Introduction
Liver cancer is the third most common cause of death
from cancer in the world. Hepatocellular carcinoma (HCC) is the
most common type of liver cancer and accounts for 90% of primary
liver cancer cases in China. The 5-year survival rate of liver
cancer patients is low and is accompanied by a high recurrence rate
after resection (1,2). One major reason for disease recurrence
originates from the metastasis of the primary tumor, tumor invasion
and growth at a new site. The metastatic potential in recurrent
tumors is determined in the original primary tumors (3,4). Thus,
the potential for metastasis, invasion and growth could be used as
parameters for judging the degree of tumor malignancy.
Migratory cancer cells display abnormal cell-cell
and cell-matrix adhesion and an extensive remodeling of their actin
cytoskeleton (5). The actin
cytoskeleton is critical in triggering cell migration by actin
network assembly and disruption (6). Tumor metastasis is attributed to a
synergy of abnormal expression of genes in the primary tumor. The
elements participating in actin network formation or regulating
actin dynamics are associated with the metastatic potential of
cancer cells. Coronin-1C, a protein with F-actin binding ability,
was found to be overexpressed in cultured HCC cell lines with high
metastatic potential when compared with cell lines with low
potential (7).
Coronin was first identified in extracts of cells
from Dictyostelium discoideum exhibiting an accumulation at
the crown-like structures on the dorsal surface of these cells
(8). Further studies on Coronin
were carried out in eukaryotic organisms, and this protein as
identified in mammalian animals was subdivided into three classes,
type I, type II and type III. Although the expression of certain
Coronin family members are tissue-specific, Coronin-1B and
Coronin-1C, the two type I homologues, are ubiquitously expressed
at high levels in most tissues with relatively lower levels for
Coronin-1C (9) which localizes to
the leading edge of cells (10).
Most of the important insights into the functions of type I Coronin
come from studies on Coronin-1A and Coronin-1B (11). Similar to other type I homologues,
Coronin-1C binds to F-actin and interacts with the Arp2/3 complex
(12). Moreover, the expression of
Coronin-1C correlates with the degree of malignancy in human
diffuse gliomas, and knockdown of Coronin-1C in glioblastoma cells
was found to retard cell proliferation, motility and invasion
(13). However, the functions of
Coronin-1C in tumor cells have not been fully understood.
The Rho family of small GTPases are involved in cell
motility by regulating cytoskeleton dynamics and cell adhesion
(14,15). In HCC, Rho family small GTPases are
implicated in tumorigenesis and metastasis (16). The migration and invasion of HCC are
suppressed by reducing Rac1 expression (17). Yet, the relationship between
Coronin-1C and Rac1 levels regarding tumor metastasis and growth
remains unknown. In this study, we aimed to ascertain whether
Coronin-1C expression levels in HCC are correlated with cell
proliferation and motility by employing Coronin-1C-overexpressing
and knockdown cell lines. We found that HCC cells with a lower
Coronin-1C level displayed less motility, invasion and
proliferation in vitro and less tumorigenetic potential
in vivo in a nude mouse model. The decreased Coronin-1C
expression was correlated with reduced Rac-1 activation.
Materials and methods
Clinical validation
Human HCC tissue and their corresponding adjacent
non-tumorous specimens from 25 patients including 14 males and 11
females, mean age 60±5 years, were obtained from The Second
Affiliated Hospital of Jilin University, Changchun, China. All
tumor tissues were subjected to analyses of the relative mRNA and
protein levels of Coronin-1C, as well as to histological staining
of Coronin-1C on sections.
Cell culture, plasmid construction,
transient transfection and establishment of the stable knockdown
cell line
Human HCC cell line BEL-7402 (18) was obtained from The Cell Bank of the
Chinese Academy of Sciences, Shanghai, China. The cells were grown
in RPMI-1640 medium containing 10% fetal bovine serum (FBS) at 37°C
in 5% CO2. For the transient transfection, an expression
plasmid containing full-length human Coronin-1C (aa 1-474;
NM_014325) was generated as described (10). Then, 2×105 cells were
seeded into a 35-mm plate for 24 h to reach ~75% confluence.
Plasmids (2 μg) were transfected using Lipofectamine 2000
transfection reagent (Invitrogen) according to the manufacturer’s
protocol. The cells with transiently overexpressed Coronin-1C were
available for further experiments after an additional 48-h culture
after transfection. For the establishment of the stable knockdown
cell line, small hairpin RNAs (shRNAs) were used to specifically
reduce the Coronin-1C expression in BEL-7402 cells. The shRNA oligo
5′-CGTCCACTACCTCAACACA TT-3′ was cloned into pLKO.1-puro. Then the
shRNA plasmids were co-transfected into 293TN cells with three
lentiviral packaging plasmids, pVSV-G, pPACKH1-GAG and pPACKH1-REV.
The resulting lentiviruses were collected and used to infect the
target BEL-7402 cells. The cells were spread in 100-mm diameter
plates 24 h after infection for a subsequent 7-day selection in
RPMI-1640 medium containing 0.75 μg/ml puromycin. Cells infected
with the pLKO.1-puro empty vector were regarded as the control
transfectants. Transient Coronin-1C overexpression and stable
knockdown of Coronin-1C expression were confirmed by western blot
analysis with an anti-Coronin-1C antibody (Proteintech Group,
Chicago, IL).
Cell viability assay
Cells (5×104 cells/well) were seeded in
24-well plates. The cell viability was determined at 12 and 48 h
after seeding using an MTT assay. Briefly, cells were washed with
PBS and replaced with 0.5 mg/ml MTT in serum-free medium for a
further 3-h incubation at 37°C. Then the resulting formazan was
extracted with isopropanol for a colorimetric measurement using a
spectrophotometer (NanoDrop, Rockland, DE, USA) at 570 nm with the
correction of interference at 690 nm. For each group, four
individual samples were collected and measured. The cell viability
was indicated as arbitrary units of absorbance at 570 nm.
