Introduction
Peptide vaccine therapies using peptides synthesized
on the basis of amino acid sequences of tumor antigens are
considered safe and efficacious in treating cancers such as
hematologic malignancies, prostatic cancer, pancreatic cancer, and
renal cancer, and such peptide vaccine therapies may represent a
new mode of treatment. In recent years, translocase of the outer
mitochondrial membrane 34 (TOMM34) and ring finger protein 43
(RNF43) have been identified as cancer-testis antigens that are
expressed in malignant colon cancer, and vascular endothelial
growth factor receptors VEGFR1 and VEGFR2 have also been identified
in malignant colon cancer through use of microarrays. In order to
evaluate the safety of cancer vaccine therapy using peptides based
on the amino acid sequences of these tumor antigens, a phase I
clinical trial of peptide vaccine therapy together with the oral
anticancer drug tegafur-uracil (UFT) plus leucovorin (LV) was
conducted to treat inoperable advanced or recurrent colorectal
cancer. The registration number was UMIN000004452.
Materials and methods
Purpose
The aim of this study was to evaluate the safety of
peptide vaccines in treating colorectal cancer.
Subjects
The subjects were patients with a human leukocyte
antigen (HLA) type of HLA-A2402 who had inoperable advanced or
recurrent colorectal cancer. All patients meeting eligibility
criteria were included and those meeting exclusion criteria were
excluded as subjects. Eligible patients had colorectal cancer
according to an imaging study and histology and a performance
status (ECOG scale) between 0 and 2. Eligible patients were between
20 and 80 years of age. Patients' tumors were not amenable to
surgical treatment by curative resection and did not respond to
guideline-based chemotherapy; eligible patients had a predicted
prognosis of at least 3 months. Eligible patients did not have
severely impaired organ function (bone marrow, liver and kidneys).
Eligible patients had blood test results that met criteria (WBC
>2000/mm3 and <10000/mm3; hemoglobin
>8.0 g/dl; platelets >10×104/mm3;
creatinine within normal limits; AST and ALT <100 U/l).
Patients' tumors were not treated at least 4 weeks prior to
vaccination. Patient consent for trial participation was confirmed
via a written consent form approved by the Institutional Review
Board of Tokyo Women's Medical University. Female patients who were
pregnant or breast feeding and patients with serious underlying
conditions (serious active infectious disease, circulatory
disorder, respiratory disorder, renal disorder, immune function
disorder, blood coagulation disorder) or severe allergies were
excluded from this trial. Patients deemed ineligible by the
clinical investigator were also excluded.
Protocol
Prior to start of the trial, the treatment was
approved by an Institutional Review Board of Tokyo Women's Medical
University. All procedures followed were in accordance with the
Helsinki Declaration. Four peptides (RNF43, TOMM34, VEGFR1 and
VEGFR2) were emulsified with Montanide and the resulting solution
was administered subcutaneously near the inguinal lymph node once a
week. Patients received the oral anticancer drug UFT+LV three times
a day every 8 h. UFT (300 mg/m2/day) and LV (75 mg/day)
were administered for 4 weeks continuously as part of one course
followed by 1 week of rest. Prior to the start of the trial and
during the 1 week rest for every course, patients received imaging
study, blood test and immune monitoring through blood test. During
the 4 weeks prior to receiving the vaccine and during the first 2
courses of vaccination (10 weeks), patients did not receive any
other anticancer treatment.
Peptides and drugs
Peptides
The peptides used in this trial were RNF43, TOMM34,
VEGFR1 and VEGFR2. The peptides were identified as cancer-testis
antigens and vascular endothelial growth factor receptors.
Cancer-testis antigens are expressed only on the cell surface of
the testicles in normal tissue. Since the cells of the testicles do
not express the HLA molecule on their surface, the body's immune
system does not attack these cells. The peptides were sterilely
manufactured in accordance with good manufacturing practice (GMP)
standards, and preclinical trials had previously confirmed that the
peptides did not produce acute toxicity.
TOMM34
Translocase exists in the inner and outer membrane
of mitochondria, and this enzyme plays an important role in
maintaining mitochondrial transmembrane function. TOMM34 is a
34-kDa translocase present on the outer membrane of mitochondria.
This enzyme transports precursor proteins and has been identified
as a new cancer antigen (1). Over
80% of colon cancers express the antigen on the cell surface.
Although the cells of the testicles and ovaries express the
antigen, other normal tissues do not. A colon cancer cell line with
TOMM34 suppressed by siRNA exhibited inhibition of cell growth,
suggesting that TOMM34 may be involved in tumor growth (1). Moreover, cytotoxic T lymphocytes
(CTLs) recognizing the TOMM34 peptide demonstrated a high cytotoxic
effect on a colon cancer cell line in vitro, suggesting that
TOMM34 may be a target cancer antigen for use in therapy.
RNF43
RNF43 was identified as a gene overexpressed in
colon cancer by profiling using cDNA microarrays (2). RNF43 exists as a protein in the
nucleus and cytoplasm and is also a secretory protein (3). Over 85% of colon cancers express
RNF43, and fetal kidneys and lungs weekly express RNF43 although
normal tissues do not express the protein (3). A cell line overexpressing RNF43 was
shown to have increased cell proliferation, and suppression of
RNF43 by siRNA inhibited cell growth, suggesting that RNF43 is
involved in tumor growth (3).
VEGFR1 and VEGFR2
VEGFR is a tyrosine kinase receptor and is
classified as VEGFR1, VEGFR2 and VEGFR3 (4). The binding of VEGF to VEGFR stimulates
angiogenesis and tumor growth. Anticancer drugs targeting VEGFR
have already been proven clinically efficacious, and vaccination
against VEGFR results in antitumor action in clinical settings
(5,6). Moreover, CTLs recognizing VEGFR are
present in the peripheral blood of cancer patients, suggesting the
potential for peptide vaccine therapy targeting VEGFR (7).
