Introduction
Pancreatic cancer is one of the most devastating
malignancies worldwide, and the fourth leading cause of
cancer-related death in the United States. It was estimated that in
2011, 44,030 new cases would be diagnosed with pancreatic cancer
and 37,660 individuals would die from this disease (1). Pancreatic cancer has the worst
prognosis of all human tumors. Only approximately 20% of pancreatic
cancer patients are eligible for surgical resection; most
pancreatic cancers are not resectable at the time of diagnosis. In
addition, there are limited treatment options available.
Chemotherapies and radiotherapies are largely ineffective, and
metastatic disease frequently recurs even after surgical resection
of primary lesions (2–4). Recently, a number of risk factors have
been identified, including age, cigarette smoking, high dietary
intake of meat and fat, low serum folate levels, obesity,
long-standing diabetes mellitus, chronic pancreatitis and family
history (5), but exactly how these
risk factors contribute to carcinogenesis remains largely unknown.
Therefore, novel strategies for the prevention of tumor progression
and metastasis are urgently needed.
The cancer stem cell (CSC) hypothesis provides novel
molecular targets and strategies for prevention of pancreatic
cancer. CSCs may be responsible for tumor onset, maintenance,
mutation accumulation and metastasis due to their ability to
express anti-apoptotic and multidrug resistance-associated
proteins, thus sustaining tumor growth (6–9).
Conventional therapeutic approaches merely kill the majority of
differentiated tumor cells, except for CSCs, which have intrinsic
detoxifying mechanisms and can easily elude these therapies.
Recently, CSCs and epithelial-mesenchymal transition (EMT)-type
cells, which share molecular characteristics with CSCs, have been
proposed to play critical roles in chemoresistance and metastasis
as demonstrated in several malignancies including pancreatic
cancer. Thus, it has become increasingly important to increase our
understanding, at the molecular level, of the features of CSCs and
EMT in pancreatic cancer. Such knowledge is likely to be helpful in
the discovery of novel molecular targets for the prevention of this
disease.
The Hedgehog (Hh) signaling pathway functions
postembryonically in the development and homeostasis of many organs
and tissues through its effects on stem or progenitor cells.
Aberrant activation of the Hh signaling pathway correlates with a
variety of human tumors where the pathway is implicated in
tumorigenesis, malignancy, metastasis and cancer stem cells
(10–13). Our previous study identified that
activation of this pathway was a key event in the histogenesis of
pancreatic cancer (14). In a
transgenic mouse model of pancreatic cancer, inhibition of the Hh
signaling pathway was found to reduce tumor-associated stromal
tissues and to ameliorate gemcitabine uptake in tumor cells
(15). Recent studies found that
activation of the Shh signaling pathway was involved in the
regulation of CSC self-renewal, differentiation and tumorigenic
potential, suggesting that the Shh signaling pathway may be a novel
therapeutic approach for the treatment of pancreatic cancer
(16–19). In the present study, the expression
of Hh molecules in human pancreatic cancer tissue samples and
pancreatic cancer cell lines was detected. In addition, the effects
of Hh signaling pathway on pancreatic cancer cell proliferation and
invasion were evaluated. Finally, the molecular mechanisms by which
inhibition of the Hh signaling pathway decreased cell
proliferation, invasion and induced apoptosis were assessed.
Materials and methods
Tissue samples and cell lines
A total of 54 human pancreatic cancer tissue samples
were collected from the Peking University First Hospital. Informed
consent of the patients was obtained before surgical resection, and
this study was approved by the Research Ethics Committee of Peking
University First Hospital. Pancreatic cancer cell lines (PANC-1,
ASPC-1 and Mia PaCa-2) were cultured in Dulbecco’s modified Eagle’s
medium (DMEM; Gibco, Invitrogen, Carlsbad, CA, USA) supplemented
with 10% fetal bovine serum (FBS; Sigma, St. Louis, MO, USA),
penicillin (100 U/ml) and streptomycin (100 μg/ml).
Establishment of pancreatic cancer cell
clones expressing SMO RNAi
The RNAi targeting the human SMO gene (GenBank
accession no. NM_005631) and the negative control sequence were
designed and constructed by GeneChem (Shanghai, China). The
sequence of the SMO RNAi was GACTCTGTCCTGCGTCATCAT, and that of the
negative control was TTCTCCGAACGTGTCACGT. Briefly, the shRNAs were
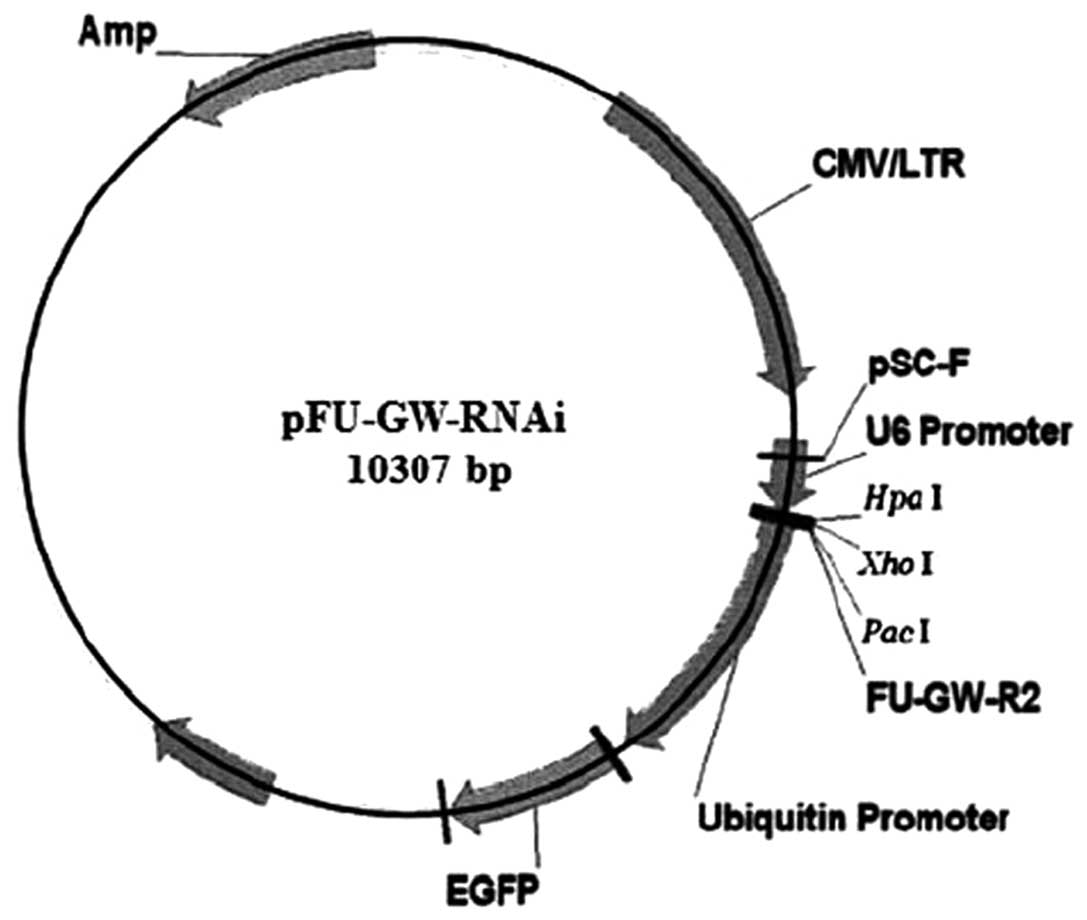
inserted into pFU-GW-RNAi lentivirus vectors containing the
HpaI and XhoI enzyme sites (Fig. 1), and all constructs were confirmed
by sequence analysis. PANC-1 cells were transfected with the
recombinant lentiviral vectors targeting the SMO gene (PANC-1-si)
or the negative control vectors (PANC-1-nc). The constructs were
stably transfected into cells to generate knockdown clones. The
cells were cultured for 48 h, and the transduction efficiency was
assessed using FACS analysis. The transfected cells were harvested
and prepared for subsequent studies.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from pancreatic cancer cells
using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Aliquots (1
μg) of RNA were DNase-treated and processed for first-strand cDNA
synthesis using the RT-PCR kit (Toyobo, Osaka, Japan). The
corresponding cDNA fragments underwent initial denaturation at 94°C
for 5 min, followed by 35 cycles of denaturation at 94°C for 30
sec, annealing at 51–57°C for 30 sec, extension at 72°C for 1 min,
and a final extension step at 72°C for 5 min. Amplified products
were separated by electrophoresis in a 2% agarose gel. The primer
sequences for RT-PCR are shown in Table
I. Three independent experiments were performed.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene name | | Sequences |
|---|
| SHH | Sense |
5′-CCAATTACAACCCCGACATC-3′ |
| Antisense |
5′-CAGTTTCACTCCTGGCCACT-3′ |
| PTCH1 | Sense |
5′-TGGGATTAAAAGCAGCGAAC-3′ |
| Antisense |
5′-TCTCCAATCTTCTGGCGAGT-3′ |
| SMO | Sense |
5′-TGCTCATCGTGGGAGGCTACTT-3′ |
| Antisense |
5′-ATCTTGCTGGCAGCCTTCTCAC-3′ |
| GLI1 | Sense |
5′-TATGGACCTGGCTTTGGA-3′ |