Immunohistochemical staining assay
Tissues were fixed with 4% paraformaldehyde,
embedded in paraffin and sectioned. The sections were stained with
rabbit anti-Coronin-1C antibody (1:100, Proteintech Group) at 4°C
overnight, and the secondary antibody for 2 h at room temperature.
The sections were finally visualized using a DAB substrate
chromogen system (DakoCytomation, Denmark) according to the
manufacturer’s instructions.
Western blot analysis
Cells were washed in PBS and lysed in RIPA buffer
(20 mM Tris, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% Na
deoxycholate, 2 mM EGTA, 2 mM EDTA, 0.1% SDS) containing protease
inhibitor cocktail (1:100; Sigma) and phosphatase inhibitor
cocktails 1 and 2 (both at 1:200; Sigma). Lysates were centrifuged
at 14,000 × g for 10 min at 4°C and the total protein
concentrations were equalized with BCA protein quantification
assay. Then equal total protein (30 μg) for each sample was loaded
onto SDS-PAGE gel and blotted with different primary antibodies
(1:500, rabbit anti-Rac-1 antibody; Santa Cruz Biotechnology, Santa
Cruz, CA; 1:1000, mouse anti-GAPDH;, Santa Cruz Biotechnology;
1:500, rabbit anti Coronin-1C, Proteintech Group) at 4°C overnight
and the corresponding secondary antibodies. Finally, the results
were visualized with enhanced chemiluminescence (ECL).
RNA extraction and semi-quantitative
RT-PCR
Total RNA was extracted from the cells or tissues
with the RNeasy Mini kit (Qiagen, Valencia, CA) following the
manufacturer’s instructions. Reverse transcription into cDNA was
carried out using 500 ng of total RNA using the SuperScript III
First-Strand Synthesis System for RT-PCR (Invitrogen). PCR was
carried out with the following primer pairs and Power SYBR-Green
Master kit according to the manufacturer’s protocol: GAPDH
(forward, 5′-AAG GTG AAG GTC GGA GTC AAC-3′; reverse, 5′-GGG GTC
ATT GAT GGC AAC AAT A-3′); Coronin-1C (forward, 5′-GCA GAA GAG TGG
TTC GAA GG-3′; reverse, 5′-TGA TCA GGT CGC ACT TCT TG-3′).
Wound healing assay
BEL-7402 cells (2×105) were seeded into
35-mm dishes and grown in RPMI-1640 medium supplemented with 1% FBS
overnight to form a confluent monolayer. A wound was produced on
the following day by scraping the cells with a pipette tip. The
cells were allowed to recover for 15 min and replenished with 10%
FBS to drive cell migration.
Transwell migration assay
Cell invasion assay was performed with self-coated
Matrigel (BD Biosciences, Sparks, MD) on the upper surface of a
Transwell chamber. The cells that had invaded through the
extracellular matrix layer to the lower surface of the membrane
were fixed with methanol and stained with DAPI. Images of three
randomly selected fields of the fixed cells were captured, and the
cells were counted. Experiments were repeated independently three
times.
Co-staining of Golgi apparatus and
α-actin
The Golgi apparatus reorientation assay was
performed as previously described (19). Briefly, wounds were created on a
monolayer of BEL-7402 cells seeded on coverslips. Cells were then
allowed to migrate for 5 h and stained with an anti-α-actin
antibody (Clone 5C5; Santa Cruz Biotechnology) and a corresponding
secondary antibody, 10 g/ml lectin HPA Alexa Fluor 488 conjugates
(Invitrogen) and DAPI to indicate the positions of actin, Golgi
apparatus and the nucleus, respectively. The migrating cells at the
wound edge were determined to be positive when the Golgi apparatus
was located in front of the nucleus in the direction towards the
wound. At least 300 cells were counted in randomly chosen fields
under a fluorescence microscope. The experiments were repeated
independently three times.
Actin staining with FITC-conjugated
phalloidin
Cells were seeded on 22×22 mm coverslips and then
fixed in 3% paraformaldehyde. The cells were then stained with
FITC-conjugated phalloidin (Sigma) according to the manufacturer’s
instructions. Finally, images were acquired with a Zeiss LSM 510
Meta confocal microscope.
Rac1 GST-PAK pull-down assay
A glutathione-S-trans-ferase (GST)-PAK-CD (PAK-CRIB
domain) fusion protein, containing the Rac1 binding region from
human PAK1B, was used to determine Rac1 activity as previously
described (20). Escherichia
coli transformed with the GST-PAK-CD construct were grown at
37°C to an absorbance of 0.3. Expression of recombinant protein was
induced by culturing with 0.1 mmol/l isopropylthiogalactoside for 2
h. Cells were homogenized, resuspended in lysis buffer [Tris-HCl 50
mmol/l (pH 7.4), NaCl 100 mmol/l, MgCl2 2 mmol/l,
benzamidine 1 mmol/l, NP-40 1%, glycerol 10%, leupeptin, pepstatin
and aprotinin 1 μg/ml, respectively] and centrifuged at 21,000 rpm
for 5 min 4°C. Equal amounts of supernatant protein were incubated
with the GST-PAK-CD fusion protein bound to glutathione-coupled
Sepharose beads at 4°C for 30 min. Beads were washed three times
with lysis buffer, eluted in loading buffer [60 mmol/l Tris (pH
6.8), 2% sodium dodecylsulfate, 10% glycerol, 0.1% bromphenol
blue], and analyzed for bound Rac1 by western blotting.
Tumor assay in nude mice
The nude mice were purchased from Shanghai SLAC
Laboratory Animal Co., Ltd., and maintained under SPF environmental
conditions. All experimental protocols were approved by the Animal
Care and Use Committee of Jilin University. In tumor xenograft
experiments, shCoronin-1C cells and control transfectants were
subcutaneously injected into nude mice. The tumor size was measured
once every week until the mice were sacrificed 5 weeks after
injection. Then each primary tumor was weighed.
