Introduction
Gastric cancer is a major public health issue
worldwide particularly in China. According to cancer statistics
published in 2011, gastric cancer is the fourth most frequently
diagnosed cancer and the third most common cause of cancer-related
mortality in men, whereas in women it is the fifth most common
malignancy in regards to incidence and mortality rate (1,2). The
highest incidence rates of gastric cancer are in Eastern Asia,
Eastern Europe and South America (1,2). In
China, gastric cancer is the third most common malignancy and the
leading cause of cancer-related death (3,4). Lack
of effective treatment options for advanced gastric cancer is
largely due to a poor understanding of the molecular mechanisms
involved in the development of gastric cancer.
Nuclear factor-κB (NF-κB) is a ubiquitously
expressed family of Rel-related transcription factors (5). Abnormal activation of NF-κB reduces
cell sensitivity to apoptotic stimuli and therefore facilitates the
survival of transformed cells (6).
NF-κB is involved in the control of cell growth and oncogenesis.
Constitutive activation of NF-κB in cancer cells is partially
responsible for the observed resistance to chemotherapy and
radiotherapy (7). As a ubiquitous
transcription factor, NF-κB regulates the expression and function
of numerous target genes, among which and of most relevance to
cancer development is E-cadherin.
E-cadherin is a major cell-cell adhesion molecule
that plays a significant role in the establishment and maintenance
of cell-cell interactions and tissue architecture (8–10). A
negative correlation between NF-κB and E-cadherin in gastric cancer
cells has recently been reported (11). It was recently shown that connective
tissue growth factor (CTGF) downregulated the expression of
E-cadherin through activation of NF-κB (11). Loss of E-cadherin expression is
associated with enhanced tumor progression, increased invasive and
metastatic potential of cancer cells and a poor overall prognosis
in patients with gastric cancer and other malignancies (12–15).
However, as gastric cancer is a multifactorial disease (16), loss of E-cadherin alone cannot
explain the increased malignant tendency of gastric cancer cells
(17). Interaction between
E-cadherin and other genes could well be involved in the
development of gastric cancer and its malignant phenotype.
We supposed that Snail may be a critical factor in
mediating the regulatory role of NF-κB on its target genes, which
has not been reported in the literature. Snail is a member of the
Snail superfamily of zinc finger transcription factors (18). It plays an important role in
embryonic development, neural differentiation, cell division and
survival (19,20). Overexpression of Snail mRNA was able
to downregulate the expression of E-cadherin in diffuse-type
gastric carcinoma (21,22). However, it is not clear whether
Snail is a critical transcription factor for the regulatory role of
NF-κB regarding its target genes.
This study aimed to evaluate whether NF-κB-mediated
changes in E-cadherin are regulated through Snail.
Materials and methods
Donor blocks and patient information
Paraffin-embedded blocks of gastric tissues
(previously fixed in 10% formaldehyde) were obtained from 189
patients with gastric cancer who underwent surgical operations at
the Wuwei Tumor Hospital, Gansu Province, China. The diagnosis of
gastric adenocarcinoma was based on the World Health Organization
(WHO) diagnostic criteria, and was confirmed by two independent
pathologists. Based on the WHO Classification of Tumors of the
Digestive System (23), there were
100 cases of poorly differentiated gastric adenocarcinoma, 44 cases
of moderately differentiated gastric adenocarcinoma, and 45 cases
of well-differentiated gastric adenocarcinoma. The patient study
population had a mean age of 55 (range, 30–73) years at the time of
operation, with an overall male to female ratio of 3.3:1. None of
the patients had received any chemotherapy and/or radiotherapy
prior to surgery. The detailed patient characteristics are
summarized in Table I.
Paraffin-embedded blocks of normal gastric mucosal tissues (n=32)
were obtained from healthy subjects who underwent gastroscopy in
the same hospital for other non-malignant gastric conditions.
Written consent from all patients was obtained prior to the study.
The study was approved by the Institutional Human Ethics Committee
of the First Clinical School of Lanzhou University.
 | Table IClinicopathological features of the
189 patients with gastric cancer. |
Table I
Clinicopathological features of the
189 patients with gastric cancer.
|
Characteristics | No. of cases | % |
|---|
| Gender |
| Female | 44 | 23.3 |
| Male | 145 | 76.7 |
| Age (years) |
| <50 | 51 | 27 |
| ≥50 | 138 | 73 |
| Tumor size
(cm) |
| <5 | 70 | 37 |
| ≥5 | 119 | 63 |
| Lymph node
metastasis |
| No | 73 | 38.6 |
| Yes | 116 | 61.4 |
| Tumor
differentiation status |
| Well/moderate | 89 | 47.1 |
| Poor | 100 | 52.9 |
| Depth of tumor
invasion |
| Without serosal
invasion | 46 | 24.3 |
| Serosal
invasion | 143 | 75.7 |
| Lauren
classification |
| Intestinal
type | 97 | 51.3 |
| Diffuse type | 85 | 48.7 |
Tissue microarray (TMA) construction
The collected paraffin blocks were used as donor
blocks to make eight TMA recipient blocks. In each donor block,
morphologically representative areas were chosen and marked on
their respective H&E slides. A tissue core of 0.6 mm in
diameter from each donor block was taken using a cylindrical tissue
puncher (Beecher, Beecher Instruments, Silver Spring, MD, USA) and
transferred into the hole on the recipient paraffin block. The
distance between each recipient hole was kept constant at 1 mm.
Duplicate tissue cores from each donor tissue were positioned side
by side. The detailed matrix plan for the arrangement of the
constructed TMA was recorded for correct tissue identification.