Montanide ISA-51VG
Montanide is a sterile vaccine adjuvant manufactured
by SEPPIC Co. (Puteaux, France) in accordance with GMP standards
and is also known as incomplete Freund's adjuvant. Montanide is
currently used as an adjuvant in vaccine therapy worldwide, and
serious adverse events due to Montanide have yet to be reported.
Montanide has been used as an adjuvant in clinical trials by many
facilities in Japan and was thus used as an adjuvant for the
peptides in the present trial. Since no adverse events due to
Montanide have been reported, it is deemed safe for
administration.
UFT+LV
UFT and LV are oral anticancer drugs marketed in
Japan and approved for treatment of colorectal cancer. UFT+LV has
the same response rate as fluorouracil (5-FU)+LV. UFT+LV inhibits
DNA synthesis and RNA function in cancer cells and has anticancer
action (8,9).
Evaluation of safety
Adverse events resulting from the peptide vaccine
were evaluated using the National Cancer Institute's Common
Terminology Criteria for Adverse Events (NCI-CTCAE) v3.0.
Evaluation of antitumor action and
endpoint
The present clinical trial evaluated safety, thus
antitumor action was not evaluated. However, changes in tumor
markers and tumor size were recorded during the trial. The primary
endpoint was evaluation of the safety of peptide vaccines, and
secondary endpoints were tumor size as determined by an imaging
study in accordance with the RECIST guideline (10), the number of CTLs according to an
ELISpot assay, and phenotypes of T cells in peripheral blood during
treatment.
Enzyme-linked immunospot (ELISpot)
assay
Specific CTL response was estimated by ELISpot assay
following in vitro sensitization. Frozen peripheral blood
mononuclear cells (PBMCs) derived from the same patient were thawed
at the same time, and the viability was confirmed to be >90%.
PBMCs (5×105/ml) were cultured with 10 μg/ml of
respective peptide and 100 IU/ml of interleukin-2 (Novartis,
Emeryville, CA, USA) at 37°C for 2 weeks. The peptide was added
into the culture on Day 0 and on Day 7. Following CD4+
cell depletion using the Dynal CD4 Positive Isolation kit
(Invitrogen, Carlsbad, CA, USA), interferon-γ (IFN-γ) ELISpot assay
was performed using the Human IFN-γ ELISpot Plus kit (Mabtech AB,
Nacka Strand, Sweden) according to the manufacturer's instructions.
Briefly, HLA-A2402-positive B-lymphoblast TISI cells (IHWG Cell and
Gene Bank, Seattle, WA, USA) were incubated with 20 μg/ml of
vaccinated peptides overnight, then the residual peptide in the
media was washed out to prepare peptide-pulsed TISI cells as the
stimulator cells. Prepared CD4- cells were cultured with
peptide-pulsed TISI cells (2×104 cells/well) at 1/1,
1/2, 1/4 and 1/8 mixture ratio of responder cells and stimulator
cells (R/S ratio) on a 96-well plate (Millipore, Bedford, MA, USA)
at 37°C overnight. Non-peptide-pulsed TISI cells were used as
negative control stimulator cells. To confirm IFN-γ productivity,
responder cells were stimulated with phorbol 12-myristate
13-acetate (PMA) (66 ng/ml) and ionomycin (3 μg/ml) overnight, and
