Introduction
At present, therapies for cancer mainly include
surgical treatment and non-surgical therapeutic modalities such as
chemotherapy and radiotherapy. Both drug resistance and toxicity
limit the efficacy of non-surgical therapies. Therefore,
oncologists are seeking new therapeutic approaches. Natural
products, including traditional Chinese medicine (TCM), have been
used for thousands of years as important remedies for a variety of
diseases, including cancer. Due to their relatively fewer
side-effects, there has always been an interest in the use of
natural products for the treatment of cancer (1–3).
According to the theory of TCM, cancer is caused by
the accumulation of foreign toxins that are harmful to the human
body. For this reason, heat clearing and detoxification herbs
(Qingre Jiedu herbs) such as Hedyotis Diffusa Willd (HDW),
Sophora flavescens (SF), Psuedobulbus Cremastrae
(PC), Prunella Bidens and Ban Zhi Lian (4–6) are
commonly used to treat cancer in China. Pharmacological studies
have shown that these herbs may contain ingredients that can
inhibit proliferation and induce apoptosis of tumor cells (7–9).
Angiogenesis is the formation of new blood
capillaries from existing vessels, supplying nutrients and oxygen
to and removing waste products from cells that are distant from
existing blood vessels (10).
Physiological angiogenesis is involved in wound healing,
reproduction and embryonic development (11). In addition to its normal
physiological role, angiogenesis contributes to the pathology of a
number of diseases including tumor progression and metastasis
(12,13). As all cells, cancer cells require a
constant supply of nutrients and oxygen in order to grow and
divide. Without an adequate blood supply tumors do not grow.
The induction of angiogenesis is mediated by a
variety of molecules secreted from the cells within the tumor.
Vascular endothelial growth factor (VEGF) is one of the most
effective biological inducers of angiogenesis. VEGF expression is
upregulated by hypoxia (14) and it
serves as a major angiogenic factor in vascular development in
tumor (15). VEGF exhibits high
affinity binding to vascular endothelial growth factor receptor
(VEGFR), two distinct receptor tyrosine kinases (RTK) on
endothelial cells. An essential role of VEGF in tumor angiogenesis
has been demonstrated in animal models showing that neutralizing
VEGF antibodies and dominant-negative VEGFR inhibit both
angiogenesis and the progression of tumor (16). Angiogenesis is also related to
metastasis as tumors with higher densities of blood vessels are
more likely to spread. Thus, anti-angiogenesis therapy is
considered to be an important approach that can enhance the
efficiency of chemotherapy (17).
Studies have shown that some TCM herbs can inhibit angiogenesis by
downregulating VEGF or VEGFR expression. For example, Li et
al reported that brucine significantly reduced VEGF expression
and microvessel density and inhibited the growth of breast cancer
and bone metastasis in a nude mouse model (18). Shen et al reported that Pien
Tze Huang suppressed the expression of VEGF-A and basic fibroblast
growth factor (bFGF) at both mRNA and protein levels to inhibit
tumor angiogenesis in chick chorioallantoic membrane (CAM) and
human umbilical vein endothelial cells (HUVECs) (19).
Jiedu Xiaozheng Yin (JXY) is a polyherbal formula of
TCM established according to the theory of Chinese medicine. It is
composed of HDW (30 g), Prunella (15 g), PC (15 g) and SF
(15 g). These herbs are capable of heat-clearing and
detoxification. Our previous clinical studies showed that JXY can
prolong the overall survival time of patients and improve their
quality of life (20). We
demonstrated that HDW extract may inhibit angiogenesis, induce
tumor cell apoptosis by the mitochondrion-dependent pathway
(7,8) and inhibit proliferation of cancer
cells by regulating the cell cycle (9). However, the precise mechanism of its
anticancer activity remains to be fully elucidated.
In the present study, we elucidated the underlying
mechanism of JXY’s inhibitory effect on tumor growth. For this
purpose, effects of ethanol extract of Jiedu Xiaozheng Yin (EE-JXY)
on tube formation of HUVECs and angiogenesis of a CAM and hepatoma
mouse xenograft model were investigated. We also explored the
effects of EE-JXY on angiogenesis-related signal pathways.
Materials and methods
Reagents
Roswell Park Memorial Institute medium-1640
(RPMI-1640), Dulbecco’s modified Eagle’s medium (DMEM), fetal
bovine serum (FBS), penicillin-streptomycin, trypsin-EDTA and
TRIzol reagents were purchased from Invitrogen (Carlsbad, CA, USA).
The cell cycle assay kit was from BD Biosciences (San Jose, CA,
USA). SuperScript II reverse transcriptase was obtained from
Promega (Madison, WI, USA). The in vitro angiogenesis assay
kit was purchased from Millipore (Billerica, MA, USA). Human VEGF-A
and VEGFR-2 (KDR) ELISA were obtained from R&D Systems
(Minneapolis, MN, USA). All other chemicals were obtained from
Sigma Chemicals (St. Louis, MO, USA).
Preparation of ethyl acetate extract from
JXY
JXY is composed of HDW (30 g), Prunella (15
g), PC (15 g) and SF (15 g). Four herbs of JXY were purchased from
Guo Yi Tang Hospital of Fujian University of Traditional Chinese
Medicine (Fuzhou, China). JXY (7.5 kg) was refluxed with 75%
ethanol for 2×3 h to obtain total extract. The alcohol was removed
under vacuum using a rotary evaporator. The residue was suspended
in water, which was partitioned sequentially with petroleum ether,
chloroform, ethanol and n-BuOH to afford extracts. Four extracts
were evaporated in vacuum and stored at 4°C prior to use. EE-JXY
was diluted in DMSO into 200 mg/ml for in vitro experiments.
For in vivo study, EE-JXY was dissolved in normal saline to
a final concentration of 6 mg/ml.
Cell culture
HUVECs and the human hepatoma cell line HepG2 were
obtained from the American Type Culture Collection (ATCC, Manassas,
VA, USA). HUVECs and HepG2 cells were grown in RPMI-1640 and DMEM,
respectively. Both RPMI-1640 and DMEM were supplemented with 10%
(v/v) FBS, 100 U/ml penicillin and 100 μg/ml streptomycin with 5%
CO2 at 37°C in a humidified environment.
