Introduction
T cells provoke the antitumor immune response;
therefore, T cell-mediated immunotherapy is regarded as a crucial
approach in cancer treatment. The adoptive cell transfer (ACT)
combined with lymphodepletion has led to clinical tumor suppression
in 50–70% of patients with metastatic melanoma (1). However, the large number of activated
and expanded T cells required in the laboratory (more than
1×1010) makes ACT a costly and labor-intensive treatment
(2–4).
Owing to the marked effects in the treatment of
metastatic melanoma and the evident impact on cancer regression in
approximately 50% of patients, development of ACT therapy utilizing
autologous tumor-infiltrating lymphocytes (TIL) is gaining momentum
(5,6). In addition, although some ACT-treated
patients achieved long-term survival, the majority experienced
cancer relapse (7). Therefore,
strategies aimed at improving the migration of T cells into tumor
sites are likely to enhance the efficacy of ACT therapy.
Immune cells trafficking to inflammation sites are
usually affected by chemokines. Through chemotaxis, cells that
express appropriate chemokine receptors can migrate along with a
chemokine gradient to specific tissues or infection sites (8). Chemokines also play an important role
in T cell-mediated antitumor immune responses (9–11). A
variety of immunocytes have strong chemotactic effects after being
regulated upon the activation of normal T-cell expressed and
secreted RANTES/CCL5 that chemotactically attracts a huge number of
immunocytes to tumor tissues to exert antitumor efficacy (12–17).
As a result of immune response unable to eradicate
tumor cells, cancer develops. There may be mechanisms significantly
involved in tumor development, which allow tumor cells to escape
immune surveillance from natural killer (NK) and other immune cells
(18–20). NK cells represent distinct subsets
of lymphoid cells with innate immune functions (21). NK cells are derived from the bone
marrow, they circulate in the blood and then become activated by
cytokines or upon encountering target cells expressing ligands for
NK cell receptors (22). NK cells
provide one of the first lines of defense against virus-infected
and tumor cells. In general, no immune receptor gene rearrangement
occurs in them and their cytotoxicity is not MHC-restricted. NK
cells are able to mediate the spontaneous killing of various tumor
cells without prior sensitization. To date, not all NK cell lines
can be established from patients with large granular lymphoma
(23) and, with regard to the in
vitro tumoricidal properties, these NK cell lines show
considerable differences, although some of them have been shown to
maintain cytotoxicity (23,24).
Isolated from peripheral lymphocytes of a
non-Hodgkin’s lymphoma patient in 1992 (25), NK-92 was successfully set up as a
natural anti-IL-2 dependent cell line, with similar functional
characteristics as human NK cells. Research demonstrated that NK-92
cells showed high cytotoxic activity against tumor cell lines from
leukemia (26) and malignant
melanoma (27). Additionally, in
vivo application of NK-92 was tested in immune deficiency
(SCID) mouse models, xenografted with patient-derived leukemia
(T-ALL, AML) (24) or human
malignant melanoma cell lines (27). Tumor burden was reduced or
undetectable and the survival of mice was significantly improved.
NK-92 cells are constantly being developed for adoptive
immunotherapy in cancer treatment and have entered clinical trials
(28).
The most widely-used oncolytic adenoviruses in
cancer therapy are based on adenovirus serotype 5 (Ad5), which
belongs to subgroup C and Coxsackie-adenovirus receptor (CAR) on
target cells are necessary for successful transduction (29,30).
However, expression of CAR is generally low or lost in the process
of the malignant progression of certain tumors, including
hepatocellular carcinoma (HCC) (31–33),
limiting the transduction of Ad5-based vectors in some tumor cells
and resulting in impaired antitumor effects (31). By contrast, CD46, a cellular
receptor for Ad11 (subgroup B) (34), is highly expressed in various types
of malignant tumor cells including HCC cells (35–38).
Therefore, to address the issue of CAR-dependent cell entry, novel
fiber chimeric adenoviral vectors have recently been designed by
switching the knob and shaft of the Ad5 fiber to those of the Ad11
fiber that can recognize the abundant CD46 receptor on tumor cells
(39). Previous studies have
indicated that viral entry and antitumor activity could be greatly
improved by 5/11 fiber chimeric oncolytic adenoviruses compared
with Ad5-based viruses (40,41).
Nevertheless, the tumor-suppressing capacity of 5/11 fiber chimeric
oncolytic adenoviruses in HCC have yet to be fully explored.
Genetic modification of signal pathways promoting
cell growth and survival leads to the emergence of tumor cells,
whose expansion depends on nutrient supply. Oxygen limitation is
crucial for controlling angiogenesis, glucose metabolism, survival
and tumor spread. This effect is orchestrated by hypoxia-inducible
factor (HIF), a main transcriptional factor in nutrient stress
signal. Variations in oxygen tension (pO2)
are not directly sensed by HIF but by a class of
2-oxoglutarate-dependent and iron-dependent dioxygenases which
belong to the family of non-haem oxidizing enzymes. Due to strict
control of pO2 to the activity, these
enzymes are the true oxygen-sensing molecules controlling the
hypoxic response. Two types of oxygen sensors control HIF action;
the first are known as the prolyl hydroxylase domain (PHD)
proteins. PHDs hydroxylate two prolyl residues (P402 and/or P564)
in the human HIF-1α region considered the oxygen-dependent
degradation domain (ODD). This HIF-α modification specifies rapid
interaction with the tumor-suppressor protein von Hippel-Lindau
(VHL), a component of an E3 ubiquitin ligase complex. As a result,
HIF-α subunits are marked with polyubiquitin chains driving them to
destruction by the proteasomal system. ODD is closely related to
the rapid degradation of intracellular proteins (42). Some proteins with ODD display
instability under normoxia, but stability under hypoxia. Hypoxia
exists within most solid tumors (43–46).
Hence, as a regulatory element, the translated ODD can lead to a
concentration of certain proteins at a specific high level.
Therefore, we constructed a chimeric adenovirus
SG511-CCL5-ODD carrying RANTES-ODD fusion gene. We hypothesized
that in hypoxia of HCC, RANTES is highly expressed under the
control of ODD and attracts a large number of immunocytes to tumor
tissues to exert antitumor efficacy. NK92 cells were also
administered by tail vein injection and they can be attracted to
the tumor site by RANTES. In the HCC tumor site, SG511-CCL5-ODD and
NK92 cells jointly exerted an enhanced antitumor activity.