then applied to the IFN-γ ELISpot assay (2.5×103
cells/well) without stimulator cells. All ELISpot assays were
performed using triplicate wells. The plates were analyzed by an
automated ELISpot reader, ImmunoSpot S4 (Cellular Technology Ltd.,
Shaker Heights, OH, USA) and ImmunoSpot Professional Software
version 5.0 (Cellular Technology Ltd.). The number of
peptide-specific spots was calculated by subtracting the spot
number in the control well from the spot number in the well with
the peptide-pulsed TISI cells. Sensitivity of our ELISpot assay was
estimated to be approximately equal to the average level by the
ELISpot panel of the Cancer Immunotherapy Consortium [CIC
(http://www.cancerresearch.org/consortium/assay-panels/)].
Statistical analysis
Overall survival (OS) and progression-free survival
(PFS) were analyzed by the Kaplan-Meier method, and the statistical
differences were analyzed by the log-rank method. The Student's
t-test was performed to compare results between the two groups.
Data were considered to be statistically significant when a P-value
of <0.05 was achieved.
Results
The subjects consisted of 8 males and 2 females,
ages 40 to 70 years (average age, 58.4 years). The lesions
evaluated were from 7 metastatic liver tumors, 3 metastatic lung
tumors, 1 metastatic lesion to pleura, 2 lesions indicating local
recurrence in the pelvis and 2 lesions indicating peritoneal
dissemination (patients may have had more than one type of lesion)
(Table I). The 10 patients were
administered the vaccine 8 to 28 times (148 times in total) from
July 2008 to December 2009. The dosage was increased to 0.5 mg for
3 patients, 1 mg for 3 and 3 mg for 4. All doses were safely
administered. During the therapy, 1 patient suffered from hematuria
and urinary retention. These events were observed in a patient
suffering from local recurrence of sigmoid colon cancer, thus these
events were attributed to the tumor itself. The other adverse event
was grade 1 pyrexia, which was successfully treated on an
outpatient basis. All of the patients had a skin reaction at the
vaccination site. The skin reactions were grade 1 redness,
induration, and bullosis in 8 patients and grade 2 ulceration of
the vaccination site in 2 patients. All skin reactions were
adequately treated and managed on an outpatient basis. In all
patients, CTLs in the peripheral blood were evaluated by IFN-γ
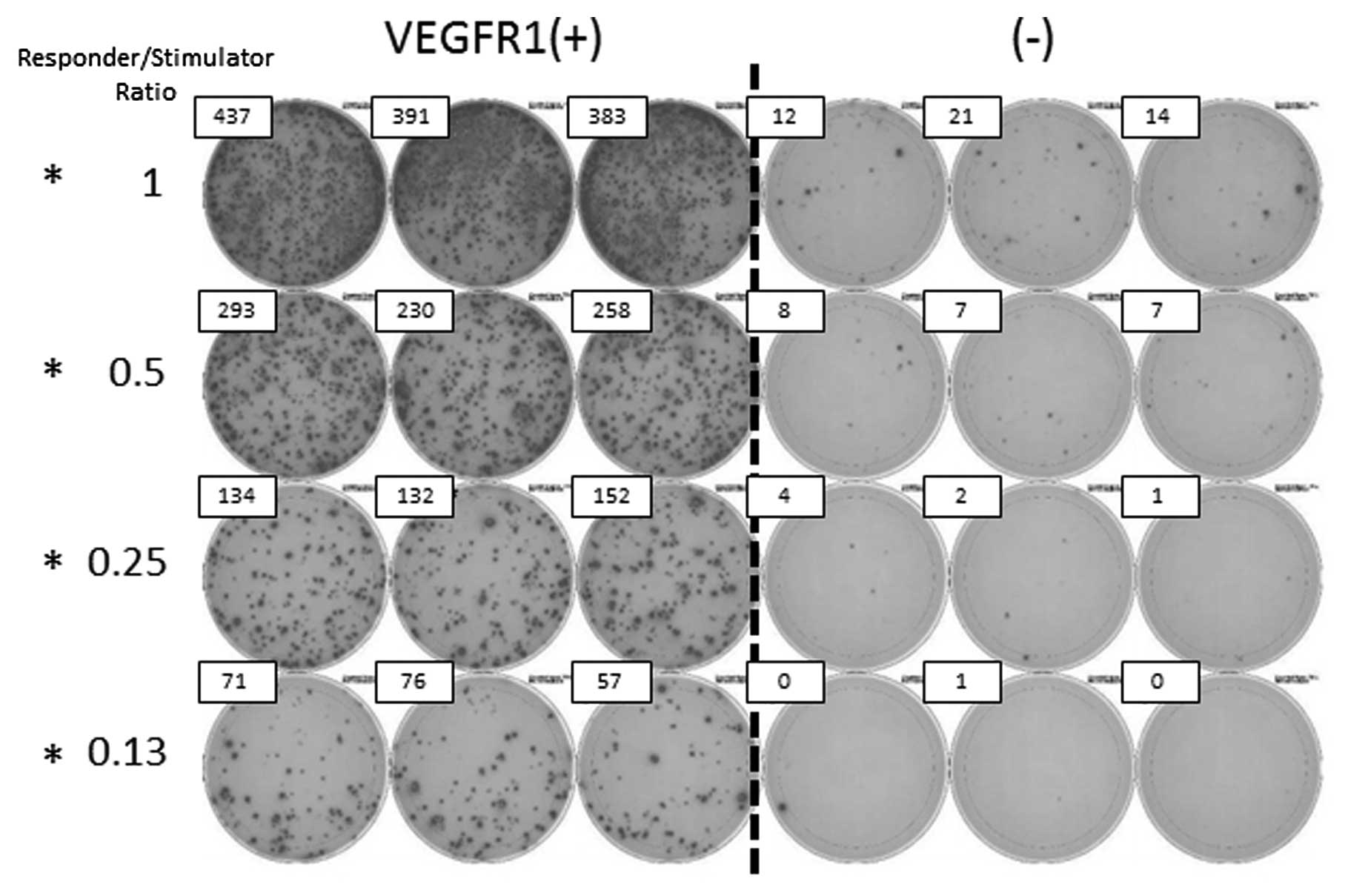
ELISpot assay. CTLs tended to be induced by VEGFR1 in patients with
a partial response (PR) and stable disease (SD). In the patient
with PR, CTLs recognizing RNF43, TOMM34 and VEGFR2 were also
induced. In the patient with progressive disease (PD), a limited
CTL response was observed (Fig. 1)
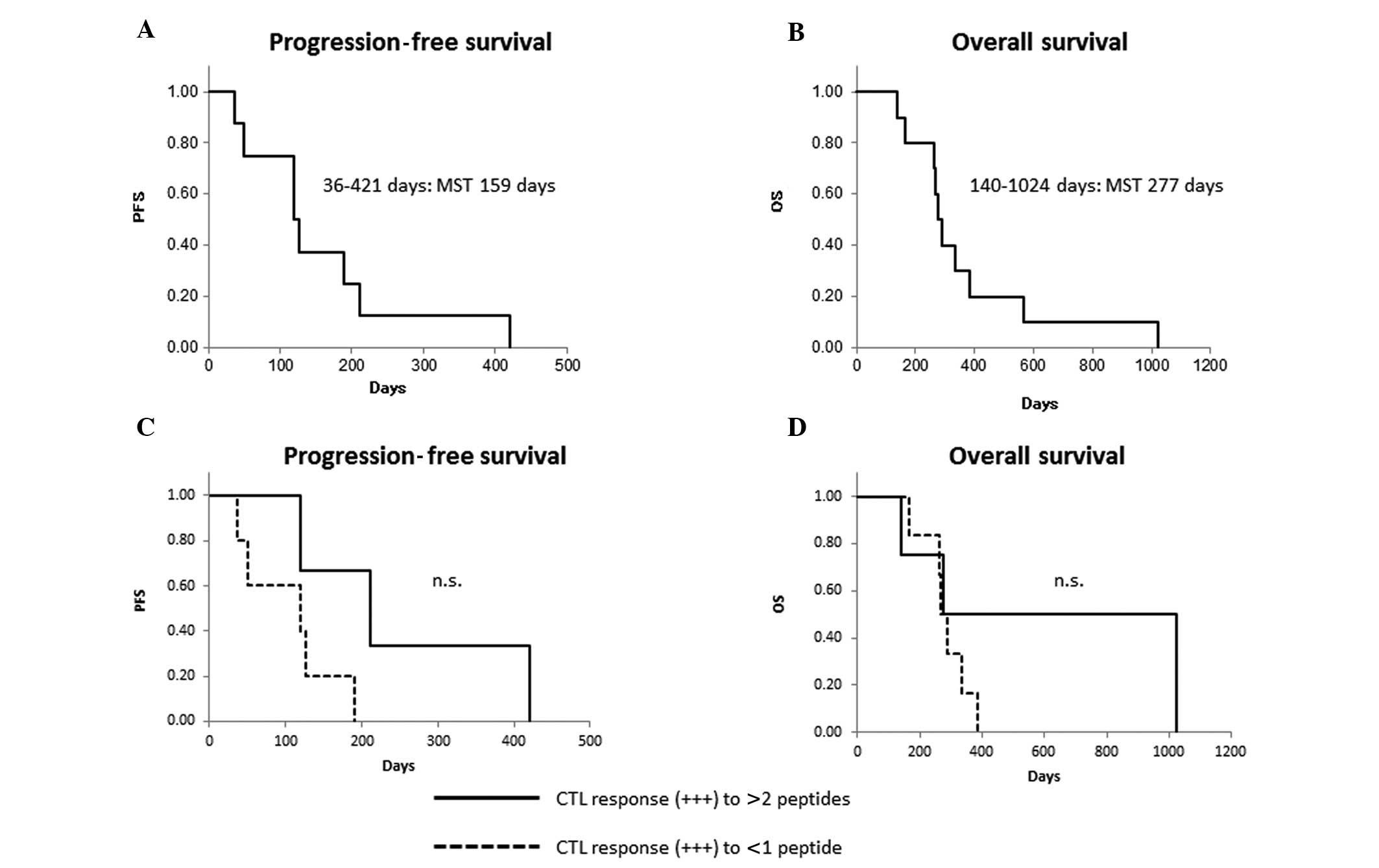
(Table II). PFS ranged from 36 to
421 days [median survival time (MST), 159 days] and OS ranged from
140 to 1,024 days (MST, 277 days). Kaplan-Meier analysis indicated
that patients who had a strong CTL reaction (+++) to more than 2
peptides had a longer PFS and OS, but the difference was not
statistically significance (Fig.