Statistical analysis
Data from at least three independent experiments
were analyzed with the two-tailed Student’s t-test and are
represented as the means ± SD. ‘n’ means the sample size, namely
the number of the nude mice that received the subcutaneous cell
injection. P-values ≥0.05 were considered to indicate a
statistically significant difference.
Results
Coronin-1C is increased in hepatocellular
carcinoma tissues
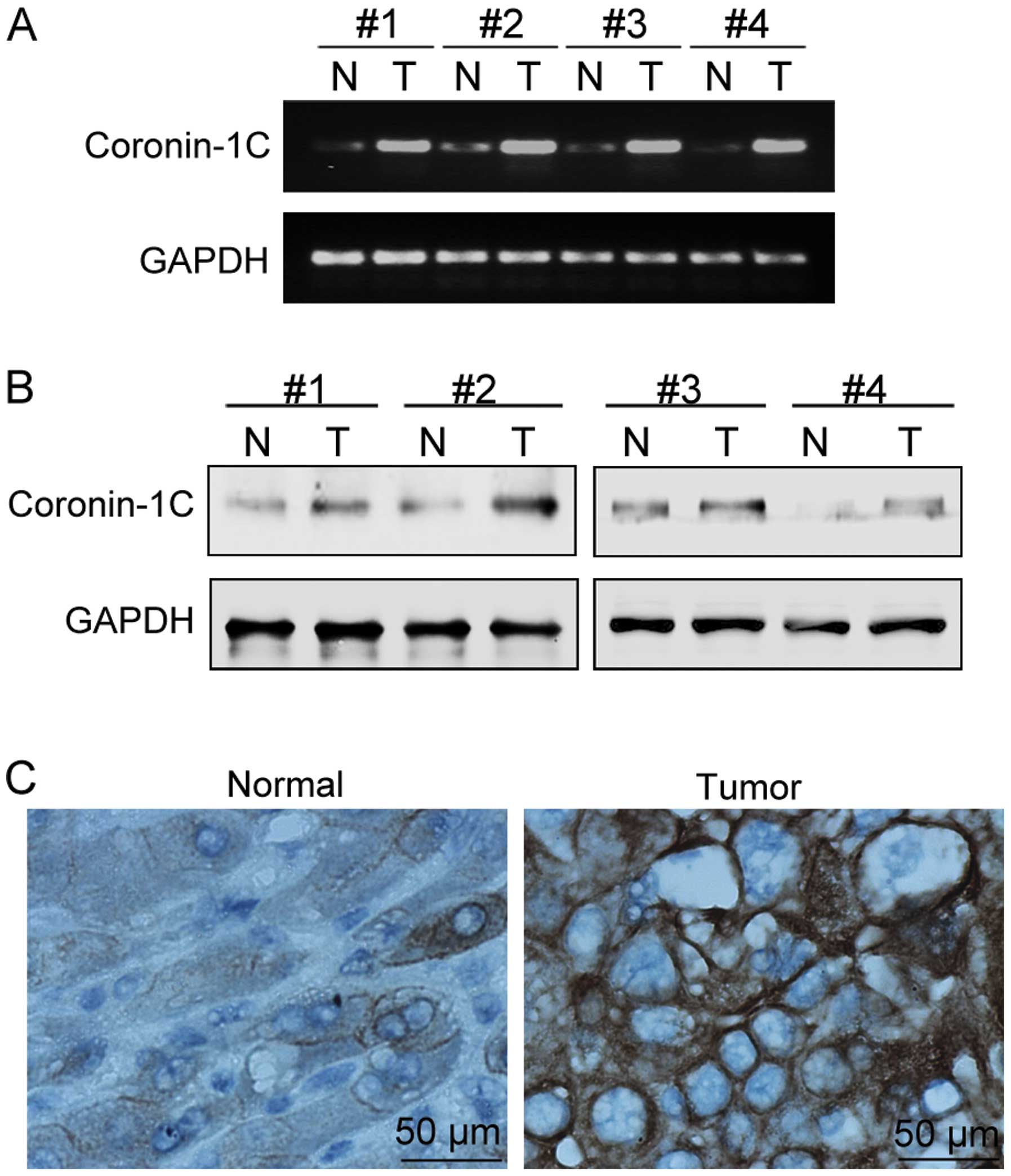
The Coronin-1C mRNA levels (via semi-quantitative
RT-PCR) and protein levels (via both western blot analysis and
immunohistological staining of Coronin-1C) were detected in paired
specimens of human HCC tissues and their corresponding non-tumorous
adjacent tissues from 25 patients (14 male and 11 female, mean age
60±5 years). Coronin-1C was found to be overexpressed at the mRNA
and protein levels in 22 (13 males and 9 females) and 20 (12 males
and 8 females) HCC cases, respectively. The representative results
from 4 patients are shown in Fig. 1A
and B. In the immunohistological assay with the anti-Coronin-1C
antibody, highly positive Coronin-1C staining was noted in the
tumor tissues but not in the adjacent non-tumorous tissues
(Fig. 1C). Furthermore, patient
gender was not associated with the tendency of increased Coronin-1C
in HCC.
