Introduction
Although there has been a sharp decline in both the
worldwide incidence and mortality due to gastric cancer during the
second half of the 20th century, gastric cancer continues to be a
major health issue (1). To date,
surgery is the most common treatment method, whereas chemotherapy
to treat gastric cancer has a limited success rate as this cancer
is not particularly sensitive to chemotherapeutic drugs. Thus,
early detection is the key to successful treatment to ensure the
long-term survival of patients since it is impossible to completely
surgically resect gastric cancer in the advanced stage. The risk
factors of gastric cancer include Helicobacter pylori
infection, consumption of smoked foods, salted fish and meat and
pickled vegetables, as well as obesity, tobacco smoke, chronic
gastritis and blood type A. These risk factors alter the expression
and function of critical cell growth-related genes. Therefore, it
is important to develop novel strategies for the prevention,
treatment, and prediction of prognosis of gastric cancer. In
addition, understanding the molecular mechanisms responsible for
gastric cancer development and progression will help to identify
useful biomarkers to predict disease progression or provide a means
to prevent or delay this disease from occurring.
To this end, the family of S100 proteins is involved
in the regulation of various cellular processes, such as cell cycle
progression and cell differentiation. S100 proteins are localized
in the cytoplasm and/or nucleus of a wide range of cells, and they
include ~25 members clustering on human chromosome 1q21, whose
encoding proteins contain two EF-hand calcium-binding motifs. Each
member contains two EF-hands connected by a central hinge, which
are S100 family-specific in the N-terminus and canonical in the
C-terminus. Among these proteins, 11-kDa acidic S100A2 is directly
involved in protein phosphorylation, cell cycle regulation, growth,
motility, differentiation, survival and chemoattraction (2). The S100A2 promoter has been shown to
be transcriptionally activated by wild-type p53, but not by mutated
p53, suggesting that S100A2 is a p53 target gene (3,4).
Previous studies have demonstrated the loss of S100A2 expression in
breast cancer and several other types of cancer (2). Indeed, ectopic overexpression of
S100A2 suppressed oral squamous cancer cells to grow, migrate,
invade in Matrigel, and form colonies in soft agar or grow tumors
in nude mice (5). In contrast,
knockdown of S100A2 expression restrained head and neck cancer cell
migration (6). However, other
studies have shown the opposite effect; for example, S100A2
overexpression has been detected in pancreatic carcinoma (7), non-small cell lung carcinomas
(8) and thyroid carcinoma (9). Therefore, the aim of this study was to
further clarify the association between S100A2 expression and
clinicopathological data. We determined the expression of S100A2
mRNA and protein in a large number of gastric cancer and
precancerous lesions and then associated its expression with
clinical significance in gastric cancer.
Materials and methods
Study population and pathological
diagnosis
In the present study, we collected gastric cancer
tissues from gastrectomy, gastric biopsies from gastritis, and
gastric intestinal metaplasia (IM) and adenoma from endoscopy from
patients at Department of Surgery, Shengjing Hospital of China
Medical University between January 1995 and January 2005. None of
the patients underwent chemotherapy, radiotherapy, or adjuvant
treatment before surgery. The tissue specimens were routinely fixed
in 10% neutral formalin, embedded in paraffin, and cut into 4-μm
section. These sections were then stained with hematoxylin and
eosin (H&E) to carry out the pathological diagnosis. In
addition, part of the tissue samples was snap-frozen in liquid
nitrogen and then stored at −80°C for later protein extraction and
RNA isolation. The tumor-node-metastasis (TNM) stage for each
gastric cancer specimen was evaluated according to the Union
Internationale Contre le Cancer (UICC) system for the extent of
tumor spread (10). Histological
architecture of gastric cancer was expressed in terms of Lauren's
classification (11,12). In this study, we also included
additional clinicopathological data, such as tumor size, depth of
invasion, and lymphatic and venous invasion. In addition, lymphatic
and venous invasion of gastric cancer cells was diagnosed using
H&E staining and D2-40 immunostaining and EvG staining,
respectively. The Ethics Committee of China Medical University
approved our research protocol, and each patient or their guardian
provided a consent form for participation in this study. The
patients were followed up through their medical records and
telephone conversations.
Quantitative real-time reverse
transcriptase-polymerase chain reaction (qRT-PCR)
Total RNA was extracted from tissue specimens using
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer's instructions. These RNA samples were reversely
transcribed into cDNA using RevertAid™ reverse transcriptase
(Takara, Dalian, China). Real-time PCR was then performed using
these cDNA samples in an ABI PRISM 7500 Sequence Detection system
(Applied Biosystems, Foster City, CA, USA), and the qPCR conditions
were 50°C for 2 min and 95°C for 10 min, followed by 50 cycles of
95°C for 15 sec, and 60°C for 1 min. The primers for GAPDH (135 bp,
201–335, NM_002046.3) were 5′-CAATGACCCCTTCATTGACC-3′ (sense) and
5′-TG GAAGATGGTGATGGGATT-3′ (antisense). The primers for S100A2
(140 bp, 440–579, NM_005978.3) were 5′-GAAGGA ACTTCTGCACAAGG-3′
(sense) and 5′-GTGCCAGGAAA ACAGCATAC-3′ (antisense). GAPDH mRNA was
used as an internal control for loading and handling of samples.
Each assay was performed in triplicate, the average was then
calculated, and the level of S100A2 mRNA was expressed as
2−ΔΔCt, where ΔCt = Ct (S100A2) - Ct (GAPDH) and ΔΔCt =
ΔCt (carcinoma) - ΔCt (adjacent non-neoplastic mucosa).