Immunohistochemistry assays
The above-constructed TMA blocks were cut into
sections of 4-μm thickness, dewaxed in xylene and rehydrated in
graded alcohols. The slides were boiled for 30 min in citrate
buffer (10 mM; pH 6.0) in a microwave oven at 250–300 W and then
cooled to room temperature. Before immunohistochemical staining,
the slides were incubated with 3% H2O2 in PBS
for 10 min to quench the endogenous peroxidase activity, followed
by incubation with 3% BSA for 15 min to block the non-specific
binding of the antibody.
For immunohistochemical staining, the slides were
incubated for 1 h at 37°C with primary antibody against E-cadherin
(monoclonal, dilution 1:250, Abcam, USA), NF-κB p65 (monoclonal,
dilution 1:200, Abcam), and Snail (polyclonal, dilution 1:200,
Abcam). The slides were then washed with PBS for three times,
incubated with biotin-conjugated secondary antibody (1:150, Abcam)
for 40 min at 37°C, washed with PBS, and then incubated with
streptavidin-horseradish peroxidase (SHRP) (Thermo Fisher
Scientific, USA) for 40 min at room temperature. DAB
(2,3-diaminobenzidine tetrahydrochloride) (Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd., China) was used to develop the
peroxidase reaction, and the slides were counterstained with
hematoxylin. The experimental validity was confirmed by using
negative controls in which the primary antibody was replaced by 5%
BSA. The slides were reviewed independently by two pathologists,
and the staining for each protein was scored according to the
criteria established in Table II
and as previously reported (24).
Representative areas were photographed for data presentation.
 | Table IIScoring criteria for
immunohistochemistry. |
Table II
Scoring criteria for
immunohistochemistry.
| Criteria | Score |
|---|
| Staining
positivity |
| Positive in <5%
of the cells | 0 |
| Positive in 5–25%
of the cells | 1 |
| Positive in 26–50%
of the cells | 2 |
| Positive in
>50% of the cells | 3 |
| Staining
intensity |
| Negative (no
staining) | 0 |
| Weak (light
yellow) | 1 |
| Moderate
(brown) | 2 |
| High (dark
brown) | 3 |
| Sum of positivity
and intensity scores |
| Negative | 0–2 |
| Weak positive | 3–4 |
| Strong
positive | 5–6 |
Culture of gastric cancer cells and
treatment with NF-κB inhibitor PDTC
SGC7901 cells (a human gastric cancer cell line;
Shanghai Institutes for Biological Sciences, Chinese Academy of
Sciences, Shanghai, China) were cultured in RPMI-1640 medium
(Gibco, Carlsbad, CA, USA) supplemented with 1% penicillin and
streptomycin (Gibco) and 10% heat-inactivated fetal bovine serum
(Hangzhou Sijiqing Biological Engineering Materials Co., Ltd.,
Hangzhou, China) at 37°C in a humidified atmosphere containing 5%
carbon dioxide.
To block the activity of NF-κB, cells were treated
with 50 μM of a chemical inhibitor of NF-κB, pyrrolidine
dithiocarbamate (PDTC). This optimal dose was based on our
preliminary study by sulforhodamine B (SRB) assay, which revealed
that 50 μM of PDTC was able to effectively block the expression and
activity of the NF-κB subunit p65 in gastric cancer cells. The SRB
assay was performed as previously reported (25,26).
Quantitative real-time PCR (qPCR)
Total RNA of the treated cells was extracted using
the Ze Spin Column of the Total RNA Isolation kit (Takara, Dalian,
China). Total RNA (1 μg) was reverse-transcribed into cDNA using
the PrimeScript™ RT Reagent kit (Takara) according to the
manufacturer’s instructions. The synthesized cDNA samples were
subjected to qPCR using SYBR® Premix Ex Taq™ reagent
(Takara). All qPCR reactions were performed using Rotor-Gene 3000
(Corbett, Australia), with each PCR cycle consisting of
denaturation for 15 sec at 95°C, annealing for 45 sec at 62°C and
extension for 30 sec at 72°C. β-actin was used as the internal
reference. The qPCR primers were as follows: E-cadherin (sense:
5′-TTAAACTCCTGGCCTCAAGCAATC-3′, antisense:
5′-TCCTATCTTGGGCAAAGCAACTG-3′), NF-κB/P65 (sense:
5′-TCAGTCAGCGCATCCAGACC-3′, antisense: 5′-CAGAGCCGCACAGCATTCA-3′),
Snail (sense: 5′-CGC GCTCTTTCCTCGTCAG-3′, antisense: 5′-TCCCAGATGA
GCATTGGCAG-3′), β-actin (sense: 5′-TGGCACCCAGCA CAATGAA-3′,
antisense: 5′-CTAAGTCATAGTCCGCCTAG AAGCA-3′). For data analysis,
fold induction relative to internal controls was calculated by the
Δ Ct evaluation method.
Western blot assay
Total protein from the treated cells was extracted
using RIPA buffer (Beyotime, Shanghai, China) supplemented with
phenylmethylsulfonyl fluoride (PMSF) and protease inhibitor
cocktails (Roche, Germany), and the protein concentrations were
measured by a BCA protein quantitative assay kit (Applygen,
Beijing, China). The cell lysates were cleared by centrifugation at
10,000 × g for 5 min at 4°C. Equal amounts of total proteins were
resolved on 10% polyacrylamide gels (SDS-PAGE) and transferred to
PVDF membranes, which were incubated with primary antibodies
(E-cadherin, NF-κB and Snail) at a dilution of 1:1,000 overnight at
4°C. The membranes were then incubated with HRP-conjugated
secondary antibody (1:10,000) for 1 h at room temperature and
exposed using an enhanced chemiluminescence (ECL) detection system
(Applygen) and visualized by autoradiography. β-actin was used as
the internal reference.