Tumor xenograft
Sixteen male BALB/c nude mice weighing 18–22 g were
injected with HepG2 cell suspension at the right flank. After 7
days, mice were randomly divided into two groups, the vehicle and
the EE-JXY group. The vehicle group was given normal saline; the
EE-JXY group was administered EE-JXY at a dose of 0.06 g/kg. Tumor
size was measured daily and volume was calculated according to the
following formula: tumor volume (TV; mm3)
=d2xD/2, where d and D were the shortest and longest
diameters, respectively. On Day 21, the tumor was excised and
weighed. The animals were maintained in a pathogen-free facility
(23±2°C, 55±5% humidity). Food and water were provided ad
libitum. All procedures on treating mice were performed
according to Animal Care Guidelines issued by Ministry of Science
and Technology of the People’s Republic of China and the Animal
Care Committee of Fu Jian University of Traditional Chinese
Medicine approved our protocols.
Cell viability assay
Cell viability was evaluated by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
colorimetric assay. HUVECs (1×104 cells/well) were
seeded into 96-well plates. The cells were treated with different
concentrations of EE-JXY for different times. At the end of the
treatment, 20 μl MTT (5 mg/ml) was added to each well. After 4 h,
MTT was discarded and 100 μl dimethyl sulfoxide (DMSO) was added to
each well. The absorbance was measured at 490 nm with a microplate
reader (Biotek, USA). Cell viability was calculated according to
the following formula: cell viability (%) = average absorbance of
EE-JXY group/average absorbance of control group × 100%.
Capillary-like tube formation assay
Tube formation by HUVECs was evaluated using a
commercially available angiogenesis assay kit (In Vitro
Angiogenesis Assay kit, Millipore), according to the manufacturer’s
instructions. After HUVECs were treated with EE-JXY, cells were
harvested and diluted (1×104 cells) in 50 μl medium. The
cells were then seeded onto a solid gel of basement proteins
(ECMatrix gel) within 12-well plates and incubated for 9 h at 37°C.
Cellular morphology and the development of capillary tube networks
were evaluated using a phase-contrast inverted microscope. Images
were captured at magnification, ×100.
VEGF-A and VEGFR-2 ELISA assay
Either HUVECs or HepG2 cells (2×105
cells) were seeded into 6-well plates and treated with various
concentrations of EE-JXY for 24 h. Cell supernatant was collected
to measure the level of VEGF-A and cell lysates were used to
determine the protein expression of VEGFR-2 in HUVECs. Measurement
was performed using Quantikine ELISA kits (R&D, USA), according
to the manufacturer’s instructions.
CAM assay
A CAM assay was performed to determine the in
vitro anti-angiogenic activity of EE-JXY. Briefly, 10 μl of
EE-JXY (10 mg/ml) was loaded onto a 0.5-cm diameter Whatman filter
paper. The filter was then applied to the CAM of a 7-day embryo.
Following incubation for 72 h at 37°C, angiogenesis around the
filter was photographed with a digital camera. The number of blood
vessels in a circular perimeter surrounding the implants, at a
distance of 0.25 cm from the edge of the filter, was counted
manually.
Immunohistochemical assay
Tumor samples fixed in 10% buffered formalin for 24
h were processed conventionally for paraffin-embedded tumor
sections. Sections were subjected to antigen retrieval and blocking
of endogenous peroxidase activity. For immunostaining, sections
were incubated with the primary antibodies mouse monoclonal
anti-CD31 (R&D Systems), mouse monoclonal anti-VEGFR2 (R&D
Systems) and rabbit polyclonal anti-VEGF (R&D Systems).
Sections were then incubated with biotinylated appropriate
secondary antibody followed by conjugated horseradish peroxidase
(HRP)-streptavidin (Maixin Bio, China). Then 3,3′-diaminobenzidine
(DAB; Sigma) was added, incubated at room temperature and
counterstained with diluted Harris hematoxylin (Sigma). Cells were
quantified by counting positive cells and total number of cells at
five arbitrarily selected fields from each tumor at magnification,
×100. Data are presented as percentage of positive cells.
VEGF-A and VEGFR-2 RT-PCR analysis
Total RNA was isolated with TRIzol reagent
(Invitrogen) from tumor. Oligo(dT)-primed RNA (1 μg) was
reverse-transcribed with SuperScript II reverse transcriptase
(Promega), according to the manufacturer’s instructions. The
obtained cDNA was used to determine the amount of VEGF-A or VEGFR-2
mRNA by PCR with Taq DNA polymerase (Fermentas) using the following
primers: VEGF-A forward: 5′-GCCTTGCCTTGCTGCTCTA-3′, reverse:
5′-GATGTCCACCAGGGTCTCG-3′; VEGFR-2 forward:
5′-ACGCCGATTATGTGAGA-3′, reverse: 5′-AGGCAGGAGT TGAGTATGT-3′; GAPDH
forward: 5′-GTCATCCATGACAA CTTTGG-3′, reverse:
5′-GAGCTTGACAAAGTGGTCGT-3′.
Statistical analysis
All data are the means of three determinations
except for the CAM assays in which 10 determinations were made for
each data point. The data were analyzed using the SPSS package for
Windows (Version 11.5). Statistical analysis of the data was
performed with the Student’s t-test and analysis of variance.
Differences with P<0.05 were considered statistically
significant.