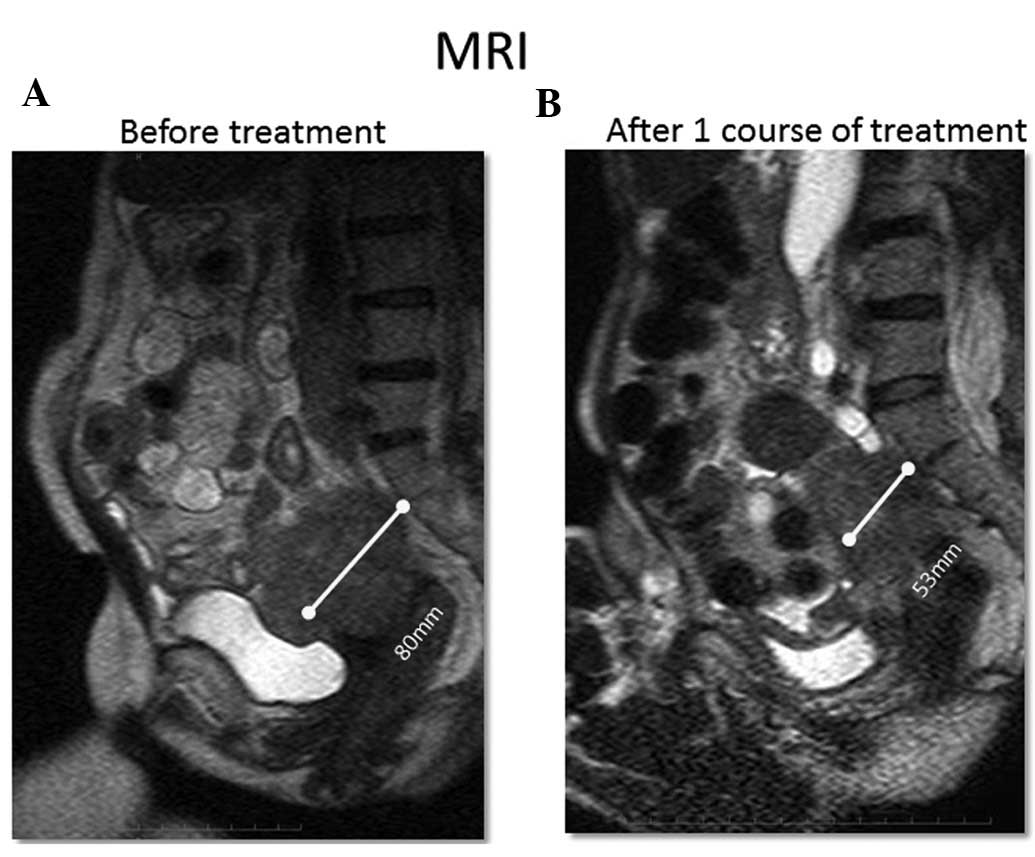
2). Evaluation of tumor size via an imaging study after 1
course of treatment revealed that 1 patient had a PR, 7 had a SD
and 2 had a PD. In the patient with PR, a tumor in the pelvis
decreased more than 30% in size (Fig.