| Antisense |
5′-CCTTGTAGACCCAGAAAC-3′ |
| E-cadherin | Sense |
5′-GCCTCCTGAAAAGAGAGTGGAAG-3′ |
| Antisense |
5′-TGGCAGTGTCTCTCCAAATCCG-3′ |
| N-cadherin | Sense |
5′-TCGCTCTCGAGCTCTCCGCCTCCATGTGCCGG-3′ |
| Antisense |
5′-AAGGGTCACCTGAAGTTCAGTCATCAC-3′ |
| Vimentin | Sense |
5′-AGGCAAAGCAGGAGTCCACTGA-3′ |
| Antisense |
5′-ATCTGGCGTTCCAGGGACTCAT-3′ |
| Fibronectin | Sense |
5′-ACAACACCGAGGTGACTGAGAC-3′ |
| Antisense |
5′-GGACACAACGATGCTTCCTGAG-3′ |
| Snail | Sense |
5′-TGCCCTCAAGATGCACATCCGA-3′ |
| Antisense |
5′-GGGACAGGAGAAGGGCTTCTC-3′ |
| Slug | Sense |
5′-ATCTGCGGCAAGGCGTTTTCCA-3′ |
| Antisense |
5′-GAGCCCTCAGATTTGACCTGTC-3′ |
| GAPDH | Sense |
5′-ACGGATTTGGTCGTATTGGG-3′ |
| Antisense |
5′-TGATTTTGGAGGGATCTCGC-3′ |
Real-time reverse
transcription-polymerase chain reaction (real-time RT-PCR)
Total RNA was extracted from human pancreatic cancer
tissue samples using TRIzol reagent (Invitrogen). Aliquots (1 μg)
of RNA were DNase-treated and processed for first-strand cDNA
synthesis using the RT-PCR kit. Real-time RT-PCR was carried out on
an Applied Biosystems 7300 Fast Real-time PCR System (Applied
Biosystems, Foster City, CA, USA) using real-time PCR Master Mix
(Toyobo). Quantification of gene expression was calculated by the
2−ΔΔCt method. GAPDH was used as an internal control.
The primer sequences for real-time RT-PCR are shown in Table I.
Western blot analysis
Pancreatic cancer cell lines were seeded in 10-cm
cell culture plates in complete medium and allowed to reach 80%
confluency. Then, cells were lysed and the proteins were obtained
using the Total Protein Extraction kit (KeyGen, Nanjing, China).
Total protein (70 μg/sample) was separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After being
transferred to nitrocellulose membranes, blots were incubated with
the SMO antibody (Chemicon International, Temecula, CA, USA) and
β-actin antibody (Zsbio, Beijing, China), and the washed blots were
then incubated with secondary antibodies conjugated with
horseradish peroxidase. To detect the activation of the PI3K/AKT
pathway, pancreatic cancer cells (PANC-1-si and PANC-1-nc) were
treated with Shh (1 μg/ml, Abcam, Cambridge, UK), and then western
blot analysis was performed with the phospho-AKT antibody (Cell
Signaling Technology, Beverly, MA, USA). Three independent
experiments were performed.
MTT assays
Pancreatic cancer cells were propagated in 96-well
plates at a density of 5.0×103 cells/well for 72 h prior
to the proliferation assays. MTT (20 μl) (5 mg/ml, Sigma) was added
to each well and incubated for 4 h at 37°C. Then culture medium was
removed, 200 μl dimethyl sulfoxide (DMSO) was added and thoroughly
mixed for 15 min to resolve the cellular formazan. Optical density
was measured at 570 nm (OD570) with an ELISA plate
reader (Bio-Rad Laboratories, Hercules, CA, USA). The cell growth
inhibition rate (GIR) was calculated as follows: GIR = (1 -
OD570 of treated cells/OD570 of untreated
cells) × 100%. Three independent experiments were performed.
Colony formation assays
Cells (3.0×103 cells/plate) were seeded
into 60-mm culture dishes and allowed to grow for 14 days. Colonies
were fixed in methanol and visible colonies containing ~50 or more
cells were counted. Three independent experiments were
performed.
Flow cytometric analysis
An Annexin V-PE Apoptosis Detection kit (BD
Biosciences, Franklin Lakes, NJ, USA) was used to measure the
apoptosis of pancreatic cancer cells (wild-type, PANC-1-si and
PANC-1-nc). The cells were seeded in 6-well plates and incubated
until 70% confluency. The cells were then harvested, washed twice
with cold phosphate-buffered saline (PBS), and resuspended in 1X
binding buffer at a concentration of 1.0×106 cells/ml.
Solution (100 μl) (1.0×105 cells) was transferred to a
5-ml culture tube. A total of 5 μl of Annexin V-PE and 5 μl of
7-AAD were added, and the mixture was incubated for 15 min at room
temperature in the dark. The labeled cells (1.0×104
cells/sample) were analyzed using FACScan flow cytometer (BD
Biosciences) in conjunction with CellQuest software. To detect the
expression of cancer stem cell marker CD133, pancreatic cancer
cells (PANC-1-si and PANC-1-nc) were collected, washed with PBS and
suspended in PBS at a concentration of 1.0×106 cells/ml,
then labeled with CD133-PE (Miltenyi Biotec, Germany) and analyzed
using FACScan flow cytometer. Three independent experiments were
performed.
Transwell invasion assays
Cells (1.0×105 cells/well) were seeded
into the upper chamber of a Transwell insert (Costar, Cambridge,
MA, USA), which was precoated with the extracellular matrix (ECM)
substitute Matrigel (BD Biosciences) in phenol red-free medium. In
the lower chamber, phenol red-free DMEM supplemented with 5% of
steroid-depleted fetal bovine serum (Biochrom, Berlin, Germany)
acted as the chemoattractant. The cells were then cultured for an
additional 48 h. Non-migrating cells were removed from the upper
chamber by scraping, and cells remaining on the lower surface of
the insert were stained using 0.1% crystal violet, and the cell
number was counted under a microscope. Three independent
experiments were performed.
Nude mouse xenograft model
Six-week-old nude mice (BALB/c AnN Crl-nu BR) were
maintained in a barrier facility on high efficiency particulate
air-filtered racks and fed by breeders from the Department of
Laboratory Animal Science, Peking University Health Science Center.
Pancreatic cancer cells (wild-type, PANC-1-si and PANC-1-nc) were
subcutaneously injected into the right axilla of nude mice. Mice
were monitored for tumor formation every 3 days. Tumor size was
measured in two dimensions, and the volume was determined by the
equation V = l × w2 × 0.5 (where V is the volume; l,
length; w, width). Four weeks later, all mice were sacrificed, and
tumors were excised. All animal studies were reviewed and approved
by the Ethics Committee for Animal Studies at the Peking
University, China.
Statistical analysis
Results are presented as the means ± SD. Statistical
analyses were undertaken using SPSS version 13.0. Student’s t-test
(for two groups) and one-way analysis of variance (ANOVA) test (for
multiple groups) followed by the Student-Newman-Keuls (SNK) test
were used to assess the differences. P<0.05 was considered to
indicate a statistically significant result.