Results
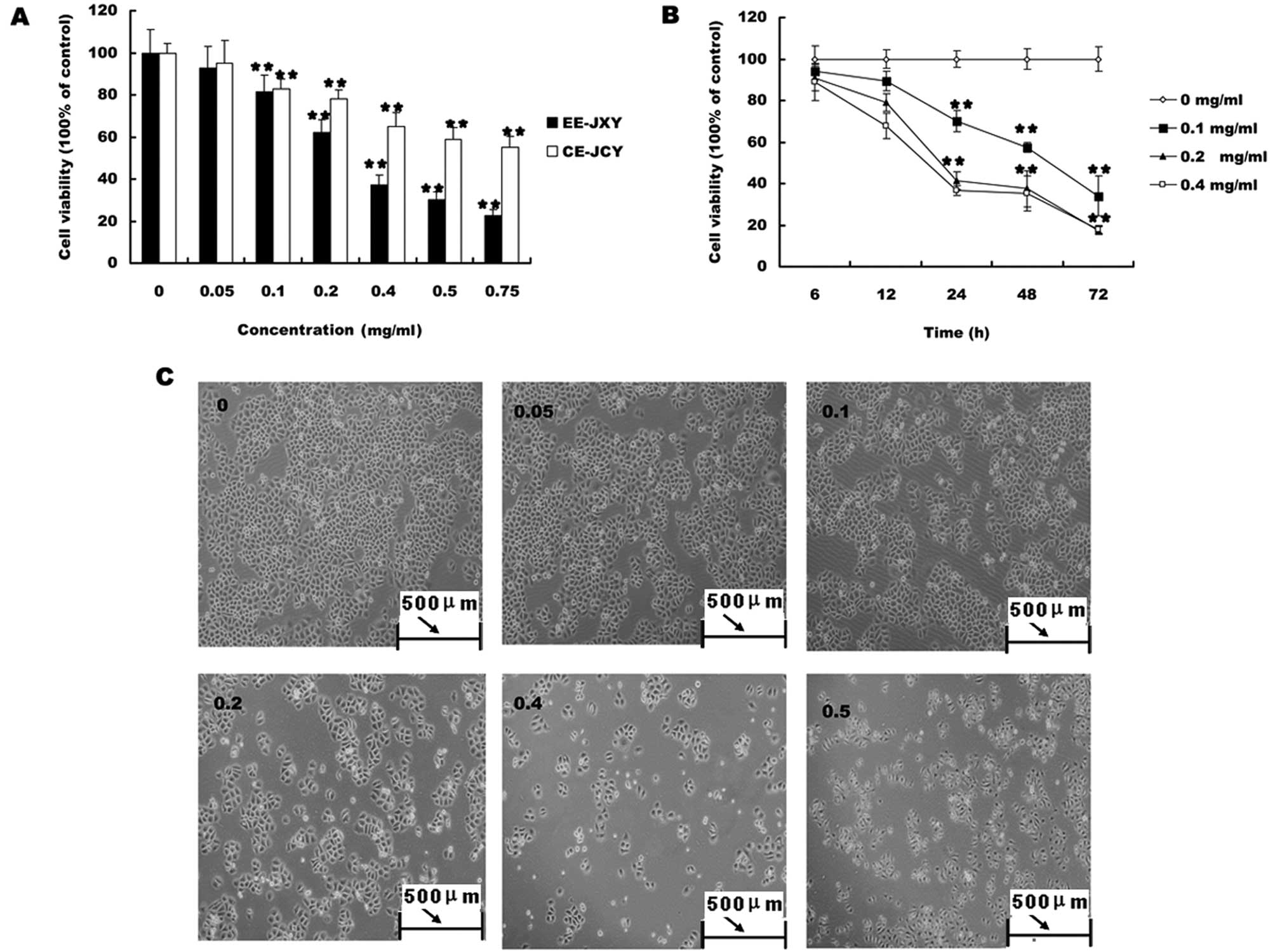
EE-JXY inhibits proliferation of
HUVECs
We first evaluated the effect of EE-JXY on the
growth of HUVECs. HUVEC viability was determined by MTT assay
following treatment with various concentrations of EE-JXY at
different time-points. As shown in Fig.
1, treatment with 0.05–0.25 mg/ml of EE-JXY for 12, 24 and 48 h
reduced cell viability by 16–78%, compared to untreated control
cells, in a dose- and time-dependent manner (P<0.01; Fig. 1A).
EE-JXY inhibits tube formation of
HUVECs
To test the effect of EE-JXY on endothelial
capillary tube formation, HUVECs were grown on a solid gel
containing mouse basement proteins (ECMatrix, Millipore). This gel
induces cultured endothelial cells to rapidly align and form hollow
tube-like structures. As shown in Fig.
1B, untreated HUVECs formed elongated tube-like structures. By
contrast, EE-JXY treatment dose-dependently decreased capillary
tube formation (P<0.01).
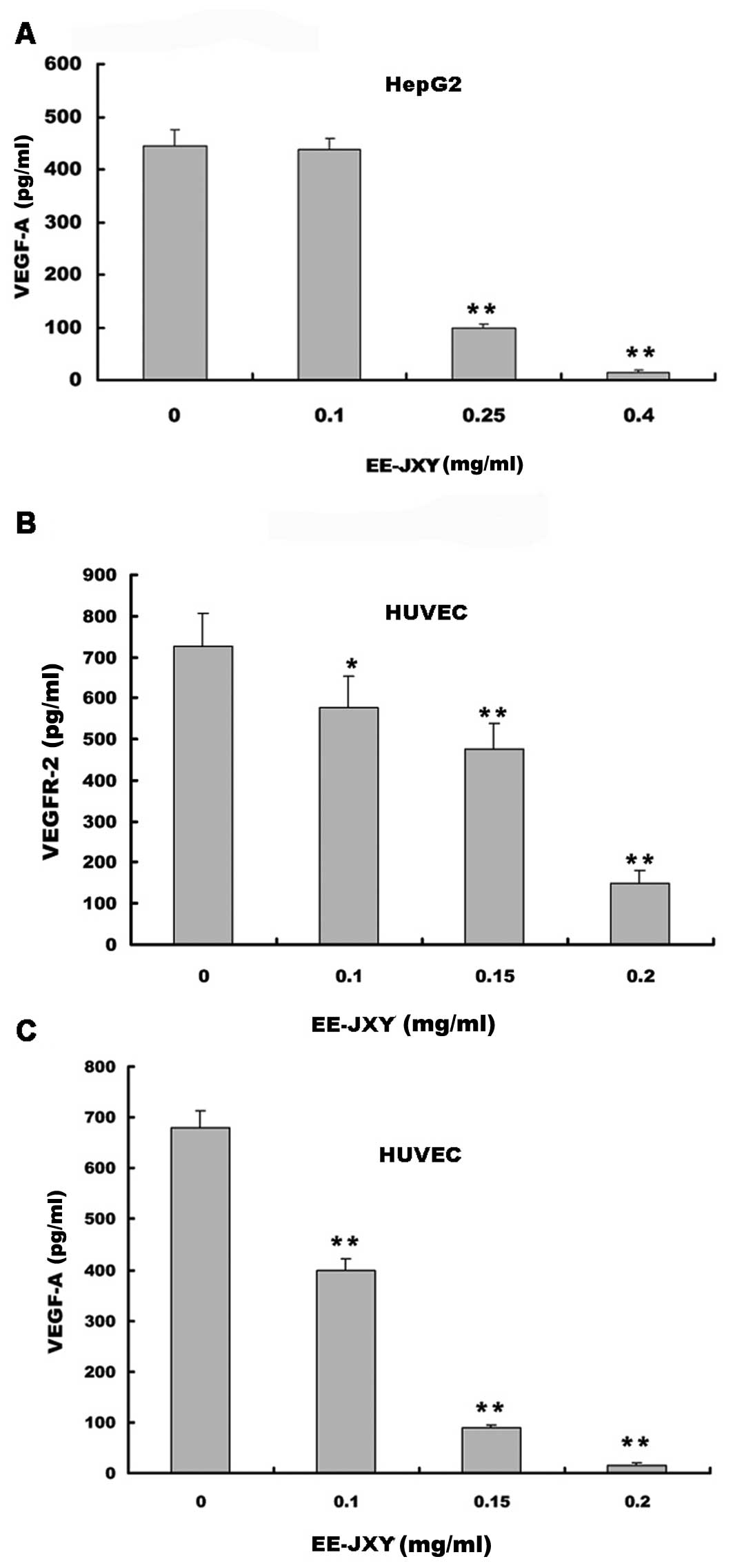
Effects of EE-JXY on VEGF-A and VEGFR-2
expression
We examined the effects of EE-JXY on VEGF-A
secretion by HUVECs and HepG2 human hepatoma cells and on the
expression of VEGFR-2 in HUVECs. The results of the ELISA assay
showed that EE-JXY treatment dose-dependently reduced VEGF-A
secretion by both HepG2 cells (P<0.01; Fig. 2A)and HUVECs (P<0.01; Fig. 2B). In addition, it suppressed
VEGFR-2 expression in HUVECs (P<0.01; Fig. 2C).
