Introduction
Malignant tumours consist of a heterogeneous
population of cells that differ in their expression of markers and
growth capacities (1,2). The cancer stem cell (CSC) hypothesis
provides new insight into the heterogeneity of malignant tumours.
Heterogeneity is determined, at least in part, by the presence of
CSCs (1–6). Therefore, it is important to improve
screening methods to obtain sufficient amounts of CSCs for further
study of the biological treatment of malignant tumours.
Stem cells and committed cells can form distinct
clones in vitro(7). CSCs can
be obtained by monoclonal morphology screening in some cancer cell
lines. In the glioma cell line, U251, clones are characterised as
tight, intermediate, or loose. The clones with a round and compact
shape (tight clones) consist of CSCs, while the irregular
(intermediate clones) and loose-shaped clones did not show any CSC
properties (8). Therefore, the
intermediate- and loose-shaped clones have not been extensively
studied. Similar clone patterns have been observed in malignant
cell lines, such as head and neck squamous cell carcinoma, breast
carcinoma and prostate carcinoma cell lines, in which only the
cells of tight clones were capable of self-renewal (9–11).
These studies also indicated that the majority of the clones were
intermediate (61.0%) and loose (27.1%), and only a few were tight
(11.9%) (8).
In the present study, both suspended and adherent
cells were present in each clonal subpopulation grown under the
same culture conditions. Cells were collected from the intermediate
and loose clones, which were hypothesised to contain CSCs, and
cultured in neurobasal medium supplemented with growth factors. The
suspended cells from these populations displayed significant
‘stemness’, while the adherent cells did not. In this experiment, a
new and efficient screening method for isolating CSCs based on
their growth state was implemented to enrich for CSCs from the
glioma cell line, U251. This method provided a shorter detection
time and simplified the procedures; future studies using these
cells are required.
Materials and methods
Cell culture
Human glioma cells (U251) were cultured in DMEM/F12
(Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine
serum (FBS; Hangzhou Sijiqing Biological Engineering Materials Co.,
Ltd., China). The U251 cells were thoroughly dissociated with
0.125% trypsin to prepare single-cell suspensions and then cultured
in 100-mm dishes at a density of 30–50
cells/cm2(8). After
incubation for two weeks at 37°C with 5% CO2 and 100%
humidity, clones of different morphological types were observed.
Subsequently, the single-cell suspensions were cultured in 24-well
plates at a clonal density (8). The
intermediate and loose clones were selected and the cells were
gently blown using a glass dropper to obtain the suspended cells.
The same numbers of suspended and adherent cells were used in the
subsequent experiments.
Self-renewal assay and induction of
differentiation
The suspended cells (1×104 cells/ml) were
cultured in neurobasal medium, which consisted of serum-free
DMEM/F12 supplemented with 20 ng/ml basic fibroblast growth factor
(bFGF; Peprotech, Rocky Hill, NJ, USA), 20 ng/ml epidermal growth
factor (EGF; Peprotech), and 20 μl/ml B27 (Invitrogen, Carlsbad,
CA, USA), and primary clone spheres were formed. Subsequently,
primary clone spheres were dissociated with stem cell accutase
(Gibco) to prepare single-cell suspensions from which secondary
clone spheres were formed. For immunofluorescence staining,
individual suspended cells and clone spheres were cultured in
serum-free DMEM/F12 for no more than 2 h to allow attachment to the
slide. To induce differentiation, the clone spheres were cultured
in DMEM/F12 with 10% FBS. Immunofluorescence staining was performed
after 72 h.
Carboxyfluorescein succinimidyl ester
(CFSE) labelling
CFSE has been widely used in the study of cell
proliferation, including measurement of the percentage of
proliferation (12). A CFSE stock
(20 mM in DMSO; Dojindo, Kumamoto, Japan) stored at −20°C was
thawed and diluted in PBS without Ca2+ and
Mg2+ to the desired working concentration (10 μM). The
labelled cells were cultured in DMEM/F12 with 10% FBS for four and
seven days. The CFSE contents of the cultured cells were estimated
with a FACSCalibur flow cytometer and CellQuest software (BD
Biosciences, San Diego, CA, USA).
MTT assay
The suspended and adherent cells were each seeded
into 96-well plates (9–10×104 cells/ml) in DMEM/F12 with
10% FBS. The growth curves were determined by an MTT (5 mg/ml;
Sigma, St. Louis, MO, USA) assay that measures absorbance at a
wavelength of 570 nm.