Materials and methods
Cell lines and culture
Human primary HCC cell lines Hep3B and SK-Hep-1 and
human normal fibroblast cell lines BJ were purchased from the
American Type Culture Collection (ATCC, Manassas, VA, USA). Human
HCC cell lines, SMMC-7721, BEL-7404 and BEL-7405 and human normal
hepatocellular cell lines L02 were obtained from the Institutes of
Cell Biology, Chinese Academy of Sciences (Shanghai, China). The
human embryonic kidney (HEK) 293 cell line was obtained from
Microbix Biosystem, Inc. (Toronto, ON, Canada). NK-92 was purchased
from the American Type Culture Collection, and was maintained in α
medium (Gibco BRL, Gaithersburg, MD, USA) supplemented with 2 mM
L-glutamine, 0.2 mM i-inositol, 20 mM folic acid, 0.1 mM
2-mercaptoethanol, 12.5% fetal bovine serum (FBS) and 12.5% horse
serum containing 100 U human IL-2 (Sigma). L02, SMMC-7721, BEL-7404
and BEL-7405 were maintained in RPMI-1640 with 10% FBS and HEK293,
SK-Hep-1 and Hep3B were maintained in Dulbecco’s modified Eagle’s
medium (Gibco BRL) in a 5% CO2 atmosphere at 37°C,
containing 10% FBS. BJ was maintained in modified Eagle’s medium
(Gibco BRL) in a 5% CO2 atmosphere at 37°C, containing
10% FBS. All media were supplemented with 100 U/ml penicillin and
100 μg/ml streptomycin. Hypoxic conditions (1% O2) were
achieved with a three-gas incubator (Thermo Scientific).
Construction of adenovirus
pCMV-SPORT6 vector containing human RANTES gene was
purchased from Proteintech Group, Inc. Ad5/F11 mosaic adenovirus
plasmids, pPE3-F11-ccdB, pSG500 (47), pPE3-F11, were modified in our lab.
pPE3-F11 was constructed by switching the knob and shaft of the
pBHGE3 (Microbix Biosystem) fiber to those of the Ad11 fiber.
pPE3-F11-ccdB was inserted ccdB (ccdB is negative selection in
E. coli following recombination and transformation) into E3
area of pPE3-F11. We used the primers 1–4 as follows: primer 1:
CGGAATTCACCATGAAGGTCTCCG CGGCAG; primer 2: GCTAACATCTCCAAGTCTAAGCT
CATCTCCAAAGAG; primer 3: GTCATCATCCATTGGGAT ATAGGGAGCTAACATCTCCAAG;
primer 4: CGGGAT CCCTATAACTGGAAGTCATCATCCATTG. The RANTES gene and
ODD domain were fused and amplified by overlapping PCR, in which
the restriction endonuclease sites, EcoR I and BamH
I, were introduced into the upstream and downstream of the fused
gene. The PCR fragment was inserted into pENTR12 (Invitrogen) and
pDC315 (Microbix). After confirming by sequencing, the generated
plasmids were labeled as pENTR12-CCL5-ODD, pDC315-CCL5-ODD.
pENTR12-CCL5-ODD was recombined with adenovirus backbone plasmid
pPE3-F11-ccdB by Gateway recombination in DH5α; the generated
adenovirus backbone vector was identified by restriction
endonuclease digestion and labeled pPE3-F11-CCL5-ODD. Adenovirus
shuttle plasmid pSG500 with E1a controlled by the hTERT promoter
and E1b controlled by HRE was transfected together with
pPE3-F11-CCL5-ODD into HEK293 cells by Lipofectamine 2000 reagent,
to generate a tumor-selective proliferating adenovirus,
SG511-CCL5-ODD. Plasmid pDC315-CCL5-ODD was transfected together
with pPE3-F11 into HEK293 cells by Lipofectamine 2000 reagent, to
generate a tumor-selective proliferating adenovirus,
AD5/11-CCL5-ODD. The viral titers of SG511-CCL5-ODD and
AD5/11-CCL5-ODD were measured with the tissue culture 50% infective
dose (TCID50) method.
In vitro viral replication assay
Cells in contact-inhibition phase and cancer cells
in log-phase were seeded in 6-well plates and infected with the
recombinant adenoviruses at a multiplicity of infection (MOI) of
5.0 plaque-forming units (pfu)/cell. Cells were then washed twice
with PBS and incubated at 37°C, 1% O2 for 0, 48 or 96 h.
Cell lysates were prepared with three cycles of freezing and
thawing. Serial dilutions of the lysates were titered in HEK293
cells, with the TCID50 method, normalized with that at
the beginning of infection and reported as multiples.
ELISA for CCL5 expression
In the in vitro experiments, SMMC-7721,
BEL-7404 and BEL-7405 were seeded in 6-well plates at a density of
5×105 cells per well. To investigate the CCL5 expression
in hepatoma cells, SMMC-7721, BEL-7404 and BEL-7405 were infected
with SG511-CCL5-ODD at an MOI of 5 pfu/cell in normoxia and
hypoxia, respectively. At 72 h after infection, the supernatants
were collected to detect CCL5 expression by ELISA (R&D).
In vitro cell viability assay
3-(4,5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide (MTT) assay was performed to
determine cell viability at various viral MOIs. Cells were plated
at a density of 1×104 in 96-well plates (Gibco); 24 h
later, cells were infected with SG511-CCL5-ODD at a wide range of
MOI from 0.001 to 100 pfu/cell. After 7 days of incubation, cell
viability was measured by MTT assay using the non-radioactive cell
proliferation kit (Roche Molecular Biochemicals), according to the
kit protocol, and the spectrophotometrical absorbance of the
samples was read on a microtiter plate reader at 570 nm in a
reference wavelength of 650 nm. The percentage of cell survival was
calculated using the formula: Cell survival (%) = (A value of
infected cells/A value of uninfected control cell) × 100%. Eight
replicating samples were obtained at each MOI and each experiment
was performed at least three times.
Transwell chamber chemotactic assay
Serum-free MEM/αM (containing 2×105
cell/200 μl) was added to the upper compartment of the invasion
chamber. Conditioned medium (1 ml) was added to the lower
compartment of the invasion chamber. The lower compartment of the
invasion chamber of the control and the standard group were
supplemented with 1 ml serum-free MEM/αM and 1 ml containing 1
ng/ml RANTES serum-free MEM/αM, respectively. The invasion chambers
were incubated at 37°C for 24 h, and the inserts and cells on the
upper side of the filter were then removed. Cells that invaded the
underside of the filter were counted. Each experiment was repeated
three times. The values obtained were calculated by averaging the
total number of cells from triplicate determinations.