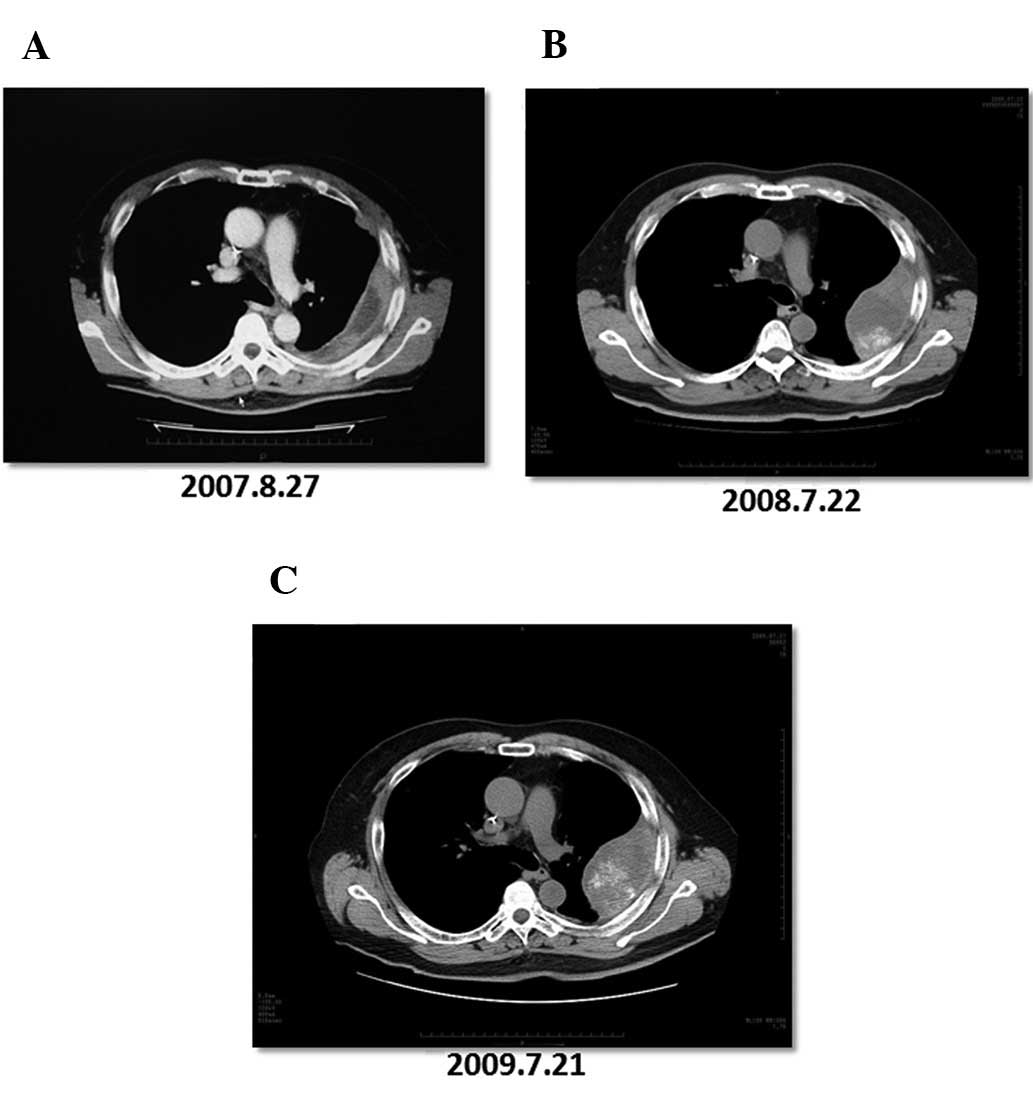
3). One patient had an extended SD (patient no. 4). The patient
was diagnosed with pleural metastasis of rectal cancer in August
2007 (Fig. 4A). After the
diagnosis, the patient underwent chemotherapy [FOLFOX+bevacizumab,
FOLFIRI+bevacizumab and S-1 (a combination of tegafur, gimeracil,
and oteracil potassium) + oxaliplatin] for ~10 months. Based on an
imaging study, the patient was deemed to have PD in July 2008
(Fig. 4B). After enrollment in the
clinical trial, the patient was treated with the peptide vaccine
and UFT+LV for ~1 year. The tumor grew slightly after the
vaccination, but the patient was deemed to have SD in July 2009
(Fig. 4C). After a CT examination
confirmed SD, the tumor remained a consistent size for 421 days and
the OS of the patient after enrollment in this trial was 1,024
days. At the end of the investigation (December 2009), 8 patients
were diagnosed with PD. The reasons for disease progression were
lesion enlargement in 7 patients and discovery of a new lesion in 1
(data not shown). No changes in the ratios of regulatory T cells
and CD8+ T cells (naïve, memory, effector memory and
effector) were observed in the patients during therapy (data not
shown).
 | Table IPatient data. |
Table I
Patient data.
| Patient no. | Age (years) | Gender | Evaluated lesion | Peptide dose
(mg) | No. of times the
vaccine was administered | Adverse events CTCAE
(grade) | Skin reaction CTCAE
(grade) | TTP (day) | OS (day) | Evaluation after 1
course of treatment |
|---|
| 1 | 55 | F | Local recurrence of
sigmoid colon cancer in the pelvis | 0.5 | 12 | Hematuria, urinary
retention (G-2) | Redness, induration
(G-1) | - | 140 | PR |
| 2 | 70 | M | Local recurrence of
rectal cancer in the pelvis, metastatic lung tumor | 0.5 | 8 | | Redness (G-1) | 36 | 290 | PD |
| 3 | 40 | F | Liver metastasis of
sigmoid colon cancer, peritoneal dissemination | 0.5 | 8 | Pyrexia (G-1) | Redness (G-1) | - | 335 | SD |
| 4 | 58 | M | Metastasis of rectal
cancer to pleura | 1 | 28 | | Redness (G-1) | 421 | 1,024 | SD |
| 5 | 65 | M | Liver metastasis of
transverse colon cancer, peritoneal dissemination | 1 | 10 | | Redness (G-1) | 127 | 267 | SD |
| 6 | 57 | M | Liver and lung
metastasis of sigmoid colon cancer | 1 | 15 | Pyrexia (G-1) | Skin ulcer (G-2) | 190 | 264 | SD |
| 7 | 64 | M | Liver metastasis of
rectal cancer | 3 | 12 | | Induration (G-1) | 50 | 167 | PD |
| 8 | 64 | M | Liver metastasis of
sigmoid colon cancer | 3 | 20 | | Redness (G-1) | 120 | 277 | SD |
| 9 | 67 | M | Liver and lung
metastasis of rectal cancer | 3 | 12 | | Redness, bullosis
(G-1) | 120 | 385 | SD |
| 10 | 44 | M | Liver metastasis of
sigmoid colon cancer | 3 | 23 | | Redness, skin ulcer
(G-2) | 211 | 568 | SD |
 | Table IICTL assessment (IFN-γ ELISpot
assay). |
Table II
CTL assessment (IFN-γ ELISpot
assay).