Statistical analysis
SPSS 15.0 was used for data analysis. All values are
expressed as means ± SD. The Student’s t-test was used to evaluate
the difference between mean values. Immunohistochemical staining
was quantitated and differences between groups were assessed by the
χ2 test. A P-value of <0.05 was considered to
indicate a statistically significant result.
Results
Expression pattern of E-cadherin
E-cadherin was detected in all tissues tested,
including normal gastric epithelial tissues, adjacent non-cancerous
gastric epithelial tissues and gastric cancer tissues. In normal
gastric mucosa, strong expression of E-cadherin was present as a
membranous protein, with some weak staining in the cytoplasmic
compartment. In gastric cancer tissues, E-cadherin was largely
expressed in cytoplasmic compartments with weak expression on the
membrane. Normal gastric mucosal tissues expressed a higher level
of E-cadherin (Fig. 1A) than
gastric cancer tissues (Fig. 1B-D).
Among the gastric cancer tissues, a higher level of E-cadherin was
detected in the well/moderately differentiated cancer tissues
(Fig. 1B and C) than in poorly
differentiated cancer tissues (Fig.
1D). Overall, E-cadherin was detected in 22% (41/189) of
gastric cancer tissues, 55.6% (30/54) of matched non-cancerous
gastric tissues, and 100% (32/32) of normal gastric mucosa. By
Chi-square (χ2) test, gastric cancer tissues expressed a
reduced level of E-cadherin compared to the matched non-cancerous
gastric tissues (χ2=22.382, P=0.000), and normal gastric
mucosa (χ2=74.33, P=0.000). Of note, reduced expression
of E-cadherin was observed in matched non-cancerous gastric tissues
when compared with tha normal gastric mucosa (χ2=19.728,
P=0.000). As shown in Table III,
increased E-cadherin expression in gastric cancer tissues strongly
correlated with a better differentiation status (P=0.000) and less
invasion (P=0.004). By Lauren classification, higher expression
level of E-cadherin was found in tumors of intestinal type than in
tumors of diffuse type (P=0.002). The expression of E-cadherin did
not appear to be associated with age, gender, tumor size and lymph
node metastasis.
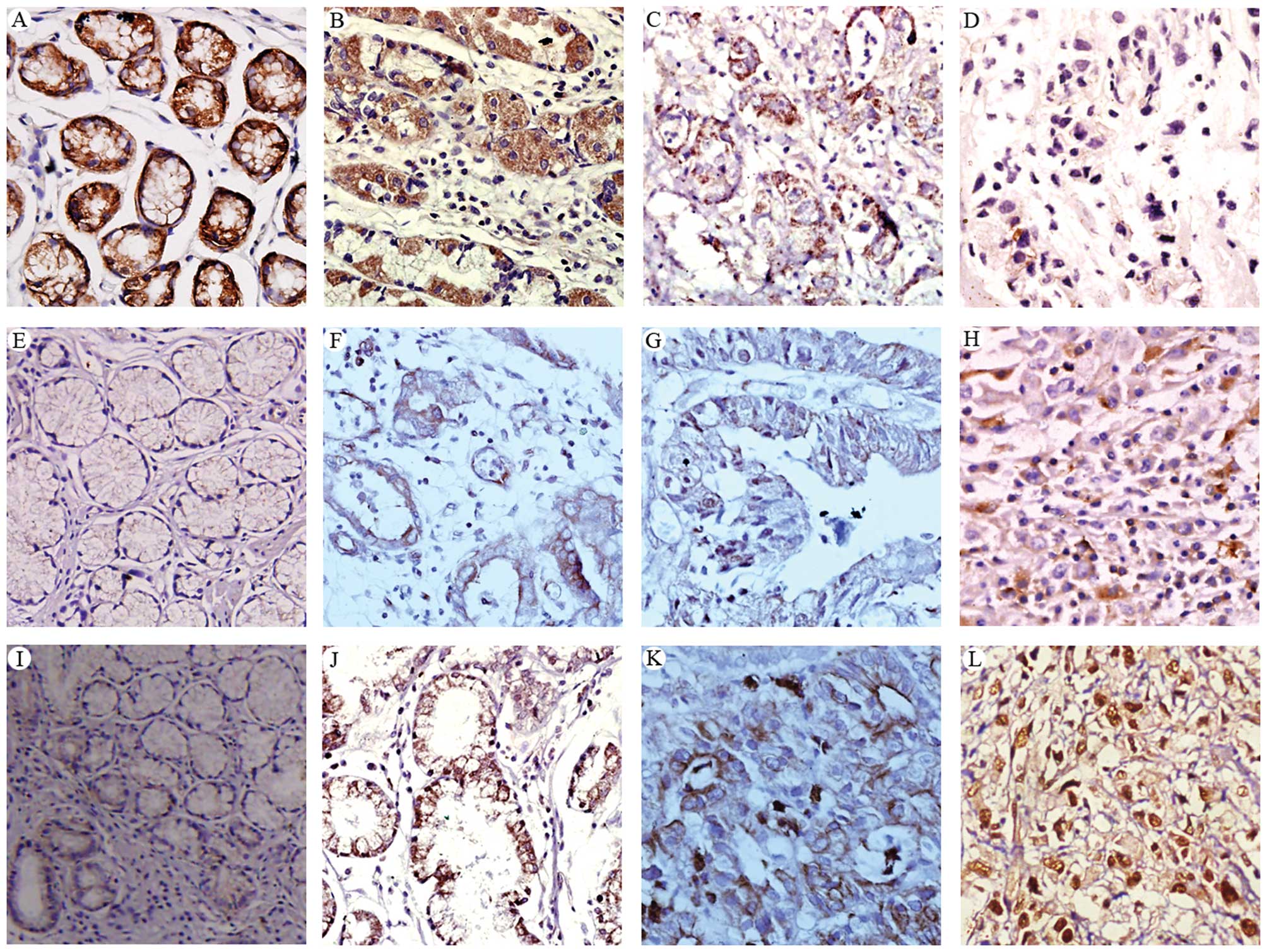 | Figure 1Immunohistochemical staining of
E-cadherin (A-D), NF-κB (E-H), and Snail (I-L) in normal gastric
mucosa (A, E, I) and gastric cancer tissues (B-D, F, G, H, J, K and
L). The expression levels of these proteins were examined in
well-differentiated (B, E-cadherin; F, NF-κB; J, Snail), moderately
differentiated (C, E-cadherin; G, NF-κB; K, Snail), and poorly
differentiated (D, E-cadherin; H, NF-κB; L, Snail) gastric cancer
tissues. Representative images are shown. Original magnification,
×200. |
 | Table IIIRelationship between E-cadherin
expression and clinicopathological factors in 189 patients with
gastric cancer. |
Table III
Relationship between E-cadherin
expression and clinicopathological factors in 189 patients with
gastric cancer.