Coronin-1C level is related with the
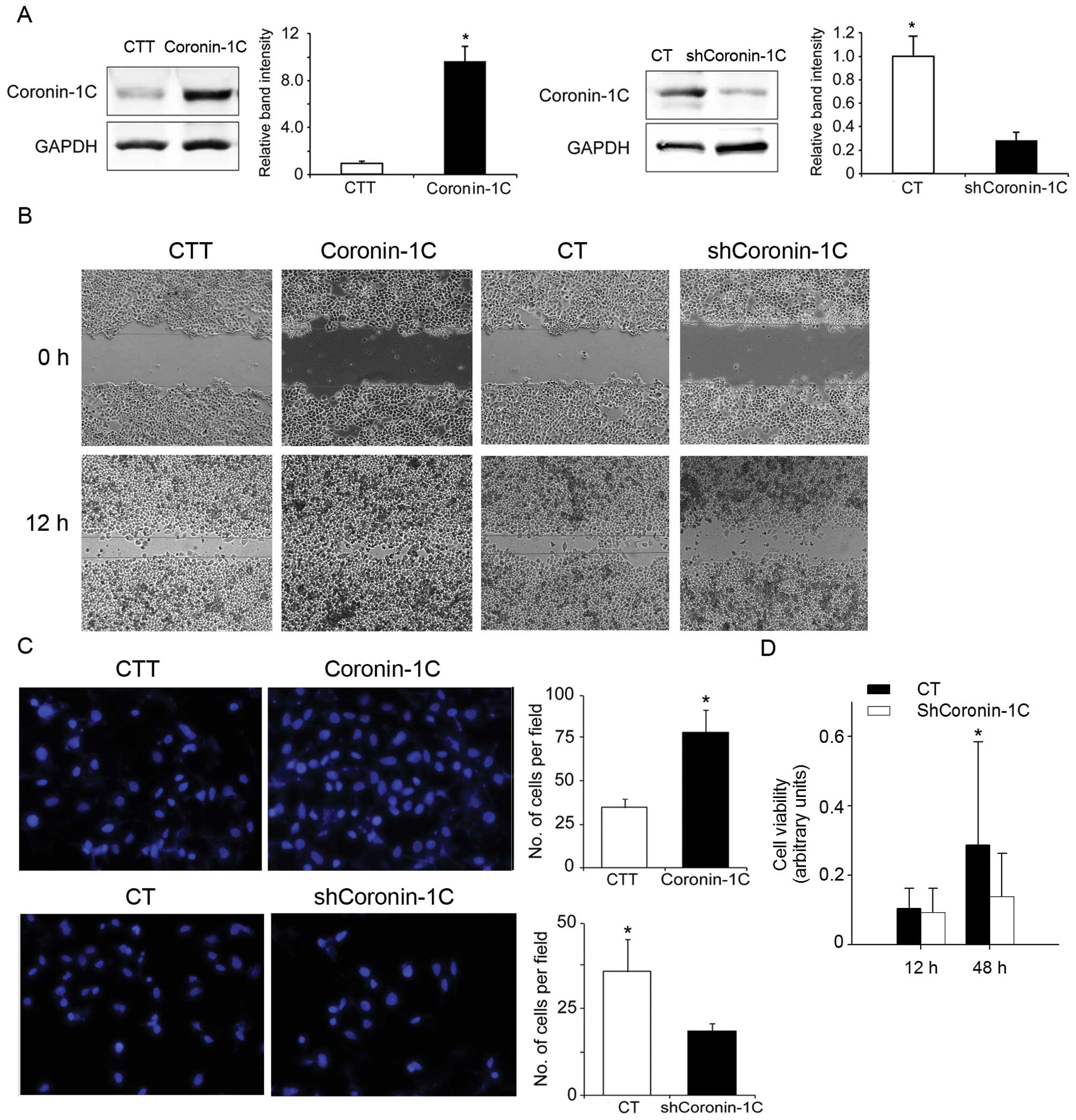
degree of malignancy of BEL-7402 cells
BEL-7402 is a cell line derived from human HCC
(18). In order to observe the
effects of Coronin-1C on BEL-7402 cell behavior related to tumor
malignancy, including cell migration, invasion and proliferation,
we transiently transfected BEL-7402 cells with transfecting
plasmids containing full-length human Coronin-1C causing
overexpression in the BEL-7402 cells. The cells transfected with
the vector plasmids were taken as control transfectants for
transient overexpression (CTT). A BEL-7402 cell line with stable
knockdown of Coronin-1C (shCoronin-1C), as well as its
corresponding control transfectant (CT), was also employed. All
transfectants were submitted to western blot analysis to detect the
Coronin-1C levels. Compared with the corresponding control
transfectant, Coronin-1C-overexpressing BEL-7402 cells and
shCoronin-1C cells displayed a 9-fold increase and a 60% decrease
in Coronin-1C levels, respectively (Fig. 2A). For the wound healing assay, the
cells were seeded and after 24 h were subjected to a scratch-wound.
At 18 h after wounding, the cells were observed and evaluated for
their cellular ability to close the scratched area. In the
Coronin-1C-overexpressing cells, the scratched area was occupied by
migrating cells to a 60–80% confluence. The wound of the control
CTT cells remained unmerged but showed a reduced area between the
two wound edges. However, the wound area in the shCoronin-1C cells
was larger than the area in the corresponding control cells (CT).
This indicates a slower cell migration in the shCoronin-1C cells
(Fig. 2B).
Similar to the results of the scratch healing assay,
the cells overexpressing Coronin-1C displayed rapid invasion
through the extracellular matrix layer to the lower surface of the
membrane in the Transwell migration assay. The slowest invasion was
observed in the shCoronin-1C cells (Fig. 2C). However, in the cell viability
assay, lower Coronin-1C expression correlated with a lower cell
proliferation rate in the shCoronin-1C cells (Fig. 2D). These data clearly indicate that
Coronin-1C enhances cell motility and cell proliferation in HCC
cells.