| | | CTL response | |
|---|
| | |
| |
|---|
| Peptide dose | Patient no. | Course | RNF43 | TOMM34 | VEGFR1 | VEGFR2 | Positive
control | Evaluation after 1
course of treatment |
|---|
| 0.5 mg | 1 | Pre | + | − | − | +++ | +++ | PR |
| | Post1 | + | + | +++ | +++ | +++ | |
| | Post2 | + | − | +++ | ++ | +++ | |
| | Post3 | − | ++ | +++ | + | +++ | |
| 2 | Pre | − | − | + | − | + | PD |
| | Post1 | − | − | + | + | + | |
| | Post2 | − | − | + | − | + | |
| 3 | Pre | − | + | − | + + | SD | |
| | Post1 | + | + | + | + | + | |
| | Post2 | − | − | +++ | − | ++ | |
| 1 mg | 4 | Pre | + | + | − | + | +++ | SD |
| | Post1 | − | − | +++ | + | +++ | |
| | Post2 | − | − | +++ | + | +++ | |
| | Post2 (resume) | − | − | ++ | +++ | +++ | |
| | Post3 | + | − | − | + | +++ | |
| | Post5 | + | − | − | + | +++ | |
| | Post6 | − | − | + | + | +++ | |
| 5 | Pre | + | + | + | − | + | SD |
| | Post1 | − | − | +++ | − | +++ | |
| | Post2 | − | − | + | − | ++ | |
| 6 | Pre | − | − | − | − | +++ | SD |
| | Post1 | − | − | ++ | + | +++ | |
| | Post2 | − | − | ++ | + | +++ | |
| 3 mg | 7 | Pre | − | − | + | − | +++ | PD |
| | Post1 | − | − | ++ | − | +++ | |
| | Post2 | − | − | ++ | − | +++ | |
| | Post3 | − | − | +++ | + | +++ | |
| 8 | Pre | + | − | + | + | +++ | SD |
| | Post1 | + | − | + | + | +++ | |
| | Post2 | − | − | ++ | − | +++ | |
| | Post3 | + | − | +++ | − | +++ | |
| | Post4 | − | − | ++ | ++ | +++ | |
| | Post5 | + | − | +++ | +++ | +++ | |
| 9 | Pre | − | + | − | − | +++ | SD |
| | Post1 | − | − | − | − | +++ | |
| | Post2 | + | − | +++ | + | +++ | |
| | Post3 | − | − | ++ | + | +++ | |
| 10 | Pre | ++ | + | ++ | ++ | +++ | SD |
| | Post1 | ++ | − | ++ | + | +++ | |
| | Post2 | ++ | − | +++ | ++ | +++ | |
| | Post3 | + | + | − | +++ | +++ | |
| | Post4 | ++ | − | + | +++ | +++ | |
| | Post5 | + | − | ++ | +++ | +++ | |
Discussion
Vaccine therapy using dendritic cells has been
reported to be clinically effective (11), therefore cancer treatment
capitalizing on the body's immunocompetence should be possible.
However, Nagorsen and Thiel (12)
conducted a meta-analysis of tumor-specific immune therapies used
to treat colon cancer in 2006. They reported a low clinical
response rate (0.9%) to these therapies despite a cellular immune
response being induced in 44% of patients after therapy (12). Moreover, Rosenberg et
al(13) also reported that
vaccine therapy alone did not provide a sufficient clinical
response. Given these facts, new breakthroughs in cancer
immunotherapies are expected. The search for cancer antigens
through exhaustive gene profiling using microarrays, a technique
that has recently been put to extensive use, has identified
potential new peptide vaccine therapies for cancer. Clinical trials
of these therapies are actively underway at a number of facilities.
In fact, a report of patients responding well to peptide vaccines
has recently emerged (14).
TOMM34 and RNF43 peptides are cancer antigens that
are created artificially based on gene profiling data. We
previously used real-time PCR to analyze TOMM34 and RNF43 gene
expression in samples prepared from paraffin-embedded colon cancer
tissues, revealing TOMM34 expression in 78.9% and RNF43 expression
in 63.2% of cases (data not shown). The cancer antigens that should
be used in vaccines are: i) those antigens expressed only by cancer
cells and not expressed by normal cells; ii) those antigens that
sustain cancer cell function and that lead to cancer cell death
when diminished; and iii) those antigens that effectively induce
CTLs. TOMM34 and RNF43 may satisfy these criteria. Under normal
conditions, cancerous cells are destroyed by immune surveillance
when they initially appear. However, cancer cells may lose their
antigens and thus escape immune surveillance. One way cancer cells
escape surveillance is by losing HLA molecules. Even if CTLs
recognizing TOMM34 and RNF43 are induced by a peptide vaccine in
vivo, they may ignore cancer cells when the cancer cells lose
HLA molecules and escape immune surveillance. Thus, VEGFR1 and
VEGFR2 peptides were used in the vaccination to inhibit tumor
vascular growth in addition to TOMM34 and RNF43. The vaccine was
intended to have antitumor action even in the event that the cancer
cells had escaped immune surveillance.
Oral anticancer drugs were used in this trial even
though these drugs are thought to inhibit the immune system in
vivo. In fact, numerous reports have mentioned achieving
enhanced immunity by approaches such as reducing tumor volume
through the use of antitumor agents, stimulation of tumor antigens
in cancer cells, increasing the CTL response to cancer cells,
decreasing regulatory T cells in vivo, inhibiting production
of immune inhibitory cytokines (e.g. TGF-β) and increasing
accumulation of immune effector cells in tumor tissue (15–24).
In the present trial, therapy was followed by UFT+LV in
anticipation of the added immunological response to those agents.
UFT+LV is taken orally and does not differ from intravenous 5FU+LV
in terms of OS and PFS. Furthermore, no grade 3 or 4 serious
adverse effects have been reported as a result of UFT+LV (8,9). Since
the patients in the present clinical trial had inoperable advanced
or recurrent colon cancer and were unable to undergo chemotherapy,
the therapy was debilitating and had little marked effect. However,
CTL induction after vaccination was noted and PR was achieved in a
patient with CTLs induced to recognize a number of peptides. The
patients who had a strong CTL reaction to more than 2 peptides had
a clinical response (PR as gauged by tumor size and extended SD).