| E-cadherin | | | | |
|---|
|
| | | | |
|---|
| Variables | Positive | Negative | Total | Positive rate
(%) | χ2 | P-value |
|---|
| Gender | | | | | 0.416 | 0.519 |
| Female | 8 | 36 | 44 | 18.2 | | |
| Male | 33 | 112 | 145 | 22.8 | | |
| Age (years) | | | | | 0.673 | 0.412 |
| <50 | 9 | 42 | 51 | 17.6 | | |
| ≥50 | 32 | 106 | 138 | 23.2 | | |
| Tumor size
(cm) | | | | | 2.382 | 0.123 |
| <5 | 19 | 51 | 70 | 27.1 | | |
| ≥5 | 21 | 98 | 119 | 17.6 | | |
| Lymph node
metastasis | | | | | 2.948 | 0.086 |
| No | 21 | 52 | 73 | 28.8 | | |
| Yes | 21 | 95 | 116 | 18.1 | | |
| Differentiation
status | | | | | 14.294 | 0.000 |
| Well/moderate | 30 | 59 | 89 | 33.7 | | |
| Poor | 11 | 89 | 100 | 11.0 | | |
| Depth of tumor
invasion | | | | | 8.338 | 0.004 |
| Without serosal
invasion | 17 | 29 | 46 | 37.0 | | |
| Serosal
invasion | 24 | 119 | 143 | 16.8 | | |
| Lauren
classification | | | | | 9.702 | 0.002 |
| Intestinal
type | 30 | 67 | 97 | 30.9 | | |
| Diffuse type | 10 | 75 | 85 | 11.8 | | |
Expression pattern of NF-κB
NF-κB was detected in the cytoplasmic and nuclear
portions of cells in normal gastric mucosa, matched non-cancerous
gastric tissues and gastric cancer tissues to a various extent.
Unlike E-cadherin, gastric cancer tissues (Fig. 1F-H) expressed a significantly higher
level of NF-κB than non-cancerous gastric tissues (data not shown)
and normal gastric mucosa (Fig.
1E). Among the gastric cancer tissues, a higher level of NF-κB
was detected in poorly differentiated cancer tissues (Fig. 1H) than in well/moderately
differentiated cancer tissues (Fig. 1G
and F). Overall, NF-κB was detected in 75.1% (142/189) of
gastric cancer tissues, 42.6% (23/54) of matched non-cancerous
gastric tissues, and 15.6% (5/32) of normal gastric mucosal
tissues. By χ2 test, the expression of NF-κB was
significantly higher in gastric cancer tissues compared to that in
the matched non-cancerous gastric tissues (χ2=20.404,
P=0.000) and normal gastric mucosa (χ2=43.511, P=0.000).
Matched non-cancerous gastric tissues also expressed a higher level
of NF-κB than the normal gastric mucosa (χ2=6.655,
P=0.010). In patients with gastric cancers, increased expression of
NF-κB was found to be strongly correlated with an increased
tendency for lymph node metastasis (P=0.018), deeper tumor invasion
(P=0.010), poor tumor differentiation (P=0.021), and diffuse type
of cancer histology (P=0.007) (Table
IV). The expression of NF-κB was, however, not associated with
gender, age and tumor size.
 | Table IVRelationship between NF-κB expression
and clinicopathological factors in 189 patients with gastric
cancer. |
Table IV
Relationship between NF-κB expression
and clinicopathological factors in 189 patients with gastric
cancer.