Protein extraction and western blot
analysis
Protein was extracted from tissue samples using a
homogenizer in RIPA lysis buffer. These protein samples were
concentrated using the BAC method (Bio-Rad Laboratories, Hercules,
CA, USA). After denaturation, the protein samples were separated by
electrophoresis on an SDS-polyacrylamide gel (15% acrylamide) and
then transferred onto Hybond membranes (Amersham, Freiburg,
Germany). The membranes were then incubated overnight at 4°C in 5%
skim milk in TBS-T (10 mM Tris-HCl, 150 mM NaCl and 0.1% Tween 20).
For immunoblotting, the membrane was then incubated for 1 h with a
rabbit antibody against S100A2 (Abcam, Cambridge, UK; 1:500). After
rinsing with TBS-T, the membrane was further incubated with an
anti-rabbit or anti-mouse IgG conjugated to horseradish peroxidase
(Dako, Carpinteria, CA, USA) at a dilution of 1:1,000 for 1 h.
Then, positive protein bands were visualized by incubating the
membrane with ECL-Plus detection reagents (Santa Cruz
Biotechnology, Santa Cruz, CA, USA) and exposed to X-ray film
(Fuji, Japan). Next, the membrane was washed with WB Stripping
Solution (pH 2.0–3.0; Nacalai, Tokyo, Japan) for 1 h, and then the
western blot procedures were repeated for detection of GAPDH
protein expression using an anti-GADPH antibody from Sigma (St.
Louis, MO, USA) at a dilution of 1:10,000 as an internal control
antibody. Densitometric quantification of S100A2 protein expression
in gastric samples was performed using Scion Image software (Scion
Corp., Frederick, MD, USA) and was compared to GADPH levels.
Tissue microarray (TMA) and
immunohistochemistry
Representative areas of gastric lesions were first
identified in H&E-stained sections, and a 2-mm diameter tissue
core per donor block was punched out and transferred into a
recipient block, with a maximum of 48 cores per block using a
manual arraying device (MTA-1; Beecher Instruments Inc., Sun
Prairie, WI, USA). After being re-embedded into the paraffin
blocks, 4-μm consecutive sections were incised from the recipient
blocks and mounted onto polylysine-coated glass slides. After
confirmation with H&E staining, these TMA sections were used
for immunohistochemistry experiments.
For the immunohistochemistry experiments, the
sections were first deparaffinized in xylene and rehydrated through
graded concentrations of alcohol. The sections were then subjected
to blockage of endogenous peroxidase activity in 1.5% hydrogen
peroxide/methanol at room temperature for 10 min and subjected to
antigen retrieval using a microwave oven (Oriental Rotor Ltd., Co.,
Tokyo, Japan) and retrieval solution (Target Retrieval Solution;
Dako, Carpinteria, CA, USA) for 15 min. Next, the sections were
incubated with a rabbit polyclonal S100A2 antibody at a dilution of
1:200 for 1 h at room temperature. After washing three times with
TBS-T, the sections were further incubated with an anti-rabbit IgG
antibody conjugated to horseradish peroxidase (Dako) at a dilution
of 1:1,000 for 1 h. The final color was visualized by exposing the
sections to 0.5 mg/ml 3,3′-diaminobenzidine and 0.005% hydrogen
peroxide for ~5 min. After counterstaining with Mayer's
hematoxylin, the sections were dehydrated, cleared and mounted.
Negative control sections were incubated with TBS-T instead of the
primary antibody.
The stained TMA sections were subsequently reviewed
and scored by two experienced pathologists without any knowledge of
the clinicopathological data. For each tissue core, the number of
cells positively staining for S100A2 was counted in five fields at
a magnification of ×200. The percentage of positively stained cells
was calculated and scored as follows: 0–5%, negative (−); 6–25%,
weakly positive (+); 26–50%, moderately positive (++); and >50%,
strongly positive (+++). Finally, these two scores were combined to
indicate the semi-quantitative expression of S100A2 protein, i.e.,
−, +, ++ and +++.
Statistical analysis
Spearman's correlation test was performed to analyze
the rank data, and the Student's t-test was used to compare the
means of different groups. Kaplan-Meier survival plots were
generated, and comparisons between survival curves were tested
using log-rank analysis. Cox's proportional hazards model was
employed for multivariate analysis. SPSS 10.0 software (SPSS Inc.,
Chicago, IL, USA) was used to generate all statistical data, and
P<0.05 was considered to indicate a statistically significant
result.