Cell cycle and clonogenic assay
The cells (5–10×105 cells) were
trypsinised and washed twice with 2.5 ml ice-cold PBS and then
re-suspended in 1 ml PBS and fixed with 2 ml ethanol. The cell
cycle stage was analysed with a FACSCalibur flow cytometer and
CellQuest software (BD Biosciences). For analysis of secondary
clone formation, the suspended and adherent cells were plated in
100-mm dishes at a density of 150–200 cells/cm2(8) and cultured in DMEM/F12 with 10% FBS.
Subsequently, the clones containing 100–200 cells were counted.
Immunofluorescence microscopy and
dyes
The single suspended cells and clone spheres (before
and after differentiation) were fixed in 4% paraformaldehyde for 30
min, permeabilised with 1% Triton X-100 in PBS for 15 min, and
blocked with 3% BSA for 20 min before adding the following primary
antibodies: mouse anti-human Nestin (1:100; Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA), rabbit anti-human CD133
(1:100; Abcam, Cambridge, MA, USA), rabbit anti-human glial
fibrillary acidic protein (GFAP; 1:100; Zhongshan Bio-Tech Co.,
Zhongshan, Guangdong, China), rabbit anti-human
βIII-tubulin (1:2000; Sigma-Aldrich), and mouse
anti-human MBP (1:100; Santa Cruz Biotechnology Inc.). Hoechst
33258 (Invitrogen) was used to stain the cell nuclei. Images were