| NF-κB | | | | |
|---|
|
| | | | |
|---|
| Variables | Positive | Negative | Total | Positive rate
(%) | χ2 | P-value |
|---|
| Gender | | | | | 1.483 | 0.223 |
| Female | 30 | 14 | 44 | 68.2 | | |
| Male | 112 | 33 | 145 | 77.2 | | |
| Age (years) | | | | | 1.034 | 0.309 |
| <50 | 41 | 10 | 51 | 80.4 | | |
| ≥50 | 101 | 37 | 138 | 73.2 | | |
| Tumor size
(cm) | | | | | 1.567 | 0.211 |
| <5 | 49 | 21 | 70 | 70.0 | | |
| ≥5 | 93 | 26 | 119 | 78.2 | | |
| Lymph node
metastasis | | | | | 5.600 | 0.018 |
| No | 48 | 25 | 73 | 65.6 | | |
| Yes | 94 | 22 | 116 | 81.0 | | |
| Differentiation
status | | | | | 5.361 | 0.021 |
| Well/moderate | 60 | 29 | 89 | 67.4 | | |
| Poorly | 82 | 18 | 100 | 82.0 | | |
| Depth of tumor
invasion | | | | | 6.619 | 0.010 |
| Without serosal
invasion | 28 | 18 | 46 | 60.9 | | |
| Serosal
invasion | 114 | 29 | 143 | 79.7 | | |
| Lauren
classification | | | | | 7.284 | 0.007 |
| Intestinal
type | 64 | 33 | 97 | 66.0 | | |
| Diffuse type | 71 | 14 | 85 | 83.5 | | |
Expression pattern of Snail
Snail had a similar expression pattern as NF-κB in
that it was detected in the cytoplasmic and nuclear compartments of
cells in normal gastric mucosa, matched non-cancerous gastric
tissues, and gastric cancer tissues. Gastric cancer tissues
(Fig. 1J-L) expressed a
significantly higher level of Snail than non-cancerous gastric
tissues (data not shown) and normal gastric mucosa (Fig. 1I). Among the gastric cancer tissues,
a higher level of Snail was detected in poorly differentiated
cancer tissues (Fig. 1L) than in
well/moderately differentiated cancer tissues (Fig. 1J and H). Overall, Snail was detected
in 75.7% (143/189) of gastric cancer tissues, 48.45% (26/54) of
matched non-cancerous gastric tissues, and 18.75% (6/32) of normal
gastric mucosal tissues. By χ2 test, the expression of
Snail was significantly higher in gastric cancer tissues compared
to that in the matched non-cancerous gastric tissues
(χ2=23.67, P=0.000) and that in normal gastric mucosa
(χ2=55.95, P=0.000). Matched non-cancerous gastric
tissues also expressed a higher level of Snail than that in the
normal gastric mucosa (χ2=7.89, P=0.010).
As shown in Table V,
in patients with gastric cancer, increased expression of Snail was
found to be strongly correlated with increased potential for lymph
node metastasis (P=0.03), increased tumor invasion (P=0.018), poor
tumor differentiation (P=0.032), and diffuse type of cancer
histology (P=0.003). Similar to NF-κB, the expression of Snail was
not associated with gender, age and tumor size.
 | Table VRelationship between Snail expression
and clinicopathological factors in the 189 patients with gastric
cancer. |
Table V
Relationship between Snail expression
and clinicopathological factors in the 189 patients with gastric
cancer.
| Snail | | | | |
|---|
|
| | | | |
|---|
| Variables | Positive | Negative | Total | Positive rate
(%) | χ2 | P-value |
|---|
| Gender | | | | | 0.054 | 0.816 |
| Female | 32 | 12 | 44 | 72.7 | | |
| Male | 111 | 34 | 145 | 76.6 | | |
| Age (years) | | | | | 0.691 | 0.406 |
| <50 | 40 | 11 | 51 | 78.4 | | |
| ≥50 | 103 | 35 | 138 | 74.6 | | |
| Tumor size
(cm) | | | | | 3.035 | 0.081 |
| <5 | 48 | 22 | 70 | 68.6 | | |
| ≥5 | 95 | 24 | 119 | 79.8 | | |
| Lymph node
metastasis | | | | | 4.708 | 0.03 |
| No | 49 | 24 | 73 | 67.1 | | |
| Yes | 94 | 22 | 116 | 81.0 | | |
| Differentiation
status | | | | | 4.586 | 0.032 |
| Well/moderate | 61 | 28 | 89 | 68.5 | | |
| Poor | 82 | 18 | 100 | 82.0 | | |
| Depth of tumor
invasion | | | | | 5.549 | 0.018 |
| Without serosal
invasion | 29 | 17 | 46 | 63.0 | | |
| Serosal
invasion | 114 | 29 | 143 | 79.7 | | |
| Lauren
classification | | | | | 8.563 | 0.003 |
| Intestinal
type | 67 | 30 | 97 | 69.1 | | |
| Diffuse type | 70 | 15 | 85 | 82.4 | | |
Effect of NF-κB blockade on the
expression of E-cadherin and Snail
The above results showed that in gastric cancer
tissues, there was a close correlation between the expression of
NF-κB, E-cadherin and Snail. We proposed that NF-κB may regulate
the expression of E-cadherin via the transcription factor Snail. In
order to examine for this, we chose gastric cancer cell line
SGC7901 as a model to investigate whether modulation of NF-κB in
this cell line could affect the expression of E-cadherin and
Snail.