Results
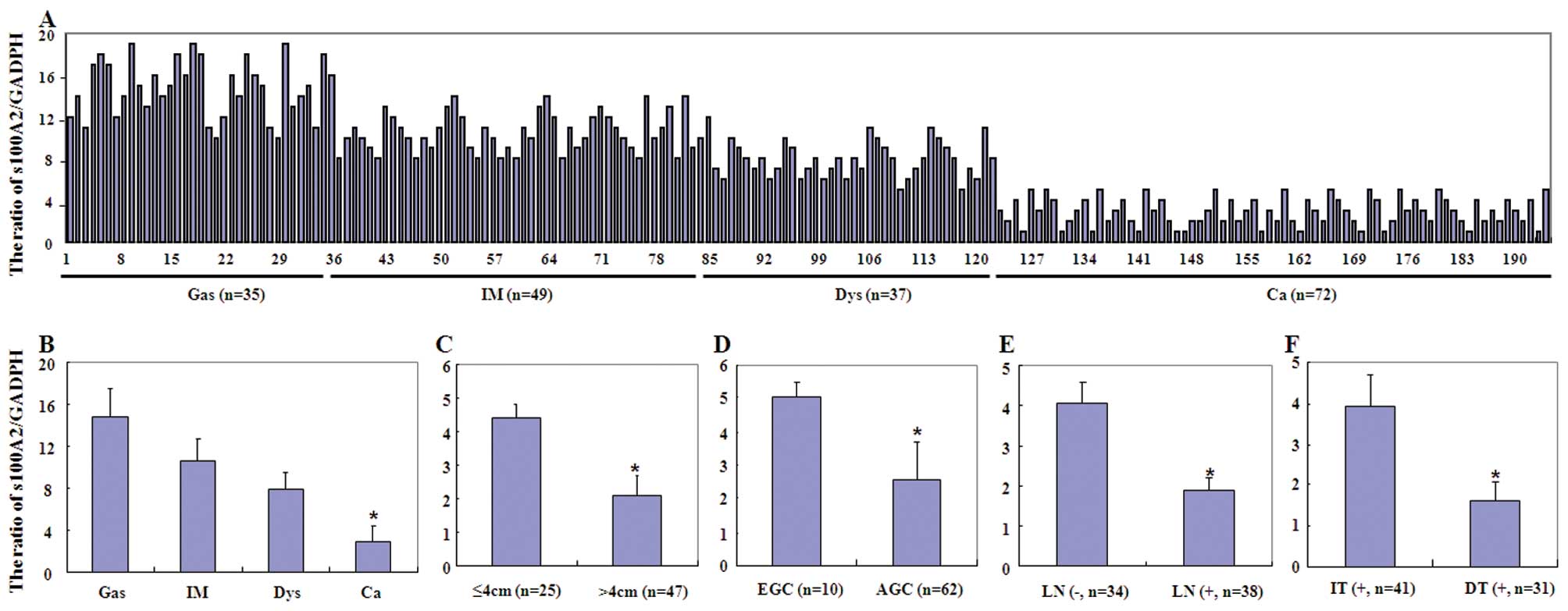
In the present study, we first assessed S100A2 mRNA
levels using qRT-PCR in a subset of gastric tissue specimens.
Reduced expression of S100A2 mRNA was observed from gastritis,
intestinal metaplasia and dysplasia to cancer tissue samples
(P<0.001, Fig. 1). Loss of
S100A2 mRNA expression occurred more frequently in gastric cancer
with a larger tumor size, deeper invasive depth and lymph node
metastasis and in intestinal-type carcinoma (P<0.05). Similar
data were also observed for S100A2 protein expression in these
samples analyzed by western blot analysis (Fig. 2).
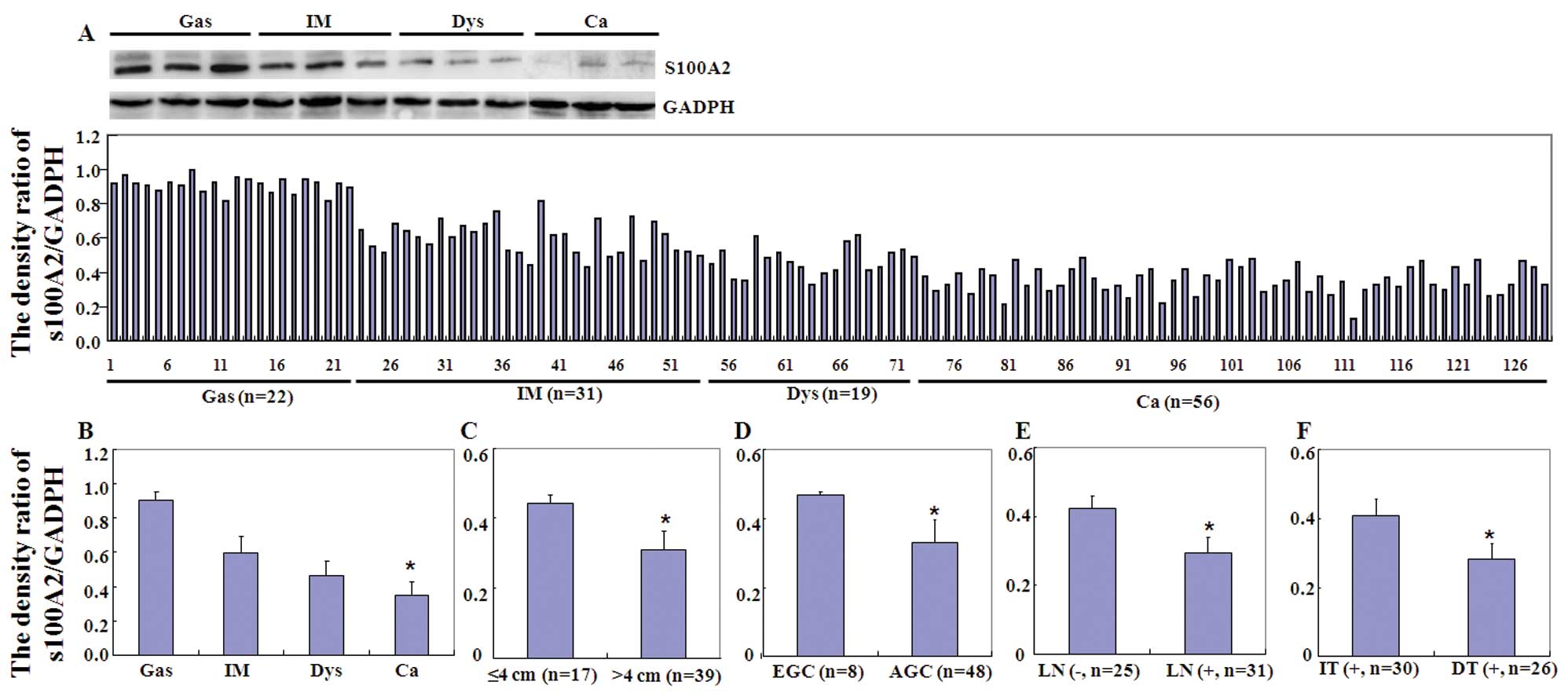 | Figure 2Expression of S100A2 protein in
different gastric tissue specimens to determine its association
with clinicopathological features of gastric cancer. (A) Western
blot analysis. Tissue lysate was loaded and probed with the
anti-S100A2 antibody (upper panel, 29 kDa) or GAPDH antibody (lower
panel, 37 kDa). Densitometric analysis was performed, and the data
indicated that S100A2 protein was detectable in gastritis (Gas),
intestinal metaplasia (IM), dysplasia (Dys) and carcinoma samples.