obtained with an Olympus fluorescence microscope. The contrast and
brightness of the micrographs were then adjusted using Adobe
Photoshop CS for data presentation.
Statistical analyses
The one-way ANOVA test or the Student’s t-test was
used to determine statistical significance using SPSS 16.0
software. P-values <0.05 were considered to indicate
statistically significant differences. All quantitative data are
presented as the means ± standard deviation.
Results
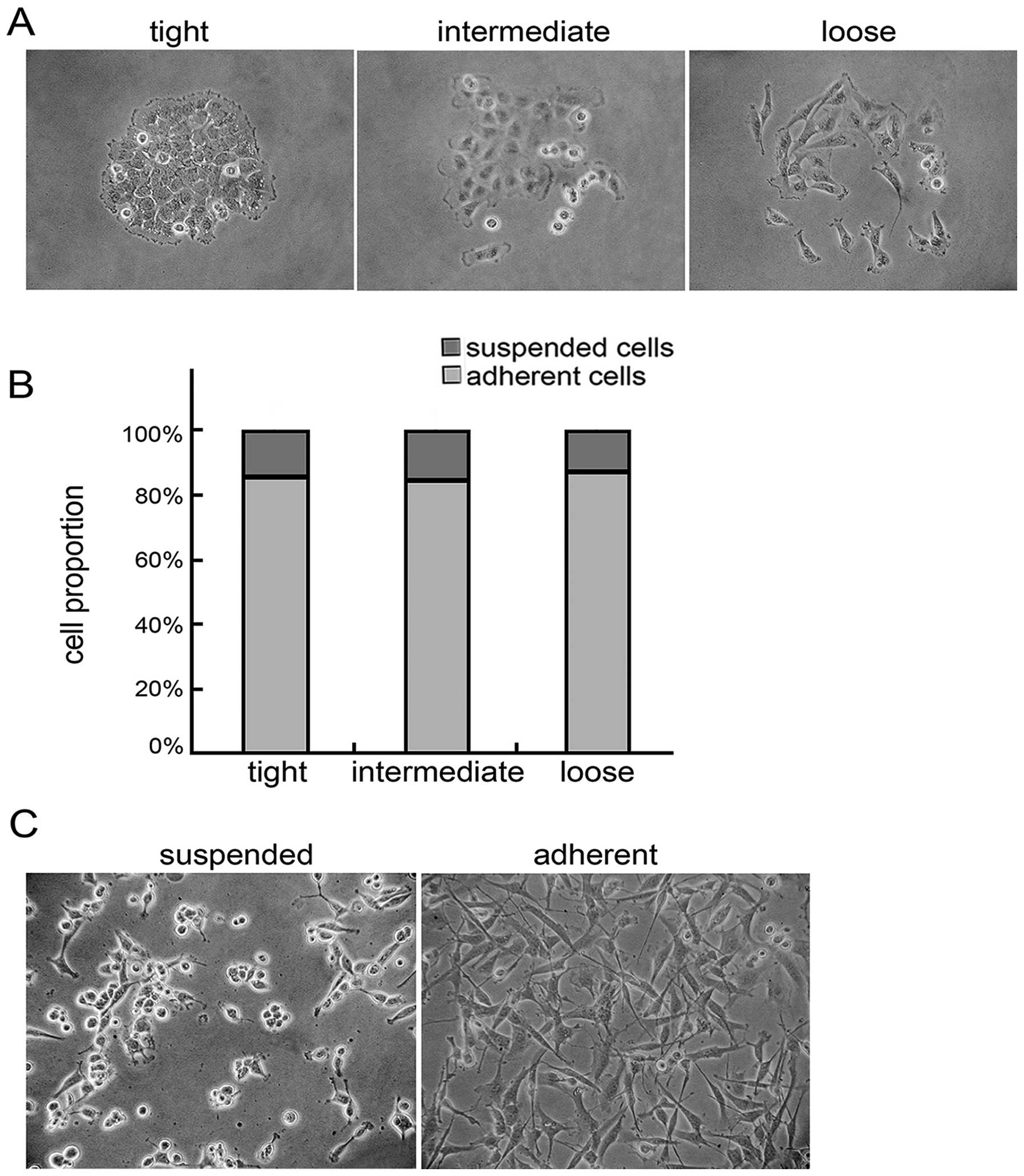
Suspended cells are present in the tight,
intermediate and loose clones
U251 cells formed three morphological types of
clones in low-density culture conditions: tight, intermediate and
loose clones. The tight clones with a regular and compact shape
contained tightly packed cells. The intermediate clones were not as
regular as the tight clones, with the inner cells being tightly
packed, while the peripheral cells were loosely aligned and had
multiple shapes. The loose clones contained spindle-shaped or flat
cells that were loosely aligned. Some suspended cells were
contained in the clones (Fig. 1A).
The tight, intermediate and loose clones comprised 13.97, 45.25 and
40.78% of the total clonal population, respectively. The suspended
cells comprised 14.06±7.61, 15.16±6.52 and 12.84±4.48% of the