Following treatment of SGC7901 cells with 50 μM of
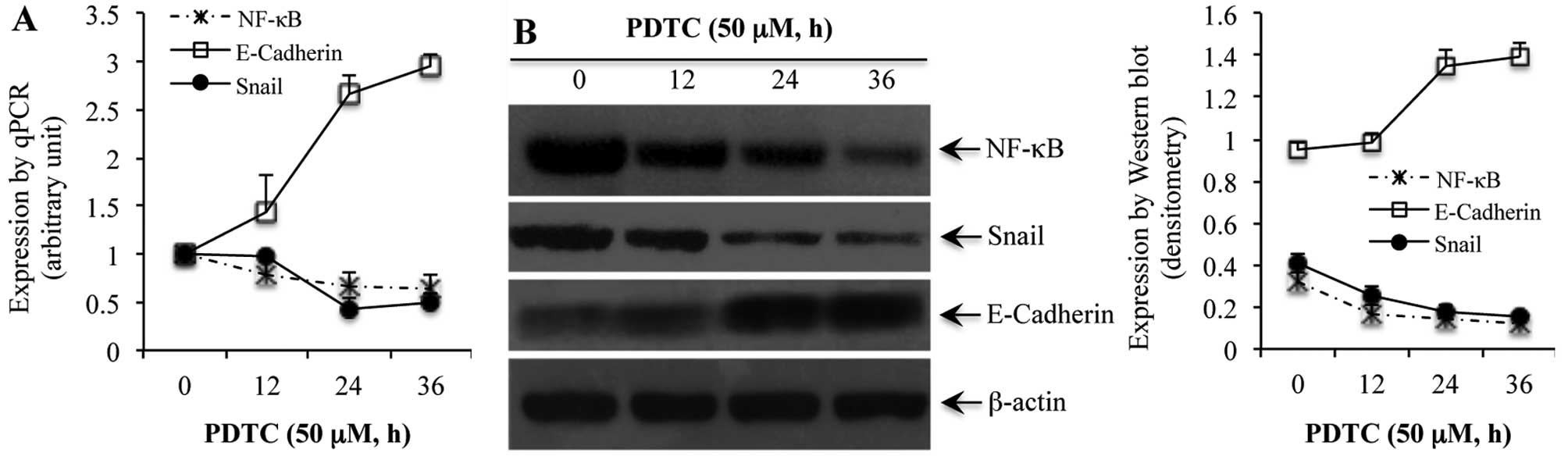
the NF-κB inhibitor PDTC, for 0, 12, 24 and 36 h, a time-dependent
reduction in NF-κB was noted at the mRNA (Fig. 2A) and protein (Fig. 2B) levels. Similarly, PDTC-induced
reduction of NF-κB in SGC7901 cells was associated with a reduced
expression of Snail in a time-dependent manner at both the mRNA and
protein levels (Fig. 2). On the
other hand, blockade of NF-κB with PDTC rendered a time-dependent
increase in the expression of E-cadherin at both the mRNA and
protein levels (Fig. 2).
Discussion
Gastric cancer is a multifactorial disease. Despite
numerous studies, the molecular mechanisms for gastric cancer
development have not yet been clarified. Our previous studies
demonstrated that loss of E-cadherin contributes to the local and
distant spread of gastric cancer (15,27).
The expression and function of E-cadherin can be regulated by many
factors such as β-catenin and NF-κB (15,28,29).
Our current study suggests that in gastric cancer, increased
expression and activity of NF-κB may contribute to the observed
loss of E-cadherin, and this biological change may be caused
through NFκB-mediated alteration in the expression of Snail.
NF-κB is a critical transcription factor involved in
the regulation of many signaling pathways that are important in
inflammation, the immune response and cancer development (30,31).
The importance of NF-κB in the development of gastric cancer has
been well-documented (32–34). In the present study, we found a
reverse correlation between the expression of E-cadherin and NF-κB
in normal and malignant gastric tissues. High expression level of
E-cadherin in normal gastric mucosa was correlated with a low level
of NF-κB, whereas in malignant gastric tissues, loss of E-cadherin
was correlated with an increased activity of NF-κB.
E-cadherin is a cell-cell adhesion molecule that
plays an important role in the formation of cell polarity and
tissue architecture (8,9). Although studies on
E-cadherin-deficient mice have provided little support concerning
the role of E-cadherin in the development of gastric adenocarcinoma
(17), numerous studies have shown
that loss of E-cadherin is closely related to increased tumor cell
migration, more aggressive invasion and metastasis, and poor
prognosis of gastric cancer (35,36).
Additionally, E-cadherin expression negatively controls the
transcriptional activity of NF-κB (29). We speculated that the inverse
relationship between these two molecules may be an important
mechanism in gastric cancer formation and metastasis.
The inverse correlation between E-cadherin and NF-κB
was recapitulated in our in vitro study in gastric cancer
cells. When NF-κB was blocked using its chemical inhibitor PDTC in
SGC7901 cells (as shown by a time-dependent decrease in the NF-κB
subunit p65 at the mRNA and protein levels), we observed a
time-dependent increase in the expression of E-cadherin. Such an
inverse correlation between NF-κB and E-cadherin may be regulated
by NF-κB-regulated Snail activity, as blockade of NF-κB was also
followed by a time-dependent inhibition of Snail. As blockade of
NF-κB has been shown to inhibit the growth of cancer cells
(37–40), we believe that NF-κB-mediated cancer
cell growth may be regulated through the transcription factor
Snail.
Snail is an important transcription factor that has
been shown to regulate many extracellular matrix genes (20,41,42).
Several studies have demonstrated that Snail functions as a direct
inhibitor for the transcription of E-cadherin (43,44),
particularly in malignant tumors (45). In addition, Snail was recognized as
an independent marker for the prognosis of patients with gastric
carcinoma (46). Our study
indicates that in gastric cancer cells, the regulatory effect of
NF-κB on its target genes such as E-cadherin is likely mediated
through Snail. To support this finding, previous studies have shown
the presence of the NF-κB binding sequence on the promoter of the
Snail gene (47,48).
As NF-κB plays an important role in the control of
growth and survival of cancer cells, and loss of E-cadherin is
closely related to the development of gastric cancer and its
metastasis, our data not only provide a new mechanism of how NF-κB
may regulate E-cadherin in gastric cancer, but also potentially
opens a new avenue for possible therapeutic targeting. If Snail is
a critical intermediating factor between NF-κB and its targets,
then specific targeting of Snail may be of therapeutic benefit.