(B) The S100A2 expression level gradually decreased from gastritis
(Gas), intestinal metaplasia (IM), and dysplasia (Dys) to carcinoma
(Ca) samples (P<0.001). Lower S100A2 protein expression was
associated with (C) a larger tumor size, (D) deeper invasive depth,
(E) frequent lymph node metastasis, and (F) non-intestinal type of
cancer. EGC, early gastric cancer; AGC, advanced gastric cancer;
LN, lymph node metastasis; IT, intestinal type; DT, diffuse
type. |
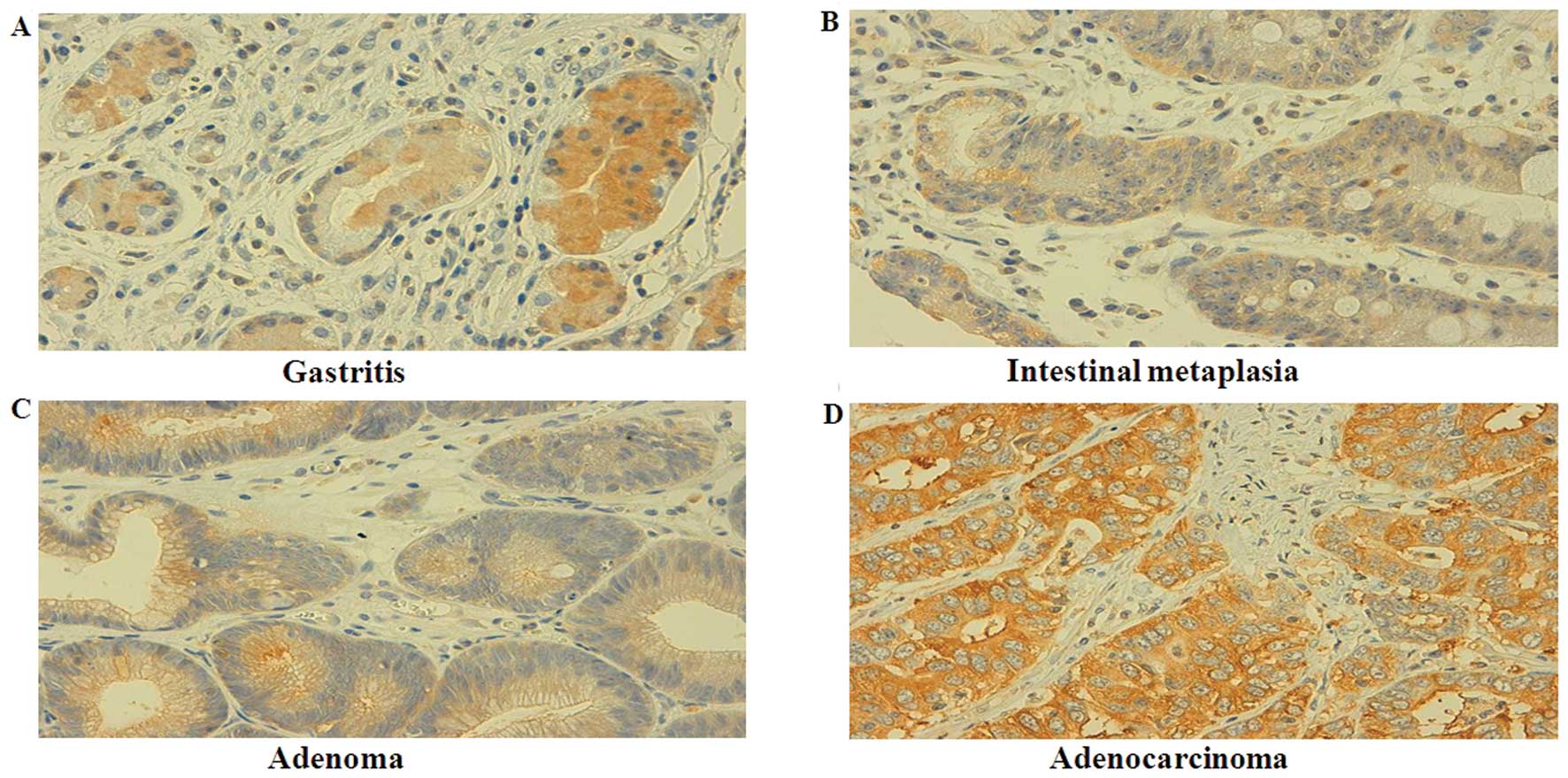
We immunostained the tissue samples for S100A2
expression and found that S100A2 protein was positively expressed
in the cytoplasm of the gastric epithelial cells and in the
intestinal metaplasia, adenomatous dysplasia, and carcinoma tissues
(Fig. 3). Specifically, S100A2
protein was expressed in all 73 cases of gastritis, 90.7% (78/86)
of intestinal metaplasia, 73.0% (46/63) of adenomatous dysplasia,
and 48.9% (170/348) of gastric cancer tissues. There was a
statistically significant difference in the level of the S100A2
protein between gastritis, intestinal metaplasia, and dysplasia
samples and cancer tissues (P<0.001, Table I). In gastric cancer, the expression
of S100A2 protein was frequently absent in younger patients
compared to that of older patients (P<0.05), and S100A2
expression was inversely associated with tumor size, depth of
invasion, lymphatic and venous invasion, lymph node metastasis and
TNM stage (P<0.05). S100A2 protein was also more highly
expressed in the intestinal type of gastric cancer than in the
diffuse type (P<0.05). However, S100A2 expression was not
associated with patient gender (P>0.05; Table II).