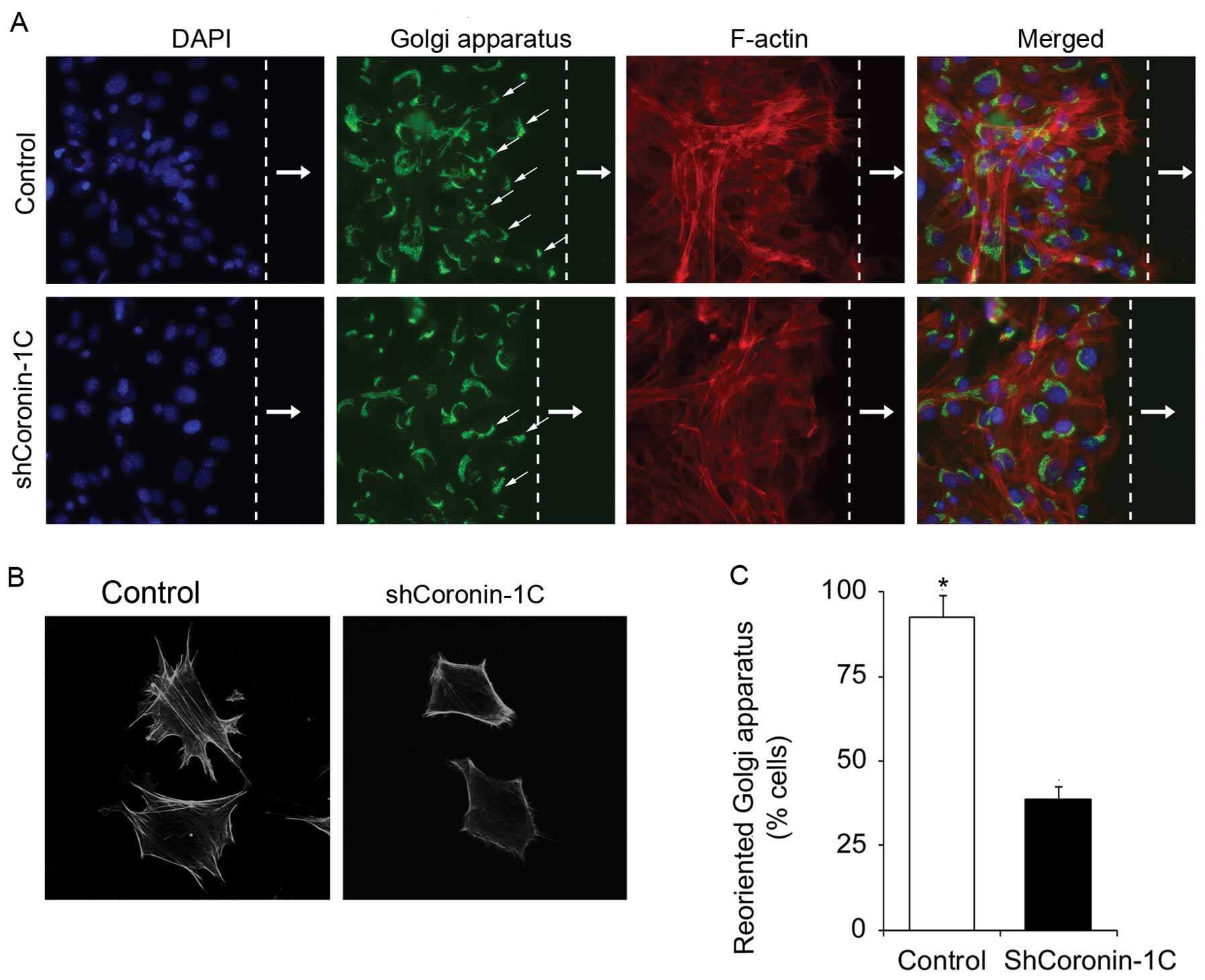
Knockdown of Coronin-1C reduces cell
polarity and disrupts the cytoskeleton
To ascertain whether Coronin-1C affects cell
polarity, we wounded the adherent shCoronin-1C and control
monolayer cells and observed the cellular morphology and the
positions of the Golgi apparatus in the cells at the scratch edge.
Compared with the control cells, the shCoronin-1C cells displayed a
reduced stress fiber network (Fig.
3A), consistent with changes in the cell morphology showing
less lamellipodial extensions (Fig.
3B). The percentage of cells exhibiting Golgi apparatus
realignment, located in front of the nucleus towards the direction
of the scratch edge, was determined. Decreased Golgi apparatus
realignment was found in the shCoronin-1C cells but not in the
control transfected cells (Fig.
3C). These observations suggest that Coronin-1C participates in
cytoskeleton formation or stability which contributes to
enhancement of cell motility.
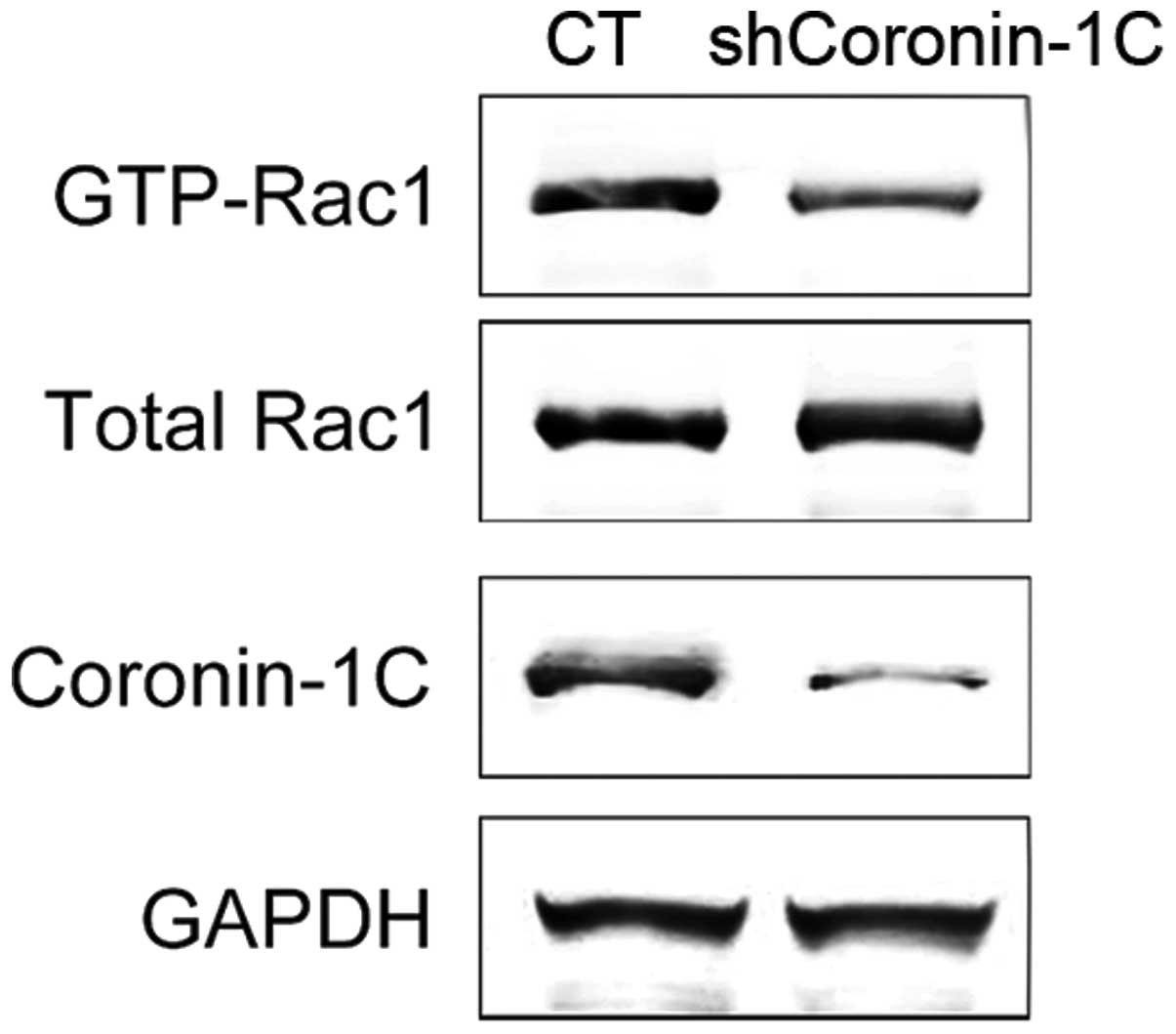
Attenuated Rac-1 activation in
Coronin-1C-knockdown cells
Coronin-1A was reported to promote Rac-1
translocation and activation (21).
Rac-1 activation was associated with the migration and tumor
metastasis in HCC (22). In