Results
Activation of the Hh signaling pathway in
pancreatic cancer tissue samples and cell lines
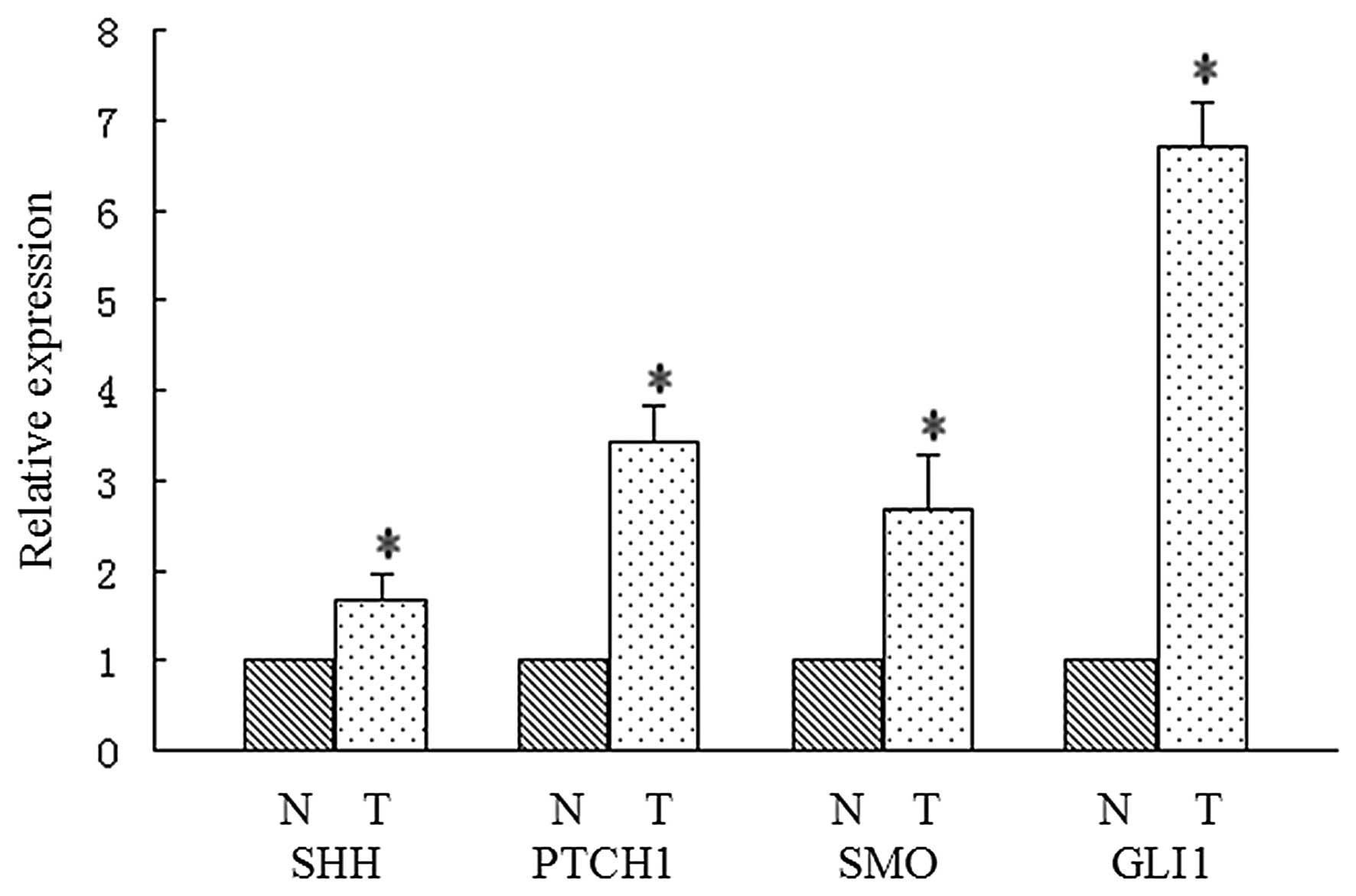
We detected SHH, PTCH1, SMO and GLI1 expression in
54 human pancreatic cancer tissue samples for the purpose of
evaluating the activation of the Hh signaling pathway. Expression
levels of Hh molecules in cancer tissues and matched adjacent
non-tumor tissues were determined by real-time RT-PCR. As shown in
Fig. 2, a significant difference in
expression of Hh molecules was noted between cancer tissues and
matched adjacent non-tumor tissues (P<0.05, respectively). In
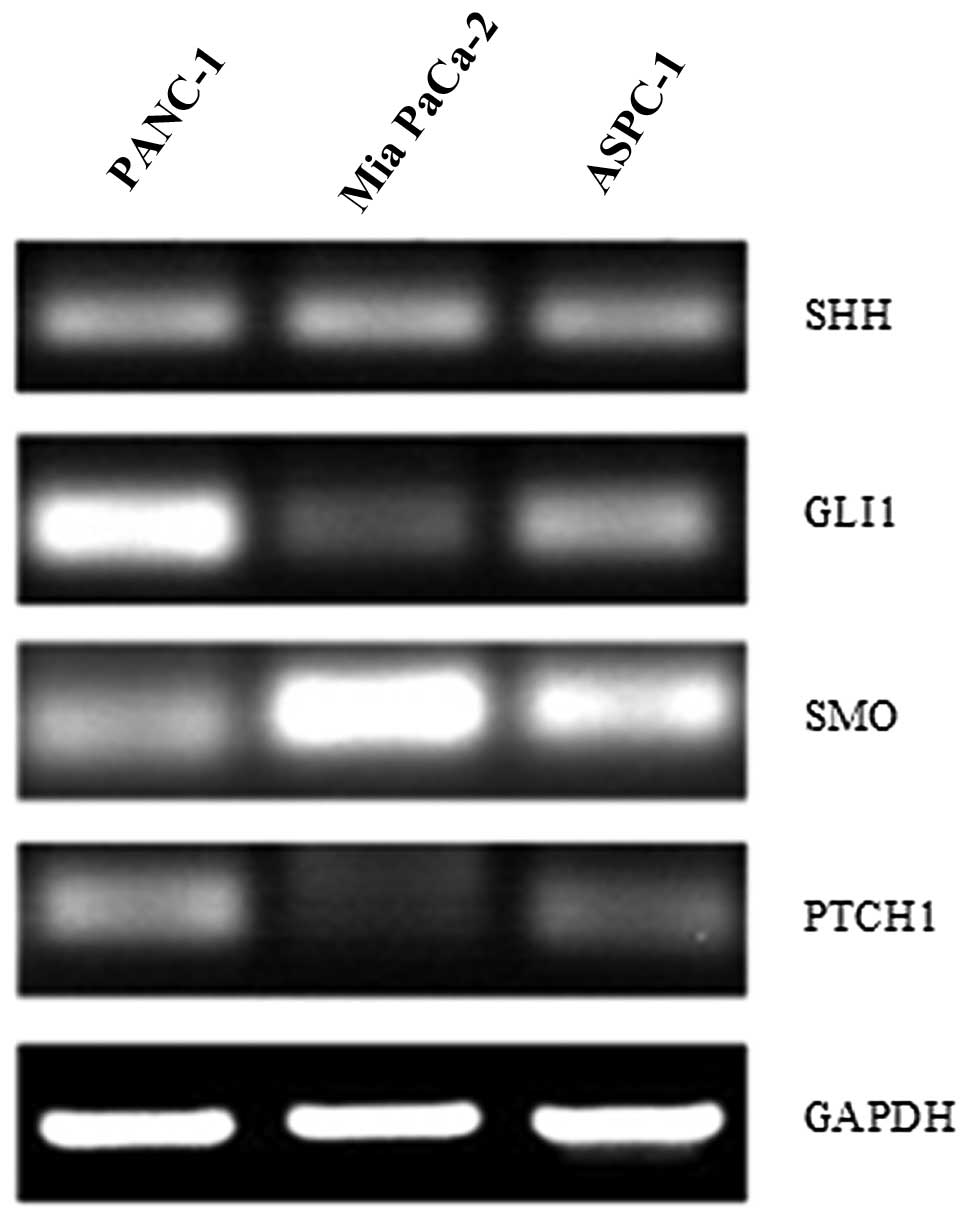
addition, to confirm the activation of the Hh signaling pathway in
pancreatic cancer, we used RT-PCR to detect the expression of SHH,
PTCH1, SMO and GLI1 in three pancreatic cancer cell lines (PANC-1,
ASPC-1 and Mia PaCa-2). As shown in Fig. 3, varied expression of Hh molecules
confirmed the general presence of an active Hh signaling pathway.
High GLI1 expression was observed in the PANC-1 cell line, which is
characterized by poor differentiation and better invasiveness. In
addition, GLI1 is the only reliable marker of Hh signaling pathway
activation. Therefore, the PANC-1 cell line was selected for
subsequent experiments.