Animal experiments
BALB/c nude mice (nu/nu) were purchased from the
Shanghai Experimental Animal Center, Chinese Academy of Sciences.
SMMC-7721 cancer cells in log phase were subcutaneously injected
into the right flanks of mice (1×107 per mouse). Two
weeks later, the tumor xenografts were established, 40 mice were
randomly assigned to four groups (SG511, AD5/11-CCL5-ODD,
SG511-CCL5-ODD and the control group, n=10 mice/group). Tumors were
then injected with 100 μl control buffer or 2×108 pfu of
viruses. The injections were repeated every other day for five
times, with a total dosage of 1×109 pfu.
Other SMMC-7721 tumor xenograft models were
established as mentioned above to evaluate the combined antitumor
efficacy of SG511-CCL5-ODD and NK-92. The mice were divided
randomly into four groups: I (SG511-CCL5-ODD), II (NK-92), III
(SG511-CCL5-ODD92) and control (10 mice/group). Group I was given a
total of 1×109 pfu viruses five times by intratumoral
injection, once every other day. The mice in group II received
intravenous inoculations of 2.5×107 NK-92 cells five
times, once every other day. Group III received intratumoral
injection of 2×108 pfu viruses in 100 μl viral
preservation solution and at the indicated time after injection,
5×106 NK-92 in 200 μl of PBS were administered by
intravenous inoculation, the animals received a series of five
doses of viruses and NK-92 cells. In the control group, 100 μl
viral preservation solution [10 mM Tris-HCl (pH 8.0), 2 mM
MgCl2, 4% sucrose] per mouse per time was injected
intratumorally. Tumor volume was estimated with the formula:
(maximal diameter) × (perpendicular diameter)2 × 0.5.
All animal experiments were performed according to the Guidelines
for the Institutional Animal Care and Use Committee of The Second
Military University (Shanghai, China).
Immunohistochemistry
Tumor samples were formalin-fixed and
paraffin-embedded and successive sections for H&E staining and
immunohistochemistry were prepared. CCL5-expressing cells were
assessed by staining with a goat anti-human CCL5 (R&D) followed
by incubation with a biotin-conjugated rabbit anti-goat IgG and a
horseradish peroxidase (HRP)-conjugated streptavidin (Southern
Biotech). NK-92 cells were assessed by staining with a mouse
anti-human CD56 (Santa Cruz) followed by incubation with a
biotin-conjugated goat anti-mouse IgG and a horseradish
peroxidase-conjugated streptavidin. The positive reaction was
visualized with 3′,3′-diaminobenzidine. The sections were examined
by two independent investigators for qualitative and
semiquantitative analysis.
Statistical analysis
Experiments were performed three times and data are
shown as the means ± SD. Student’s t-test was used to interpret the
significance of differences between every two groups. ANOVA for
repeated measurement experiments was conducted to compare the tumor
growth over time between the treated groups and the control group
in the animal experiment. P<0.05 was considered to indicate
statistically significant differences.
Results
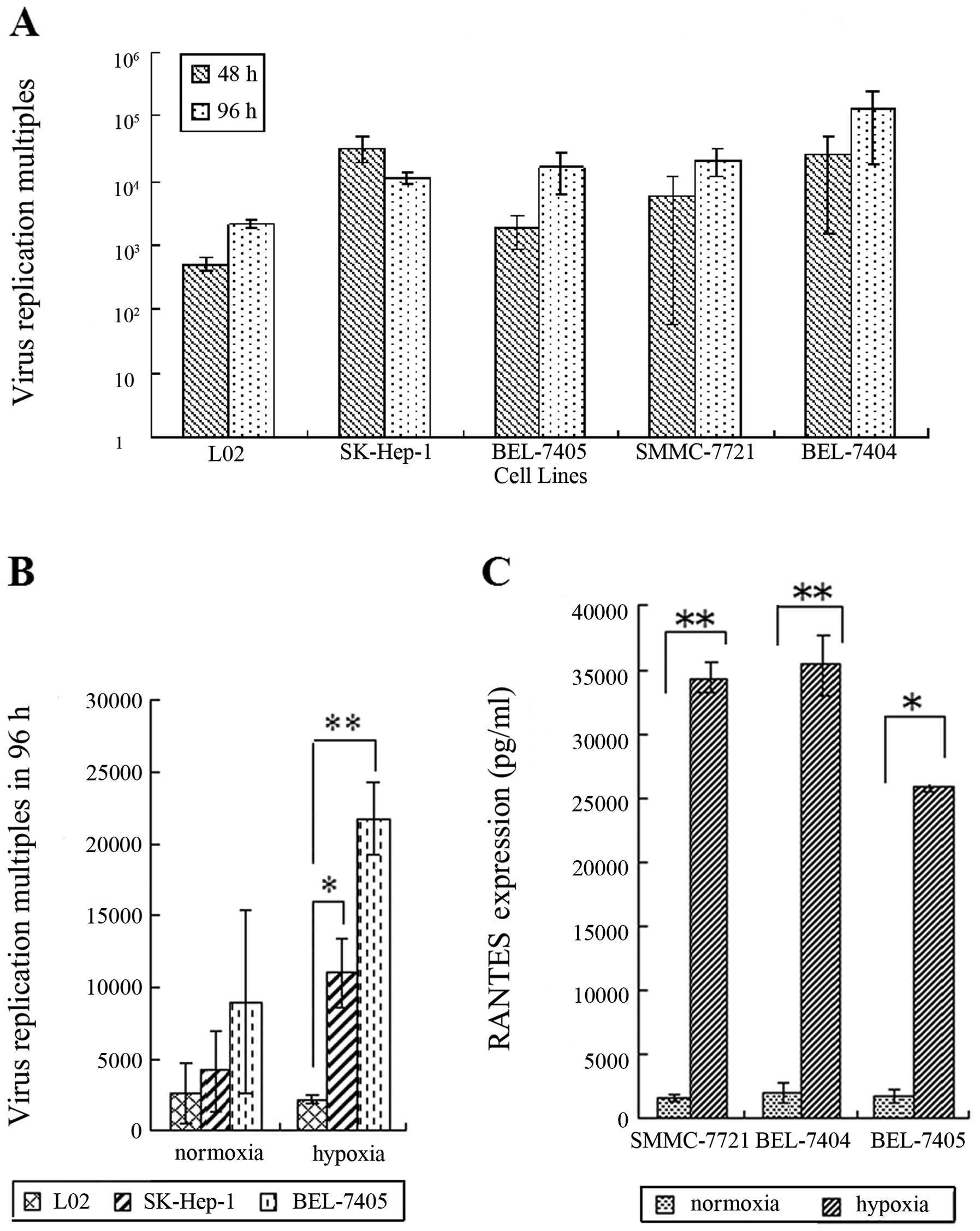
Adenovirus selectively proliferates and
mediates RANTES expression in HCC cells
The Ad5/F11 adenovirus showed a geometrical multiple
proliferation in liver cancer cell lines, particularly in BEL-7404
with the proliferation multiple of 250,000 times in hypoxia at 96
h, demonstrating the virus has strong proliferation ability in
cancer cells (Fig. 1A). The
proliferation multiple of SG511-CCL5-ODD in the normal cell line
was very low, showing that SG511-CCL5-ODD has weak proliferation
ability in normal cells (Fig.