tight, intermediate, and loose clonal populations, respectively,
and no significant difference was found among them according to
statistical analysis (Fig. 1B).
Both the suspended and adherent cells were collected from the
intermediate and loose clones and cultured in neurobasal medium for
48 h. The suspended and adherent cells maintained their former
growth patterns (Fig. 1C).
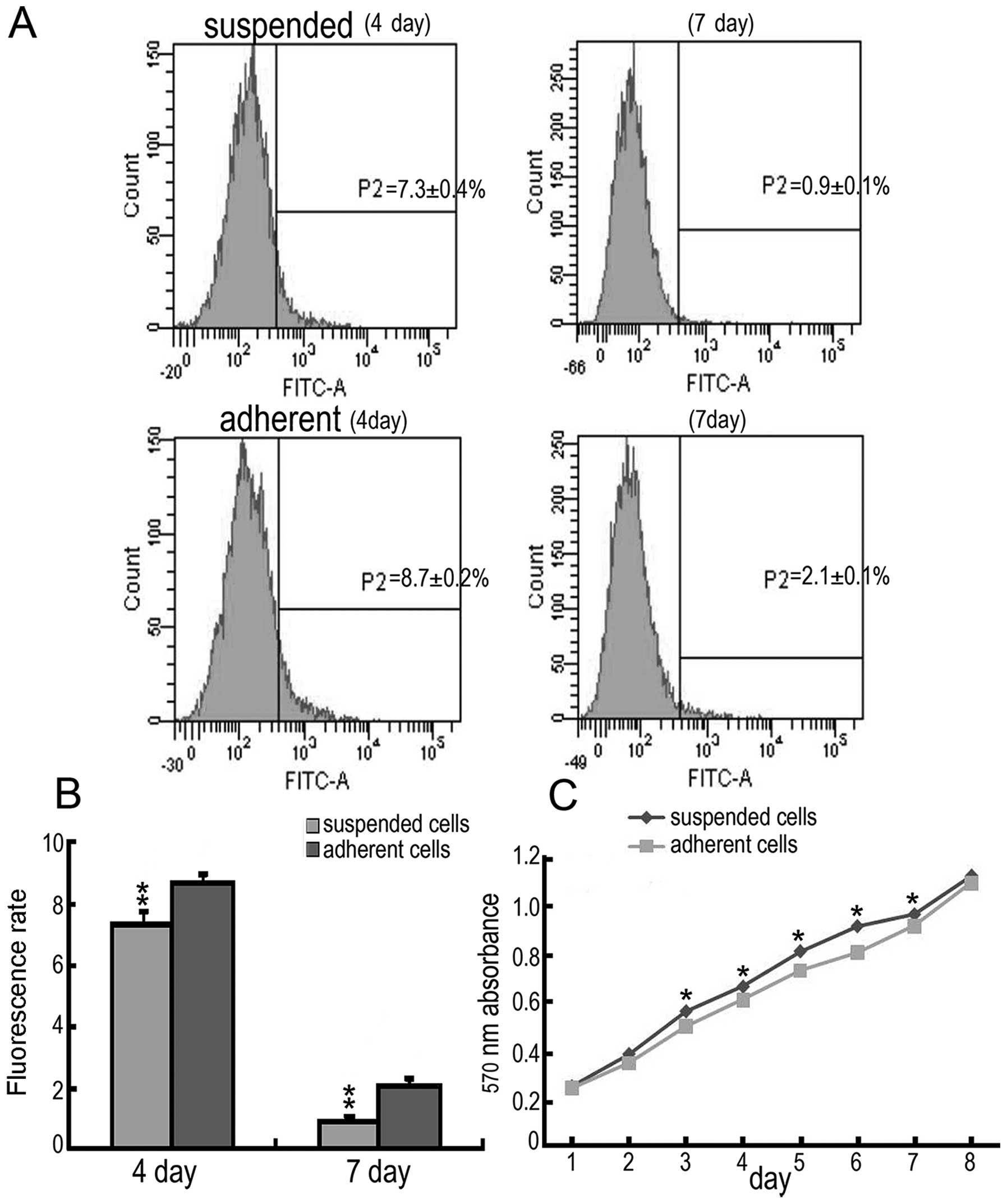
Suspended cells have a higher
proliferation capacity
CSFE is distributed to both daughter cells by
mitosis; therefore, CSFE fluorescence decreases with cell
proliferation (13). The suspended
and adherent cells were labelled by CSFE at the same time and then
were plated in culture dishes. After culturing for four and seven
days, the CSFE fluorescence of the suspended cells was
significantly less than that of the adherent cells (four-day
suspended cells, 7.3±0.4%; adherent cells, 8.7±0.2%; seven-day
suspended cells, 0.9±0.1%; adherent cells, 2.1±0.1%) (Fig. 2A and B). Furthermore, the growth
curve, measured using the MTT assay, showed a significantly higher
absorbance in the suspended cell cultures from days three to seven
compared to the adherent cells (Fig.
2C). These results indicate that suspended cells have a higher
proliferation capacity than adherent cells.