Further studies using more cell lines involving specific knockdown
of Snail (e.g., using siRNA) and appropriate in vivo studies
are needed to generate more valuable data to confirm such an
assumption.
In conclusion, our results showed that in gastric
cancer, loss of E-cadherin in gastric epithelial cells may be
regulated through NF-κB-mediated Snail signaling. Further studies
are warranted to clarify the role of the NF-κB-Snail-E-cadherin
axis in gastric cancer.
Acknowledgements
We thank Drs Zhaofeng Chen, Lina Wang, and Meikai
Zhou from the First Clinical Medical School of Lanzhou University
for their assistance in TMA construction and immunohistochemistry.
This study was funded by the National Natural Science Funding of
China (grant ID: no. 432355/041003). Dr Z. Hu’s visiting study to
the Storr Liver Unit of the Westmead Millennium Institute was
supported by the Robert W. Storr Bequest. Dr L. Qiao was supported
by the Robert W. Storr Bequest and the Career Development and
Support Fellowship Future Research Leader Grant of the NSW Cancer
Institute, NSW, Australia.
Abbreviations:
|
NF-κB
|
nuclear factor-κB
|
|
PDTC
|
pyrrolidine dithiocarbamate
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang L, Parkin DM, Ferlay J, Li L and Chen
Y: Estimates of cancer incidence in China for 2000 and projections
for 2005. Cancer Epidemiol Biomarkers Prev. 14:243–250.
2005.PubMed/NCBI
|
|
4
|
Yang L: Incidence and mortality of gastric
cancer in China. World J Gastroenterol. 12:17–20. 2006.
|
|
5
|
Baeuerle PA and Baltimore D: NF-κB: ten
years after. Cell. 87:13–20. 1996.
|
|
6
|
Bours V, Dejardin E, Goujon-Letawe F,
Merville MP and Castronovo V: The NF-kappa B transcription factor
and cancer: high expression of NF-kappa B- and I kappa B-related
proteins in tumor cell lines. Biochem Pharmacol. 47:145–149. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baldwin AS: Control of oncogenesis and
cancer therapy resistance by the transcription factor NF-κB. J Clin
Invest. 107:241–246. 2001.
|
|
8
|
Frixen UH, Behrens J, Sachs M, et al:
E-cadherin-mediated cell-cell adhesion prevents invasiveness of
human carcinoma cells. J Cell Biol. 113:173–185. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hirohashi S: Inactivation of the
E-cadherin-mediated cell adhesion system in human cancers. Am J
Pathol. 153:333–339. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gabbert HE, Mueller W, Schneiders A, et
al: Prognostic value of E-cadherin expression in 413 gastric
carcinomas. Int J Cancer. 69:184–189. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mao Z, Ma X, Rong Y, et al: Connective
tissue growth factor enhances the migration of gastric cancer
through downregulation of E-cadherin via the NF-κB pathway. Cancer
Sci. 102:104–110. 2011.PubMed/NCBI
|
|
12
|
Ghadimi BM, Behrens J, Hoffmann I, Haensch
W, Birchmeier W and Schlag PM: Immunohistological analysis of
E-cadherin, alpha-, beta-and gamma-catenin expression in colorectal
cancer: implications for cell adhesion and signaling. Eur J Cancer.
35:60–65. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pignatelli M, Ansari TW, Gunter P, et al:
Loss of membranous E-cadherin expression in pancreatic cancer:
correlation with lymph node metastasis, high grade, and advanced
stage. J Pathol. 174:243–248. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oka H, Shiozaki H, Kobayashi K, et al:
Expression of E-cadherin cell adhesion molecules in human breast
cancer tissues and its relationship to metastasis. Cancer Res.
53:1696–1701. 1993.PubMed/NCBI
|
|
15
|
Zhou Y, Li G, Wu J, et al:
Clinicopathological significance of E-cadherin, VEGF, and MMPs in
gastric cancer. Tumour Biol. 31:549–558. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kyrlagkitsis I and Karamanolis DG: Genes
and gastric cancer. Hepatogastroenterology. 51:320–327. 2004.
|
|
17
|
Mimata A, Fukamachi H, Eishi Y and Yuasa
Y: Loss of E-cadherin in mouse gastric epithelial cells induces
signet ring-like cells, a possible precursor lesion of diffuse
gastric cancer. Cancer Sci. 102:942–950. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nieto MA: The snail superfamily of
zinc-finger transcription factors. Nat Rev Mol Cell Biol.
3:155–166. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bonavida B and Baritaki S: Dual role of NO
donors in the reversal of tumor cell resistance and EMT:
downregulation of the NF-κB/Snail/YY1/RKIP circuitry. Nitric Oxide.
24:1–7. 2011.PubMed/NCBI
|
|
20
|
Wu Y and Zhou BP: Snail: more than EMT.
Cell Adh Migr. 4:199–203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rosivatz E, Becker I, Specht K, et al:
Differential expression of the epithelial-mesenchymal transition
regulators Snail, SIP1, and Twist in gastric cancer. Am J Pathol.
161:1881–1891. 2002. View Article : Google Scholar
|
|
22
|
Castro Alves C, Rosivatz E, Schott C, et
al: Slug is overexpressed in gastric carcinomas and may act
synergistically with SIP1 and Snail in the down-regulation of
E-cadherin. J Pathol. 211:507–515. 2007.PubMed/NCBI
|
|
23
|
Bosman FT: WHO classification of tumours
of the digestive system. World Health Organization. International
Agency for Research on Cancer; 4th edition. IARC Press; Lyon:
2010
|
|
24
|
Volm M, Koomagi R and Mattern J:
Prognostic value of vascular endothelial growth factor and its
receptor Flt-1 in squamous cell lung cancer. Int J Cancer.