 | Table IDifferential expression of S100A2
protein in gastric tissue specimens. |
Table I
Differential expression of S100A2
protein in gastric tissue specimens.
| | S100A2 protein
expression level | | |
|---|
| |
| | |
|---|
| Group | n | − | + | ++ | +++ | PR (%) | P-value |
|---|
| Gastritis | 73 | 0 | 16 | 21 | 36 | 100.0 | <0.001 |
| Intestinal
metaplasia | 86 | 8 | 8 | 16 | 54 | 90.7 | |
| Adenoma | 63 | 17 | 5 | 15 | 26 | 73.0 | |
| Gastric cancer | 348 | 178 | 92 | 66 | 12 | 48.9 | |
 | Table IIAssociation of S100A2 protein
expression with the clinicopathological features of the gastric
cancer patients. |
Table II
Association of S100A2 protein
expression with the clinicopathological features of the gastric
cancer patients.
| | S100A2 protein
expression level | | |
|---|
| |
| | |
|---|
| Clinicopathological
features | n | − | + | ++ | +++ | PR (%) | P-value |
|---|
| Age (years) | | | | | | | 0.013 |
| <55 | 139 | 81 | 36 | 18 | 4 | 41.7 | |
| ≥55 | 209 | 97 | 56 | 48 | 8 | 53.6 | |
| Gender | | | | | | | 0.697 |
| Male | 248 | 127 | 67 | 50 | 4 | 48.8 | |
| Female | 100 | 51 | 25 | 16 | 8 | 49.0 | |
| Tumor size (cm) | | | | | | | 0.005 |
| ≤4 | 120 | 48 | 42 | 20 | 10 | 60.0 | |
| >4 | 228 | 130 | 50 | 46 | 2 | 43.0 | |
| Depth of
invasion | | | | | | | 0.032 |
|
Tis-T1 | 33 | 13 | 8 | 6 | 6 | 60.5 | |
|
T2–T4 | 315 | 165 | 84 | 60 | 6 | 47.6 | |
| Lymphatic
invasion | | | | | | | <0.001 |
| − | 192 | 70 | 68 | 47 | 7 | 63.5 | |
| + | 156 | 108 | 24 | 19 | 5 | 30.8 | |
| Venous
invasion | | | | | | | <0.001 |
| − | 245 | 105 | 79 | 56 | 5 | 57.1 | |
| + | 103 | 73 | 13 | 10 | 7 | 29.1 | |
| Lymph node
metastasis | | | | | | | <0.001 |
| − | 138 | 14 | 64 | 52 | 8 | 89.9 | |
| + | 210 | 164 | 28 | 14 | 4 | 21.9 | |
| TNM stage | | | | | | | <0.001 |
| I | 6 | 1 | 3 | 2 | 0 | 83.3 | |
| II | 60 | 15 | 25 | 16 | 4 | 75.0 | |
| III | 104 | 46 | 30 | 24 | 4 | 55.8 | |
| IV | 178 | 116 | 34 | 24 | 4 | 34.8 | |
| Lauren's
classification | | | | | | | <0.001 |
|
Intestinal-type | 203 | 62 | 81 | 51 | 9 | 69.0 | |
| Diffuse-type | 145 | 116 | 92 | 66 | 12 | 20.0 | |
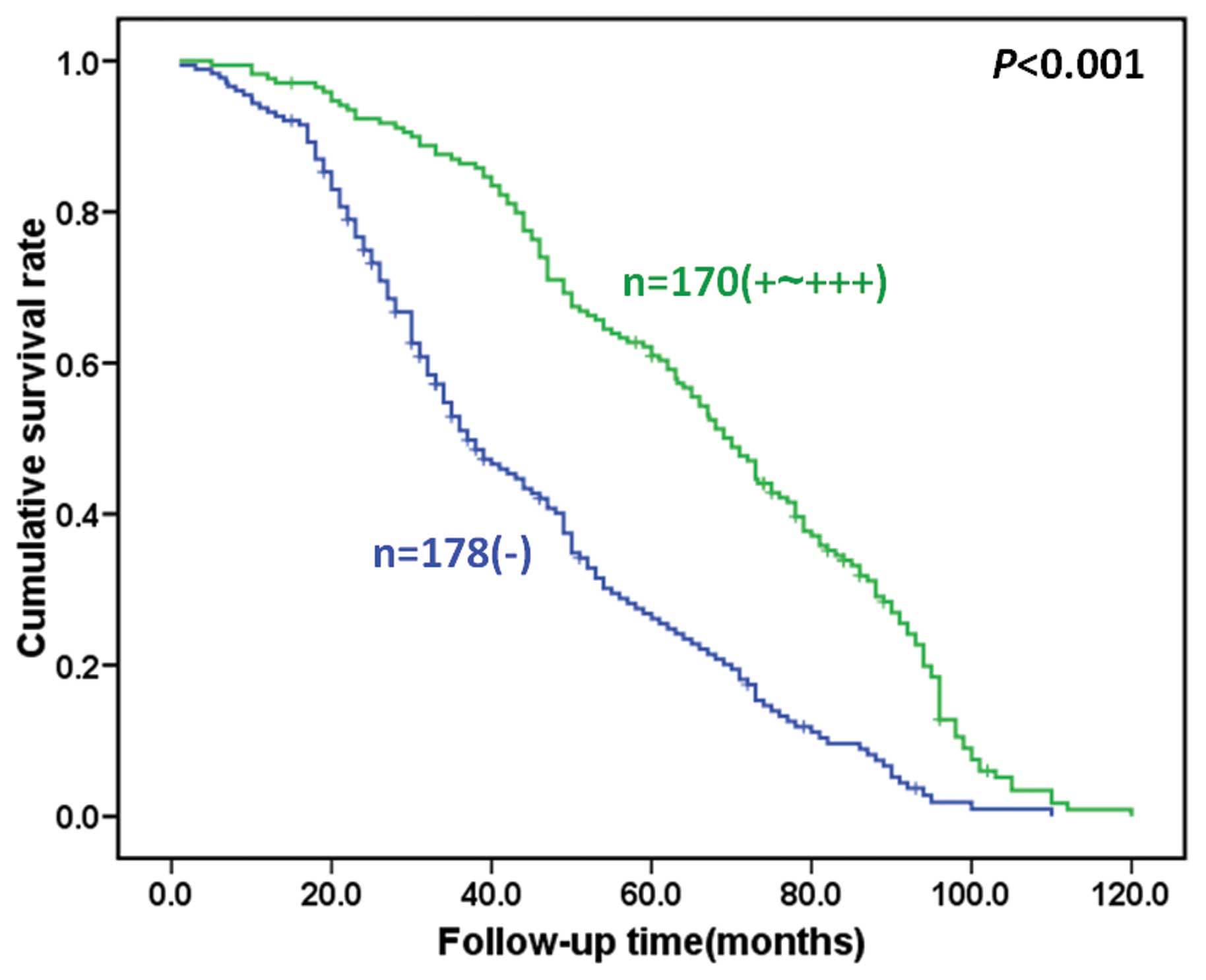
Next, we assessed the association of S100A2 protein
expression with the survival of the gastric cancer patients.
Survival data were available for all 348 gastric cancer patients
with a follow-up period ranging from 1 month to 10.1 years (median,
65.1 months). Fig. 4 indicates that
loss of S100A2 expression contributed to poorer survival of the
gastric cancer patients (P<0.05). Kaplan-Meier analysis
demonstrated that the cumulative survival rate of patients with
weak and moderate S100A2 expression was obviously higher than those
with loss of S100A2 expression. Moreover, multivariate analyses
revealed that S100A2 expression, depth of invasion, lymphatic