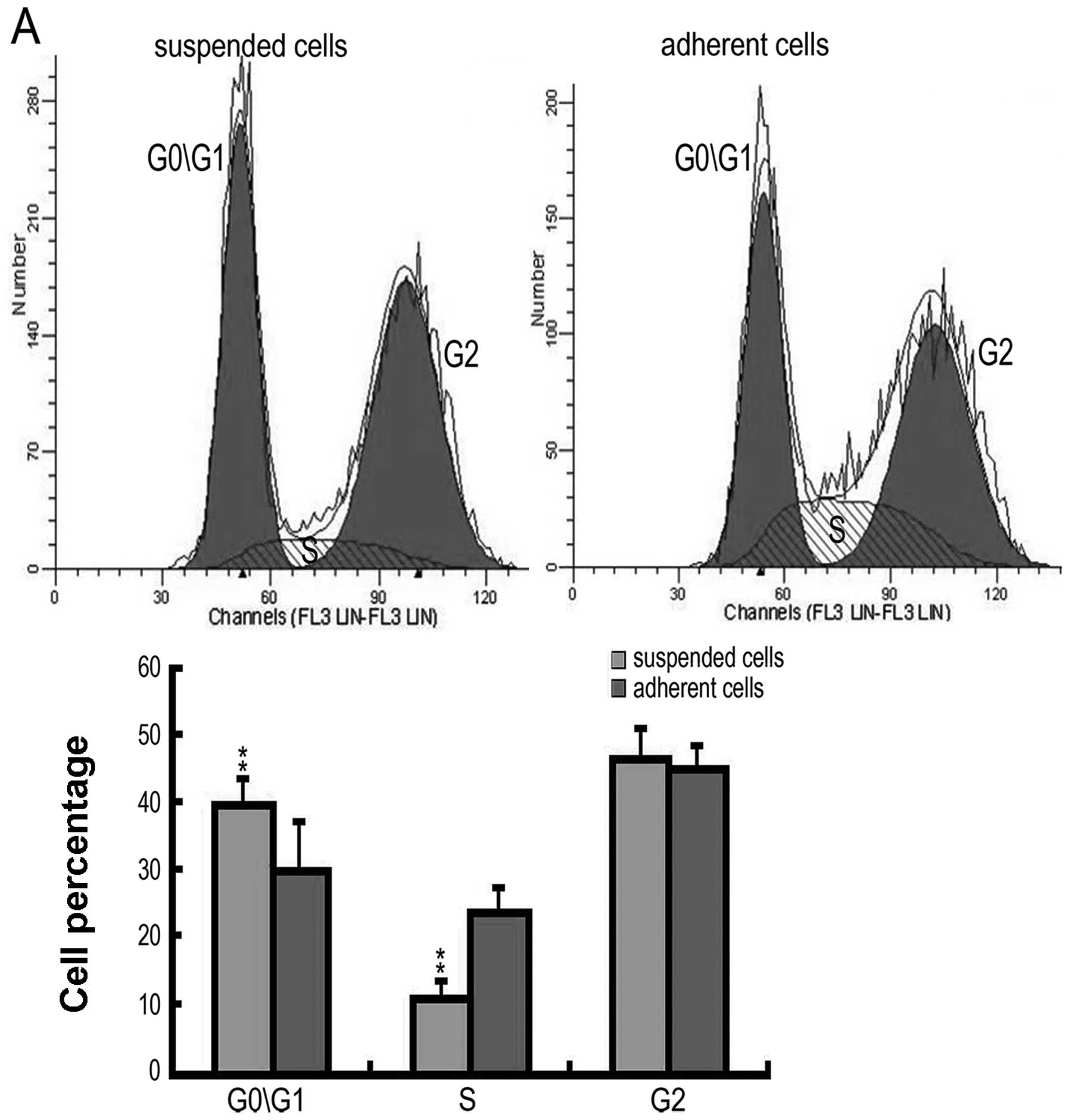
Suspended cells have the potential for
self-maintenance
To maintain a similar cell growth pattern, the
suspended cells were cultured in neurobasal medium and the adherent
cells were cultured in DMEM/F12 with 10% FBS for 48 h. The
suspended cells had a significantly higher number of cells in the
G0 and G1 phases (suspended cells, 40.5±2.2%; adherent cells,
34.69±1.67%) and a significantly lower number in the S phase
(suspended cells, 9.86±0.37%; adherent cells, 22.78±1.05%)
(Fig. 3A). A clonogenic assay was
then carried out, and it was found that 43.70% of the suspended
cells formed secondary clones, which was a significantly higher
percentage than those formed by the adherent cells (32.91%)
(Fig. 3B).