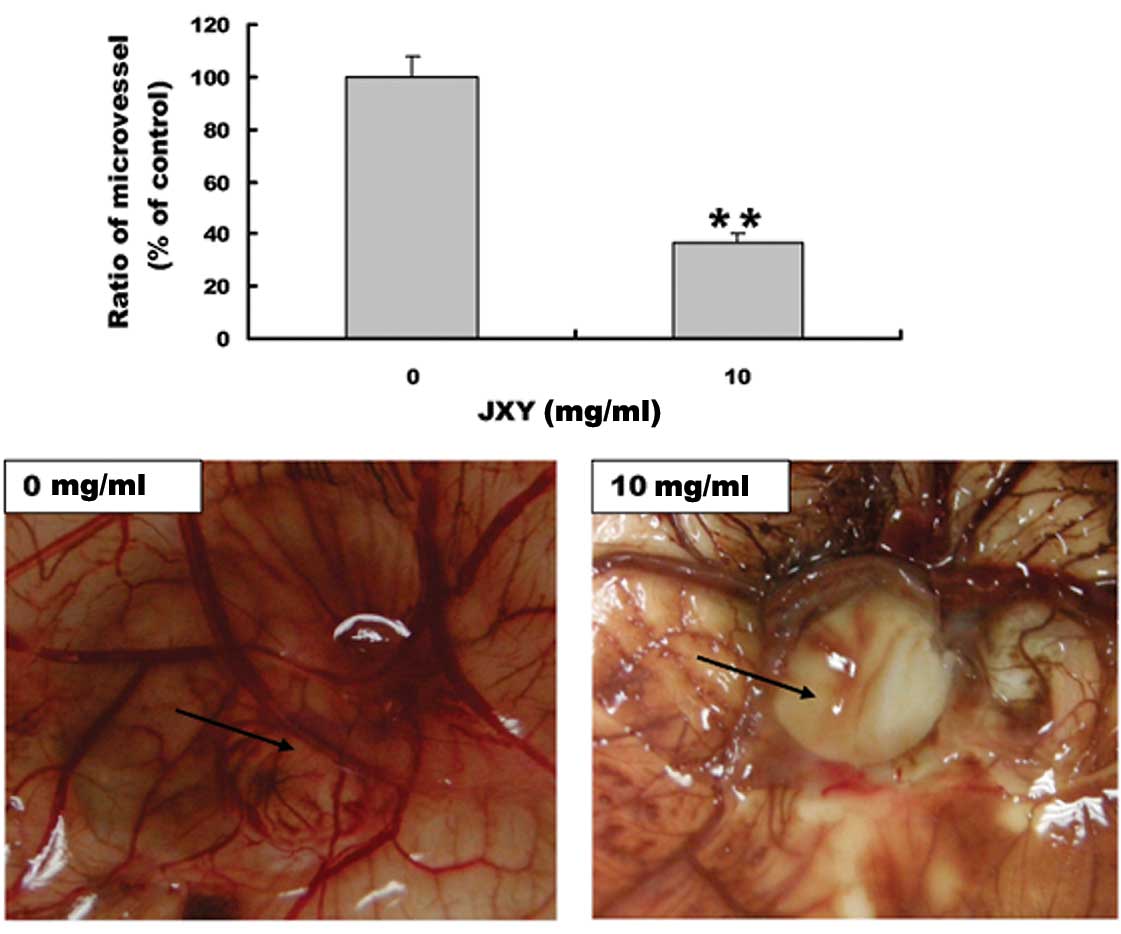
EE-JXY inhibits angiogenesis in CAM
The anti-angiogenic effect of EE-JXY was further
evaluated using a classic CAM model. EE-JXY treatment significantly
reduced the total number of blood vessels in the chicken embryos as
compared to the untreated control (P<0.01; Fig. 3), indicating that EE-JXY is able to
suppress angiogenesis in vivo.