Lentivirus-mediated shRNA targeting SMO
decreases cell proliferation and enhances cell apoptosis
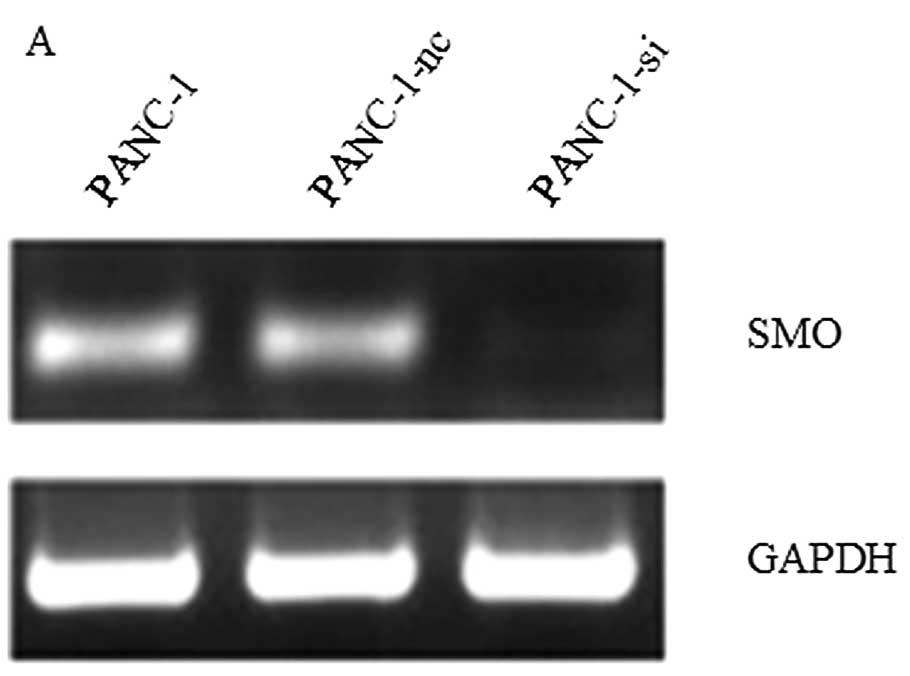
PANC-1 cells were transfected with recombinant
lentiviral vectors targeting the SMO gene (PANC-1-si) or negative
control vectors (PANC-1-nc). The effects of the lentivirus-mediated
shRNA on mRNA and protein expression of SMO were examined by RT-PCR
and western blot analysis, and the result revealed that SMO
expression was significantly downregulated in PANC-1-si cells
(Fig. 4). The effect of
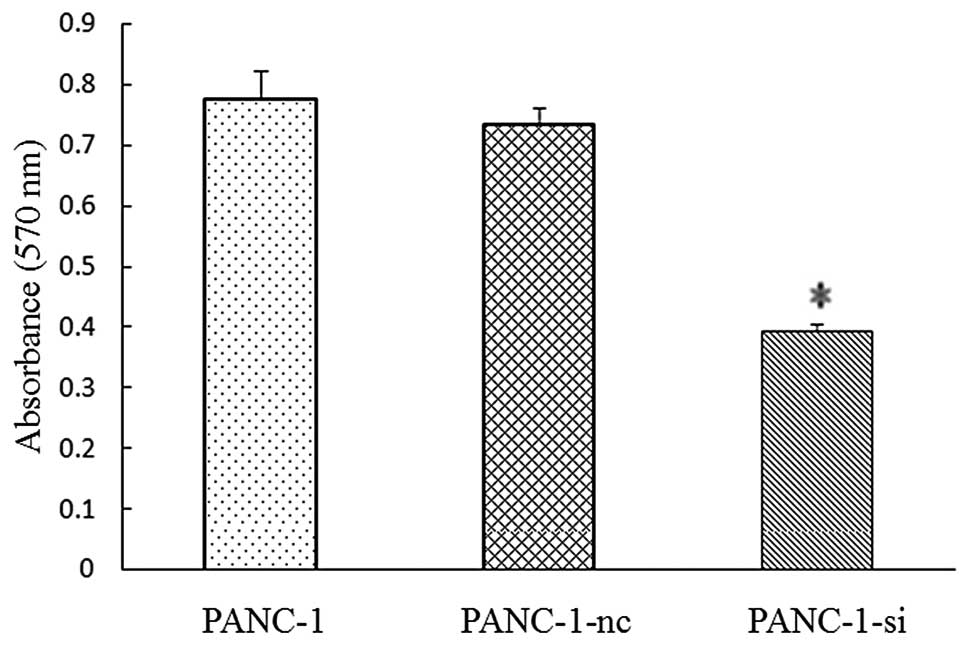
lentivirus-mediated shRNA targeting SMO on cell proliferation was
measured by MTT and colony formation assays. Pancreatic cancer
cells were propagated in 96-well plates for 72 h prior to the
proliferation assays. Inhibition of the Hh signaling pathway
significantly decreased the cell proliferation. The average
inhibitory rate was 35.7±1.34% for PANC-1-si compared with the
negative control (P<0.05) (Fig.
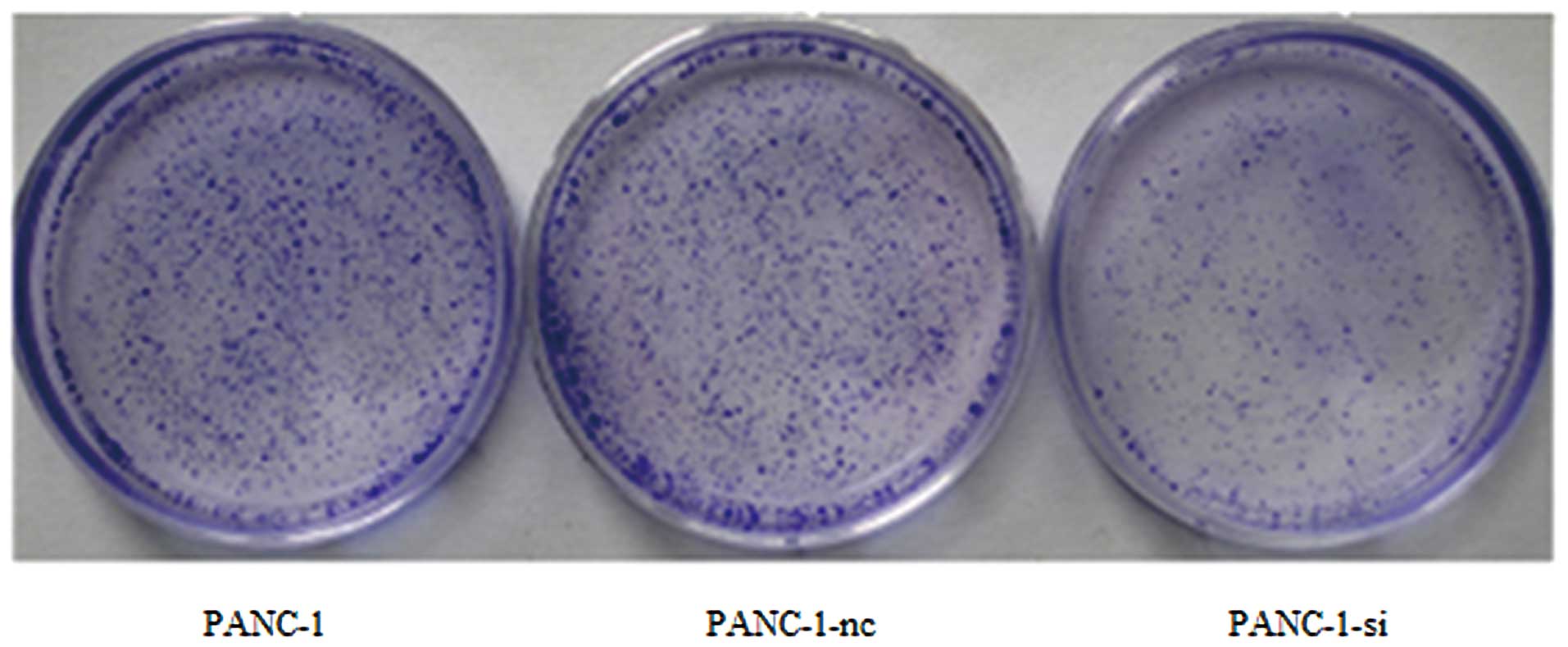
5). In addition, cells were seeded and allowed to grow for 14
days. Visible colonies containing ~50 or more cells were counted.
