenomyosis remained at low levels and could
have negative implications for the growth and survival of ectopic
endometrial tissue (72). Although
the monoclonal outgrowth of ectopic glands has been demonstrated in
endometriosis, the details of the phenomenon in adenomyosis remain
unknown. Adenomyosis has epigenetic aberration with respect to
promoter hypermethylation of progesterone receptor (73). Epigenetic changes have to be
explored in adenomyosis. However, the biological functions of
several other genes in adenomyosis remain to be determined. A
molecular continuum between the benign affection and the malignant
entity requires stronger evidence of common mutational or
methylational events.
In conclusion, adenomyosis is a common benign
disease. Numerous hypotheses have been proposed to explain the
presence of ectopic endometrial tissue and stroma in uterine
myometrium. Perhaps the most compelling mystery surrounding
adenomyosis is not why a subset of women is afflicted, but how the
majority of women can prevent adenomyosis development. In the
present review we also presented cases of malignant transformation,
reviewing the literature for data of possible associated factors
that lead to the pathogenesis of the disease. Although its course
is usually benign, adenomyosis may be the precursor of malignant
disease. However, to date, there has not been sufficient genetic
and epigenetic evidence for malignant transformation of
adenomyosis. With further research, we may come closer to
identifying the pathogenesis and pathophysiology of adenomyosis and
its malignant transformation.
Acknowledgements
This study was supported by KAKENHI [Japan Society
for the Promotion of Science (JSPS) Grant-in-Aid]. We thank all the
study participants for their time and efforts. We thank Mikiko Kita
for the editorial assistance.
References
|
1
|
Ferenczy A: Pathophysiology of
adenomyosis. Hum Reprod Update. 4:312–322. 1998. View Article : Google Scholar
|
|
2
|
Templeman C, Marshall SF, Ursin G,
Horn-Ross PL, Clarke CA, Allen M, Deapen D, Ziogas A, Reynolds P,
Cress R, Anton-Culver H, West D, Ross RK and Bernstein L:
Adenomyosis and endometriosis in the California Teachers Study.
Fertil Steril. 90:415–424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lalović N, Cvijanović R, Vladicić ND,
Marić R, Jokanović D and Skipina DB: Adenomyomatosis of the
gallbladder - case report. Med Pregl. 64:323–326. 2011.
|
|
4
|
Kayahara M, Ohta T, Kitagawa H, Miwa K,
Urabe T and Murata T: Adenomyomatosis of the papilla of Vater: a
case illustrating diagnostic difficulties. Dig Surg. 18:139–142.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matsumoto T and Shimada K: A case of
gallbladder cancer arising from the Rokitansky-Aschoff sinus. Jpn J
Clin Oncol. 39:7762009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krutsay M: Adenomyosis of the common bile
duct. Magy Onkol. 54:179–180. 2010.(In Hungarian).
|
|
7
|
Benagiano G, Brosens I and Carrara S:
Adenomyosis: new knowledge is generating new treatment strategies.
Womens Health. 5:297–311. 2009.PubMed/NCBI
|
|
8
|
Parrott E, Butterworth M, Green A, White
IN and Greaves P: Adenomyosis - a result of disordered stromal
differentiation. Am J Pathol. 159:623–630. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mori T, Ohta Y and Nagasawa H:
Ultrastructural changes in uterine myometrium of mice with
experimentally-induced adenomyosis. Experientia. 40:1385–1387.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hever A, Roth RB, Hevezi PA, Lee J,
Willhite D, White EC, Marin EM, Herrera R, Acosta HM, Acosta AJ and
Zlotnik A: Molecular characterization of human adenomyosis. Mol Hum
Reprod. 12:737–748. 2006. View Article : Google Scholar
|
|
11
|
Barcena de Arellano ML, Gericke J,
Reichelt U, Okuducu AF, Ebert AD, Chiantera V, Schneider A and
Mechsner S: Immunohistochemical characterization of
endometriosis-associated smooth muscle cells in human peritoneal
endometriotic lesions. Hum Reprod. 26:2721–2730. 2011.