invasion, venous invasion, lymph node metastasis, distant
metastasis, and TNM stage were independent predictors for gastric
cancer patient survival.
Discussion
In the present study, we analyzed the expression of
S100A2 mRNA and protein in gastritis, intestinal metaplasia,
adenomatous dysplasia, and gastric cancer tissue specimens to
determine whether the expression levels were associated with
clinical features. We found that S100A2 protein was observed in the
cytoplasm of gastric tissue specimens, which confirmed previous
data (13), whereas we did not find
nuclear localization of S100A2 protein, which was previously
observed in oral and esophageal mucosa (5,14). Our
data demonstrated that expression of S100A2 mRNA and protein
gradually decreased from gastritis, intestinal metaplasia and
adenomatous dysplasia to gastric cancer.
A previous study showed that S100A2 was expressed in
tissue specimens of patients with benign prostate hyperplasia,
while it was reduced in prostate cancer (15). Another study reported that
expression of S100A2 mRNA was positive in 77.5% of esophageal
squamous cell carcinoma tissues, which was lower than that in
normal mucosa (100%) detected by in situ hybridization
(16). However, normal esophageal
mucosa expressed S100A2 in the cell nuclei, whereas two-thirds of
Barrett's dysplasia and adenocarcinoma samples with S100A2
expression had stronger cytosolic staining of S100A2 protein
(14). An additional study
demonstrated that 2 out of 8 (25%) esophageal cancer cell lines and
14 out of 30 (47%) primary esophageal squamous cell carcinomas
exhibited S100A2 expression compared to paired normal tissues
(17), suggesting that S100A2 maybe
related to the progression of esophageal squamous cell carcinoma.
In the present study, we found gradually reduced expression of
S100A2 from gastritis, intestinal metaplasia and dysplasia to
carcinoma. We believe that our tissues covered a series of lesions
throughout gastric cancer development, and a gradual loss of S100A2
expression in these specimens indicates that S100A2 is associated
with carcinogenesis. Indeed, a previous study revealed that
intestinal metaplasia is an adaptive condition for an injured
gastric epithelium, and inflammation can develop into globoid
dysplasia and then to gastric signet ring cell carcinoma, as shown
by morphological appearance and biological characteristics
(18). Moreover, pathological and
genetic observations of intestinal dysplasia have demonstrated that
gastric dysplasia is a premalignant lesion that has a high
probability of undergoing malignant transformation (19). Thus, our data, for the first time,
demonstrated that loss of S100A2 expression may contribute to
gastric carcinogenesis. Other studies have shown that loss of
S100A2 expression is only due to promoter hypermethylation
(20–22).