The results indicated that the number of PANC-1-si colonies was
much lower than that of wild-type or negative control cells
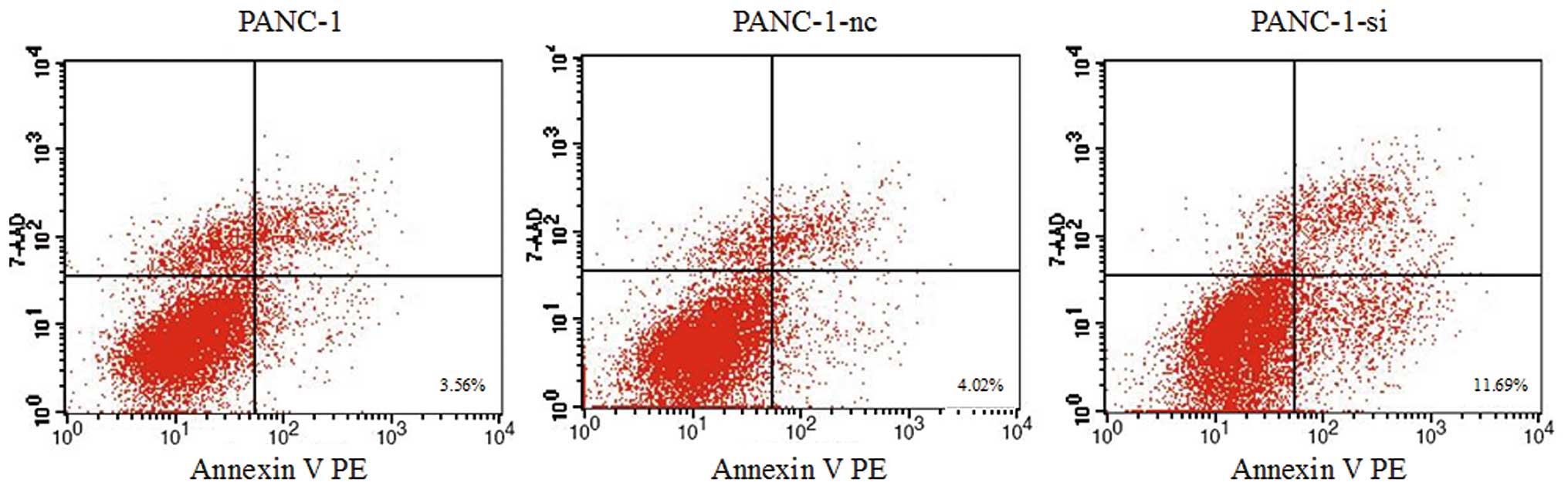
(Fig. 6). Moreover, SMO suppression
caused a moderate increase in the apoptotic rate. As shown in
Fig. 7, apoptosis of PANC-1-si
cells was markedly increased to 11.69±1.372%, significantly higher
than that of wild-type or negative control cells (P<0.05). These
findings imply that inhibition of the Hh signaling pathway
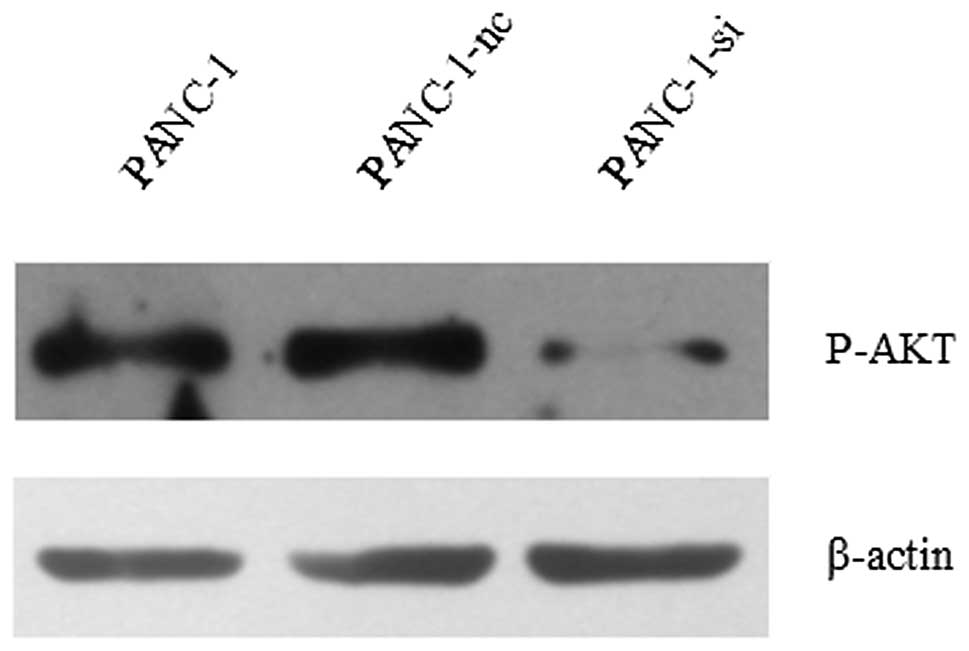
significantly decreases proliferation of PANC-1 cells. To
investigate the possible mechanisms, we analyzed activation of the
PI3K/AKT pathway in pancreatic cancer cells. Pancreatic cancer
cells (PANC-1-si and PANC-1-nc) were treated with Shh prior to
western blot assays. As shown in Fig.
8, downregulation of SMO caused a notable decrease in
phosphorylated AKT.
Lentivirus-mediated shRNA targeting SMO
inhibits cell invasion
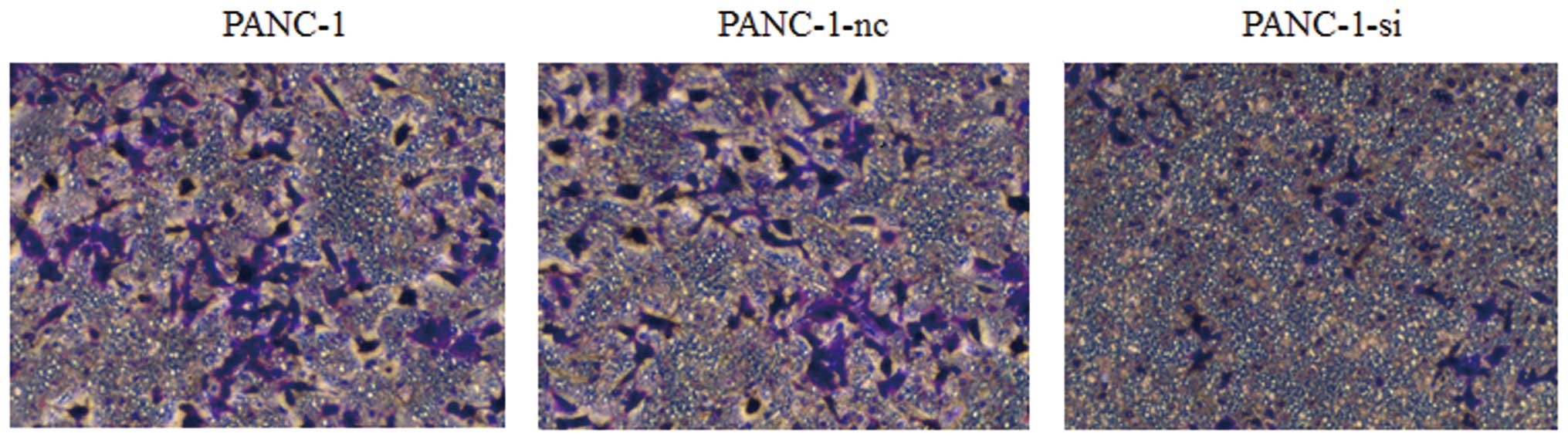
To further study the potential influence of the Hh
signaling pathway on cancer cell invasion, we performed Transwell
invasion assays. As shown in Fig.