1B).
With the viral proliferation, Ad5/F11 chimeric
adenoviruses express RANTES protein at high levels in SMMC-7721,
BEL-7404 and BEL-7405 cells, reaching up to 35427.3 pg/ml in
BEL-7404 cells in hypoxic conditions. Protein expression of the
RANTES gene in hypoxia was much higher than that in normoxia
in hepatoma cell lines; ODD can effectively regulate RANTES protein
expression (Fig. 1C,
P<0.05).
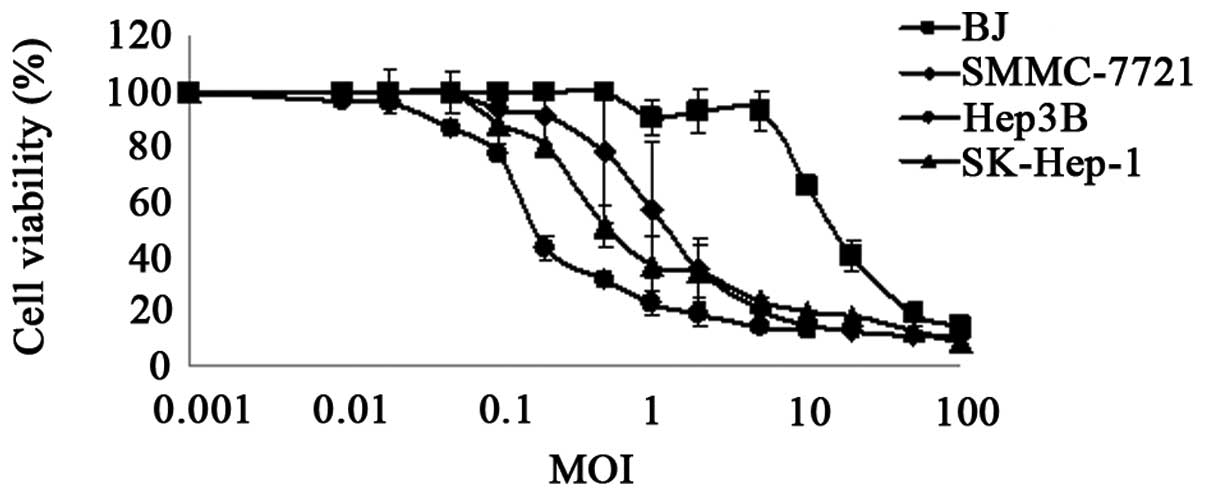
Cytotoxic specificity of SG511-CCL5-ODD
by MTT assay
MTT assay was performed to characterize the
specificity of SG511-CCL5-ODD in tumor cells. As shown in Fig. 2, SG511-CCL5-ODD caused significant
cytolysis in the Hep3B, Sk-Hep-1 and SMMC-7721 cell lines at a MOI
of 0.1 pfu/cell, 0.5 pfu/cell and 1 pfu/cell, respectively.
However, normal cells infected with SG511-CCL5-ODD showed a >50%
cell viability at an MOI of 10 pfu/cell, suggesting that
>10–100-fold of SG511-CCL5-ODD were needed to kill half of the
normal fibroblast cells compared with liver cancer cells (Fig. 2).
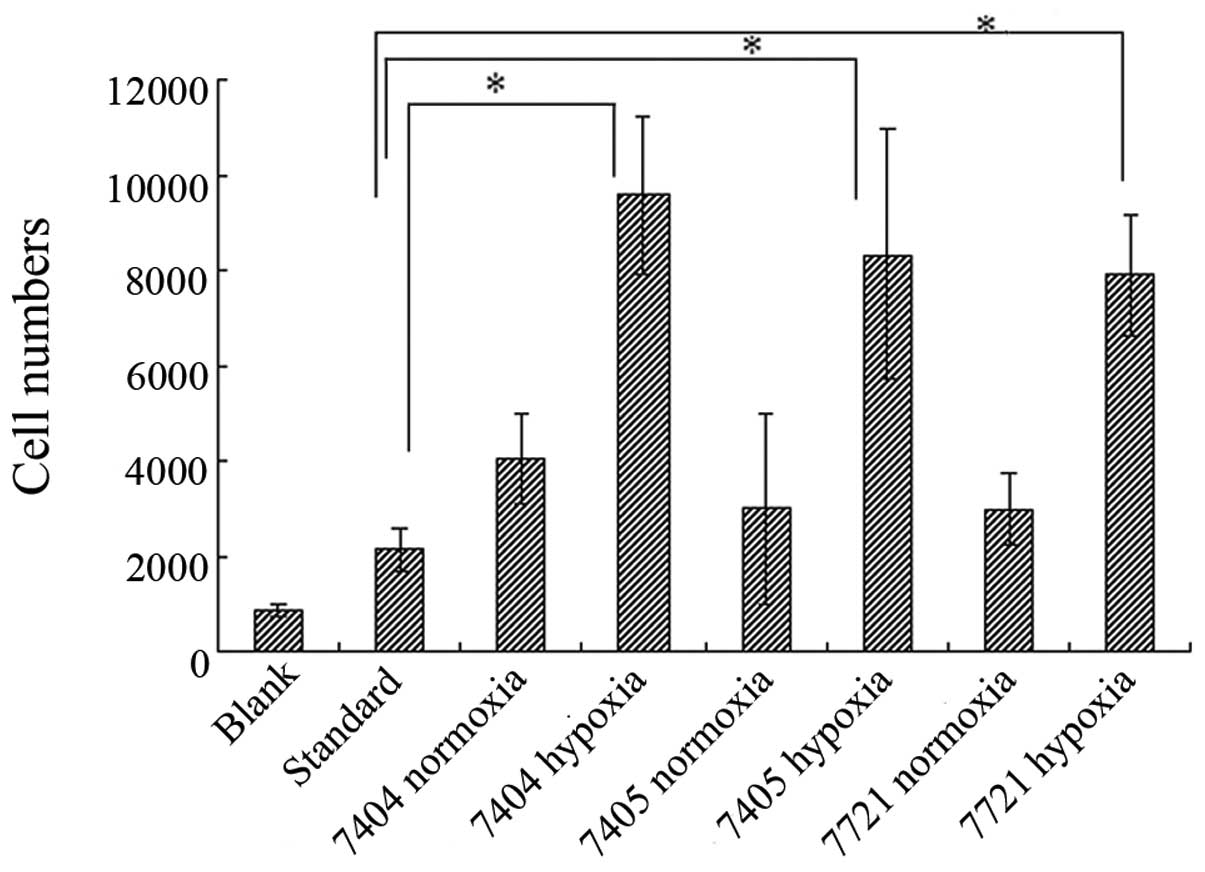
Transwell chamber chemotactic assay
The chemokine experimental results showed that,
compared with the control group and the standard group which
contained RANTES protein at 1 ng/ml in the lower compartment of the
invasion chamber, the RANTES expressed by Ad5/F11 chimeric
adenovirus presented the chemotactic activity for NK-92 cells. In
hypoxia, the chemotactic effect was higher than in normal oxygen
conditions (P<0.05) (Fig.