shCoronin-1C cells, the level of activated Rac-1, by means of GTP
bound Rac-1, was reduced. Yet, the total Rac-1 protein level was
not affected by diminished Coronin-1C (Fig. 4). Thus, Coronin-1C overexpression in
HCC cells enhanced Rac-1 activation by triggering the interaction
between GTP and Rac-1 but not by altering the Rac-1 protein
expression.
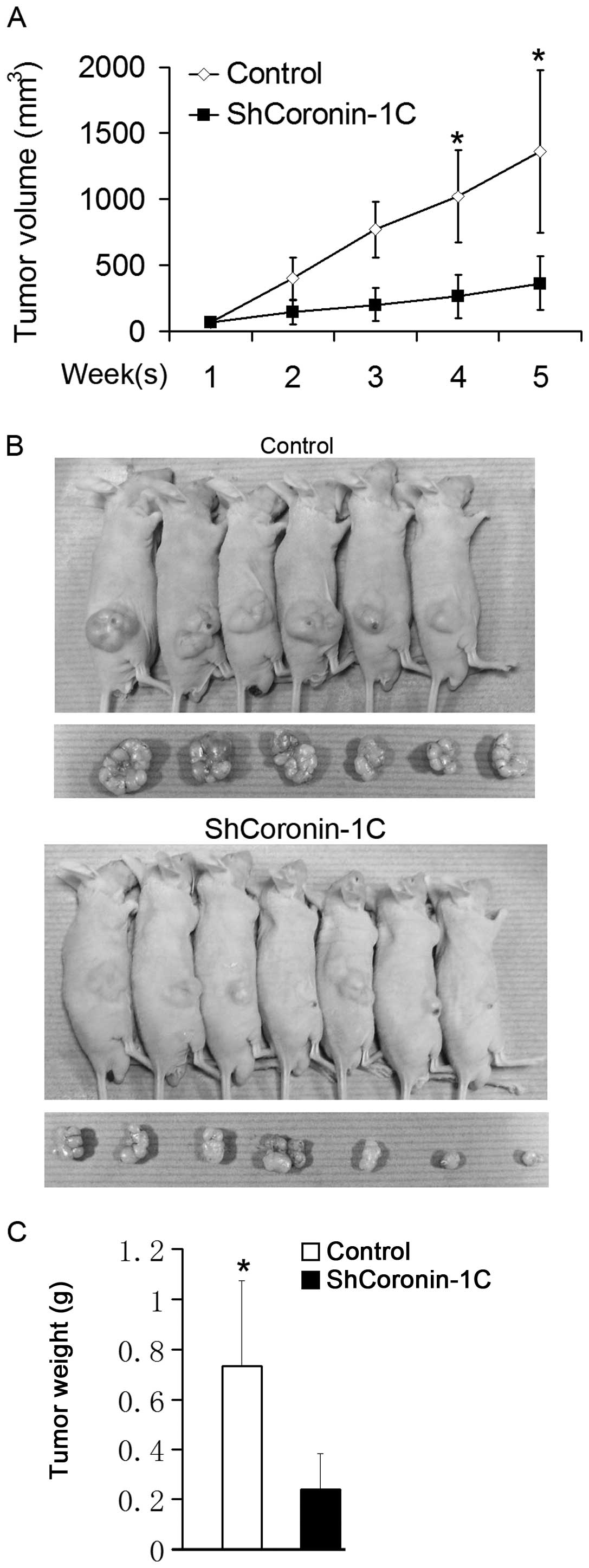
Tumorigenic potential in
Coronin-1C-knockdown BEL-7402 cells
The attenuated malignant potential of BEL-7402 cells
by Coronin-1C knockdown was also confirmed in vivo.
shCoronin-1C and control transfected cells were subcutaneously
injected into nude mice. The xenograft tumor volumes in nude mice
were measured once a week, and the mice were sacrificed for tumor
weighing 5 weeks afterwards. The xenograft tumor growth curve is
shown in Fig. 5A. Larger tumor
volume were observed in mice injected with the control-transfected
cells even one week after the injection. Differences in tumor
extension between the shCoronin-1C and control mice, including
tumor volume and weight measured after sacrifice, were noted five
weeks after the injection (Fig. 5B and
C). These results indicate that Coronin-1C silencing reduces
the rapid tumor growth in vivo.