9, the number of PANC-1-si cells that migrated through the
upper chamber of the Costar wells was less than that of wild-type
or negative control cells. Moreover, to test the possibility that
the Hh signaling pathway directly drives EMT and metastatic
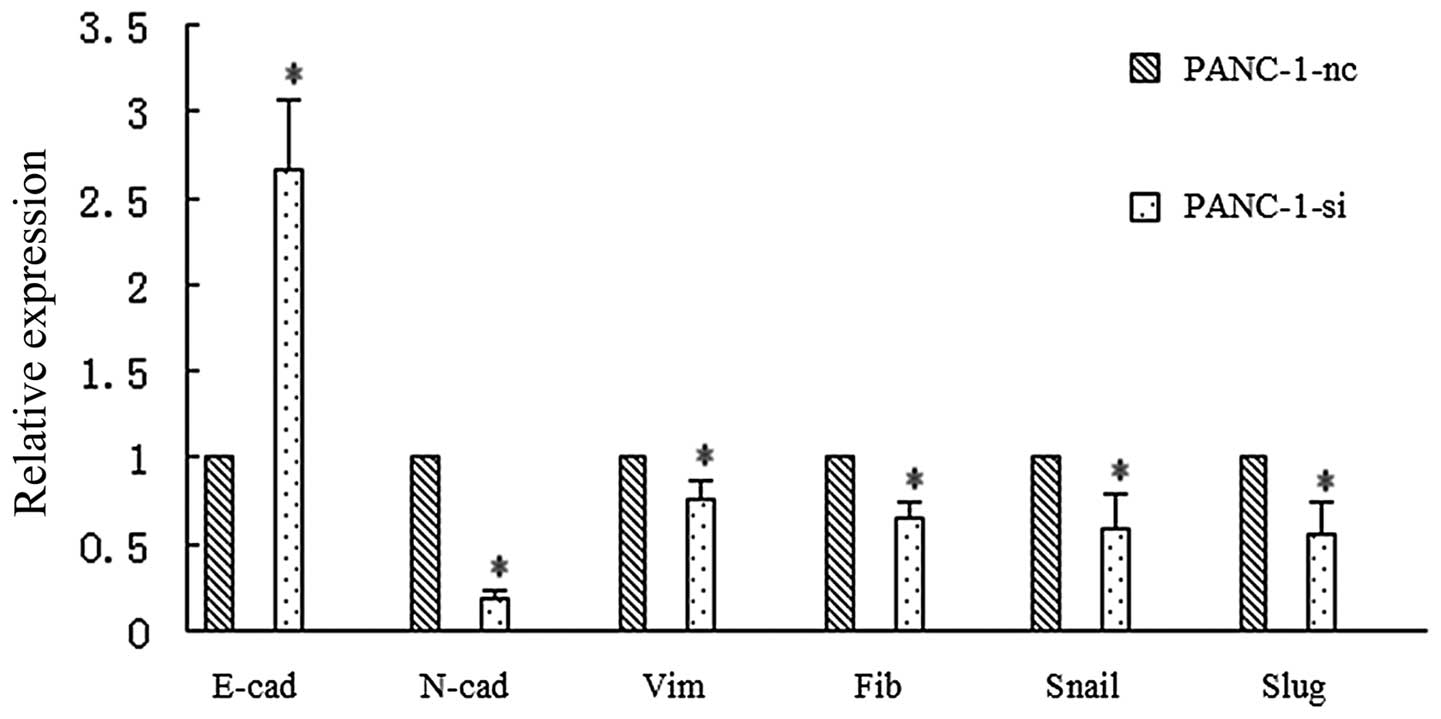
behavior in human pancreatic cancer cells, we analyzed the
EMT-related genes by real-time RT-PCR. As shown in Fig. 10, downregulation of SMO caused a
notable increase in E-cadherin and a notable decrease in
N-cadherin, vimentin and fibronectin. The expression of the
transcription factors Snail and Slug also displayed a reduction.
Collectively, these studies demonstrate that the Hh signaling
pathway could play a role in the invasion of pancreatic cancer.
Lentivirus-mediated shRNA targeting SMO
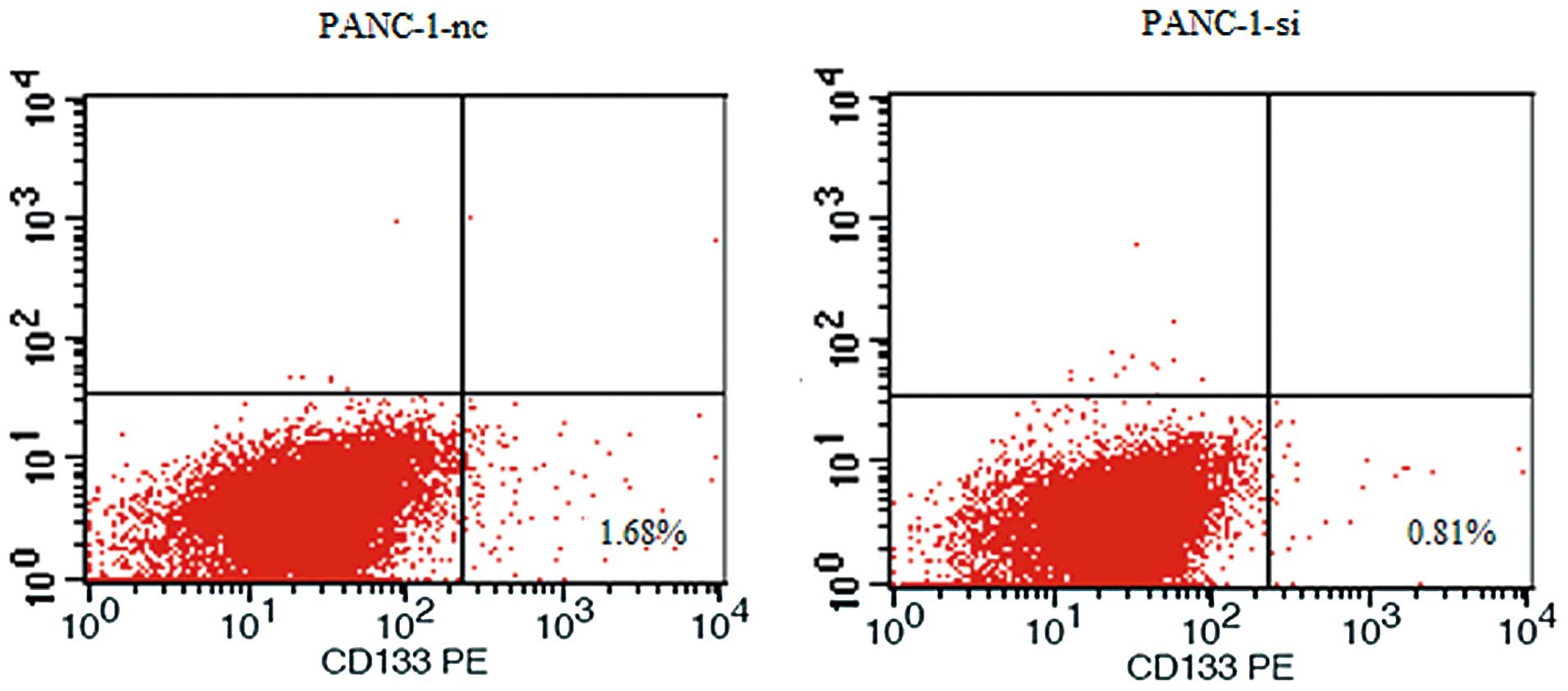
decreases the expression of a cancer stem cell marker
The involvement of the Hh signaling pathway in
proliferation and invasion suggests a possible role in the behavior
of pancreatic cancer stem cells. To test cancer stem cell behavior
in vitro, we detected the expression levels of cancer stem
cell marker CD133 in pancreatic cancer cells. As shown in Fig. 11, our data showed that SMO
suppression resulted in downregulation of the CD133+
subfraction in PANC-1-si.
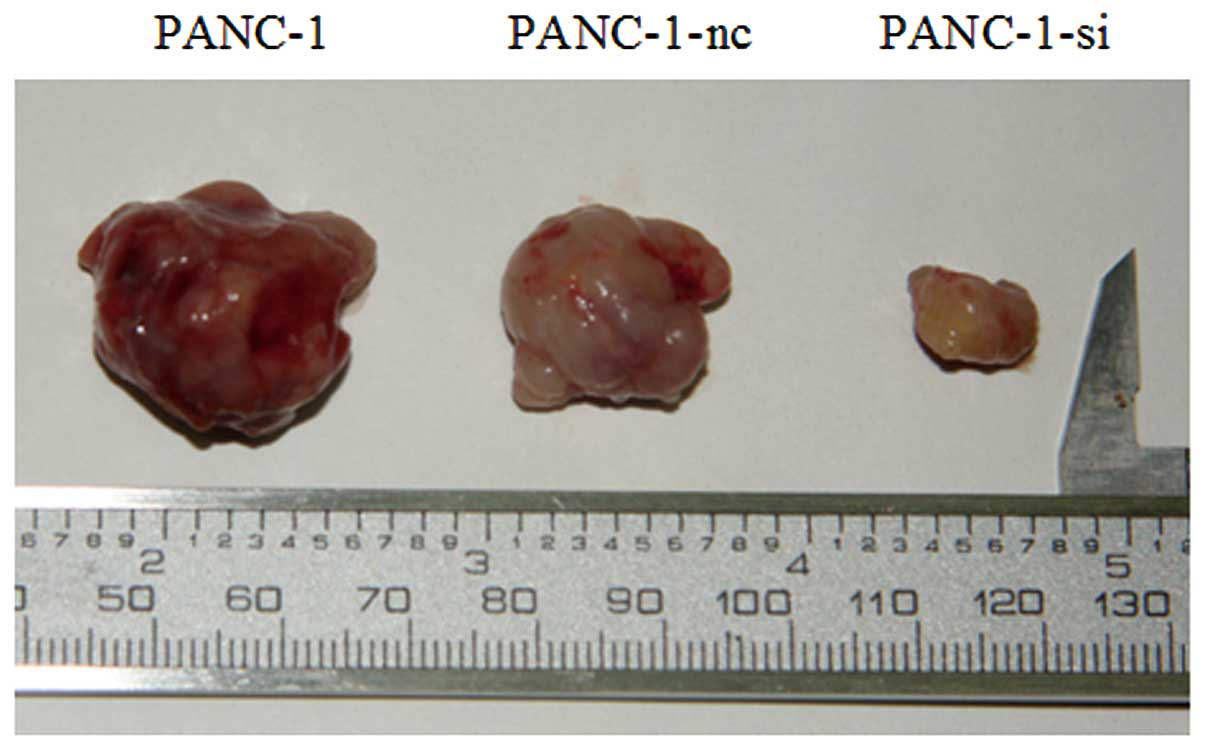
Lentivirus-mediated shRNA targeting SMO
decreases xenograft tumor growth
To investigate the antitumor effect in vivo,
we established xenograft models with PANC-1, PANC-1-nc or PANC-1-si
cells. Four weeks later, all mice were sacrificed, and tumors were
excised. As shown in Fig. 12, the
growth of PANC-1-si xenograft tumors was significantly inhibited
when compared with the control groups.