3).
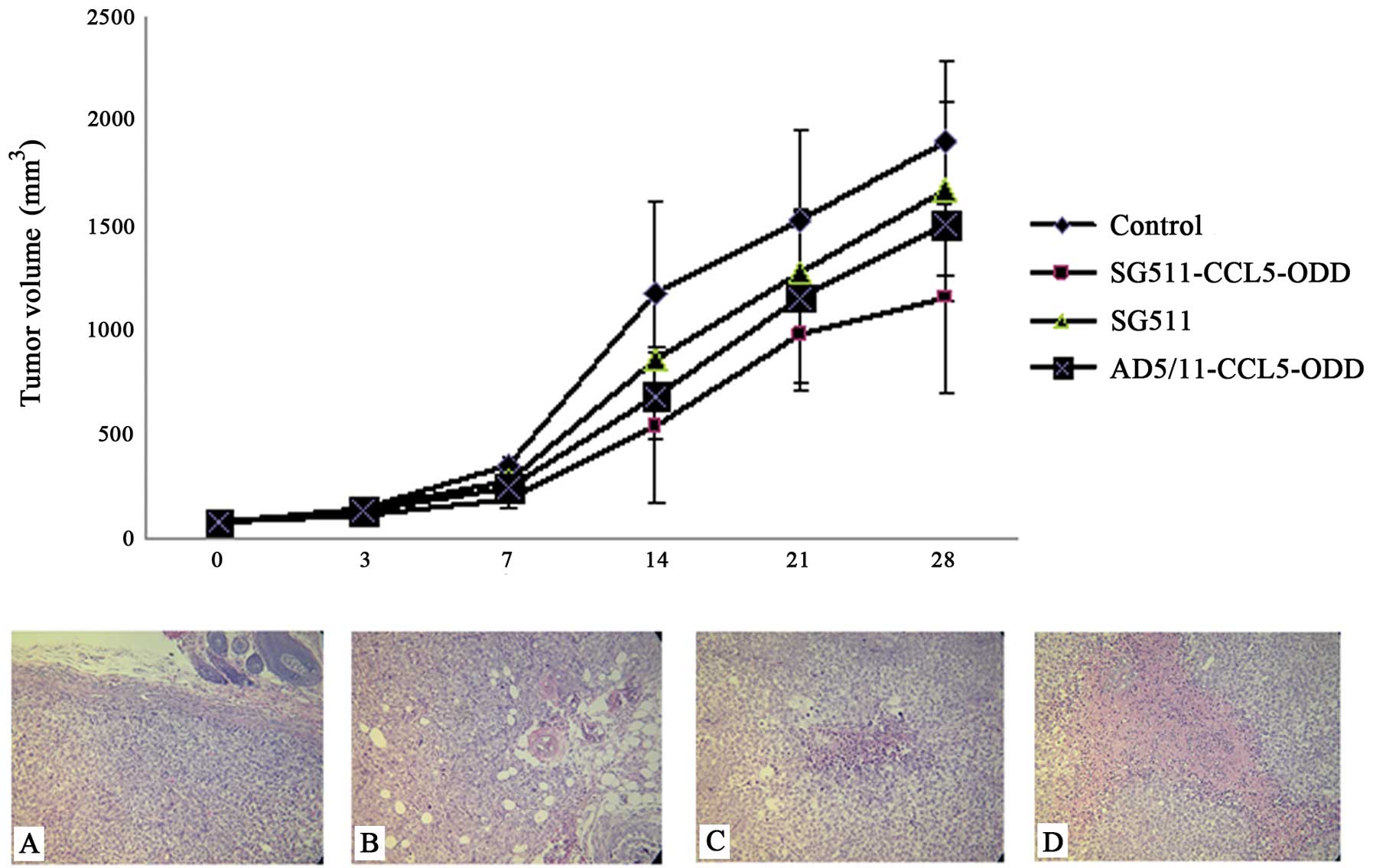
Ad5/F11 chimeric adenovirus-mediated
RANTES expression exerts antitumor potency in HCC xenograft
models
In the SMMC-7721 xenograft models, SG511-CCL5-ODD
intratumor treatment significantly inhibited tumor growth compared
with the control group from day 14 post-treatment (P<0.001).
Both SG511-CCL5-ODD and AD5/11-CCL5-ODD treatment groups showed
stronger antitumor activity than the SG511 group from day 14
post-treatment (P<0.05) (upper panel, Fig. 4). Mice were sacrificed after the
observation period. Tumors were collected and examined
pathologically by H&E staining. Several large necrotic regions
were found in tumors from each group, especially in the
SG511-CCL5-ODD-treated group. In the control group, however, cancer
cells grew unhindered with only small focal areas of necrosis
(Fig. 4A-D).