Furthermore, patients with favorable results on the CTL assay had a
longer OS and PFS, although the difference was not statistically
significant. These phenomena indicate the potential for peptide
vaccine therapy. Most patients in this trial ultimately had PD, but
the reason for PD in most of these patients was enlargement of an
existing lesion; only 1 patient was found to have a new lesion.
These findings suggest the potential for peptide vaccine therapy
together with UFT+LV to inhibit the recurrence of colorectal cancer
after curative surgery.
There were differences in CTL induction depending on
the patient, and CTLs were more readily and less readily induced by
different peptides. The reasons for these differences should be
investigated. In this trial, UFT+LV was administered following the
same schedule as for UFT+LV alone. Suitable scheduling of UFT+LV
with the peptide vaccine and selection of other anticancer drugs
besides UFT+LV are topics for future study.
The only major adverse event presumed to be related
to the peptide vaccine was pyrexia. Pyrexia could not be avoided as
the therapy induces a tumor-specific immune response in
vivo. Moreover, pyrexia did not present a problem clinically.
The other adverse event was a skin reaction at the vaccination
site. Several patients had an intense skin reaction, such as
ulceration of the vaccination site. One report indicated that an
intense skin reaction is a sign of clinical effectiveness (25). A new vaccine adjuvant mixture should
be developed to effectively induce an immune reaction without
causing skin problems.
In the present trial, an ELISpot assay was used to
predict the clinical efficacy of the therapy. Our study noted a
longer survival time with a more intense CTL reaction. However, an
ELISpot assay has drawbacks such as its expense and its demands on
personnel performing the assay. Simple methods of immune monitoring
must be developed to predict clinical efficacy. Given the
mechanisms of the immune system, immune therapy presumably requires
time to produce an effect in patients. In addition, 1 of the
current patients (patient no. 4) had an extended SD
(time-to-progression 421 days), thus new statistical techniques are
needed to analyze the survival time and progression-free
interval.
The safety of the peptide vaccine therapy together
with UFT+LV was confirmed. At present, the clinical efficacy of the
therapy is not satisfactory. However, peptide-specific CTLs were
induced in some patients, thus further study is needed. Additional
evidence is needed to overcome problems with the therapy and should
lead to a standard therapy in the future. Peptide vaccine therapy
should not be a standard therapy only for patients with inoperative
or advanced cancer, as it was in the present trial, but instead
peptide vaccine therapy should be performed as the standard
adjuvant therapy for patients with a high risk of postoperative
recurrence.
Acknowledgements
The authors would like to thank Professor Yusuke
Nakamura, Dr Takuya Tsunoda, Dr Koji Yoshida, Laboratory of
Molecular Medicine, Human Genome Center, Institute of Medical
Science, the University of Tokyo, for their excellent advice,
cooperation and provision of all the peptides.
References
|
1
|
Shimokawa T, Matsushima S, Tsunoda T,
Tahara H, Nakamura Y and Furukawa Y: Identification of TOMM34,
which shows elevated expression in the majority of human colon
cancers, as a novel drug target. Int J Oncol. 29:381–386.
2006.PubMed/NCBI
|
|
2
|
Lin YM, Furukawa Y, Tsunoda T, Yue CT,
Yang KC and Nakamura Y: Molecular diagnosis of colorectal tumors by
expression profiles of 50 genes expressed differentially in
adenomas and carcinomas. Oncogene. 21:4120–4128. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yagyu R, Furukawa Y, Lin YM, Shimokawa T,
Yamamura T and Nakamura Y: A novel oncoprotein RNF43 functions in
an autocrine manner in colorectal cancer. Int J Oncol.
25:1343–1348. 2004.PubMed/NCBI
|
|
4
|
Soker S, Takashima S, Miao HQ, Neufeld G
and Klagsbrun M: Neuropilin-1 is expressed by endothelial and tumor
cells as an isoform-specific receptor for vascular endothelial
growth factor. Cell. 92:735–745. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Niethammer AG, Xiang R, Becker JC, Wodrich
H, Pertl U, Karsten G, Eliceiri BP and Reisfeld RA: A DNA vaccine
against VEGF receptor 2 prevents effective angiogenesis and
inhibits tumor growth. Nat Med. 8:1369–1375. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Y, Wang MN, Li H, King KD, Bassi R, Sun
H, Santiago A, Hooper AT, Bohlen P and Hicklin DJ: Active
immunization against the vascular endothelial growth factor
receptor flk1 inhibits tumor angiogenesis and metastasis. J Exp
Med. 195:1575–1584. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wada S, Tsunoda T, Baba T, Primus FJ,
Kuwano H, Shibuya M and Tahara H: Rationale for antiangiogenic
cancer therapy with vaccination using epitope peptides derived from
human vascular endothelial growth factor receptor 2. Cancer Res.
65:4939–4946. 2005. View Article : Google Scholar
|
|
8
|
Douillard JY, Hoff PM, Skillings JR,
Eisenberg P, Davidson N, Harper P, Vincent MD, Lembersky BC,
Thompson S, Maniero A and Benner SE: Multicenter phase III study of
uracil/tegafur and oral leucovorin versus fluorouracil and
leucovorin in patients with previously untreated metastatic
colorectal cancer. J Clin Oncol. 20:3605–3616. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carmichael J, Popiela T, Radstone D, Falk
S, Borner M, Oza A, Skovsgaard T, Munier S and Martin C: Randomized
comparative study of tegafur/uracil and oral leucovorin versus
parenteral fluorouracil and leucovorin in patients with previously
untreated metastatic colorectal cancer. J Clin Oncol. 20:3617–3627.
2002. View Article : Google Scholar
|
|
10
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
Organization for Research and Treatment of Cancer, National Cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar
|
|
11
|
Sadanaga N, Nagashima H, Mashino K, Tahara
K, Yamaguchi H, Ohta M, Fujie T, Tanaka F, Inoue H, Takesako K,
Akiyoshi T and Mori M: Dendritic cell vaccination with MAGE peptide
is a novel therapeutic approach for gastrointestinal carcinomas.