Furthermore, the present data revealed that
expression of S100A2 mRNA and protein was inversely linked to tumor
size, depth of tumor invasion, lymphatic and venous invasion, lymph
node metastasis, and TNM stage; these findings are supported by
other studies in various types of cancer (23–25).
These findings suggest that S100A2 plays a role in the suppression
of growth, invasion and metastasis of gastric cancer. However, the
function of S100A2 may be tissue-specific. For example, in
esophageal cancer, the level of S100A2 mRNA expression has been
shown to be closely associated with de-differentiation and lymph
node metastasis (16), while
positive S100A2 expression has been significantly associated with
lymphatic invasion in lung cancer (26). In contrast, Nagy et
al(6) showed that S100A2 has a
clear inhibitory effect on cell motility by antisense
oligonucleotides and extracellular treatments. Furthermore, Tsai
et al reported that ectopic expression of S100A2 in the
human malignant squamous cell carcinoma cell line KB resulted in
significant inhibition of proliferation, migration and invasion.
Moreover, S100A2 significantly reduced the number of colonies
formed in semi-solid agar and decreased tumor growth and burden in
nude mice with oral squamous cancer (5). Taken altogether, we speculate that
downregulation of S100A2 expression is involved in the development
and progression of gastric cancer.
Although gastric cancer originates from the same
gastric epithelium, its morphological features vary substantially
for individual patients. According to Lauren's classification,
intestinal-type gastric cancer is characterized by cohesive
carcinoma cells forming gland-like tubular structures with an
expanding or infiltrative growth pattern, which includes well and
moderately differentiated adenocarcinoma (11,12).
In contrast, diffuse-type gastric cancer displays less apparent
adenocarcinoma or lacks cell adhesion and contains poorly
differentiated and signet ring cell carcinoma (11,12).
The present data demonstrated that the expression of S100A2 mRNA
and protein was higher in intestinal-type gastric cancer than in
diffuse-type, suggesting that S100A2 may contribute to the
development of diffuse-type gastric cancer but does not play a role
in intestinal-type. A previous study showed that S100A2 is more
highly expressed in well and moderately differentiated cancer than
in poorly differentiated gastric cancer (13), which supports the present data.
To date, there have been no reports describing the
prognostic significance of S100A2 expression in gastric cancer. Our
present study showed that S100A2 expression was associated with a
more favorable prognosis of gastric cancer patients. Multivariate
analysis using Cox's proportional risk analysis indicated that
depth of invasion, lymphatic or venous invasion, lymph node
metastasis, distal metastasis, TNM stage, and S100A2 expression
were independent factors for the prognosis of carcinoma patients.
These findings suggest that S100A2 is an independent prognostic
factor for gastric cancer. Others have reported that reduced or
increased expression of S100A2 is an independent predictive factor,
depending on the type of human cancer (27–30).
Nevertheless, the data from our present study using a large number
of tissue samples, demonstrated the clinical significance of S100A2
expression. Further studies will investigate how the S100A2 gene
contributes to suppression of gastric cancer development and
progression.
Acknowledgements
This study was supported, in part, by a grant from
the Natural Science Foundation of Liaoning Province
(#201202279).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer Clin. 61:69–90.
2011. View Article : Google Scholar
|
|
2
|
Wolf S, Haase-Kohn C and Pietzsch J:
S100A2 in carcinogenesis: a friend or a foe? Amino Acids.
41:849–861. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mueller A, Schäfer BW, Ferrari S, Weibel
M, Makek M, Höchli M and Heizmann CW: The calcium-binding protein
S100A2 interacts with p53 and modulates its transcriptional
activity. J Biol Chem. 280:29186–29193. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lapi E, Iovino A, Fontemaggi G, Soliera
AR, Iacovelli S, Sacchi A, Rechavi G, Givol D, Blandino G and
Strano S: S100A2 gene is a direct transcriptional target of p53
homologues during keratinocyte differentiation. Oncogene.
25:3628–3637. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsai ST, Jin YT, Tsai WC, Wang ST, Lin YC,
Chang MT and Wu LW: S100A2, a potential marker for early recurrence
in early-stage oral cancer. Oral Oncol. 41:349–357. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nagy N, Brenner C, Markadieu N, Chaboteaux
C, Camby I, Schäfer BW, Pochet R, Heizmann CW, Salmon I, Kiss R and
Decaestecker C: S100A2, a putative tumor suppressor gene, regulates
in vitro squamous cell carcinoma migration. Lab Invest. 81:599–612.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohuchida K, Mizumoto K, Miyasaka Y, Yu J,
Cui L, Yamaguchi H, Toma H, Takahata S, Sato N, Nagai E, Yamaguchi
K, Tsuneyoshi M and Tanaka M: Over-expression of S100A2 in
pancreatic cancer correlates with progression and poor prognosis. J
Pathol. 213:275–282. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartling B, Rehbein G, Schmitt WD, Hofmann
HS, Silber RE and Simm A: S100A2-S100P expression profile and
diagnosis of non-small cell lung carcinoma: impairment by advanced
tumour stages and neoadjuvant chemotherapy. Eur J Cancer.
43:1935–1943. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ito Y, Yoshida H, Tomoda C, Uruno T, Miya
A, Kobayashi K, Matsuzuka F, Kakudo K, Kuma K and Miyauchi A:
Expression of S100A2 and S100A6 in thyroid carcinomas.