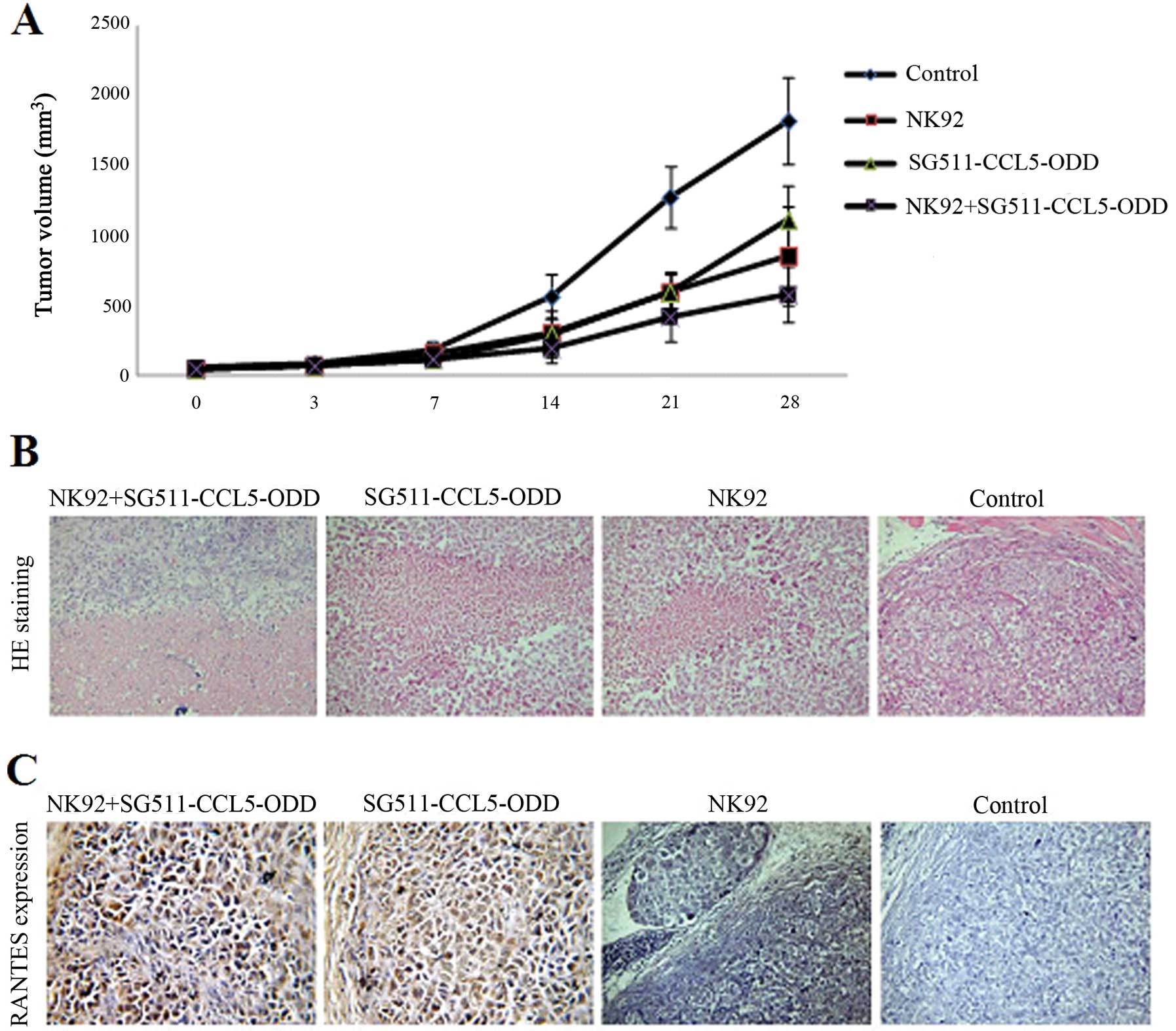
Antitumor efficacy of SG511-CCL5-ODD
combined with NK-92
The antitumor efficacy of SG511-CCL5-ODD combined
with NK-92 was evaluated on SMMC-7721 tumor xenografts established
in nude mice. Significant reduction of tumor volume was observed in
all the treatment groups, with tumor inhibition rates of 51.19,
53.28 and 34%, respectively, for Group I (SG511-CCL5-ODD), Group II
(NK-92), Group III (SG511-CCL5-ODD92), when compared with the
control group (P<0.05) on day 14 after treatment, and 46.15,
46.42 and 32.7% on day 21, 61.04, 46.81 and 31.46% on day 28,
respectively (Fig. 5A).
Mice were sacrificed 28 days later by cervical
dislocation. All tumor samples were examined histologically using
H&E staining (Fig. 5B) and
immunohistochemical staining for RANTES (Fig. 5C). In the control group, cancer
cells grew abundantly with small foci of necrosis. In the groups
treated with replicating viruses or (and) NK-92 cells, several wide
areas of necrosis were observed. There were many necrotic foci in
tumor tissues of the SG511-CCL5-ODD combined NK-92 groups. Around
the necrotic areas, most cancer cells were positive for RANTES
expression, but there were no cancer cells positive for RANTES in
the NK-92 and control groups.
Discussion
RANTES (CCL5) is an 8-kDa cytokine, with clear
chemotactic activity to the cells which are involved in the
immune/inflammatory response, such as lymphocytes,
monocytes/macrophages. RANTES can also regulate the function of
effector cells. Chemotaxis of RANTES relies on the concentration of
RANTES. Therefore, the aggregation of RANTES can enhance its role
in chemotaxis. By increasing the local concentration of RANTES, the
district can attract the cells by the concentration gradient of the
cytokine.
This study successfully constructed a selective
replication adenovirus SG511-CCL5-ODD, in which the hTERT promoter
drives the E1a gene and the hypoxia response promoter controls the
E1b gene. This adenovirus is specifically replicated in tumor
cells. ELISA assay was used to detect RANTES protein expression in
tumor cells infected with SG511-CCL5-ODD. We found RANTES protein
expression was different in both normoxic and hypoxic conditions,
when in the same MOI and at the same time. Expression in hypoxia
was significantly higher than in normoxia (P<0.05), confirming
that adenovirus SG511-CCL5-ODD in hypoxic conditions has a stronger
ability of gene expression. The results proved the RANTES gene
under the control of ODD, RANTES protein in normal oxygen
conditions is partially degraded.
The chemotaxis test is a method used to test the
effectiveness of chemokine. Compared with the control, we found the
virus in liver cancer cells could highly express RANTES protein and
had a strong chemotactic effect to NK-92 cells. The supernatant of
liver cancer cells which was infected by SG511-CCL5-ODD was more
effective to chemotaxis NK-92 cells in hypoxic conditions. The
RANTES protein was confirmed as a strong chemotaxis agent for NK-92
cells. High concentrations of local tumor chemokine RANTES protein
attract immune cells around the tumor tissue to maintain a high
concentration of immune cells, which is key to cancer biological
therapy.