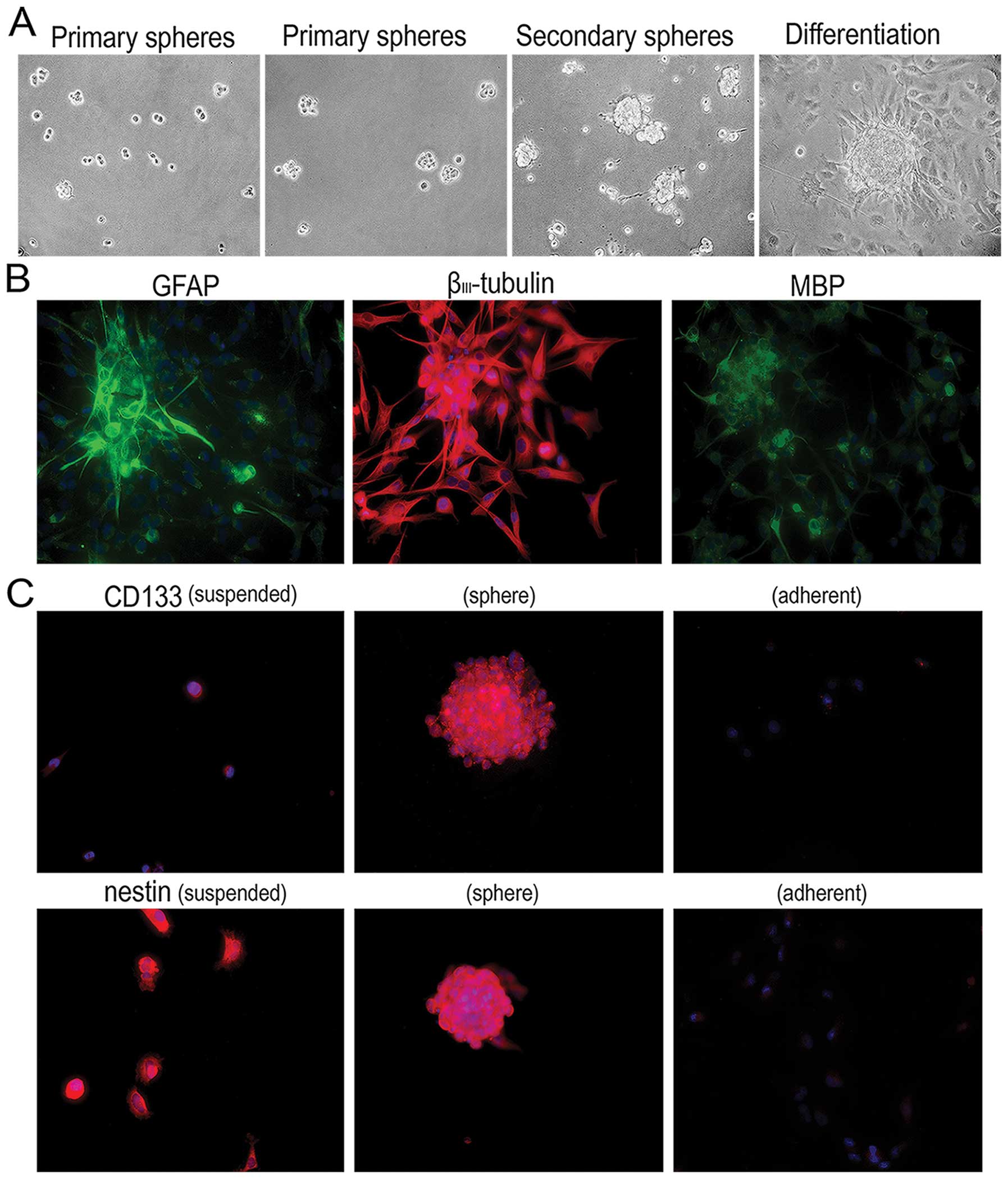
Suspended cells have the potential for
self-renewal, multilineage differentiation, and the expression of
markers of brain CSCs
We performed tests to identify the ‘stem-like’
characteristics of the suspended cells. In neurobasal culture
medium, a single suspended cell formed a primary clone sphere
within four days. These primary clone spheres gradually increased
in size. On day seven, the primary clone spheres were dissociated
into the single cells and cultured in the same neurobasal culture
medium. The secondary clone spheres had smoother edges after seven
days. The clone spheres were differentiated in DMEM/F12 with 10%
FBS. They tightly attached to the dishes, and the cells grew out
and around the spheres (Fig. 4A).
Glial fibrillary acidic protein (GFAP), βIII-tubulin and
myelin basic protein (MBP), differentiation markers for astrocytes,
neurons and oligodendrocytes, respectively, were found to be
positively expressed in the differentiated cells by indirect
immunofluoresence (Fig. 4B). These
results suggest that the suspended cells had the capacity for
self-renewal and multilineage differentiation. Furthermore, CD133
and nestin, markers of brain CSCs, were detected by indirect
immunofluoresence. As shown in Fig.
4C, CD133 and nestin were positively expressed in both the
single suspended cells and clone spheres. CD133 was negatively
expressed in the adherent cells. Nestin was weakly expressed in
parts of the adherent cells, while most of the adherent cells had a
negative nestin expression. Based on these results, the suspended
cells derived from intermediate and loose clones may be CSCs.