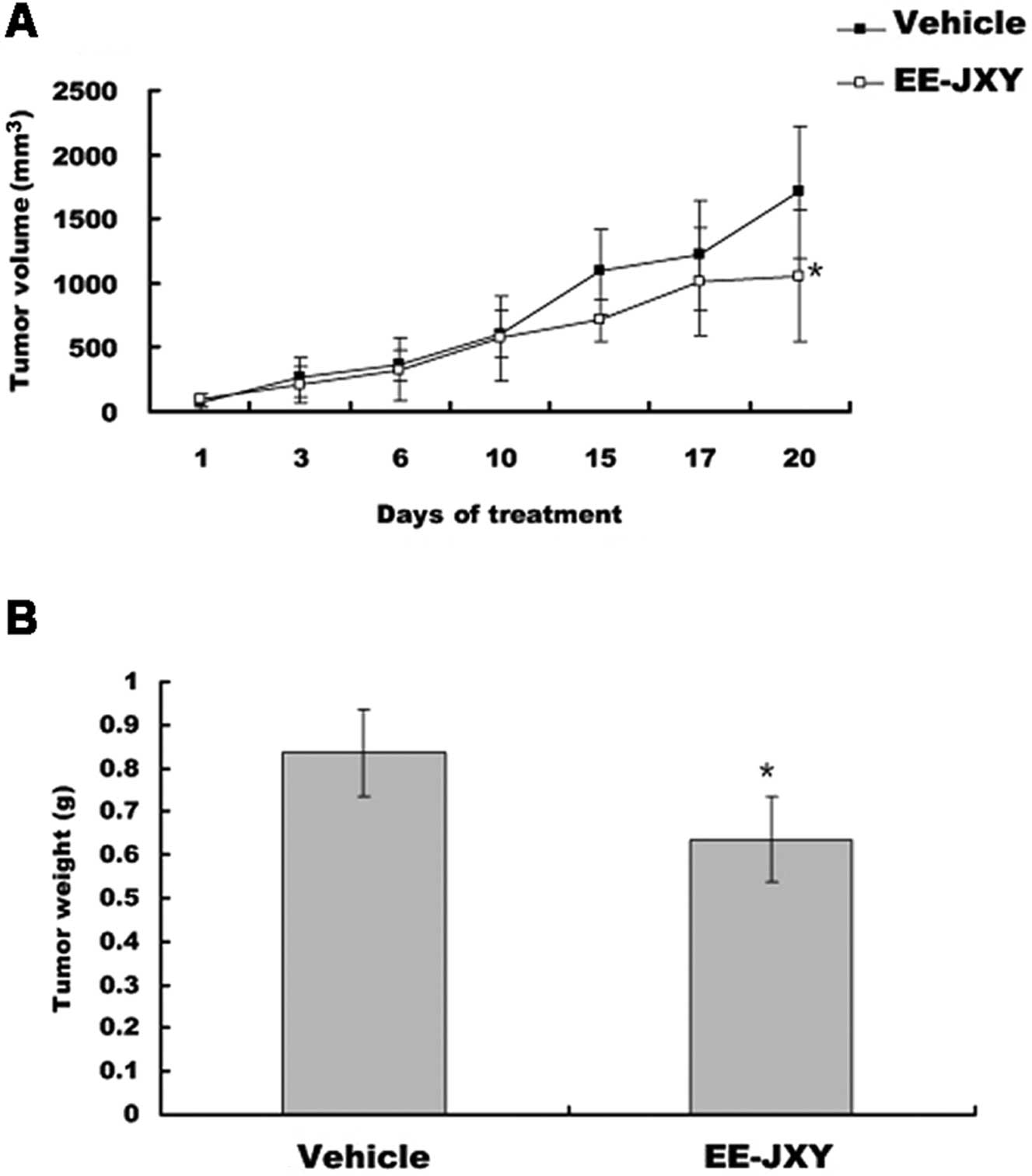
EE-JXY inhibits tumor growth in vivo
After mice were treated with EE-JXY for 20 days,
tumor volume was reduced by 39% (P<0.05) in the EE-JXY group
compared with the vehicle group (P<0.05; Fig. 4A). A comparison of tumor weight
between the EE-JXY and the vehicle group showed a similar tendency
with tumor volume (P<0.05; Fig.
4B).
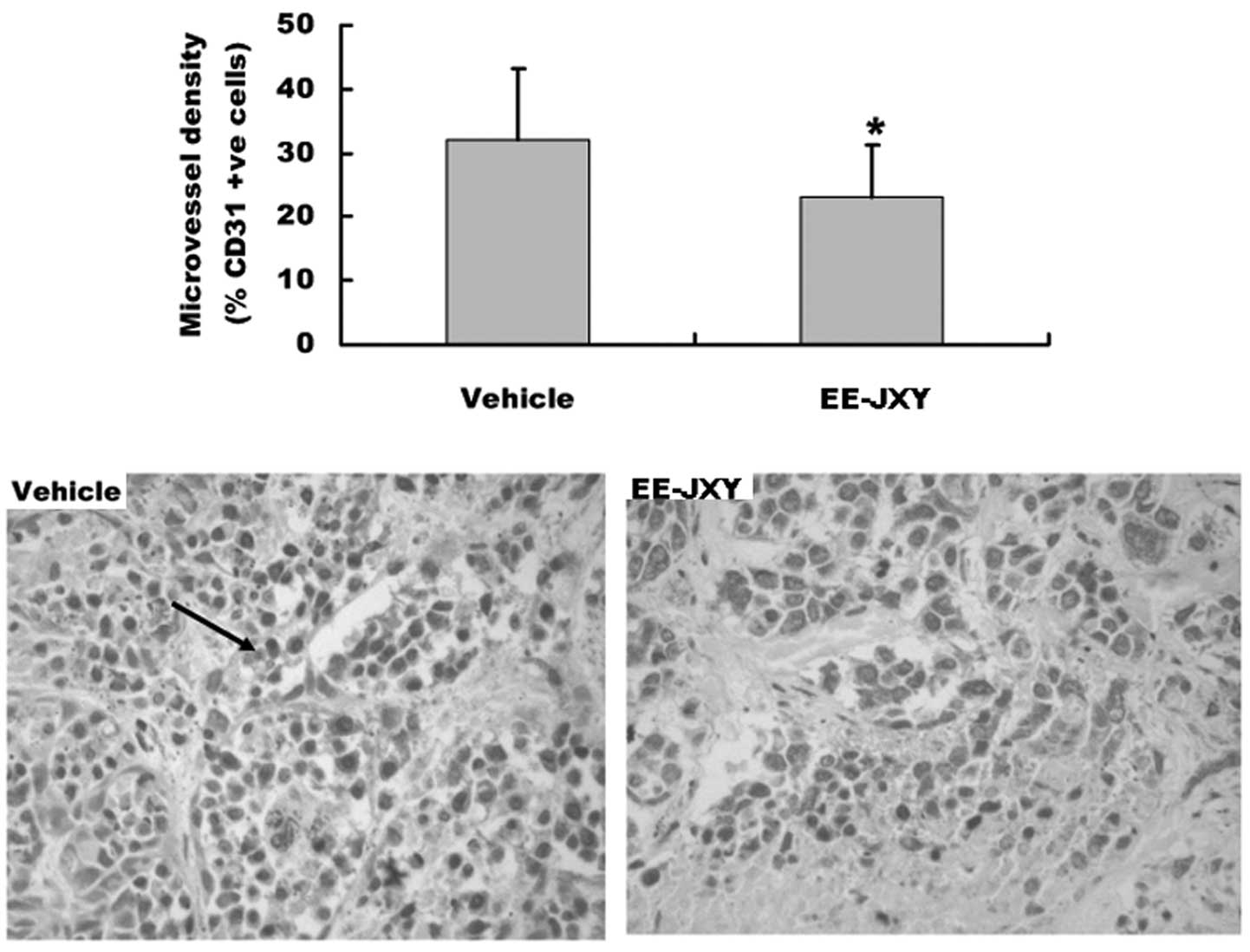
Effect of EE-JXY on microvessel density
(MVD) of tumor
We also detected tumor MVD after mice were treated
with EE-JXY for 20 days, by immunohistochemical staining assay. The
results showed that the MVD of the EE-JXY group was significantly
lower than that of the vehicle group (P<0.05) (Fig. 5).