In the liver cancer xenograft model, significant
tumor growth inhibition was demonstrated in all the treatment
groups compared with the control group. From day 14 after
treatment, the tumor volume in the mice treated with SG511-CCL5-ODD
combined with NK-92 was suppressed more than that in the mice with
any other treatment. After a 4-week treatment, when compared with
the control group, the tumor volume was reduced by 68.54 and
53.19%, respectively, in Group III (SG511-CCL5-ODD92) and Group II
(NK-92), and Group III (SG511-CCL5-ODD92) showed more tumor
repression than Group II (NK-92) (P<0.01). These results
indicate that all treatments [including I (SG511-CCL5-ODD), II
(NK-92) and III (SG511-CCL5-ODD92)] inhibited tumor growth and III
(SG511-CCL5-ODD92) is the most efficient agent among them.
Therefore, immune cell-viral biotherapy is superior to simple
immunotherapy and virotherapy.
Acknowledgements
This study was financially supported by the Key
Program of the National Natural Science Foundation of China (No.
81071850) and the National Science Funds for Distinguished Young
Scholars (No. 30925037).
References
|
1
|
Dudley ME, Yang JC, Sherry R, Hughes MS,
Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF,
Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper
J, Morton K, Laurencot C, White DE and Rosenberg SA: Adoptive cell
therapy for patients with metastatic melanoma: evaluation of
intensive myeloablative chemoradiation preparative regimens. J Clin
Oncol. 26:5233–5239. 2008. View Article : Google Scholar
|
|
2
|
Dudley ME, Wunderlich JR, Shelton TE, Even
J and Rosenberg SA: Generation of tumor-infiltrating lymphocyte
cultures for use in adoptive transfer therapy for melanoma
patients. J Immunother. 26:332–342. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dudley ME, Wunderlich JR, Yang JC, Sherry
RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE,
Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP,
Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC,
Abati A and Rosenberg SA: Adoptive cell transfer therapy following
non-myeloablative but lymphodepleting chemotherapy for the
treatment of patients with refractory metastatic melanoma. J Clin
Oncol. 23:2346–2357. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wrzesinski C, Paulos CM, Kaiser A,
Muranski P, Palmer DC, Gattinoni L, Yu Z, Rosenberg SA and Restifo
NP: Increased intensity lymphodepletion enhances tumor treatment
efficacy of adoptively transferred tumor-specific T cells. J
Immunother. 33:1–7. 2010. View Article : Google Scholar
|
|
5
|
Rosenberg SA, Yang JC, Sherry RM, Kammula
US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF,
Wunderlich JR, Morton KE, Laurencot CM, Steinberg SM, White DE and
Dudley ME: Durable complete responses in heavily pretreated
patients with metastatic melanoma using T-cell transfer
immunotherapy. Clin Cancer Res. 17:4550–4557. 2011. View Article : Google Scholar
|
|
6
|
Ripley RT, Davis JL, Klapper JA, Mathur A,
Kammula U, Royal RE, Yang JC, Sherry RM, Hughes MS, Libutti SK,
White DE, Steinberg SM, Dudley ME, Rosenberg SA and Avital I: Liver
resection for metastatic melanoma with postoperative
tumor-infiltrating lymphocyte therapy. Ann Surg Oncol. 17:163–170.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rosenberg SA and Dudley ME: Adoptive cell
therapy for the treatment of patients with metastatic melanoma.
Curr Opin Immunol. 21:233–240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pittet MJ and Mempel TR: Regulation of
T-cell migration and effector functions: insights from in vivo
imaging studies. Immunol Rev. 221:107–129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Braun SE, Chen K, Foster RG, Kim CH,
Hromas R, Kaplan MH, Broxmeyer HE and Cornetta K: The CC chemokine
CK beta-11/MIP-3 beta/ELC/Exodus 3 mediates tumor rejection of
murine breast cancer cells through NK cells. J Immunol.
164:4025–4031. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hromas R, Cripe L, Hangoc G, Cooper S and
Broxmeyer HE: The exodus subfamily of CC chemokines inhibits the
proliferation of chronic myelogenous leukemia progenitors. Blood.
95:1506–1508. 2000.PubMed/NCBI
|
|
11
|
Sharma S, Stolina M, Luo J, Strieter RM,
Burdick M, Zhu LX, Batra RK and Dubinett SM: Secondary lymphoid
tissue chemokine mediates T cell-dependent antitumor responses in
vivo. J Immunol. 164:4558–4563. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kudo T, Lu H, Wu JY, Graham DY, Casola A
and Yamaoka Y: Regulation of RANTES promoter activation in gastric
epithelial cells infected with Helicobacter pylori. Infect
Immun. 73:7602–7612. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lebovic DI, Chao VA and Taylor RN:
Peritoneal macrophages induce RANTES (regulated on activation,
normal T cell expressed and secreted) chemokine gene transcription
in endometrial stromal cells. J Clin Endocrinol Metab.
89:1397–1401. 2004. View Article : Google Scholar
|
|
14
|
Veillard NR, Kwak B, Pelli G, Mulhaupt F,
James RW, Proudfoot AE and Mach F: Antagonism of RANTES receptors
reduces atherosclerotic plaque formation in mice. Circ Res.
94:253–261. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rojas-Ramos E, Avalos AF, Pérez-Fernandez
L, Cuevas-Schacht F, Valencia-Maqueda E and Terán LM: Role of the
chemokines RANTES, monocyte chemotactic proteins-3 and -4 and
eotaxins-1 and -2 in childhood asthma. Eur Respir J. 22:310–316.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen CJ, Chen JH, Chen SY, Liao SL and
Raung SL: Upregulation of RANTES gene expression in neuroglia by
Japanese encephalitis virus infection. J Virol. 78:12107–12119.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oltmanns U, Issa R, Sukkar MB, John M and
Chung KF: Role of c-jun N-terminal kinase in the induced release of
GM-CSF, RANTES and IL-8 from human airway smooth muscle cells. Br J
Pharmacol. 139:1228–1234. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teichmann JV, Ludwig WD and Thiel E:
Cytotoxicity of interleukin 2-induced lymphokine-activated killer
(LAK) cells against human leukemia and augmentation of killing by
interferons and tumor necrosis factor. Leuk Res. 16:287–298. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pawelec G: MHC-unrestricted immune
surveillance of leukemia. Cancer Biother. 9:265–288. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dokhelar MC, Wiels J, Lipinski M, Tetaud
C, Devergie A, Gluckman E and Tursz T: Natural killer cell activity
in human bone marrow recipients: early reappearance of peripheral
natural killer activity in graft-versus-host disease.