|
|
12
|
Mechsner S, Grum B, Gericke C,
Loddenkemper C, Dudenhausen JW and Ebert AD: Possible roles of
oxytocin receptor and vasopressin-1α receptor in the pathomechanism
of dysperistalsis and dysmenorrhea in patients with adenomyosis
uteri. Fertil Steril. 94:2541–2546. 2010.
|
|
13
|
Mehasseb MK, Bell SC, Brown L, Pringle JH
and Habiba M: Phenotypic characterisation of the inner and outer
myometrium in normal and adenomyotic uteri. Gynecol Obstet Invest.
71:217–224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mehasseb MK, Panchal R, Taylor AH, Brown
L, Bell SC and Habiba M: Estrogen and progesterone receptor isoform
distribution through the menstrual cycle in uteri with and without
adenomyosis. Fertil Steril. 95:2228–2235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jaffer S, Shynlova O and Lye S: Mammalian
target of rapamycin is activated in association with myometrial
proliferation during pregnancy. Endocrinology. 150:4672–4680. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shynlova O, Kwong R and Lye SJ: Mechanical
stretch regulates hypertrophic phenotype of the myometrium during
pregnancy. Reproduction. 139:247–253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Treloar SA, Bell TA, Nagle CM, Purdie DM
and Green AC: Early menstrual characteristics associated with
subsequent diagnosis of endometriosis. Am J Obstet Gynecol.
202:534.e1–6. 2010.PubMed/NCBI
|
|
18
|
Mechsner S, Bartley J, Infanger M,
Loddenkemper C, Herbel J and Ebert AD: Clinical management and
immunohistochemical analysis of umbilical endometriosis. Arch
Gynecol Obstet. 280:235–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Kaam KJ, Schouten JP, Nap AW,
Dunselman G and Groothuis PG: Fibromuscular differentiation in
deeply infiltrating endometriosis is a reaction of resident
fibroblasts to the presence of ectopic endometrium. Hum Reprod.
23:2692–2700. 2008.PubMed/NCBI
|
|
20
|
Leyendecker G, Wildt L and Mall G: The
pathophysiology of endometriosis and adenomyosis: tissue injury and
repair. Arch Gynecol Obstet. 280:529–538. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Walter I, Handler J, Reifinger M and
Aurich C: Association of endometriosis in horses with
differentiation of periglandular myofibroblasts and changes of
extracellular matrix proteins. Reproduction. 121:581–586. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen YJ, Li HY, Huang CH, Twu NF, Yen MS,
Wang PH, Chou TY, Liu YN, Chao KC and Yang MH: Oestrogen-induced
epithelial-mesenchymal transition of endometrial epithelial cells
contributes to the development of adenomyosis. J Pathol.
222:261–270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou S, Yi T, Liu R, Bian C, Qi X, He X,
Wang K, Li J, Zhao X, Huang C and Wei Y: Proteomics identification
of annexin A2 as a key mediator in the metastasis and
proangiogenesis of endometrial cells in human adenomyosis. Mol Cell
Proteomics. 11:M112.0179882012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Greaves P and White IN: Experimental
adenomyosis. Best Pract Res Clin Obstet Gynaecol. 20:503–510. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kang S, Li SZ, Wang N, Zhou RM, Wang T,
Wang DJ, Li XF, Bui J and Li Y: Association between genetic
polymorphisms in fibroblast growth factor (FGF)1 and FGF2 and risk
of endometriosis and adenomyosis in Chinese women. Hum Reprod.
25:1806–1811. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hinoki A, Kimura K, Higuchi S, Eguchi K,
Takaguri A, Ishimaru K, Frank GD, Gerthoffer WT, Sommerville LJ,
Autieri MV and Eguchi S: p21-activated kinase 1 participates in
vascular remodeling in vitro and in vivo. Hypertension. 55:161–165.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim SR, Kim SH, Lee HW, Chae HD, Kim CH
and Kang BM: Increased expression of p21-activated kinase in
adenomyosis. Fertil Steril. 94:1125–1128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meenakshi M and McCluggage WG: Vascular
involvement in adenomyosis: report of a large series of a common
phenomenon with observations on the pathogenesis of adenomyosis.