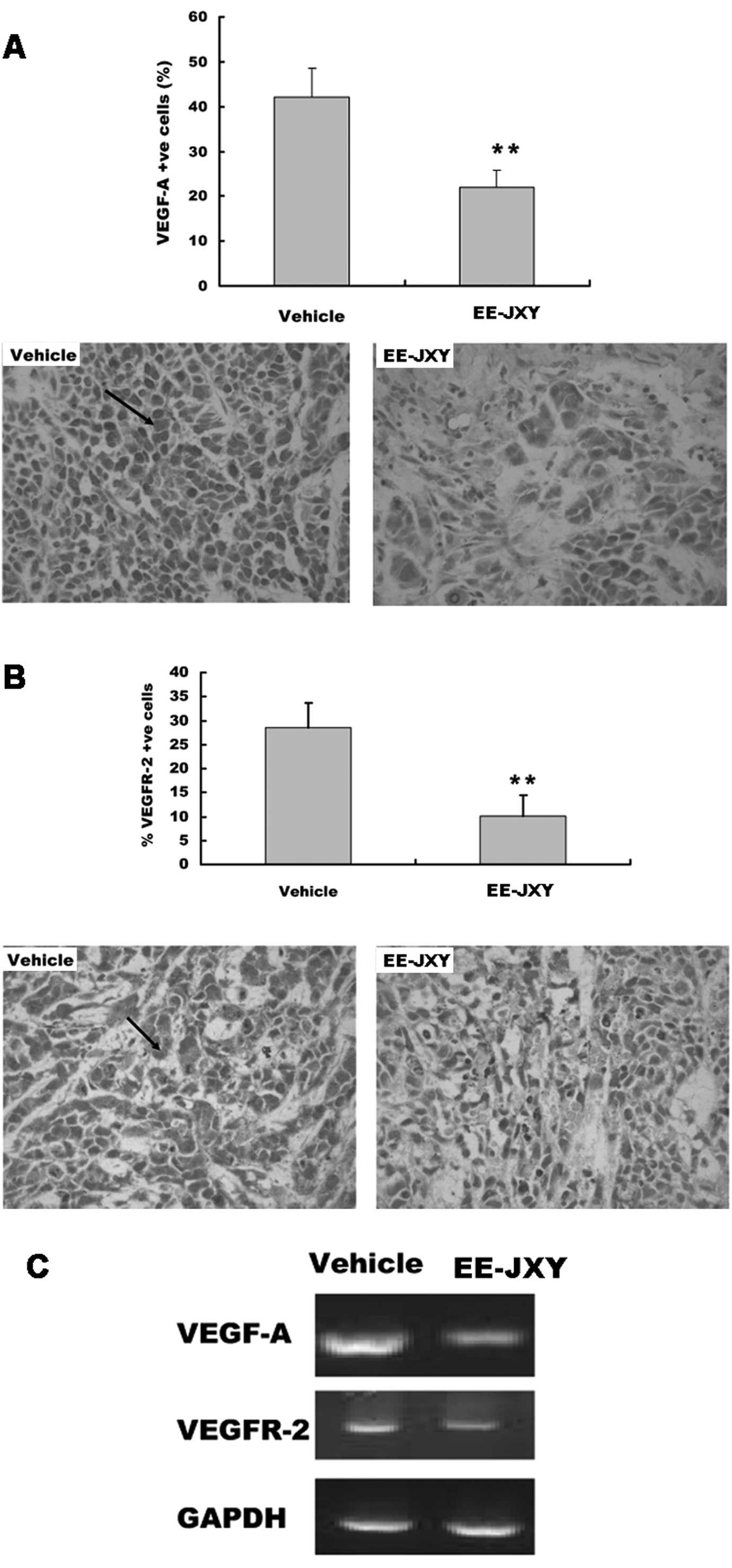
EE-JXY suppresses VEGF and VEGFR
expression
We detected the expression of VEGF and VEGFR
proteins in the tumor tissue with immunohistochemical staining.
Immunohistochemistry showed there were more cells positive for
VEGF-A and VEGFR-2 in the vehicle group than in the EE-JXY group
(P<0.01; Fig. 6A and B). The
results of the RT-PCR assay showed that EE-JXY treatment reduced
both VEGF-A mRNA and VEGFR-2 mRNA expression in the tumor (Fig. 6C).
Discussion
Angiogenesis indicates the growth of new blood
vessels from pre-existing vessels. Angiogenesis is a normal and
vital process in growth and development, as well as in wound
healing and in granulation tissue. However, it is also a
fundamental step in the transition of tumors from a dormant to a
malignant state. Tumor-associated angiogenesis allows the tumor to
maintain its growth and also facilitates metastatic spreading by
establishing connections to the existing vasculature (21). Therefore, targeting tumor
vasculature may be as important as targeting the tumor itself. With
the advent of drugs targeting the angiogenesis of cancer, patient
survival has improved for several malignancies, including
metastatic colorectal cancer (22,23).
VEGF (including VEGF-A, VEGF-B, VEGF-C and VEGF-D)
is a key regulator of physiologic angiogenesis and plays a major
role in the pathobiology of cancer. VEGF-A is highly expressed in,
and secreted from, various types of human cancer, and is associated
with cancer progression, invasion and metastasis as well as poor
patient prognosis (24). The VEGFRs
are structurally related members of the RTK family that mediate
critical signaling pathways in endothelial cells. Binding to
VEGFR-2, VEGF starts a tyrosine kinase signaling cascade that
stimulates the production of factors that variously stimulate
vessel permeability (eNOS, producing NO), endothelial cell
proliferation/survival (bFGF), migration (ICAMs/VCAMs/MMPs) and
finally differentiation into mature blood vessels (25,26).
Some TCM herbs, including Lithospermum
erythrorhizon(27), Viscum
album coloratum(28),
Chrysobalanus icaco(29),
Cassia garrettiana heartwood (30), Agaricus blazei(31), Pulsatilla koreana(32), HDW and Prunella(9) have been shown to possess
anti-angiogenic activity either in vitro or in vivo.
HDW and Prunella belong to heat-clearing and detoxification
herbs in TCM theory. JXY is composed of four heat-clearing and
detoxification herbs including HDW, Prunella, PC and SF. In
the present study we found that JXY was able to inhibit tumor
angiogenesis by downregulating VEGF-A and VEGFR-2 expression both
in vivo and in vitro. EE-JXY decreased viability of
HUVECs and their tube formation capacity. Moreover, EE-JXY
inhibited angiogenesis in CAM and decreased MVD in the xenograft
tumor. The above results demonstrate that JXY inhibited
angiogenesis at least by downregulating VEGF-A and VEGFR-2
expression.