Transplantation. 31:61–65. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cerwenka A and Lanier LL: Natural killer
cells, viruses and cancer. Nat Rev Immunol. 1:41–49. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lanier LL: NK cell recognition. Annu Rev
Immunol. 23:225–274. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Drexler HG and Matsuo Y: Malignant
hematopoietic cell lines: in vitro models for the study of natural
killer cell leukemia-lymphoma. Leukemia. 14:777–782. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yan Y, Steinherz P, Klingemann HG, Dennig
D, Childs BH, McGuirk J and O’Reilly RJ: Antileukemia activity of a
natural killer cell line against human leukemias. Clin Cancer Res.
4:2859–2868. 1998.PubMed/NCBI
|
|
25
|
Gong JH, Maki G and Klingemann HG:
Characterization of a human cell line (NK-92) with phenotypical and
functional characteristics of activated natural killer cells.
Leukemia. 8:652–658. 1994.PubMed/NCBI
|
|
26
|
Reid GS, Bharya S, Klingemann HG and
Schultz KR: Differential killing of pre-B acute lymphoblastic
leukaemia cells by activated NK cells and the NK-92 ci cell line.
Clin Exp Immunol. 129:265–271. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tam YK, Miyagawa B, Ho VC and Klingemann
HG: Immunotherapy of malignant melanoma in a SCID mouse model using
the highly cytotoxic natural killer cell line NK-92. J Hematother.
8:281–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tonn T, Becker S, Esser R, Schwabe D and
Seifried E: Cellular immunotherapy of malignancies using the clonal
natural killer cell line NK-92. J Hematother Stem Cell Res.
10:535–544. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bergelson JM, Cunningham JA, Droguett G,
Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL and
Finberg RW: Isolation of a common receptor for Coxsackie B viruses
and adenoviruses 2 and 5. Science. 275:1320–1323. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tomko RP, Xu R and Philipson L: HCAR and
MCAR: the human and mouse cellular receptors for subgroup C
adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA.
94:3352–3356. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim M, Zinn KR, Barnett BG, Sumerel LA,
Krasnykh V, Curiel DT and Douglas JT: The therapeutic efficacy of
adenoviral vectors for cancer gene therapy is limited by a low
level of primary adenovirus receptors on tumour cells. Eur J
Cancer. 38:1917–1926. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Matsumoto K, Shariat SF, Ayala GE, Rauen
KA and Lerner SP: Loss of coxsackie and adenovirus receptor
expression is associated with features of aggressive bladder
cancer. Urology. 66:441–446. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamamoto H, Itoh F, Sakamoto H, Nakajima
Y, Une Y, Hinoda Y and Imai K: Association of reduced cell adhesion
regulator messenger RNA expression with tumor progression in human
hepatocellular carcinoma. Int J Cancer. 74:251–254. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gaggar A, Shayakhmetov DM and Lieber A:
CD46 is a cellular receptor for group B adenoviruses. Nat Med.
9:1408–1412. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hara T, Kojima A, Fukuda H, Masaoka T,
Fukumori Y, Matsumoto M and Seya T: Levels of complement regulatory
proteins, CD35 (CR1), CD46 (MCP) and CD55 (DAF) in human
haematological malignancies. Br J Haematol. 82:368–373. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kinugasa N, Higashi T, Nouso K,
Nakatsukasa H, Kobayashi Y, Ishizaki M, Toshikuni N, Yoshida K,
Uematsu S and Tsuji T: Expression of membrane cofactor protein
(MCP, CD46) in human liver diseases. Br J Cancer. 80:1820–1825.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Murray KP, Mathure S, Kaul R, Khan S,
Carson LF, Twiggs LB, Martens MG and Kaul A: Expression of
complement regulatory proteins-CD 35, CD 46, CD 55 and CD 59-in
benign and malignant endometrial tissue. Gynecol Oncol. 76:176–182.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Thorsteinsson L, O’Dowd GM, Harrington PM
and Johnson PM: The complement regulatory proteins CD46 and CD59,
but not CD55, are highly expressed by glandular epithelium of human
breast and colorectal tumour tissues. APMIS. 106:869–878. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vähä-Koskela MJ, Heikkilä JE and Hinkkanen
AE: Oncolytic viruses in cancer therapy. Cancer Lett. 254:178–216.
2007.
|
|
40
|
Liu X, Qian Q, Xu P, Wolf F, Zhang J,
Zhang D, Li C and Huang Q: A novel conditionally replicating
‘armed’ adenovirus selectively targeting gastrointestinal tumors
with aberrant wnt signaling. Hum Gene Ther. 22:427–437. 2011.
|
|
41
|
Xie M, Niu JH, Chang Y, Qian QJ, Wu HP, Li
LF, Zhang Y, Li JL, Huang XJ and Ruan GR: A novel triple-regulated
oncolytic adenovirus carrying PDCD5 gene exerts potent antitumor
efficacy on common human leukemic cell lines. Apoptosis.
14:1086–1094. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Harada H, Hiraoka M and Kizaka-Kondoh S:
Antitumor effect of TAT-oxygen-dependent degradation-caspase-3
fusion protein specifically stabilized and activated in hypoxic
tumor cells. Cancer Res. 62:2013–2018. 2002.
|
|
43
|
Teicher BA: Hypoxia and drug resistance.
Cancer Metastasis Rev. 13:139–168. 1994. View Article : Google Scholar
|
|
44
|
Kung AL, Wang S, Klco JM, Kaelin WG and
Livingston DM: Suppression of tumor growth through disruption of
hypoxia-inducible transcription. Nat Med. 6:1335–1340. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Helmlinger G, Yuan F, Dellian M and Jain
RK: Interstitial pH and pO2 gradients in solid tumors in vivo:
high-resolution measurements reveal a lack of correlation. Nat Med.
3:177–182. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhong H, De Marzo AM, Laughner E, Lim M,
Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL and Simons
JW: Overexpression of hypoxia-inducible factor 1alpha in common
human cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
47
|
Zhang Q, Chen G, Peng L, Wang X, Yang Y,
Liu C, Shi W, Su C, Wu H, Liu X, Wu M and Qian Q: Increased safety
with preserved antitumoral efficacy on hepatocellular carcinoma
with dual-regulated oncolytic adenovirus. Clin Cancer Res.
12:6523–6531. 2006. View Article : Google Scholar : PubMed/NCBI
|