Discussion
CSCs determine the biological behaviour of malignant
tumours. Stem cells are rare in the majority of tissues, and they
must be carefully identified and purified based on their properties
(14). In vitro culture
systems may solve this problem. CSCs can be obtained from primary
culture and cancer cell line screening, of which, the primary
culture of fresh cancer samples is the most common method. However,
it is difficult to obtain a large amount of samples that can be
maintained for a long period of time, as the samples are often
derived from patients undergoing surgical resection. Therefore,
cell line screening may overcome this difficulty.
Cells can form distinct morphological types of
clones in vitro(7). For
example, when cloning individual epidermal keratinocytes, the
clones acquire three different morphological types (7). In epithelial cancer cell lines, clone
morphology has been used to determine whether clones originate from
CSCs or committed cells (9–11). It has also been concluded that CSCs
can be obtained from tight clones from the U251 glioma cell line
(8). In this study, to obtain a
larger amount of CSCs, the irregular and loose morphological clones
were examined. A certain proportion of the suspended cells from the
intermediate and loose clones were found to migrate elsewhere and
develop new clones comparable to the tight morphology. The
suspended cells had the ability to remain suspended in the
neurobasal medium, while the adherent cells did not. Based on
previous experiments, this may be due to the growth state of the
CSCs. Thus, we hypothesised that CSCs exist in intermediate and
loose clones and may potentially be utilised to obtain a larger
amount of CSCs.
Previous studies have shown that stem cells have the
ability to: i) proliferate, ii) exhibit self-maintenance, iii)
self-renew to generate new cells, iv) generate a large number of
progeny, and v) retain their multilineage potential over time
(15). In this study, we showed
that the suspended cells displayed significantly higher capacities
for proliferation and clonogenicity and higher proportions of cells
in the G0 and G1 phases than adherent cells. The suspended cells
positively expressed MBP, βIII-tubulin and GFAP, markers
for mature oligodendrocytes, neurons, and astrocytes, respectively,
after clonal differentiation (16).
Our results demonstrate that these cells have a multilineage
potential and may exhibit the properties of stem cells (17,18).
Some CSCs have been identified and screened based on different cell
surface markers, culture conditions, or functional criteria
(19). In neurobasal medium
supplemented with growth factors, such as epidermal growth factor
or basic fibroblast growth factor, CSCs can be propagated and
expanded indefinitely, grown into spheres, and stained for CD133
and nestin, markers of brain CSCs, whereas the majority of
differentiating or differentiated cells rapidly die (20–22).
We verified that the suspended cells formed clone spheres, while
the adherent cells did not.
Clearly, the intermediate and loose clones contained
a proportion of CSCs. The results did not contradict previous
studies. Suspended cells may be easily lost during experiments or
perhaps they are not considered as cells of interest by others. A
large number of suspended cells was carefully collected in our
experiments, so that we could observe their distinct
characteristics. Although the proportion of suspended cells
decreased and the intermediate and loose cells became more
prevalent with subsequent clonal expansion, we were able to obtain
a larger amount of CSCs. The monoclonal morphology screening method
has been used in a previous study (8). For each experiment, it was necessary
to culture single cells in low-density cultures to achieve
monoclonal morphology. Then, tight clones were selected and
cultured in neurobasal medium to form clone spheres, which have a
longer testing potential. However, a simpler and more effective
method was used in this study. The suspended cells were gently
dislodged with a glass dropper and cultured in neurobasal medium,
which resulted in the rapid formation of clone spheres. This method
provided a shorter detection time and simplified the procedures;
accidents such as cell pollution were also avoided. First, the
adherent committed cells were not repeatedly passaged to purify the
suspended CSCs. Second, monoclonal morphology screening which was
time-consuming was not required.
In the current study, only one glioma cell line
(U251) was studied. However, we found that three morphological
types of clones also developed from the C6 rat glioma cell line in
low-density culture conditions, and a certain quantity of suspended
cells was present in each clone. A similar result from other glioma
cell lines may be obtained, which may be useful for CSC culture in
the future.
Acknowledgements
We thank the members of our Laboratory of the Fourth
Military Medical University for technical assistance. This study
was supported by grants (81000171 and 8170798) from the National
Natural Science Foundation of China and the State Key Laboratory of
Cancer Biology (CBSKL 201103).