Int J Gynecol Pathol. 29:117–121. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sieiński W: Tumor-like intravascular
proliferations of the stroma in adenomyosis. Patol Pol. 44:1–4.
1993.PubMed/NCBI
|
|
30
|
Mori A, Zhai YL, Toki T, Nikaido T and
Fujii S: Distribution and heterogeneity of mast cells in the human
uterus. Hum Reprod. 12:368–372. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Menzies FM, Shepherd MC, Nibbs RJ and
Nelson SM: The role of mast cells and their mediators in
reproduction, pregnancy and labour. Hum Reprod Update. 17:383–396.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Anaf V, Chapron C, El Nakadi I, De Moor V,
Simonart T and Noël JC: Pain, mast cells, and nerves in peritoneal,
ovarian, and deep infiltrating endometriosis. Fertil Steril.
86:1336–1343. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kusakabe KT, Abe H, Kondo T, Kato K, Okada
T and Otsuki Y: DNA microarray analysis in a mouse model for
endometriosis and validation of candidate factors with human
adenomyosis. J Reprod Immunol. 85:149–160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang F, Wen Z, Li H, Yang Z, Zhao X and
Yao X: Human leukocyte antigen-G is expressed by the eutopic and
ectopic endometrium of adenomyosis. Fertil Steril. 90:1599–1604.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Choi KC, Leung PC and Jeung EB: Biology
and physiology of Calbindin-D9k in female reproductive tissues:
involvement of steroids and endocrine disruptors. Reprod Biol
Endocrinol. 3:662005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang F, Li H, Yang Z, Du X, Cui M and Wen
Z: Expression of interleukin-10 in patients with adenomyosis.
Fertil Steril. 91:1681–1685. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kang S, Zhao X, Xing H, Wang N, Zhou R,
Chen S, Li W, Zhao J, Duan Y, Sun D and Li Y: Polymorphisms in the
matrix metalloproteinase-2 and tissue inhibitor of
metalloproteinase-2 and the risk of human adenomyosis. Environ Mol
Mutagen. 49:226–231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen YJ, Li HY, Chang YL, Yuan CC, Tai LK,
Lu KH, Chang CM and Chiou SH: Suppression of migratory/invasive
ability and induction of apoptosis in adenomyosis-derived
mesenchymal stem cells by cyclooxygenase-2 inhibitors. Fertil
Steril. 94:1972–1979. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kang R, Zeh HJ, Lotze MT and Tang D: The
Beclin 1 network regulates autophagy and apoptosis. Cell Death
Differ. 18:571–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ren Y, Mu L, Ding X and Zheng W: Decreased
expression of Beclin 1 in eutopic endometrium of women with
adenomyosis. Arch Gynecol Obstet. 282:401–406. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu X, Nie J and Guo SW: Elevated
immunoreactivity against class I histone deacetylases in
adenomyosis. Gynecol Obstet Invest. 74:50–55. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Suire RA, Goodman DG, Valerio MG,
Fredrickson TN, Strandberg JD, Levitt MH, Lingeman CH, Harshbarger
JC and Dawe CJ: Female reproductive system. Pathology of Laboratory
Animals. 2. Benirschke K, Garner FM and Jones TC: Springer-Verlag;
New York, NY: pp. 1051–1262. 1978
|
|
43
|
Oehler MK, Greschik H, Fischer DC, Tong X,
Schuele R and Kieback DG: Functional characterization of somatic
point mutations of the human estrogen receptor alpha (hERalpha) in
adenomyosis uteri. Mol Hum Reprod. 10:853–860. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mehasseb MK, Bell SC and Habiba MA: The
effects of tamoxifen and estradiol on myometrial differentiation
and organization during early uterine development in the CD1 mouse.