74:64–68. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vichai V and Kirtikara K: Sulforhodamine B
colorimetric assay for cytotoxicity screening. Nat Protoc.
1:1112–1116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Woolston C and Martin S: Analysis of tumor
and endothelial cell viability and survival using sulforhodamine B
and clonogenic assays. Methods Mol Biol. 740:45–56. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou Y, Ran J, Tang C, et al: Effect of
celecoxib on E-cadherin, VEGF, microvessel density and apoptosis in
gastric cancer. Cancer Biol Ther. 6:269–275. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dentice M, Luongo C, Ambrosio R, et al:
Beta-catenin regulates deiodinase levels and thyroid hormone
signaling in colon cancer cells. Gastroenterology. 143:1037–1047.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Solanas G, Porta-de-la-Riva M, Agustí C,
et al: E-cadherin controls beta-catenin and NF-kappaB
transcriptional activity in mesenchymal gene expression. J Cell
Sci. 121:2224–2234. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sebastian K, Reza F, Christoph C, et al:
Time dependency and topography of hepatic NF-κB activation after
hemorrhagic shock and resuscitation in mice. Shock. 38:486–492.
2012.PubMed/NCBI
|
|
31
|
Bernardi FC, Felisberto F, Vuolo F, et al:
Oxidative damage, inflammation, and toll-like receptor 4 pathway
are increased in preeclamptic patients: a case-control study. Oxid
Med Cell Longev. 2012:6364192012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang Z, Wu W and Qian ML: Cellular damage
and apoptosis along with changes in NF-kappa B expression were
induced with contrast agent enhanced ultrasound in gastric cancer
cells and hepatoma cells. Cancer Cell Int. 12:82012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee KH and Kim JR: Regulation of
HGF-mediated cell proliferation and invasion through NF-κB, JunB,
and MMP-9 cascades in stomach cancer cells. Clin Exp Metastasis.
29:263–272. 2012.PubMed/NCBI
|
|
34
|
Li J, Shen L, Lu FR, et al: Plumbagin
inhibits cell growth and potentiates apoptosis in human gastric
cancer cells in vitro through the NF-κB signaling pathway. Acta
Pharmacol Sin. 33:242–249. 2012.PubMed/NCBI
|
|
35
|
Uchikado Y, Okumura H, Ishigami S, et al:
Increased Slug and decreased E-cadherin expression is related to
poor prognosis in patients with gastric cancer. Gastric Cancer.
14:41–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maehata Y, Hirahashi M, Aishima S, et al:
Significance of dysadherin and E-cadherin expression in
differentiated-type gastric carcinoma with submucosal invasion. Hum
Pathol. 42:558–567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Biswas DK, Shi Q, Baily S, et al: NF-kappa
B activation in human breast cancer specimens and its role in cell
proliferation and apoptosis. Proc Natl Acad Sci USA.
101:10137–10142. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dolcet X, Llobet D, Pallares J and
Matias-Guiu X: NF-κB in development and progression of human
cancer. Virchows Arch. 446:475–482. 2005.
|
|
39
|
Han SS, Yun H, Son DJ, et al:
NF-kappaB/STAT3/PI3K signaling crosstalk in iMyc E mu B lymphoma.
Mol Cancer. 9:972010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fang Y, Sun H, Zhai J, et al: Antitumor
activity of NF-κB decoy oligodeoxynucleotides in a prostate cancer
cell line. Asian Pac J Cancer Prev. 12:2721–2726. 2011.
|
|
41
|
Dhasarathy A, Kajita M and Wade PA: The
transcription factor snail mediates epithelial to mesenchymal
transitions by repression of estrogen receptor-alpha. Mol
Endocrinol. 21:2907–2918. 2007. View Article : Google Scholar
|
|
42
|
Harder JL, Whiteman EL, Pieczynski JN, Liu
CJ and Margolis B: Snail destabilizes cell surface Crumbs3a.
Traffic. 13:1170–1185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Becker KF, Rosivatz E, Blechschmidt K,
Kremmer E, Sarbia M and Höfler H: Analysis of the E-cadherin
repressor Snail in primary human cancers. Cells Tissues Organs.
185:204–212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Blechschmidt K, Kremmer E, Hollweck R, et
al: The E-cadherin repressor snail plays a role in tumor
progression of endometrioid adenocarcinomas. Diagn Mol Pathol.
16:222–228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Spaderna S, Schmalhofer O, Wahlbuhl M, et
al: The transcriptional repressor ZEB1 promotes metastasis and loss
of cell polarity in cancer. Cancer Res. 68:537–544. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
He H, Chen W, Wang X, et al: Snail is an
independent prognostic predictor for progression and patient
survival of gastric cancer. Cancer Sci. 103:1296–1303. View Article : Google Scholar
|
|
47
|
Bachelder RE, Yoon SO, Franci C, de
Herreros AG and Mercurio AM: Glycogen synthase kinase-3 is an
endogenous inhibitor of Snail transcription: implications for the
epithelial-mesenchymal transition. J Cell Biol. 168:29–33. 2005.
View Article : Google Scholar
|
|
48
|
Julien S, Puig I, Caretti E, et al:
Activation of NF-kappaB by Akt upregulates Snail expression and
induces epithelium mesenchyme transition. Oncogene. 26:7445–7456.
2007. View Article : Google Scholar : PubMed/NCBI
|