References
|
1
|
Dalerba P, Cho RW and Clarke MF: Cancer
stem cells: models and concepts. Annu Rev Med. 58:267–284. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vermeulen L, Sprick MR, Kemper K, Stassi G
and Medema JP: Cancer stem cells - old concepts, new insights. Cell
Death Differ. 15:947–958. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heppner GH: Tumor heterogeneity. Cancer
Res. 44:2259–2265. 1984.PubMed/NCBI
|
|
4
|
Lapidot T, Sirard C, Vormoor J, Murdoch B,
Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA and
Dick JE: A cell initiating human acute myeloid leukaemia after
transplantation into SCID mice. Nature. 367:645–648. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
7
|
Barrandon Y and Green H: Three clonal
types of keratinocyte with different capacities for multiplication.
Proc Natl Acad Sci USA. 84:2302–2306. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou ZH, Ping YF, Yu SC, Yi L, Yao XH,
Chen JH, Cui YH and Bian XW: A novel approach to the identification
and enrichment of cancer stem cells from a cultured human glioma
cell line. Cancer Lett. 281:92–99. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harper LJ, Piper K, Common J, Fortune F
and Mackenzie IC: Stem cell patterns in cell lines derived from
head and neck squamous cell carcinoma. J Oral Pathol Med.
36:594–603. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Locke M, Heywood M, Fawell S and Mackenzie
IC: Retention of intrinsic stem cell hierarchies in
carcinoma-derived cell lines. Cancer Res. 65:8944–8950. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li H, Chen X, Calhoun-Davis T, Claypool K
and Tang DG: PC3 human prostate carcinoma cell holoclones contain
self-renewing tumor-initiating cells. Cancer Res. 68:1820–1825.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Banks HT, Sutton KL, Thompson WC, et al: A
new model for the estimation of cell proliferation dynamics using
CFSE data. J Immunol Methods. 373:143–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Evrard B, Dosgilbert A, Jacquemot N,
Demeocq F, Gilles T, Chassagne J, Berger M and Tridon A: CFSE flow
cytometric quantification of lymphocytic proliferation in
extracorporeal photopheresis: use for quality control. Transfus
Apher Sci. 42:11–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reynolds BA and Weiss S: Clonal and
population analyses demonstrate that an EGF-responsive mammalian
embryonic CNS precursor is a stem cell. Dev Biol. 175:1–13. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Campos B, Wan F, Farhadi M, Ernst A,
Zeppernick F, Tagscherer KE, Ahmadi R, Lohr J, Dictus C, Gdynia G,
Combs SE, Goidts V, Helmke BM, Eckstein V, Roth W, Beckhove P,
Lichter P, Unterberg A, Radlwimmer B and Herold-Mende C:
Differentiation therapy exerts antitumor effects on stem-like
glioma cells. Clin Cancer Res. 16:2715–2728. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hemmati HD, Nakano I, Lazareff JA,
Masterman-Smith M, Geschwind DH, Bronner-Fraser M and Kornblum HI:
Cancerous stem cells can arise from pediatric brain tumors. Proc
Natl Acad Sci USA. 100:15178–15183. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vermeulen L, Todaro M, de Sousa Mello F,
Sprick MR, Kemper K, Perez Alea M, Richel DJ, Stassi G and Medema
JP: Single-cell cloning of colon cancer stem cells reveals a
multi-lineage differentiation capacity. Proc Natl Acad Sci USA.
105:13427–13432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tabatabai G and Weller M: Glioblastoma
stem cells. Cell Tissue Res. 343:459–465. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Günther HS, Schmidt NO, Phillips HS,
Kemming D, Kharbanda S, Soriano R, Modrusan Z, Meissner H, Westphal
M and Lamszus K: Glioblastoma-derived stem cell-enriched cultures
form distinct subgroups according to molecular and phenotypic
criteria. Oncogene. 27:2897–2909. 2008.PubMed/NCBI
|
|
22
|
Shi CJ, Gao J, Wang M, Wang X, Tian R, Zhu
F, Shen M and Qin RY: CD133(+) gallbladder carcinoma cells exhibit
self-renewal ability and tumorigenicity. World J Gastroenterol.
17:2965–2971. 2011.
|