Reproduction. 138:341–350. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Green AR, Styles JA, Parrott EL, Gray D,
Edwards RE, Smith AG, Gant TW, Greaves P, Al-Azzawi F and White IN:
Neonatal tamoxifen treatment of mice leads to adenomyosis but not
uterine cancer. Exp Toxicol Pathol. 56:255–263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li Y, Zhang SF, Zou SE, Xia X and Bao L:
Accumulation of nerve growth factor and its receptors in the uterus
and dorsal root ganglia in a mouse model of adenomyosis. Reprod
Biol Endocrinol. 9:302011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sampson JA: Endometrial carcinoma of the
ovary arising in endometrial tissue of that organ. Arch Surg.
10:1–72. 1925. View Article : Google Scholar
|
|
48
|
Motohara K, Tashiro H, Ohtake H, Saito F,
Ohba T and Katabuchi H: Endometrioid adenocarcinoma arising in
adenomyosis: elucidation by periodic magnetic resonance imaging
evaluations. Int J Clin Oncol. 13:266–270. 2008. View Article : Google Scholar
|
|
49
|
Kumar D and Anderson W: Malignancy in
endometriosis interna. J Obstet Gynaecol Br Emp. 65:435–437. 1958.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Colman HI and Rosenthal AH: Carcinoma
developing in areas of adenomyosis. Obset Gynecol. 14:342–348.
1959.PubMed/NCBI
|
|
51
|
Ismiil ND, Rasty G, Ghorab Z, Nofech-Mozes
S, Bernardini M, Thomas G, Ackerman I, Covens A and Khalifa MA:
Adenomyosis is associated with myometrial invasion by FIGO 1
endometrial adenocarcinoma. Int J Gynecol Pathol. 26:278–283. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kucera E, Hejda V, Dankovcik R, Valha P,
Dudas M and Feyereisl J: Malignant changes in adenomyosis in
patients with endometrioid adenocarcinoma. Eur J Gynaecol Oncol.
32:182–184. 2011.PubMed/NCBI
|
|
53
|
Hernandez E and Woodruff JD: Endometrial
adenocarcinoma arising in adenomyosis. Am J Obstet Gynecol.
138:827–832. 1980.PubMed/NCBI
|
|
54
|
Kazandi M, Zeybek B, Terek MC, Zekioglu O,
Ozdemir N and Oztekin K: Grade 2 endometrioid adenocarcinoma
arising from adenomyosis of the uterus: report of a case. Eur J
Gynaecol Oncol. 31:719–721. 2010.PubMed/NCBI
|
|
55
|
Rolly F: Über einen Fall von Adenomyoma
Uteri, Übergang in Karzinom und Metastasenbildung. Virchows Arch.
150:5551897.(In German).
|
|
56
|
Abushahin N, Zhang T, Chiang S, Zhang X,
Hatch K and Zheng W: Serous endometrial intraepithelial carcinoma
arising in adenomyosis: a report of 5 cases. Int J Gynecol Pathol.
30:271–281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Puppa G, Shozu M, Perin T, Nomura K,
Gloghini A, Campagnutta E and Canzonieri V: Small primary
adenocarcinoma in adenomyosis with nodal metastasis: a case report.
BMC Cancer. 7:1032007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Takeuchi K, Yamanaka Y, Hamana S, Ohara N
and Maruo T: Invasive adenocarcinoma arising from uterine
adenomyosis involving the rectosigmoid colon. Int J Gynecol Cancer.
14:1004–1006. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Izadi-Mood N, Samadi N, Sarmadi S and
Eftekhar Z: Papillary serous carcinoma arising from adenomyosis
presenting as intramural leiomyoma. Arch Iran Med. 10:2582–2560.
2007.PubMed/NCBI
|
|
60
|
Hirabayashi K, Yasuda M, Kajiwara H,
Nakamura N, Sato S, Nishijima Y, Mikami M and Osamura RY: Clear
cell adenocarcinoma arising from adenomyosis. Int J Gynecol Pathol.