Targeted therapies represent a new perspective in
the treatment of cancer. In contrast to conventional chemotherapy
which kills both cancer cells and normal tissues, targeted drugs
aim at cancer cells in a more specific manner (33). Molecules controlling cell
proliferation and death, such as RTKs for growth factors, are among
the best targets for this type of therapeutic approach. The era of
targeted therapy began with the approval of trastuzumab, a
monoclonal antibody against human epidermal growth factor receptor
2 (HER2), for the treatment of metastatic breast cancer, and
imatinib, a small tyrosine kinase inhibitor targeting BCR-Abl, in
chronic myeloid leukemia (34).
Despite the initial enthusiasm for the efficacy of these
treatments, cancer often develops resistance to trastuzumab due to
the activation of alternative pathways. Similarly, in a mouse model
of pancreatic islet carcinogenesis, inhibition of VEGFR2 markedly
disrupted angiogenesis and initial tumor growth. However, in
late-stage tumors, phenotypic resistance to VEGFR2 blockade emerged
via reactivation of tumor angiogenesis, independent of VEGF, and
associated with hypoxia-mediated induction of other proangiogenic
factors, including members of the FGF family (35). In this view, the rationale at the
basis of targeting drugs is radically shifting. There is a general
agreement that molecules interfering simultaneously with multiple
targets might be more effective than single target agents.
Sorafenib and unitinib-targeting VEGFR, platelet-derived growth
factor receptor (PDGFR), fms-like tyrosine kinase receptor-3
(FLT-3) and c-kit, are among these examples (36,37).
Our previous studies demonstrated that JXY inhibits
tumor growth by targeting several pathways, such as by promoting
apoptosis by the mitochondrial pathway and inhibiting proliferation
by the cell cycle pathway (38,39).
By using molecular docking simulation, Zheng et al showed
that some components in HDW, Prunella and PC can combine
with Bcl-xL, TNF-α, Cdk2, IL-2 and CDK2 (40). Thus, our studies indicate that TCM
formulas, composed of several herbs and therefore targeting
multiple pathways (such as JXY), warrant further investigation.
Acknowledgements
This study was supported by the Chen Ke-ji
Integrative Medicine Development Fund (CKJ2010020), the
International Science Joint Project of the Ministry of Science and
Technology of the People’s Republic of China (2008DFA32200), the
Key Project of Fujian Province Department of Science &
Technology (2008KJB-01) and the Fujian Province Natural Science
Foundation (2012J01393). National Natural Science Foundation of
China (81102582).
Abbreviations:
|
HDW
|
Hedyotis Diffusa Willd
|
|
SF
|
Sophora flavescens
|
|
PC
|
Psuedobulbus Cremastrae
|
|
EE-JXY
|
ethanol extract of Jiedu Xiaozheng
Yin
|
|
DMSO
|
dimethyl sulfoxide
|
|
TCM
|
traditional Chinese medicine
|
|
VEGF-A
|
vascular endothelial growth factor
A
|
|
VEGFR-2
|
vascular endothelial growth factor
receptor 2
|
|
bFGF
|
basic fibroblast growth factor
|
|
RPMI-1640
|
Roswell Park Memorial Institute
medium-1640
|
|
DMEM
|
Dulbecco’s modified Eagle’s medium
|
|
FBS
|
fetal bovine serum
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
CAM
|
chick chorioallantoic membrane
|
|
DAB
|
3,3′-diaminobenzidine
|
|
HRP
|
horseradish peroxidase
|
|
RTK
|
receptor tyrosine kinase
|
|
TV
|
tumor volume
|
|
PDGFR
|
platelet-derived growth factor
receptor
|
|
FLT-3
|
fms-like tyrosine kinase
receptor-3
|
References
|
1
|
Zhao J, Jiang P and Zhang WD: Molecular
networks for the study of TCM Pharmacology. Brief Bioinform.
11:417–430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ji HF, Li XJ and Zhang HY: Natural
products and drug discovery. Can thousands of years of ancient
medical knowledge lead us to new and powerful drug combinations in
the fight against cancer and dementia? EMBO Rep. 10:194–200.
2009.PubMed/NCBI
|
|
3
|
Longley DB, Allen WL and Johnston PG: Drug
resistance, predictive markers and pharmacogenomics in colorectal
cancer. Biochim Biophys Acta. 1766:184–196. 2006.PubMed/NCBI
|
|
4
|
Yan Y, Cook J, McQuillan J, Zhang G,
Hitzman CJ, Wang Y, Wiedmann TS and You M: Chemopreventive effect
of aerosolized polyphenon E on lung tumorigenesis in A/J mice.
Neoplasia. 9:401–405. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fang Y, Zhang Y, Chen M, Zheng H and Zhang
K: The active component of Hedyotis diffusa Willd. Chin Trad
Plant Med. 26:577–579. 2004.
|
|
6
|
Wu YG and Song LR: Shanghai Science and
Technology Press. Zhong Hua Ben Cao. 25:530–533. 1998.(In
Chinese).
|
|
7
|
Lin J, Wei L, Xu W, Hong Z, Liu X and Peng
J: Effect of Hedyotis Diffusa Willd extract on tumor
angiogenesis. Mol Med Rep. 4:1283–1288. 2011.
|
|
8
|
Lin J, Chen Y, Wei L, Chen X, Xu W, Hong
Z, Sferra TJ and Peng J: Hedyotis Diffusa Willd extract
induces apoptosis via activation of the mitochondrion-dependent
pathway in human colon carcinoma cells. Int J Oncol. 37:1331–1338.
2010.
|
|
9
|
Chen XZ, Cao ZY, Chen TS, Zhang YQ, Liu
ZZ, Su YT, Liao LM and Du J: Water extract of Hedyotis
Diffusa Willd suppresses proliferation of human HepG2 cells and
potentiates the anticancer efficacy of low-dose 5-fluorouracil by
inhibiting the CDK2-E2F1 pathway. Oncol Rep. 28:742–748. 2012.
|
|
10
|
Folkman J: Tumor angiogenesis: therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Folkman J: Angiogenesis. Annu Rev Med.
57:1–18. 2006. View Article : Google Scholar
|
|
12
|
Fava RA, Olsen NJ, Spencer-Green G, Yeo
TK, Yeo KT, Berse B, Jackman RW, Senger DR, Dvorak HF and Brown JF:
Vascular permeability factor/endothelial growth factor (VPF/VEGF):
accumulation and expression in human synovial fluids and rheumatoid
synovial tissue. J Exp Med. 180:341–346. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McLaren J, Prentice A, Charmock-Jones DS,
Millican SA, Muller KH, Sharkey AM and Smith SK: Vascular
endothelial growth factor is produced by peritoneal fluid
macrophages in endometriosis and is regulated by ovarian steroids.