Discussion
Hepatocellular carcinoma (HCC) is the third most
common cause of cancer-related mortality in the world (23). Most of the HCC cases occur in Asia
and sub-Saharan Africa (24). The
hospital mortality rate of hepatectomy for HCC has improved in past
20 years; however, the long-term survival rate remains
unsatisfactory (2). Metastasis is a
major cause of high mortality in HCC patients after surgical
resection. More and more biomarkers to determine the degree of
tumor malignancy and metastasis have been identified to cope with
this challenge (25). Coronin-1C
was considered to be a biomarker for HCC cells with high metastatic
potential. We found Coronin-1C protein levels were increased in 20
of the 25 HCC patient specimens suggesting that an increase in
Coronin-1C tumors may be a general biomarker for HCC occurrence.
Although the functions for Coronin-1C are not fully understood,
studies of other type I Coronin homologues have indicated that this
protein is able to bind F-actin and acts as an inhibitor for the
Arp2/3 complex (10,12). Overexpression of Coronin-1B mutants
causing enhanced Arp2/3 interaction led to increased cell motility,
whereas overexpression of mutants containing less interactional
affinity suppressed cell motility. Coronin-Arp2/3 interaction was
found to be regulated by Coronin phosphorylation (26). By cooperating with Arp2/3 activator
WASP or its more widely expressed homologue N-WASP, type I Coronins
regulate Arp2/3 nucleation tightly and precisely (27–29).
These results have demonstrated that the regulative functions of
type I Colonin proteins on cell motility depend on modulating the
Arp2/3 complex.
During cell migration, a cell first extends its
protrusions such as lamellipodia and filopodia to the front, forms
adhesion at the leading edge and finally retracts its trail at the
trailing edge. This process results from the treadmilling of actin
filaments based on F-actin dynamics: F-actin assembly followed by
F-actin extension and degradation (6). The proteins regulating the actin
filament polymerization and depolymerization control the force on
protrusions. One hypothesis suggests that Coronin is a coordinating
factor between Arp2/3-based actin assembly and Cofilin-mediated
disassembly (28,30). Accumulation of Coronin 1A was
associated with more rapid F-actin dynamics (31). As an inhibitor to Arp2/3 activation,
Coronin replaces Arp2/3 at the actin branch, facilitates actin
debranching or creates more flexible actin branches which
consequentially guarantees the recycling of actin monomers in the
middle and rear of the lamellipodium and supports the actin
assembly at the front (32). More
flexible actin branches may contribute to the genesis of stress
fiber network. These previous studies offer relevant explanations
for the reduced stress fiber network noted in Coronin-1C-depleted
cells.
Rac-1 is a Rho family small GTPase participating in
many cell events including cell growth, cytoskeletal
reorganization, cell migration and invasion (33). A negative microRNA for RAC1 was
found to diminish Rac-1 expression and suppress migration and
invasion of HCC cells (17). This
may explain why the proliferation rate was reduced in shCoronin-1C
cells which have less activated Rac-1. The activation of Rac-1
depends on its translocation from the cytosol to the membrane and
release from its inhibitor RhoGDI (34). The manner by which Coronin-1C
modulates Rac-1 activation might be similar to its homologue
Coronin-1A, which facilitates Rac-1 activation by promoting Rac-1
membrane translocation and dissociation from RhoGDIα via a
cytoskeleton-based feedback loop (21). What is more, another possible effect
of Coronin-1C on modulating cell motility is its affect on Arp2/3
activator WASP or N-WASP. It is not yet clear whether there are
direct interactions between WASP/N-WASP and Rho family small
GTPases or not. Yet, WASP and N-WASP were reported to be activated
by Rho family small GTPase Cdc 42 and Rac-1. Rac-1 displayed a more
potent affect for activating N-WASP (35).
In this study, we found that Coronin-1C was
overexpressed in hepatocellular carcinoma tissues. HCC cells
displayed enhanced cell migration, invasion and proliferation which
were impaired in Coronin-1C-deficient cells with a coordinated
attenuation in Rac-1 activation. The upregulated Coronin-1C in
tumor cells enhanced the metastasis by promoting a more rapid actin
dynamics which was mediated by Arp2/3 complex inhibition and the
events relating to Rac-1 activation. Moreover, overexpression of
Coronin-1C triggered a more rapid proliferation rate in HCC cells.
All these changes endow HCC cells with high tumorigenic potential.
Thereby, Coronin-1C may be used as a diagnostic marker for tumor
malignancy and a target for tumor therapy in the future.
Acknowledgements
This study was supported by a grant from the Jilin
Provincial Science and Technology Department, no. 200705199.
References
|
1
|
Jemal A, Thomas A, Murray T and Thun M:
Cancer statistics, 2002. CA Cancer J Clin. 52:23–47. 2002.
View Article : Google Scholar
|
|
2
|
Fan ST, Mau Lo C, Poon RT, et al:
Continuous improvement of survival outcomes of resection of
hepatocellular carcinoma: a 20-year experience. Ann Surg.
253:745–758. 2011.PubMed/NCBI
|
|
3
|
Tang ZY, Ye SL, Liu YK, et al: A decade’s
studies on metastasis of hepatocellular carcinoma. J Cancer Res
Clin Oncol. 130:187–196. 2004.
|
|
4
|
Tang Z, Zhou X, Lin Z, et al: Surgical
treatment of hepatocellular carcinoma and related basic research
with special reference to recurrence and metastasis. Chin Med J.
112:887–891. 1999.PubMed/NCBI
|
|
5
|
Yilmaz M and Christofori G: Mechanisms of
motility in metastasizing cells. Mol Cancer Res. 8:629–642. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bugyi B and Carlier MF: Control of actin
filament treadmilling in cell motility. Annu Rev Biophys.