28:262–266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Jha P, Ansari C, Coakley FV, Wang ZJ, Yeh
BM, Rabban J and Poder L: Case report: imaging of Mullerian
adenosarcoma arising in adenomyosis. Clin Radiol. 64:645–648. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ohta Y, Hamatani S, Suzuki T, Ikeda K,
Kiyokawa K, Shiokawa A, Kushima M and Ota H: Clear cell
adenocarcinoma arising from a giant cystic adenomyosis: a case
report with immunohistochemical analysis of laminin-5 gamma2 chain
and p53 overexpression. Pathol Res Pract. 204:677–682. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Couto D, Mota F, Silva T and de Oliveira
C: Adenocarcinoma arising in adenomyosis: report of an unusual
case. Acta Obstet Gynecol Scand. 83:406–408. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Woodruff JD, Erozan YS and Genadry R:
Adenocarcinoma arising in adenomyosis detected by atypical
cytology. Obstet Gynecol. 67:145–148. 1986.PubMed/NCBI
|
|
65
|
Koshiyama M, Suzuki A, Ozawa M, Fujita K,
Sakakibara A, Kawamura M, Takahashi S, Fujii H, Hirano T, Okagaki
A, Nagano T and Ban C: Adenocarcinomas arising from uterine
adenomyosis: a report of four cases. Int J Gynecol Pathol.
21:2392–2345. 2002. View Article : Google Scholar
|
|
66
|
Kuwashima Y, Uehara T, Kishi K, Tajima H,
Shiromizu K, Matsuzawa M and Takayama S: Intramural adenocarcinoma
of the uterus, arisen from adenomyosis uteri, showing unique
histologic appearances. Report of two cases. Eur J Gynaecol Oncol.
15:418–423. 1994.
|
|
67
|
Rubod C, Narducci F, Delattre C, Decocq J,
Verbert A and Delahousse G: Endometrioid adenocarcinoma arising
from adenomyosis. A case report and literature review. J Gynecol
Obstet Biol Reprod. 33:140–144. 2004.PubMed/NCBI
|
|
68
|
Takai N, Akizuki S, Nasu K, Etoh Y and
Miyakawa I: Endometrioid adenocarcinoma arising from adenomyosis.
Gynecol Obstet Invest. 48:141–144. 1999. View Article : Google Scholar
|
|
69
|
Heo SH, Lee KH, Kim JW and Jeong YY:
Unusual manifestation of endometrioid adenocarcinoma arising from
subserosal cystic adenomyosis of the uterus: emphasis on MRI and
positron emission tomography CT findings. Br J Radiol. 84:e210–212.
2011.
|
|
70
|
Boes AS, Tousseyn T, Vandenput I,
Timmerman D, Vergote I, Moerman P and Amant F: Pitfall in the
diagnosis of endometrial cancer: case report of an endometrioid
adenocarcinoma arising from uterine adenomyosis. Eur J Gynaecol
Oncol. 32:431–434. 2011.PubMed/NCBI
|
|
71
|
Goumenou AG, Arvanitis DA, Matalliotakis
IM, Koumantakis EE and Spandidos DA: Loss of heterozygosity in
adenomyosis on hMSH2, hMLH1, p16Ink4 and GALT loci. Int
J Mol Med. 6:667–671. 2000.PubMed/NCBI
|
|
72
|
Jones RK, Searle RF and Bulmer JN:
Apoptosis and bcl-2 expression in normal human endometrium,
endometriosis and adenomyosis. Hum Reprod. 13:3496–3502. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Nie Jichan, Liu Xishi and Guo SW: Promoter
hypermethylation of progesterone receptor isoform B (PR-B) in
adenomyosis and its rectification by a histone deacetylase
inhibitor and a demethylation agent. Reprod Sci. 17:995–1005. 2010.
View Article : Google Scholar : PubMed/NCBI
|