Discussion
The Hedgehog (Hh) signaling pathway was originally
identified through the genetic analysis of segmentation in
Drosophila and is highly conserved from insects to
vertebrates (20,21). In mammals, there are three Hh ligand
proteins: Sonic hedgehog (SHH), Indian hedgehog (IHH) and Desert
hedgehog (DHH). They exert their molecular and cellular effects
through two transmembrane protein receptors: Patched (PTCH) and
Smoothened (SMO), two key regulators of the Hh signaling pathway.
PTCH is a 12-transmembrane protein that acts as a negative
regulator of SMO, which is a 7-transmembrane protein. When this
pathway is activated, Hh ligand proteins activate SMO, complex with
coactivator or corepressor proteins, and bind to transcriptional
factor GLI in the regulatory regions of target genes to regulate
the transcription of GLI-responsive genes (22,23).
Aberrant activation of the Hh signaling pathway has
been demonstrated during the progression of human pancreatic
cancer. Berman et al(24)
analyzed the expression of PTCH mRNA in freshly resected pancreatic
tumors by quantitative RT-PCR, and found that PTCH mRNA levels were
69–5,044 times higher in pancreatic tumors (mean, 448; n=15) than
in adjacent normal tissue. Kayed et al(25) examined the expression of Hh
molecules (IHH, PTCH and SMO) in pancreatic cancer tissues by
quantitative RT-PCR, revealing that IHH, PTCH and SMO mRNA levels
were increased 35-, 1.2- and 1.6-fold, respectively, compared to
normal pancreatic tissues.
In the present study, we evaluated the expression
levels of Hh molecules in human pancreatic cancer tissue samples
and pancreatic cancer cell lines. Consistent with recent published
data (25–28), our results revealed that expression
of Hh signaling pathway-related molecules were at a higher level in
the cancer tissues than in the matched adjacent non-tumor tissues
and existed in all pancreatic cancer cell lines. Although
pancreatic cancer tissue samples and cell lines expressed
detectable levels of the mRNA of the molecules, the expression
levels varied. Little is understood about the exact mechanism of Hh
molecules in pancreatic cancer cell proliferation and invasion.
Further analysis showed that the therapeutic effects of SMO
suppression were correlated with the induction of apoptosis in
pancreatic cancer cells. The mechanism of this effect has not yet
been fully elucidated. Our results showed that the phosphorylation
levels of AKT were markedly reduced in PANC-1-si cells after Shh
stimulation compared to control groups (Fig. 8). The results suggests that the
suppression of PI3K/AKT activity might be responsible for the
apoptosis-inducing effect of SMO suppression. As reported by
several authors, the PI3K/AKT pathway has also been reported to
play important roles in the Hh signaling pathway-mediated
metastasis and chemoresistance (29–31).
CSCs have been identified in many different types of
solid tumors (32–37). Recent evidence indicates that the Hh
signaling pathway is recruited to stimulate CSC growth. It is one
of the key pathways that regulates stem cells in the adult body
(38,39). Several studies have also shown that
the Hh signaling pathway plays an important role in maintaining the
biological characteristics of pancreatic cancer stem cells. Hermann
and colleagues (40) successfully
isolated a different highly tumorigenic subpopulation of pancreatic
cancer cells. They found that cells expressing CD133 have the
characteristics that defined CSCs, and compared to
CD133− pancreatic cancer cells, CD133+ cells
showed significantly enhanced resistance to gemcitabine. Dembinski
and Krauss (41) found that
compared with pancreatic cancer cells, SHH was increased nearly
4-fold in BxPC-3 CSCs and 2-fold in Panc03.27 CSCs, and the zinc
finger transcription factor GLI1 was upregulated 2.5-fold in BxPC-3
and 1.5-fold in the Panc03.27 CSC populations. Furthermore,
Feldmann et al(42)
inhibited the Hh signaling pathway by means of a small-molecule SMO
inhibitor, cyclopamine, and found that the treatment preferentially
reduced aldehyde dehydrogenase (ALDH)-expressing populations in
pancreatic cancer cells; ALDH overexpression is characteristic for
stem cells.
In the present study, we found that the Hh signaling
pathway may play a role in the regulation of self-renewal in
pancreatic cancer stem cells. CD133 expression was analyzed using
FACScan flow cytometer in pancreatic cancer cells, which is
reported to be a putative marker of CSCs in pancreatic cancer. We
demonstrated that the percentage of CD133+ cells
significantly differed between PANC-1-si and PANC-1-nc cells. The
results revealed that inhibition of the Hh signaling pathway by
knockdown of SMO affects the pancreatic CSC populative.
Moreover, recent evidence indicates that the Hh
signaling pathway is recruited to orchestrate the reprogramming of
cancer cells via EMT in various types of cancer (42–44).
EMT is an embryonic program in which epithelial cells lose their
characteristics and gain mesenchymal features. Accumulating
evidence suggests that EMT plays an important role during malignant
tumor progression. Transformed epithelial cells can activate
embryonic programs of epithelial plasticity and switch from a
sessile, epithelial phenotype to a motile, mesenchymal phenotype.
Induction of EMT can, therefore, lead to invasion of surrounding
stroma, intravasation, dissemination and colonization of distant
sites. It is believed that sustained metastatic growth requires the
dissemination of CSCs from the primary tumor followed by its
reestablishment in a secondary site. Thus, EMT can confer
metastatic ability on carcinomas (45,46).
Here, we discovered that inhibition of the Hh signaling pathway can
reverse EMT in pancreatic cancer cells. After knockdown of SMO
expressions the epithelial cell surface marker E-cadherin
increased, the expression of mesenchymal markers N-cadherin,
vimentin, fibronectin and transcription factors Snail and Slug
decreased and the invasion of PANC-1 cells was inhibited. These
characteristics are consistent with the morphological changes,
molecular events and functions of mesenchymal-epithelial
transition, which is the opposite of EMT.
In conclusion, our data demonstrated that the Hh
signaling pathway is frequently activated in human pancreatic
cancer tissue samples and pancreatic cancer cells, and plays a
pivotal role in maintaining pancreatic cancer cell proliferation
and invasion. Inhibition of the Hh signaling pathway by SMO
suppression represents an effective method to decrease cell
proliferation and induce apoptosis through inhibition of PI3K/AKT
pathways and CSCs. Further studies found that inhibition of the Hh
signaling pathway significantly inhibited EMT, suggesting blockade
of signaling involved in early metastasis. These data suggest that
targeted therapy against the Hh signaling pathway may be beneficial
in the treatment of pancreatic cancer.
Acknowledgements
This research was supported by grants from the
National Natural Science Foundation of China (no. 30972897 and
81172184 to Y.M. Yang), and the Overseas Study Program of the China
Scholarship Council, Beijing, China. We thank Professor Ze-Bin Mao
of the Department of Biochemistry and Molecular Biology in the
Peking University Health Science Center for his assistance and
technical support.
References
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Magee CJ, Ghaneh P and Neoptolemos JP:
Surgical and medical therapy for pancreatic carcinoma. Best Pract
Res Clin Gastroenterol. 16:435–455. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yeo TP, Hruban RH, Leach SD, et al:
Pancreatic cancer. Curr Probl Cancer. 26:176–275. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Warshaw AL and Fernández-del Castillo C:
Pancreatic carcinoma. N Engl J Med. 326:455–465. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koorstra JB, Hustinx SR, Offerhaus GJ and
Maitra A: Pancreatic carcinogenesis. Pancreatology. 8:110–125.