Histopathology. 46:569–575. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sobin LH and Wittekind CH: TNM
Classification of Malignant Tumors. 6th edition. Hoboken NJ: John
Wiley & Sons; NJ: 2002
|
|
11
|
Zheng HC, Li XH, Hara T, Masuda S, Yang
XH, Guan YF and Takano Y: Mixed-type gastric carcinomas exhibit
more aggressive features and indicate the histogenesis of
carcinomas. Virchows Arch. 452:525–534. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng H, Takahashi H, Murai Y, Cui Z,
Nomoto K, Miwa S, Tsuneyama K and Takano Y: Pathobiological
characteristics of intestinal and diffuse-type gastric carcinoma in
Japan: an immunostaining study on the tissue microarray. J Clin
Pathol. 60:273–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo J, Zhu Y, Yang G, Gong L, Wang B and
Liu H: Loss of Reprimo and S100A2 expression in human gastric
adenocarcinoma. Diagn Cytopathol. 39:752–757. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee OJ, Hong SM, Razvi MH, Peng D, Powell
SM, Smoklin M, Moskaluk CA and El-Rifai W: Expression of
calcium-binding proteins S100A2 and S100A4 in Barrett's
adenocarcinomas. Neoplasia. 8:843–850. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kwon YW, Chang IH, Kim KD, Kim YS, Myung
SC, Kim MK and Kim TH: Significance of S100A2 and S100A4 expression
in the progression of prostate adenocarcinoma. Korean J Urol.
51:456–462. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cao LY, Yin Y, Li H, Jiang Y and Zhang HF:
Expression and clinical significance of S100A2 and p63 in
esophageal carcinoma. World J Gastroenterol. 15:4183–4188. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Imazawa M, Hibi K, Fujitake S, Kodera Y,
Ito K, Akiyama S and Nakao A: S100A2 overexpression is frequently
observed in esophageal squamous cell carcinoma. Anticancer Res.
25:1247–1250. 2005.PubMed/NCBI
|
|
18
|
Zheng HC, Xu XY, Yu M, Takahashi H, Masuda
S and Takano Y: The role of Reg IV gene and its encoding product in
gastric carcinogenesis. Hum Pathol. 41:59–69. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang YC: Geographic pathology of gastric
dysplasia in China. Semin Surg Oncol. 10:100–106. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wicki R, Franz C, Scholl FA, Heizmann CW
and Schäfer BW: Repression of the candidate tumor suppressor gene
S100A2 in breast cancer is mediated by site-specific
hypermethylation. Cell Calcium. 22:243–254. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rehman I, Cross SS, Catto JW, Leiblich A,
Mukherjee A, Azzouzi AR, Leung HY and Hamdy FC: Promoter
hyper-methylation of calcium binding proteins S100A6 and S100A2 in
human prostate cancer. Prostate. 65:322–330. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng G, Xu X, Youssef EM and Lotan R:
Diminished expression of S100A2, a putative tumor suppressor, at
early stage of human lung carcinogenesis. Cancer Res. 61:7999–8004.
2001.PubMed/NCBI
|
|
23
|
Zhang X, Hunt JL, Shin DM and Chen ZG:
Down-regulation of S100A2 in lymph node metastases of head and neck
cancer. Head Neck. 29:236–243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suzuki F, Oridate N, Homma A, Nakamaru Y,
Nagahashi T, Yagi K, Yamaguchi S, Furuta Y and Fukuda S: S100A2
expression as a predictive marker for late cervical metastasis in
stage I and II invasive squamous cell carcinoma of the oral cavity.
Oncol Rep. 14:1493–1498. 2005.PubMed/NCBI
|
|
25
|
Matsumoto K, Irie A, Satoh T, Ishii J,
Iwabuchi K, Iwamura M, Egawa S and Baba S: Expression of S100A2 and
S100A4 predicts for disease progression and patient survival in
bladder cancer. Urology. 70:602–607. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matsubara D, Niki T, Ishikawa S, Goto A,
Ohara E, Yokomizo T, Heizmann CW, Aburatani H, Moriyama S, Moriyama
H, Nishimura Y, Funata N and Fukayama M: Differential expression of
S100A2 and S100A4 in lung adenocarcinomas: clinicopathological
significance, relationship to p53 and identification of their
target genes. Cancer Sci. 96:844–857. 2005. View Article : Google Scholar
|
|
27
|
Almadori G, Bussu F, Galli J, Rigante M,
Lauriola L, Michetti F, Maggiano N, Schafer BW, Heizmann CW,
Ranelletti FO and Paludetti G: Diminished expression of S100A2, a
putative tumour suppressor, is an independent predictive factor of
neck node relapse in laryngeal squamous cell carcinoma. J
Otolaryngol Head Neck Surg. 38:16–22. 2009.PubMed/NCBI
|
|
28
|
Lauriola L, Michetti F, Maggiano N, Galli
J, Cadoni G, Schäfer BW, Heizmann CW and Ranelletti FO: Prognostic
significance of the Ca(2+) binding protein S100A2 in laryngeal
squamous-cell carcinoma. Int J Cancer. 89:345–349. 2000.
|
|
29
|
Wang H, Zhang Z, Li R, Ang KK, Zhang H,
Caraway NP, Katz RL and Jiang F: Overexpression of S100A2 protein
as a prognostic marker for patients with stage I non-small cell
lung cancer. Int J Cancer. 116:285–290. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kyriazanos ID, Tachibana M, Dhar DK,
Shibakita M, Ono T, Kohno H and Nagasue N: Expression and
prognostic significance of S100A2 protein in squamous cell
carcinoma of the esophagus. Oncol Rep. 9:503–510. 2002.PubMed/NCBI
|