39:449–470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu L, Peng CW, Hou JX, et al: Coronin-1C
is a novel biomarker for hepatocellular carcinoma invasive
progression identified by proteomics analysis and clinical
validation. J Exp Clin Cancer Res. 29:172010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Hostos EL, Bradtke B, Lottspeich F,
Guggenheim R and Gerisch G: Coronin, an actin binding protein of
Dictyostelium discoideum localized to cell surface
projections, has sequence similarities to G protein beta subunits.
EMBO J. 10:4097–4104. 1991.
|
|
9
|
Uetrecht AC and Bear JE: Coronins: the
return of the crown. Trends Cell Biol. 16:421–426. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spoerl Z, Stumpf M, Noegel AA and Hasse A:
Oligomerization, F-actin interaction, and membrane association of
the ubiquitous mammalian coronin 3 are mediated by its carboxyl
terminus. J Biol Chem. 277:48858–48867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chan KT, Creed SJ and Bear JE: Unraveling
the enigma: progress towards understanding the coronin family of
actin regulators. Trends Cell Biol. 21:481–488. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rosentreter A, Hofmann A, Xavier CP,
Stumpf M, Noegel AA and Clemen CS: Coronin 3 involvement in
F-actin-dependent processes at the cell cortex. Exp Cell Res.
313:878–895. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thal D, Xavier CP, Rosentreter A, et al:
Expression of coronin-3 (coronin-1C) in diffuse gliomas is related
to malignancy. J Pathol. 214:415–424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Parsons JT, Horwitz AR and Schwartz MA:
Cell adhesion: integrating cytoskeletal dynamics and cellular
tension. Nat Rev Mol Cell Biol. 11:633–643. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Spiering D and Hodgson L: Dynamics of the
Rho-family small GTPases in actin regulation and motility. Cell Adh
Migr. 5:170–180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xue Y, Bi F, Zhang X, et al: Role of Rac1
and Cdc42 in hypoxia induced p53 and von Hippel-Lindau suppression
and HIF1alpha activation. Int J Cancer. 118:2965–2972. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu L, Cai C, Wang X, Liu M, Li X and Tang
H: MicroRNA-142–3p, a new regulator of RAC1, suppresses the
migration and invasion of hepatocellular carcinoma cells. FEBS
Lett. 585:1322–1330. 2011.
|
|
18
|
Chen R, Zhu D, Ye X, Shen D and Lu R:
Establishment of three human liver carcinoma cell lines and some of
their biological characteristics in vitro. Sci Sin. 23:236–247.
1980.PubMed/NCBI
|
|
19
|
Wong CC, Wong CM, Tung EK, Man K and Ng
IO: Rho-kinase 2 is frequently overexpressed in hepatocellular
carcinoma and involved in tumor invasion. Hepatology. 49:1583–1594.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maack C, Kartes T, Kilter H, et al: Oxygen
free radical release in human failing myocardium is associated with
increased activity of rac1-GTPase and represents a target for
statin treatment. Circulation. 108:1567–1574. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Castro-Castro A, Ojeda V, Barreira M, et
al: Coronin 1A promotes a cytoskeletal-based feedback loop that
facilitates Rac1 translocation and activation. EMBO J.
30:3913–3927. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang CY, Lin SC, Su WH, Ho CM and Jou YS:
Somatic LMCD1 mutations promoted cell migration and tumor
metastasis in hepatocellular carcinoma. Oncogene. 31:2640–2652.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
24
|
Sell S: Mouse models to study the
interaction of risk factors for human liver cancer. Cancer Res.
63:7553–7562. 2003.PubMed/NCBI
|
|
25
|
Gonzalez SA and Keeffe EB: Diagnosis of
hepatocellular carcinoma: role of tumor markers and liver biopsy.
Clin Liver Dis. 15:297–306. vii–x. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cai L, Holoweckyj N, Schaller MD and Bear
JE: Phosphorylation of coronin 1B by protein kinase C regulates
interaction with Arp2/3 and cell motility. J Biol Chem.
280:31913–31923. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Machesky LM, Reeves E, Wientjes F, et al:
Mammalian actin-related protein 2/3 complex localizes to regions of
lamellipodial protrusion and is composed of evolutionarily
conserved proteins. Biochem J. 328:105–112. 1997.
|
|
28
|
Cai L, Marshall TW, Uetrecht AC, Schafer
DA and Bear JE: Coronin 1B coordinates Arp2/3 complex and cofilin
activities at the leading edge. Cell. 128:915–929. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yan M, Di Ciano-Oliveira C, Grinstein S
and Trimble WS: Coronin function is required for chemotaxis and
phagocytosis in human neutrophils. J Immunol. 178:5769–5778. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gandhi M, Achard V, Blanchoin L and Goode
BL: Coronin switches roles in actin disassembly depending on the
nucleotide state of actin. Mol Cell. 34:364–374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yokoyama K, Kaji H, He J, et al: Rab27a
negatively regulates phagocytosis by prolongation of the
actin-coating stage around phagosomes. J Biol Chem. 286:5375–5382.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cai L, Makhov AM, Schafer DA and Bear JE:
Coronin 1B antagonizes cortactin and remodels Arp2/3-containing
actin branches in lamellipodia. Cell. 134:828–842. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sawada N, Li Y and Liao JK: Novel aspects
of the roles of Rac1 GTPase in the cardiovascular system. Curr Opin
Pharmacol. 10:116–121. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moissoglu K, Slepchenko BM, Meller N,
Horwitz AF and Schwartz MA: In vivo dynamics of Rac-membrane
interactions. Mol Biol Cell. 17:2770–2779. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tomasevic N, Jia Z, Russell A, et al:
Differential regulation of WASP and N-WASP by Cdc42, Rac1, Nck, and
PI(4,5)P2. Biochemistry. 46:3494–3502. 2007. View Article : Google Scholar : PubMed/NCBI
|