2008. View Article : Google Scholar
|
|
6
|
Mueller MT, Hermann PC, Witthauer J,
Rubio-Viqueira B, Leicht SF and Huber S: Combined targeted
treatment to eliminate tumorigenic cancer stem cells in human
pancreatic cancer. Gastroenterology. 137:1102–1113. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tanaka H, Nakamura M, Kameda C, Kubo M,
Sato N and Kuroki S: The Hedgehog signaling pathway plays an
essential role in maintaining the
CD44+CD24−/low subpopulation and the side
population of breast cancer cells. Anticancer Res. 29:2147–2157.
2009.PubMed/NCBI
|
|
8
|
Shankar S, Nall D, Tang SN, Meeker D,
Passarini J, Sharma J and Srivastava RK: Resveratrol inhibits
pancreatic cancer stem cell characteristics in human and KrasG12D
transgenic mice by inhibiting pluripotency maintaining factors and
epithelial-mesenchymal transition. PLoS One. 6:e165302011.
View Article : Google Scholar
|
|
9
|
Srivastava RK, Tang SN, Zhu W, Meeker D
and Shankar S: Sulforaphane synergizes with quercetin to inhibit
self-renewal capacity of pancreatic cancer stem cells. Front
Biosci. 3:515–528. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beachy PA, Karhadkar SS and Berman DM:
Tissue repair and stem cell renewal in carcinogenesis. Nature.
432:324–331. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ruiz i Altaba A, Mas C and Stecca B: The
Gli code: an information nexus regulating cell fate, stemness and
cancer. Trends Cell Biol. 17:438–447. 2007.PubMed/NCBI
|
|
12
|
Ruiz i Altaba A: Therapeutic inhibition of
Hedgehog-GLI signaling in cancer: epithelial, stromal, or stem cell
targets? Cancer Cell. 14:281–283. 2008.PubMed/NCBI
|
|
13
|
Bailey JM, Singh PK and Hollingsworth MA:
Cancer metastasis facilitated by developmental pathways: Sonic
hedgehog, Notch, and bone morphogenic proteins. J Cell Biochem.
102:829–839. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang Y, Tian X, Xie X, Zhuang Y, Wu W and
Wang W: Expression and regulation of hedgehog signaling pathway in
pancreatic cancer. Langenbecks Arch Surg. 395:515–525. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Olive KP, Jacobetz MA, Davidson CJ, et al:
Inhibition of Hedgehog signaling enhances delivery of chemotherapy
in a mouse model of pancreatic cancer. Science. 324:1457–1461.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee CJ, Dosch J and Simeone DM: Pancreatic
cancer stem cells. J Clin Oncol. 26:2806–2812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang SN, Fu J, Nall D, Rodova M, Shankar S
and Srivastava RK: Inhibition of sonic hedgehog pathway and
pluripotency maintaining factors regulate human pancreatic cancer
stem cell characteristics. Int J Cancer. 131:30–40. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yao J, An Y, Wie JS, et al: Cyclopamine
reverts acquired chemoresistance and down-regulates cancer stem
cell markers in pancreatic cancer cell lines. Swiss Med Wkly.
141:w132082011.PubMed/NCBI
|
|
19
|
Strand MF, Wilson SR, Dembinski JL, et al:
A novel synthetic smoothened antagonist transiently inhibits
pancreatic adenocarcinoma xenografts in a mouse model. PLoS One.
6:e199042011. View Article : Google Scholar
|
|
20
|
Nüsslein-Volhard C and Wieschaus E:
Mutations affecting segment number and polarity in
Drosophila. Nature. 287:795–801. 1980.PubMed/NCBI
|
|
21
|
Hooper JE and Scott MP: Communicating with
Hedgehogs. Nat Rev Mol Cell Biol. 6:306–317. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kalderon D: Transducing the hedgehog
signal. Cell. 103:371–374. 2000. View Article : Google Scholar
|
|
23
|
Kayed H, Kleeff J, Osman T, Keleg S,
Büchler MW and Friess H: Hedgehog signaling in the normal and
diseased pancreas. Pancreas. 32:119–129. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Berman DM, Karhadkar SS, Maitra A, et al:
Widespread requirement for Hedgehog ligand stimulation in growth of
digestive tract tumors. Nature. 425:846–851. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kayed H, Kleeff J, Keleg S, et al: Indian
hedgehog signaling pathway: expression and regulation in pancreatic
cancer. Int J Cancer. 110:668–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thayer SP, di Magliano MP, Heiser PW, et
al: Hedgehog is an early and late mediator of pancreatic cancer
tumorigenesis. Nature. 425:851–856. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao J, Li Z, Chen Z, et al: Antisense Smo
under the control of the PTCH1 promoter delivered by an adenoviral
vector inhibits the growth of human pancreatic cancer. Gene Ther.
13:1587–1594. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu XF, Guo CY, Liu J, et al: Gli1
maintains cell survival by up-regulating IGFBP6 and Bcl-2 through
promoter regions in parallel manner in pancreatic cancer cells. J
Carcinog. 8:132009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoo YA, Kang MH, Lee HJ, et al: Sonic
hedgehog pathway promotes metastasis and lymphangiogenesis via
activation of Akt, EMT, and MMP-9 pathway in gastric cancer. Cancer
Res. 71:7061–7070. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Buonamici S, Williams J, Morrissey M, et
al: Interfering with resistance to smoothened antagonists by
inhibition of the PI3K pathway in medulloblastoma. Sci Transl Med.
2:51ra702010.PubMed/NCBI
|
|
31
|
Chen X, Lingala S, Khoobyari S, Nolta J,
Zern MA and Wu J: Epithelial mesenchymal transition and hedgehog
signaling activation are associated with chemoresistance and
invasion of hepatoma subpopulations. J Hepatol. 55:838–845. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
33
|
Di Fiore R, Santulli A, Ferrante RD, et
al: Identification and expansion of human osteosarcoma-cancer-stem
cells by long-term 3-aminobenzamide treatment. J Cell Physiol.
219:301–313. 2009.PubMed/NCBI
|
|
34
|
Tirino V, Desiderio V, d’Aquino R, et al:
Detection and characterization of CD133+ cancer stem
cells in human solid tumours. PLoS One. 3:e34692008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mahller YY, Williams JP, Baird WH, et al:
Neuroblastoma cell lines contain pluripotent tumor initiating cells
that are susceptible to a targeted oncolytic virus. PLoS One.
4:e42352009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pode-Shakked N, Metsuyanim S, Rom-Gross E,
et al: Developmental tumourigenesis: NCAM as a putative marker for
the malignant renal stem/progenitor cell population. J Cell Mol
Med. 13:1792–1808. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Walter D, Satheesha S, Albrecht P, et al:
CD133 positive embryonal rhabdomyosarcoma stem-like cell population
is enriched in rhabdospheres. PLoS One. 6:e195062011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Han YG, Spassky N, Romaguera-Ros M,
Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S and
Alvarez-Buylla A: Hedgehog signaling and primary cilia are required
for the formation of adult neural stem cells. Nat Neurosci.
11:277–284. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Singh BN, Fu J, Srivastava RK and Shankar
S: Hedgehog signaling antagonist GDC-0449 (Vismodegib) inhibits
pancreatic cancer stem cell characteristics: molecular mechanisms.
PLoS One. 6:e273062011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hermann PC, Huber SL, Herrler T, Aicher A,
Ellwart JW and Guba M: Distinct populations of cancer stem cells
determine tumor growth and metastatic activity in human pancreatic
cancer. Cell Stem Cell. 1:313–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dembinski JL and Krauss S:
Characterization and functional analysis of a slow cycling stem
cell-like subpopulation in pancreas adenocarcinoma. Clin Exp
Metastasis. 26:611–623. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Feldmann G, Dhara S, Fendrich V, et al:
Blockade of hedgehog signaling inhibits pancreatic cancer invasion
and metastases: a new paradigm for combination therapy in solid
cancers. Cancer Res. 67:2187–2196. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Maitah MY, Ali S, Ahmad A, Gadgeel S and
Sarkar FH: Up-regulation of sonic hedgehog contributes to
TGF-β1-induced epithelial to mesenchymal transition in NSCLC cells.
PLoS One. 6:e160682011.PubMed/NCBI
|
|
44
|
Liao X, Siu MK, Au CW, et al: Aberrant
activation of hedgehog signaling pathway in ovarian cancers: effect
on prognosis, cell invasion and differentiation. Carcinogenesis.
30:131–140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Olmeda D, Jordá M, Peinado H, Fabra A and
Cano A: Snail silencing effectively suppresses tumour growth and
invasiveness. Oncogene. 26:1862–1874. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: an alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|