Clin Cancer Res. 7:2277–2284. 2001.PubMed/NCBI
|
|
12
|
Nagorsen D and Thiel E: Clinical and
immunologic responses to active specific cancer vaccines in human
colorectal cancer. Clin Cancer Res. 12:3064–3069. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rosenberg SA, Yang JC and Restifo NP:
Cancer immunotherapy: moving beyond current vaccines. Nat Med.
10:909–915. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kono K, Mizukami Y, Daigo Y, Takano A,
Masuda K, Yoshida K, Tsunoda T, Kawaguchi Y, Nakamura Y and Fujii
H: Vaccination with multiple peptides derived from novel
cancer-testis antigens can induce specific T-cell responses and
clinical responses in advanced esophageal cancer. Cancer Sci.
100:1502–1509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Correale P, Cusi MG, Tsang KY, Del Vecchio
MT, Marsili S, Placa ML, Intrivici C, Aquino A, Micheli L, Nencini
C, Ferrari F, Giorgi G, Bonmassar E and Francini G:
Chemo-immunotherapy of metastatic colorectal carcinoma with
gemcitabine plus FOLFOX 4 followed by subcutaneous granulocyte
macrophage colony-stimulating factor and interleukin-2 induces
strong immunologic and antitumor activity in metastatic colon
cancer patients. J Clin Oncol. 23:8950–8958. 2005.
|
|
16
|
Correale P, Aquino A, Giuliani A,
Pellegrini M, Micheli L, Cusi MG, Nencini C, Petrioli R, Prete SP,
De Vecchis L, Turriziani M, Giorgi G, Bonmassar E and Francini G:
Treatment of colon and breast carcinoma cells with 5-fluorouracil
enhances expression of carcinoembryonic antigen and susceptibility
to HLA-A(*)02.01 restricted, CEA-peptide-specific cytotoxic T cells
in vitro. Int J Cancer. 104:437–445. 2003.PubMed/NCBI
|
|
17
|
Dauer M, Herten J, Bauer C, Renner F,
Schad K, Schnurr M, Endres S and Eigler A: Chemosensitization of
pancreatic carcinoma cells to enhance T cell-mediated cytotoxicity
induced by tumor lysate-pulsed dendritic cells. J Immunother.
28:332–342. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Correale P, Cusi MG, Del Vecchio MT,
Aquino A, Prete SP, Tsang KY, Micheli L, Nencini C, La Placa M,
Montagnani F, Terrosi C, Caraglia M, Formica V, Giorgi G, Bonmassar
E and Francini G: Dendritic cell-mediated cross-presentation of
antigens derived from colon carcinoma cells exposed to a highly
cytotoxic multidrug regimen with gemcitabine, oxaliplatin,
5-fluorouracil, and leucovorin, elicits a powerful human
antigen-specific CTL response with antitumor activity in vitro. J
Immunol. 175:820–828. 2005.
|
|
19
|
Sato Y, Fujiwara T, Mine T, Shomura H,
Homma S, Maeda Y, Tokunaga N, Ikeda Y, Ishihara Y, Yamada A, Tanaka
N, Itoh K, Harada M and Todo S: Immunological evaluation of
personalized peptide vaccination in combination with a
5-fluorouracil derivative (TS-1) for advanced gastric or colorectal
carcinoma patients. Cancer Sci. 98:1113–1119. 2007. View Article : Google Scholar
|
|
20
|
Correale P, Del Vecchio MT, Di Genova G,
Savellini GG, La Placa M, Terrosi C, Vestri M, Urso R, Lemonnier F,
Aquino A, Bonmassar E, Giorgi G, Francini G and Cusi MG:
5-Fluorouracil-based chemotherapy enhances the antitumor activity
of a thymidylate synthase-directed polyepitopic peptide vaccine. J
Natl Cancer Inst. 97:1437–1445. 2005. View Article : Google Scholar
|
|
21
|
Weihrauch MR, Ansén S, Jurkiewicz E,
Geisen C, Xia Z, Anderson KS, Gracien E, Schmidt M, Wittig B, Diehl
V, Wolf J, Bohlen H and Nadler LM: Phase I/II combined
chemoimmunotherapy with carcinoembryonic antigen-derived
HLA-A2-restricted CAP-1 peptide and irinotecan, 5-fluorouracil, and
leucovorin in patients with primary metastatic colorectal cancer.
Clin Cancer Res. 11:5993–6001. 2005. View Article : Google Scholar
|
|
22
|
Harrop R, Drury N, Shingler W, Chikoti P,
Redchenko I, Carroll MW, Kingsman SM, Naylor S, Melcher A, Nicholls
J, Wassan H, Habib N and Anthoney A: Vaccination of colorectal
cancer patients with modified vaccinia Ankara encoding the tumor
antigen 5T4 (TroVax) given alongside chemotherapy induces potent
immune responses. Clin Cancer Res. 13:4487–4494. 2007. View Article : Google Scholar
|
|
23
|
Yuan L, Kuramitsu Y, Li Y, Kobayashi M and
Hosokawa M: Restoration of interleukin-2 production in
tumor-bearing rats through reducing tumor-derived transforming
growth factor beta by treatment with bleomycin. Cancer Immunol
Immunother. 41:355–362. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Backus HH, Dukers DF, van Groeningen CJ,
Vos W, Bloemena E, Wouters D, van Riel JM, Smid K, Giaccone G,
Pinedo HM and Peters GJ: 5-Fluorouracil induced Fas upregulation is
associated with apoptosis in liver metastases of colorectal cancer
patients. Ann Oncol. 12:209–216. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sato Y, Shomura H, Maeda Y, Mine T, Une Y,
Akasaka Y, Kondo M, Takahashi S, Shinohara T, Katagiri K, Sato M,
Okada S, Matsui K, Yamada A, Yamana H, Itoh K and Todo S:
Immunological evaluation of peptide vaccination for patients with
gastric cancer based on pre-existing cellular response to peptide.
Cancer Sci. 94:802–808. 2003. View Article : Google Scholar : PubMed/NCBI
|