J Clin Invest. 98:482–489. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shweiki D, Itin A, Soffer D and Keshet E:
Vascular endothelial growth factor induced by hypoxia may mediate
hypoxia-initiated angiogenesis. Nature. 359:843–845. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shalaby F, Rossant J, Yamaguchi TP,
Gertsenstein M, Wu XF, Britman ML and Schuh AC: Failure of
blood-island formation and vasculogenesis in Flk-1-deficient mice.
Nature. 376:62–66. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim KF, Li B, Winer J, Armanin M, Gillet
N, Philips HS and Ferrara N: Inhibition of vascular endothelial
growth factor-induced angiogenesis suppresses tumour growth in
vivo. Nature. 36:841–844. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yano S, Shinohara H, Herbst RS, Kuniyasu
H, Bucana CD, Ellis LM, Davis DW, McConkey DJ and Fidler IJ:
Expression of vascular endothelial growth factor is necessary but
not sufficient for production and growth of brain metastasis.
Cancer Res. 60:4959–4967. 2000.PubMed/NCBI
|
|
18
|
Li P, Zhang M, Ma WJ, Sun X and Jin FP:
Effects of brucine on vascular endothelial growth factor expression
and microvessel density in a nude mouse model of bone metastasis
due to breast cancer. Chin J Integr Med. 18:605–609. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen AL, Hong F, Liu LY, Lin JM, Zhuang
QC, Hong ZF and Peng J: Effects of Pien Tze Huang (PTH) on
angiogenesis in vivo and in vitro. Chin J Integr Med. 18:431–436.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen LW, Lin J, Chen W and Zhang WP:
Effect of Chinese herbal medicine on patients with primary hepatic
carcinoma in III stage during perioperational period: a report of
42 cases. Zhongguo Zhong Xi Yi Jie He Za Zhi. 25:832–834. 2005.(In
Chinese).
|
|
21
|
Ellis LM and Hicklin DJ: VEGF-targeted
therapy: mechanisms of antitumour activity. Nat Rev Cancer.
8:579–591. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kerbel RS, Yu J, Tran J, Man S,
Viloria-Petit A, Klement G, Coomber BL and Rak J: Possible
mechanisms of acquired resistance to antiangiogenic drugs:
implications for the use of combination therapy approaches. Cancer
Metastasis Rev. 20:79–86. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miller KD, Sweeney CJ and Sledge GW: Can
tumor angiogenesis be inhibited without resistance? EXS. 95–112.
2005.PubMed/NCBI
|
|
24
|
Carmeliet P: VEGF as a key mediator of
angiogenesis in cancer. Oncology. 69:4–10. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee S, Chen TT, Barber CL, Jordan MC,
Murdock J, Desai S, Ferrara N, Nagy A, Roos KP and Iruela-Arispe
ML: Autocrine VEGF signaling is required for vascular homeostasis.
Cell. 130:691–703. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kerbel RS: Tumor angiogenesis. N Engl J
Med. 358:2039–2049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hisa T, Kimura Y, Takada K, Suzuki F and
Takigawa M: Shikonin: an ingredient of Lithospermum
erythrorhizon, inhibits angiogenesis in vivo and in vitro.
Anticancer Res. 18:783–790. 1998.
|
|
28
|
Yoon TJ, Yoo YC, Choi OB, Do MS, Kang TB,
Lee SW, Azuma I and Kim JB: Inhibitory effect of Korean mistletoe
(Viscum album coloratum) extract on tumour angiogenesis and
metastasis of haematogenous and non-haematogenous tumour cells in
mice. Cancer Lett. 97:83–91. 1995.
|
|
29
|
Alves De Paulo S, Teruszkin Balassiano I,
Henriques Silva N, Oliveira Castilho R, Coelho Kaplan MA, Currie
Cabral M and da Costa Carvalho MG: Chrysobalanus icaco L.
extract for antiangiogenic potential observation. Int J Mol Med.
5:667–669. 2000.
|
|
30
|
Kim MS, Lee YM, Moon EJ, Kim SE, Lee JJ
and Kim KW: Anti-angiogenic activity of torilin, a sesquiterpene
compound isolated from Torilis japonica. Inl J Cancer.
87:269–275. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kimura Y, Baba K and Okuda H: Inhibitory
effects of active substances isolated from Cassia
garrettiana heartwood on tumor growth and lung metastasis in
Lewis lung carcinoma-bearing mice (part 1). Anticancer Res.
20:2899–2906. 2000.PubMed/NCBI
|
|
32
|
Takaku T, Kimura Y and Okuda H: Isolation
of an antitumor compound from Agaricus blazei Murill and its
mechanism of action. J Nutr. 131:1409–1413. 2001.PubMed/NCBI
|
|
33
|
Casaletto JB and McClatchey AI: Spatial
regulation of receptor tyrosine kinases in development and cancer.
Nat Rev Cancer. 12:387–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sastry SK and Elferink LA: Checks and
balances: interplay of RTKs and PTPs in cancer progression. Biochem
Pharmacol. 82:435–440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Casanovas O, Hicklin DJ, Bergers G and
Hanahan D: Drug resistance by evasion of antiangiogenic targeting
of VEGF signaling in late-stage pancreatic islet tumors. Cancer
Cell. 8:299–309. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Faivre S, Ronot M, Dreyer C, Serrate C,
Hentic O, Bouattour M, Bruno O, Couvelard A, Vilgrain V and Raymond
E: Imaging response in neuroendocrine tumors treated with targeted
therapies: the experience of sunitinib. Target Oncol. 7:127–133.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Escudier B, Szczylik C, Porta C and Gore
M: Treatment selection in metastatic renal cell carcinoma: expert
consensus. Nat Rev Clin Oncol. 9:327–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zheng LP, Hu HX, Lin W, Huang YM, Zhao JY
and Chen WL: The effect of serum of Jiedu Xiaozheng Yin on
proliferation of gastric carcinoma cell line. Pharmacology and
Clinics of Chinese Materia Medica. 25:91–93. 2009.(In Chinese).
|
|
39
|
Chen XZ and Du J: The effect of Jiedu
Xiaozheng drink on HepG2 cells mitochondrial membrane potential.
Journal of Fujian University of TCM. 19:22–23. 2009.(In
Chinese).
|
|
40
|
Chen L, Zheng C and Du J: Study on
antitumor mechanism of Qingre Xiaozheng drink by molecular docking
method. Clin Pharmacol Ther. 12:324–328. 2007.
|